ABSTRACT
Introduction
P2Y12-inhibitor monotherapy following 1–3 months of dual antiplatelet therapy (DAPT) reduces (major) bleeding without an apparent increase in ischemic events and has therefore emerged as an alternative to 6–12 months of DAPT following percutaneous coronary intervention (PCI). However, there are important differences between the available P2Y12-inhibitors (clopidogrel, prasugrel, and ticagrelor) as agents of choice for P2Y12-inhibitor monotherapy.
Areas covered
This review critically appraises the evidence for P2Y12-inhibitor monotherapy after PCI using either clopidogrel, prasugrel, or ticagrelor. Furthermore, we discuss ongoing trials and future directions for research.
Expert opinion
P2Y12-inhibitor monotherapy following 1–3 months of DAPT is an alternative to 6–12 months of DAPT following PCI. Ticagrelor may be considered the current preferred option due to its reliable effect on platelet reactivity and its predominant use in clinical trials. Prasugrel could serve as a useful substitute for those not tolerating ticagrelor, but more research into prasugrel monotherapy is warranted. Alternatively, clopidogrel can be used, although there are concerns of high platelet reactivity, especially when genotyping and/or platelet function testing are not used. Future research will need to address the minimal duration of DAPT before switching to P2Y12-inhibitor monotherapy and what the optimal antithrombotic therapy beyond 12 months is.
1. Introduction
Dual antiplatelet therapy (DAPT), consisting of aspirin and a P2Y12-inhibitor, has been the standard of care to prevent both stent-related and non-stent-related ischemic events following percutaneous coronary intervention (PCI) for over 30 years [Citation1–4]. However, the reduction in ischemic events as a result of antiplatelet therapy is in part counterbalanced by a concomitant increase in bleeding events [Citation5,Citation6]. Moreover, treatment beyond 6 months after PCI for chronic coronary syndrome (CCS) or 12 months after PCI for acute coronary syndrome (ACS) has not always translated into a significant reduction of ischemic events and in some settings has even been associated with worse net clinical outcomes [Citation7–10]. Therefore, a P2Y12-inhibitor is prescribed as short as possible alongside aspirin. Alternatively, recent large randomized controlled trials (RCTs), including over thirty thousand patients, have evaluated the effects of early aspirin withdrawal instead of P2Y12-inhibitor withdrawal, after a short course of DAPT [Citation11–16]. In these RCTs, P2Y12-inhibitor monotherapy was associated with a reduction in clinically relevant bleeding compared to 12 months of DAPT without an increase in both stent-related and non-stent-related ischemic events [Citation17,Citation18]. Yet, it remains unclear which of the commercially available P2Y12-inhibitors (clopidogrel, prasugrel, or ticagrelor) is most suitable for a P2Y12-inhibitor monotherapy strategy. Therefore, the present review summarizes the pharmacological and clinical evidence for P2Y12-inhibitor monotherapy using any of these three drugs and discusses ongoing and future clinical trials investigating P2Y12-inhibitor monotherapy following PCI.
2. Pharmacology of platelet inhibition
Both thromboxane A2 (TxA2) and the platelet P2Y12-receptor play a pivotal role in platelet activation and aggregation and are therefore attractive pharmacological targets for preventing the formation of arterial thrombi () [Citation19–22]. Under physiological circumstances, the intact coronary artery endothelium prevents the adherence of platelets to the endothelium through the release of antiplatelet agents, such as nitric oxide and prostacyclin. Endothelium erosion or disruption triggers binding of the platelets’ glycoprotein receptors to subendothelial collagen, von Willebrand factor, and fibrinogen. Subsequently, TxA2 is released after conversion of arachidonic acid by cyclo-oxygenase 1 (COX-1) and thromboxane synthase. In response to TxA2, adenosine diphosphate (ADP) is released from dense granules in the platelets, which further activates and amplifies platelet aggregation via P2Y12-receptors. Aspirin exerts its antithrombotic properties through irreversible inhibition of COX-1 and subsequent blockade of TxA2 generation [Citation23]. Clopidogrel, prasugrel, and ticagrelor all target the P2Y12-receptor, though in different ways and to a different extent ().
