1. Introduction
Practical questions concerning the sequence, timing, initial doses, and subsequent titration strategies of foundational drugs constituting comprehensive disease-modifying therapy (CDMT) in patients with heart failure and reduced ejection fraction (HFrEF) remain unclear. Such therapy is also known as “quadruple” or “four pillars” therapy, and it consists of an angiotensin-converting enzyme inhibitor (ACE-I) or angiotensin receptor-neprilysin inhibitor (ARNi), beta-blocker, mineralocorticoid receptor antagonist (MRA), and sodium-glucose co-transporter 2 (SGLT2) inhibitor. This pharmacologic armamentarium nowadays should represent a standard of care for HFrEF syndrome as these agents significantly curbed mortality and HF-related hospitalizations in randomized clinical trials. Similar to patients with acute myocardial infarction (AMI), patients with decompensated chronic or de novo HFrEF are at increased risk of sudden cardiac death (SCD) and readmission in the early post-discharge period – also known as the “vulnerable phase” [Citation1]. For this reason, avoidance of unnecessary delays and rapid initiation of life-saving therapies are of paramount importance to mitigate syndrome progression and improve prognosis. In fact, according to the conceptual framework provided by Abdin and colleagues “time-to-treatment initiation” should be acknowledged as an important modifiable risk factor in HFrEF, and once this timeframe is narrowed, optimization of initiated treatments (meaning up-titration to target doses reached in pivotal trials or maximally tolerated doses) is crucial to gain most of clinical benefits [Citation1]. Therefore, each HF hospitalization episode should be perceived as a teachable moment and opportunity to initiate, optimize, and/or switch life-saving therapies to improve post-discharge outcomes of patients with HFrEF [Citation2].
2. An argument for comprehensive vs. conventional disease-modifying therapy in HFrEF
There is solid evidence derived from real-world data and randomized studies reinforcing the concept that initiation and maintenance/optimization of CDMT provides substantial mortality and morbidity benefits in the HFrEF population. Equally so, accumulating data shows that early in-hospital initiation of these drugs can be a feasible and safe management strategy that should be employed on an individual patient basis and strongly considered at least before patient discharge from the hospital [Citation2]. Of note, a seminal analysis by Vaduganathan et al. recently examined the impact of CDMT consisting of ARNI, beta-blocker, MRA, and SGLT2 inhibitor versus conventional standard of care consisting of ACE-I or angiotensin-receptor blocker (ARB) plus beta-blocker on event-free and overall survival among patients with HFrEF [Citation3]. The results clearly demonstrated the robust gain in survival as HFrEF patients treated with CDMT compared to those treated conventionally gained 2.7–8.3 additional years free from cardiovascular death or first HF-related hospitalization, while their overall survival was prolonged by 1.4–6.3 years.
3. An argument for persistent up-titration and optimization of CDMT in HFrEF
The aim to uptitrate and optimize HF therapies in a sequential fashion is a well-substantiated and proven concept established from before; however, data show that it is often not reached in contemporary clinical practice. For example, a US-based study showed that only 13% of discharged HFrEF patients were treated with triple guideline-directed medical therapy, while 41% of patients were prescribed two drugs, thus implicating that nearly 1 in 2 of patients with HFrEF were discharged with monotherapy or no therapy [Citation4]. Similarly, nearly half (46%) of those patients who received post-discharge CDMT did not have any dose escalation, while at the same time, the consistent trend of reduction in death or rehospitalizations was observed as the number of CDMT agents increased during the follow-up period. Data from the same registry also showed that most eligible HFrEF patients did not receive target doses of life-saving therapies at any point during follow-up, while less than 1% of all patients were simultaneously treated with a target dose of ACE-I/ARB/ARNI, beta-blocker, and MRA [Citation5]. Poor patterns of dose titration and high drug discontinuation rates were also confirmed in a large multinational observational analysis that examined data from health care databases in Sweden, the UK, and the US, thus showing that the traditional approach of the sequential and prolonged introduction of CDMT in HFrEF is largely inadequate and exposes patients to excess risk of death and clinical worsening [Citation6]. Taken together, coverage of all foundational therapies and persistent up-titration and optimization of CDMT post-discharge is a crucial step and urgently needed in HFrEF as it is strongly associated with improved clinical outcomes.