Figure 1. Platelet activation mechanisms and targets of antiplatelet therapy.
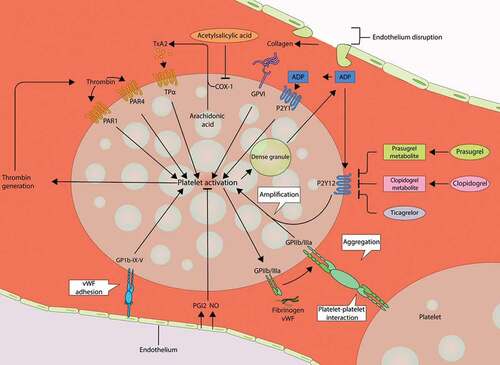
Table 1. Pharmacological characteristics of P2Y12-inhibitors used after PCI.
2.1. Clopidogrel
The thienopyridine prodrug clopidogrel is converted in a two-step process into its active metabolite by cytochrome P450 enzymes [Citation20,Citation24]. The active metabolite of clopidogrel irreversibly binds to the P2Y12-receptor and thereby prevents ADP from binding to the receptor [Citation20,Citation24]. This prevents the ADP-mediated activation of the glycoprotein IIb/IIIa complex, effectively inhibiting platelet aggregation [Citation20,Citation24]. Due to the irreversible binding of the active metabolite of clopidogrel to the P2Y12-receptor, platelet aggregation is inhibited for the remainder of the lifespan of the affected platelets (approximately 7–10 days) [Citation20]. Importantly, in 40% of patients, the conversion of clopidogrel to its active metabolite is less effective most often due to loss-of-function polymorphisms of CYP2C19, the gene encoding for the key enzyme in the metabolism of clopidogrel [Citation24–27]. These loss-of-function polymorphisms are associated with a lower pharmacodynamic effect of clopidogrel and an increase in adverse ischemic events [Citation27–29]. In turn, CYP2C19 gain-of-function polymorphisms may be associated with an increased pharmacodynamic response and some, but not all, studies have reported an increased bleeding risk in patients with a CYP2C19 gain-of-function polymorphism [Citation27,Citation28]. Also, clopidogrel–drug interactions are common and may affect the pharmacological effect of clopidogrel [Citation30,Citation31]. Most notably, the interaction between proton-pump inhibitors and clopidogrel has been the subject of much debate [Citation32–36]. Proton-pump inhibitors inhibit CYP2C19 and may theoretically reduce the platelet inhibition potency of clopidogrel. Based on pharmacological evidence, this interaction is least likely to occur with pantoprazole and more likely with omeprazole or esomeprazole [Citation37]. However, the clinical implications of this interaction seem to be limited. The COGENT (Clopidogrel and the Optimization of Gastrointestinal Events Trial) investigators randomly assigned 3,873 patients treated with aspirin and clopidogrel to either omeprazole or placebo [Citation38]. At 180 days, prophylactic use of omeprazole significantly reduced the rate of upper gastrointestinal bleeding (1.1% vs. 2.9%, hazard ratio [HR] 0.34, 95%-confidence interval [CI]: 0.18–0.63, P < 0.01) [Citation38]. There was no apparent cardiovascular interaction between clopidogrel and omeprazole (4.9% vs. 5.7%, HR 0.99, 95%-CI: 0.68–1.44, P = 0.96), although the investigators did not perform a formal non-inferiority analysis and the confidence interval around the hazard ratio for cardiovascular events was wide due to a low event rate [Citation38]. Interestingly, pharmacological studies have shown that clopidogrel inhibits the release of thromboxane A2 and can decrease physiological thromboxane A2 levels even without concurrent aspirin [Citation39]. Still, levels of thromboxane A2 in clopidogrel-treated patients are higher compared to the levels in aspirin-treated patients, indicating that clopidogrel monotherapy (at least in part) leaves the TxA2-mediated platelet aggregation intact [Citation40].
2.2. Prasugrel
The thienopyridine prodrug prasugrel is also metabolized by cytochrome P450 enzymes, but unlike clopidogrel, genetic variance in cytochrome P450 enzyme activity has little effect on this process. More specifically, the metabolism of prasugrel is primarily facilitated by CYP3A4 and CYP2B6, and to a lesser extent by CYP2C9 and CYP2C19, which are more dominant in the metabolism of clopidogrel [Citation24]. After the two-step metabolism process, the active metabolite of prasugrel irreversibly binds to the P2Y12-receptor [Citation20,Citation21]. Prasugrel has a more predictable effect on platelet aggregation compared to clopidogrel. The TRIGGER-PCI (Testing Platelet Reactivity In Patients Undergoing Elective Stent Placement on Clopidogrel to Guide Alternative Therapy With Prasugrel) investigators demonstrated that switching to prasugrel yielded effective platelet inhibition in previously clopidogrel-treated patients with high on-treatment platelet reactivity after elective PCI [Citation41]. In the vast majority of patients who switched to prasugrel (94.1%) the platelet reactivity dropped below the pre-specified and internationally recognized threshold for high on-treatment platelet reactivity after 90 days [Citation25,Citation41]. Other studies have reiterated that high on-treatment platelet reactivity with prasugrel is rare both after PCI for CCS and ACS [Citation42,Citation43]. Of note, patients in the TRIGGER-PCI trial were concomitantly treated with aspirin. Using platelet-rich plasma from healthy volunteers, Armstrong et al. demonstrated that prasugrel causes potent inhibition of platelet aggregation even without aspirin [Citation44]. Interestingly, the addition of aspirin to prasugrel did not significantly increase inhibition of platelet aggregation [Citation44]. These in vitro observations support the hypothesis that potent P2Y12-inhibitor monotherapy might provide sufficient reduction in platelet reactivity to prevent ischemic events to the same extent as with concomitant aspirin. Prasugrel also has a more rapid onset of action compared to clopidogrel, which can be further accelerated by the administration of crushed prasugrel instead of integral prasugrel [Citation45]. This is especially relevant for patients presenting with ST-segment elevation myocardial infarction (STEMI) but might become increasingly relevant for less acute patients since the administration of an oral loading dose of P2Y12-inhibitor pre-PCI is not recommended by the most recent European Society of Cardiology (ESC) non-ST-segment elevation acute coronary syndrome (NSTE-ACS) guidelines [Citation3,Citation46].
2.3. Ticagrelor
In contrast to clopidogrel and prasugrel, ticagrelor is a cyclopentyl-triazolopyrimidine that does not require metabolic activation [Citation20,Citation21]. The binding between ticagrelor and the P2Y12-receptor is reversible and prevents ADP-induced signal transduction leading to inhibition of platelet aggregation [Citation20,Citation21]. Like prasugrel, ticagrelor has a rapid and predictable effect on platelet aggregation [Citation21,Citation47]. Due to the reversible binding and relatively short half-life of the ticagrelor, the offset of the effect is faster than clopidogrel and prasugrel [Citation48]. Ticagrelor has a more consistent and less variable effect on platelet reactivity compared to clopidogrel [Citation49,Citation50]. The effect of ticagrelor and prasugrel is similar early after the loading dose, but some reports have suggested that ticagrelor decreases platelet reactivity to a greater extent than prasugrel during long-term maintenance therapy [Citation51–55]. Interestingly, ex vivo data on thrombogenicity under dynamic flow conditions have shown that the antithrombotic potency of ticagrelor monotherapy was similar to that of ticagrelor combined with aspirin in high-risk patients who underwent PCI with drug-eluting stents (DES) included in a pharmacodynamic substudy of the TWILIGHT (Ticagrelor With Aspirin or Alone in High-Risk Patients After Coronary Intervention) trial [Citation56]. These findings suggest that the antithrombotic potency of ticagrelor monotherapy is similar to that of ticagrelor combined with aspirin and provide a mechanistic basis for the clinical observations in RCTs evaluating P2Y12-inhibitor monotherapy, especially those using ticagrelor as the agent of choice.