4. An argument for in-hospital or earliest possible initiation of CMDT in HFrEF
Expert initiatives proposing a faster and simpler strategy of CDMT introduction for HFrEF have emerged recently with blueprints for rapid sequence or simultaneous initiation of life-saving drugs with subsequent optimization. This concept is not yet largely embraced in clinical practice due to the interplay of complex factors such as clinical inertia and clinician’s fear of adverse events, heterogeneous, and ambiguous perceptions of hemodynamic stability, but also patient characteristics such as intolerance or allergies to certain drugs, increased risk of drug interactions, presence of renal dysfunction, hyperkalemia, symptomatic hypotension, and/or sociodemographic paramedical factors (e.g. reimbursement issues, insurance coverage for novel therapies, etc). Nevertheless, as responsible clinicians, we should strive to offer our HFrEF patients the best possible protection from future adverse events by covering them with foundational therapies, whenever and as earliest as possible. For example, Khan et al. proposed simultaneous initiation of all four foundational drugs in low doses for treating HFrEF with an algorithm that allows for dose up-titration and optimization to be reached in as fast as 6 weeks versus a conventional sequential approach that usually takes 28–56 weeks before the CDMT is fully optimized [Citation7]. Similarly, Packer and McMurray provided a rapid sequencing algorithm that allows for patients with HFrEF to receive quadruple foundational therapy within 4 weeks in three flexible steps followed by the dose optimization [Citation8]. A similar or combined approach of initiation, optimization, and reassessment of CDMT in patients with HFrEF has been distilled and is proposed in .
Figure 1. A proposed scheme depicting common barriers to in-hospital initiation of comprehensive disease-modifying pharmacotherapies for heart failure with reduced ejection fraction (HFrEF) and a pathway showing the possibility of in-hospital initiation of all four foundational classes of drugs in the lowest and/or most-tolerable doses for the individual patient. The proposed management plan consists of three steps: 1) in-hospital initiation, 2) post-discharge optimization, and 3) assessment of treatment (potential escalation/de-escalation, introduction of additional therapies if needed).
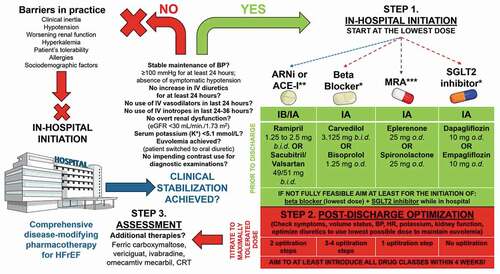
Available randomized data from PIONEER-HF and TRANSITION trials showed that initiation of sacubitril/valsartan following hemodynamic stabilization in HFrEF patients hospitalized for acute decompensation event was safe and feasible and could be started in-hospital or shortly after discharge [Citation9,Citation10]. Convincing results from the PIONEER-HF trial taught us that the initiation of treatment with sacubitril-valsartan at the median of 68 hours after initial presentation to hospital for acute decompensated HF was associated with a significantly greater reduction in circulating natriuretic peptides, compared to enalapril treatment [Citation9]. Such dynamics were also accompanied by a reduction in levels of high-sensitivity cardiac troponin T, which is an established biomarker of myocardial injury associated with poor prognosis in patients with HF. Likewise, rates of rehospitalizations for HF following 8 weeks post-discharge were lower in the sacubitril-valsartan group compared to the group randomized to enalapril with no difference in safety outcomes between examined groups. Similar findings were observed in the real-life non-selected HFrEF population in whom sacubitril/valsartan was initiated during hospitalization [Citation11]. The early initiation of SGLT2 inhibitor sotagliflozin before or within 3 days following discharge was tested in the population of patients with recent worsening of HF and diabetes mellitus (SOLOIST-WHF trial), thus showing that such use of SGLT2 inhibitor was safe and associated with significant mortality and morbidity benefits [Citation12]. This multicenter, double-blind randomized trial showed that administration of the first dose of sotagliflozin before hospital discharge or at the median of 2 days after discharge was associated with a 33% relative risk reduction in the rate of primary endpoint consisting of death from cardiovascular causes and hospitalizations or urgent visits for HF, compared to placebo treatment. It is important to highlight that beneficial sotagliflozin-mediated effects were observed early and were achieved irrespective of baseline ejection fraction and on top of high coverage with other established life-saving therapies for HF. Promising data also comes from the EMPA-RESPONSE-AHF trial conducted in the acute HF (AHF) setting, in which initiation of 10 mg empagliflozin on top of standard-of-care treatment was started within 24 hours of hospitalization [Citation13]. Of note, more than half of patients in this trial experienced an acute exacerbation of chronic HF. This trial demonstrated that early empagliflozin initiation was safe and associated with increased urinary output and reduced combined endpoint of worsening HF, rehospitalization for HF, or death at 60 days. Furthermore, a propensity score-matched analysis showed that in-hospital initiation of MRAs among heterogeneous patients with AHF event (of which ≈40% were those with chronic HF) was safe and associated with significantly reduced in-hospital mortality, while this effect was independent of known prognostic factors and co-administered intravenous and oral HF therapies [Citation14]. Similarly, a recent analysis from the Kyoto Congestive Heart Failure registry demonstrated that early heart rate (HR) modulation in hemodynamically stabilized patients with acute decompensated HF with beta-blockers was associated with significant in-hospital mortality reduction, while no safety concerns were raised [Citation15]. Finally, additional therapies that could further improve morbidity and mortality outcomes and increase quality of life, beyond foundational pharmacotherapy, should also be strongly considered for selected patients with HFrEF. These treatments include intravenous ferric carboxymaltose, vericiguat, omecamtiv mecarbil, ivabradine, and cardiac resynchronization therapy (CRT); however, these are out of the scope of this article.