3. Clinical trials evaluating P2Y12-inhibitor monotherapy
To date, six RCTs have investigated the efficacy and safety of P2Y12-inhibitor monotherapy after 1–3 months of DAPT in patients undergoing PCI () [Citation11–16]. In total, 35,120 patients were included in these trials (only 23.2% women) of whom approximately six out of ten patients presented with ACS. Most of the patients (76.1%) in the P2Y12-inhibitor monotherapy arm of these trials were treated with ticagrelor (). A minority of patients (23.5%) was treated with clopidogrel and prasugrel was used in only 0.4% of patients. Five out of six trials showed a statistically significant relative reduction in clinically relevant bleeding, ranging from 36% to 74% compared to standard treatment [Citation12–16]. Only in the GLOBALLEADERS trial, ticagrelor monotherapy for 23 months after 1 month of DAPT was not associated with a reduction of major bleeding defined as BARC type 3 or 5 bleeding compared to aspirin monotherapy for 12 months after 12 months of DAPT in both the intention‐to‐treat and per‐protocol analysis [Citation11,Citation57]. Three out of six trials conducted a non-inferiority analysis with regard to the ischemic complications, most often defined as the composite of all-cause or cardiovascular mortality, myocardial infarction (MI) (including stent thrombosis), and stroke [Citation12,Citation13,Citation16]. All three trials met their prespecified criteria for non-inferiority, but the non-inferiority margins in both the SMART-CHOICE (Comparison Between P2Y12 Antagonist Monotherapy and Dual Antiplatelet Therapy After DES) and STOPDAPT-2 (ShorT and OPtimal Duration of Dual AntiPlatelet Therapy-2) trial were relatively wide [Citation12,Citation13,Citation16]. More recently, Watanabe et al. published the results of the STOPDAPT-2 ACS (ShorT and OPtimal Duration of Dual AntiPlatelet Therapy-2 Study for the Patients With ACS) trial [Citation14]. In the STOPDAPT-2 ACS trial, 4,169 patients undergoing PCI with cobalt-chromium everolimus-eluting stents for ACS were randomized to clopidogrel monotherapy after 1–2 months of DAPT or 12 months of DAPT. Of note, 1,161 patients came from the ACS subgroup of the STOPDAPT-2 trial. Clopidogrel monotherapy did not meet non-inferiority with regard to the primary endpoint consisting of cardiovascular death, MI, stroke, stent thrombosis, and TIMI major or minor bleeding (3.2% vs. 2.8%, HR 1.14, 95%-CI: 0.80–1.62, PNI = 0.06). Importantly, the composite of cardiovascular death, MI, stroke, and stent thrombosis was numerically higher in the clopidogrel monotherapy group (2.8% vs. 1.9%, HR 1.50, 95%-CI: 0.99–2.26). Clopidogrel monotherapy was associated with a reduction in TIMI major or minor bleeding (0.5% vs. 1.2%, HR 0.46, 95%-CI: 0.23–0.94). A recent meta-analysis by Giacoppo et al. of five out of these six RCTs confirmed that P2Y12-inhibitor monotherapy was associated with a reduction in major bleeding defined as BARC type 3 or 5 bleeding (random-effects model: HR 0.63, 95%-CI: 0.45–0.86) [Citation18]. No significant differences between groups treated with or without DAPT were observed in terms of non-stent-related ischemic events (i.e. all-cause mortality, MI, and stroke) and stent thrombosis with an absolute event rate of less than 0.5% in patients treated with P2Y12-inhibitor monotherapy [Citation18]. Notably, in an exploratory sub-group analyses by Valgimigli et al., a significant interaction by sex (P for interaction = 0.02) was observed suggesting that P2Y12-inhibitor monotherapy reduces the risk of death, MI, and stroke in women (HR 0.64, 95%-CI: 0.46–0.89) but not in men (HR 1.00, 95%-CI 0.83–1.19) [Citation58]. The mechanism for this interaction is unclear and needs further investigation [Citation58].
Figure 2. P2Y12-inhibitor of choice in the experimental arms of RCTs evaluating P2Y12-inhibitor monotherapy after PCI.
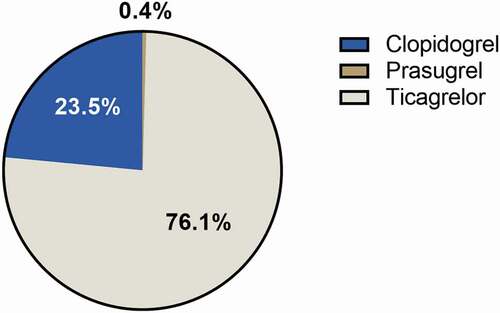
Table 2. Bleeding and ischemic events in RCTs evaluating P2Y12-inhibitor monotherapy after PCI.