5. Conclusions
Accumulating data show that it is critically important to mitigate substantial risks of death and rehospitalizations in the HFrEF population as soon as possible, and this can only be achieved by prompt initiation and persistent up-titration of all foundational life-saving therapies in all eligible patients, preferably during hospitalization or at least before discharge. Such treatment strategy calls for the shift of the paradigm from slow and sequential to rapid and efficacious targeting of key pathophysiological culprits in HFrEF to improve both quantity and the quality of life of these patients.
Expert opinion
Based on the accumulating evidence from both randomized and registry-derived data, in my opinion, the early implementation of disease-modifying pharmacotherapy in heart failure with reduced ejection fraction (HFrEF) should be embraced in contemporary clinical practice. This treatment approach should integrate foundational life-prolonging medications including beta-blockers, angiotensin receptor-neprilysin inhibitors or angiotensin-converting enzyme inhibitors, mineralocorticoid receptor antagonists, and sodium-glucose co-transporter 2 inhibitors. These medications should be initiated, whenever possible, in the lowest dose to simultaneously target several pathophysiological pathways that contribute to HFrEF syndrome progression. Once successfully initiated, these medications should be up titrated to maximally tolerated doses in a rapid sequence rather than a conventional sequence approach.
Declaration of interest
The author has no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Abdin A, Anker SD, Butler J, et al. ‘Time is prognosis’ in heart failure: time-to-treatment initiation as a modifiable risk factor. ESC Heart Fail. 2021;8:4444–4453. Online ahead of print.
- Bhagat AA, Greene SJ, Vaduganathan M, et al. Initiation, continuation, switching, and withdrawal of heart failure medical therapies during hospitalization. JACC Heart Fail. 2019;7(1):1–12.
- Vaduganathan M, Claggett BL, Jhund PS, et al. Estimating lifetime benefits of comprehensive disease-modifying pharmacological therapies in patients with heart failure with reduced ejection fraction: a comparative analysis of three randomised controlled trials. Lancet. 396(10244): 121–128. 2020.
- Wirtz HS, Sheer R, Honarpour N, et al. Real-world analysis of guideline-based therapy after hospitalization for heart failure. J Am Heart Assoc. 2020;9(16):e015042.
- Greene SJ, Fonarow GC, DeVore AD, et al. Titration of medical therapy for heart failure with reduced ejection fraction. J Am Coll Cardiol. 2019;73(19):2365–2383.
- Savarese G, Bodegard J, Norhammar A, et al. Heart failure drug titration, discontinuation, mortality and heart failure hospitalization risk: a multinational observational study (US, UK and Sweden). Eur J Heart Fail. 2021;23(9):1499–1511.
- Khan MS, Butler J, Greene SJ. Simultaneous or rapid sequence initiation of medical therapies for heart failure: seeking to avoid the case of ‘too little, too late.’ Eur J Heart Fail. 2021;23(9):1514–1517.
- Packer M, McMurray JJV. Rapid evidence-based sequencing of foundational drugs for heart failure and a reduced ejection fraction. Eur J Heart Fail. 2021;23(6):882–894.
- Velazquez EJ, Morrow DA, DeVore AD, et al. Angiotensin-neprilysin inhibition in acute decompensated heart failure. N Engl J Med. 380(6): 539–548. 2019.
- Wachter R, Senni M, Belohlavek J, et al. Initiation of sacubitril/valsartan in haemodynamically stabilised heart failure patients in hospital or early after discharge: primary results of the randomised TRANSITION study. Eur J Heart Fail. 2019;21(8):998–1007.
- López-Azor JC, Vicent L, Valero-Masa MJ, et al. Safety of sacubitril/valsartan initiated during hospitalization: data from a non-selected cohort. ESC Heart Fail. 2019;6(6):1161–1166.
- Bhatt DL, Szarek M, Steg PG, et al. Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med. 2021;384(2):117–128.
- Damman K, Beusekamp JC, Boorsma EM, et al. Randomized, double-blind, placebo-controlled, multicentre pilot study on the effects of empagliflozin on clinical outcomes in patients with acute decompensated heart failure (EMPA-RESPONSE-AHF). Eur J Heart Fail. 2020;22(4):713–722.
- Bistola V, Simitsis P, Farmakis D, et al. Association of mineralocorticoid receptor antagonist use and in-hospital outcomes in patients with acute heart failure. Clin Res Cardiol. 2018;107(1):76–86.
- Tamaki Y, Yaku H, Morimoto T, et al. Lower In-hospital mortality with beta-blocker use at admission in patients with acute decompensated heart failure. J Am Heart Assoc. 2021;10(13):e020012.