Some limitations of the aforementioned trials should be acknowledged. Five out of six trials randomized patients directly after PCI and not at the time of aspirin discontinuation (after 1 or 3 month[s]) [Citation11–15]. Therefore, early bleeding and ischemic events (i.e. during the first 1 to 3 month[s] after PCI) were included in the analyses, while both groups were still using DAPT. This may have diluted the effect of P2Y12-inhibitor monotherapy on reducing bleeding but also masked a possible effect of omitting aspirin on increasing ischemic events. However, all of these trials performed a landmark analysis from aspirin discontinuation confirming that the rate of ischemic events did not diverge after aspirin withdrawal [Citation11–15]. Furthermore, only TWILIGHT used a placebo-controlled design [Citation16]. Still, all trials except GLOBAL LEADERS adjudicated the primary outcomes by a clinical event committee blinded to the treatment allocation, limiting the risk of (reporting) bias.
Thus far, ticagrelor has been predominantly used in clinical trials evaluating P2Y12-inhibitor monotherapy, and experience with prasugrel monotherapy in the setting of P2Y12-inhibitor monotherapy has been limited. However, prasugrel could be an alternative to ticagrelor when ticagrelor is prematurely discontinued due to side effects, such as dyspnea [Citation59,Citation60]. The mechanism of ticagrelor-induced dyspnea remains unclear, but current hypotheses include overstimulation of pulmonary vagal C fibers due to increased levels of adenosine caused by inhibition of adenosine reuptake and the inhibition of P2Y12-receptors of sensory neurons [Citation60]. Although ticagrelor-induced dyspnea is usually transient and does not appear to be associated with differences concerning any efficacy or safety outcomes, some patients require an alternative treatment strategy [Citation59,Citation60]. Prasugrel has not been associated with increased dyspnea compared to clopidogrel and can therefore be a useful substitute for ticagrelor in these patients [Citation61]. In turn, prasugrel is contraindicated in patients with a prior transient ischemic attack or stroke and a reduced prasugrel dose is recommended in older and low-weight patients. If both ticagrelor and prasugrel are contraindicated, clopidogrel monotherapy can be considered. Still, the results of STOPDAPT-2-ACS indicate that clopidogrel monotherapy is less suitable for patients undergoing PCI for ACS, especially if patients do not undergo CYP2C19 genotyping prior to aspirin withdrawal [Citation62]. The POPular Genetics trial demonstrated that genotype-guided P2Y12-inhibition (de-escalation from ticagrelor or prasugrel to clopidogrel based on CYP2C19 genetic testing) was non-inferior to ticagrelor or prasugrel for 12 months in terms of the primary outcome net clinical benefit, and led to a significant reduction in bleeding in STEMI patients undergoing primary PCI [Citation63]. Whether CYP2C19 genetic testing can enhance the efficacy of clopidogrel monotherapy in ACS patients remains unclear. Further, until now clopidogrel monotherapy has primarily been evaluated in East Asian patients, who have a unique risk profile [Citation12,Citation13]. Therefore, caution should be taken when extrapolating these trials’ results in other ethnicities.
As mentioned previously, all RCTs included at least 1–3 months of DAPT before switching to P2Y12-inhibitor monotherapy, when most (major) bleeding events occur [Citation6]. Recently, the ASET (Acetyl Salicylic Elimination Trial) pilot has shown that a completely aspirin-free prasugrel monotherapy strategy directly following PCI was feasible in CCS patients, opening the door to RCTs investigating direct P2Y12-inhibitor monotherapy following PCI [Citation64]. In turn, directly omitting aspirin could not only improve clinical outcomes and reduce bleeding-related health-care costs but also reduce polypharmacy and therefore improve medication adherence [Citation65].
4. Ongoing and future clinical trials investigating P2Y12-inhibitor monotherapy
Current guidelines already state that stopping aspirin after 3–6 months should be considered in patients undergoing PCI, depending on the balance between the ischemic and bleeding risk (Class IIa Level A recommendation) [Citation3]. However, for P2Y12-inhibitor monotherapy to become the standard of care, possibly after a short period of concurrent aspirin use, several remaining questions need to be answered. Ongoing and planned RCTs evaluating different aspects of P2Y12-inhibitor monotherapy after PCI are summarized in .
Table 3. Ongoing or future RCTs evaluating P2Y12-inhibitor monotherapy after PCI.
First, several clinical trials are evaluating whether and for how long concomitant aspirin use is necessary before switching to P2Y12-inhibitor monotherapy. In line with the ASET pilot, which showed the feasibility of direct prasugrel monotherapy in CCS patients, the OPTICA (Optical Coherence Tomography-Guided PCI with Single-Antiplatelet Therapy, NCT04766437) pilot is investigating the feasibility and safety of prasugrel and ticagrelor monotherapy directly after stent implantation in 75 patients with NSTE-ACS. All patients undergo platelet function testing after receiving a loading dose of either prasugrel or ticagrelor and the first 35 patients undergo optical coherence tomography (OCT) to ensure optimal stent result. The primary ischemic and bleeding endpoints are the composite of all-cause mortality, MI, definite or probable stent thrombosis, and stroke at 6 months and BARC type 2, 3 or 5 bleeding at 6 months, respectively. Results are expected in September 2022. Ultimately, larger RCTs are needed to confirm the efficacy and safety of direct P2Y12-inhibitor monotherapy compared with traditional DAPT following PCI. The NEOMINDSET (PercutaNEOus Coronary Intervention Followed by Monotherapy INstead of Dual Antiplatelet Therapy in the SETting of Acute Coronary Syndromes, NCT04360720) and LEGACY (Less Bleeding by Omitting Aspirin in Non-ST-segment Elevation Acute Coronary Syndrome Patients, NCT05125276) trials randomly assign ACS patients undergoing PCI to direct P2Y12-inhibitor monotherapy or conventional DAPT. Of note, these RCTs will also provide eagerly awaited data on the efficacy and safety of prasugrel monotherapy. The ULTIMATE-DAPT trial (NCT03971500) has a similar design but will include 1 month of concurrent aspirin use before switching to ticagrelor monotherapy. As part of the 2 by 2 factorial design of the trial, patients will also be randomized between IVUS- or angiography-guided PCI, providing relevant information on the role of intracoronary imaging for guiding antiplatelet therapy.
Furthermore, the optimal antithrombotic strategy during the chronic maintenance period following PCI (i.e. after 6–12 months) remains the subject of debate. Current guidelines recommend aspirin for long-term treatment, insinuating that patients on P2Y12-inhibitor monotherapy should switch to aspirin after 6-12 months [Citation2–4]. However, the HOST-EXAM (Harmonizing Optimal Strategy for Treatment of Coronary Artery Stenosis-EXtended Antiplatelet Monotherapy) investigators recently demonstrated that clopidogrel monotherapy was superior to aspirin monotherapy during the chronic maintenance period among patients who had successfully completed the required duration of DAPT with regard to net adverse clinical events (5.7% vs. 7.7%, HR 0.73, 95%-CI: 0.59–0.90, P < 0.01) [Citation66]. Clopidogrel monotherapy was also associated with a reduction in ischemic events (3.7% vs. 5.5%, HR 0.68, 95%-CI: 0.52–0.87, P < 0.01) and bleeding (2.3% vs. 3.3%, HR 0.70, 95%-CI: 0.57–0.98, P = 0.04) [Citation66]. Currently, several other RCTs are evaluating the optimal antithrombotic regimen after 12 months, but most trials compare P2Y12-inhibitor monotherapy using clopidogrel to prolonged DAPT instead of aspirin monotherapy. Adding a second antithrombotic agent for extended long-term secondary prevention should only be considered in patients with high ischemic risk and without increased risk of major or life-threatening bleeding and should therefore only be considered after careful risk assessment [Citation3,Citation67]. Of note, there are currently no completed randomized studies available comparing (continued) P2Y12-inhibitor monotherapy to aspirin monotherapy beyond 1 year after conventional DES implantation. Only, the ongoing SMART-CHOICE 3 (SMart Angioplasty Research Team: CHoice of Optimal Anti-Thrombotic Strategy in Patients Undergoing Implantation of Coronary Drug-Eluting Stents, NCT04418479) and STOPDAPT-2 ACS trials compare P2Y12-inhibitor monotherapy to aspirin monotherapy after 12 months in patients at high risk for recurrent ischemic events and patients presenting with ACS, respectively. Importantly, trials using ticagrelor as agent of choice for P2Y12-inhibitor monotherapy during the chronic maintenance period following PCI should carefully monitor compliance with the twice daily dosing regimen.
5. Conclusion
P2Y12-inhibitor monotherapy following a short period of DAPT is an alternative to standard DAPT after PCI. Currently, ticagrelor seems the most viable agent of choice for P2Y12-inhibitor monotherapy, but prasugrel and clopidogrel could serve as useful substitutes in specific clinical scenarios. Importantly, more clinical trials directly comparing the different P2Y12-inhibitors used as monotherapy are warranted.
6. Expert opinion
P2Y12-inhibitor monotherapy following 1–3 months of DAPT is a viable alternative for 6 months of DAPT after CCS or 12 months of DAPT after PCI for ACS. Several pharmacological studies have demonstrated that P2Y12-inhibitor monotherapy not only inhibits the ADP-mediated platelet aggregation but also (in part) constrains the TxA2-mediated platelet aggregation and thus mimics the effect of aspirin. Other pharmacological studies have produced in vitro and ex-vivo evidence suggesting that the antithrombotic potency of P2Y12-inhibitor monotherapy is similar compared to the potency of P2Y12-inhibitor combined with aspirin. Most importantly, in clinical practice P2Y12-inhibitor monotherapy after 1 or 3 months of concurrent aspirin use has been associated with a reduction in (major) bleeding without an apparent increase in stent-related or non-stent-related ischemic events.
Whether to choose clopidogrel, prasugrel or ticagrelor as agent of choice for P2Y12-inhibitor monotherapy in daily practice depends on several clinical circumstances. For now, ticagrelor seems to be the preferred option in most cases, due to its reliable effect on platelet reactivity and its predominant use in clinical trials evaluating P2Y12-inhibitor monotherapy. However, in some patients, ticagrelor is associated with side effects such as dyspnea and therefore an alternative treatment strategy may be required. Prasugrel could serve as a useful substitute in those patients not tolerating ticagrelor, but more research is needed into prasugrel monotherapy. Data from pharmacological studies provide support for prasugrel monotherapy, but prasugrel use has so far been very limited in clinical trials evaluating P2Y12-inhibitor monotherapy. Alternatively, clopidogrel can be used, although there are concerns of high on-treatment platelet reactivity, especially when CYP2C19 genotyping is not performed before switching to P2Y12-inhibitor monotherapy. Future studies will have to address whether genotype-guided de-escalation from ticagrelor or prasugrel monotherapy to clopidogrel monotherapy is possible, especially in patients presenting with ACS. Platelet function testing may also play a role in the decision for (or against) clopidogrel monotherapy, although the added value of platelet function testing in patients treated with P2Y12-inhibitor monotherapy has not yet been evaluated in clinical trials. Furthermore, clopidogrel monotherapy has only been evaluated in East Asian patients, who have a unique risk profile. Therefore, caution should be taken when extrapolating these trials’ results in other ethnicities.
In general, there is sufficient clinical evidence that P2Y12-inhibitor monotherapy after 1–3 months of DAPT is safe and even earlier aspirin withdrawal might be possible. So far, all RCTs evaluating P2Y12-inhibitor monotherapy included 1–3 months of DAPT before switching to P2Y12-inhibitor monotherapy. Future research will need to address whether or how long concomitant aspirin use is necessary before switching to P2Y12-inhibitor monotherapy and whether there are clinical (e.g. PCI for CCS versus ACS) or procedural characteristics (e.g. stent result) that should guide this decision. Additionally, future research will need to address the optimal antithrombotic strategy in the chronic maintenance period beyond 6 or 12 months after PCI. For now, guidelines recommend lifelong aspirin in the chronic maintenance period, but there is some evidence that (continued) P2Y12-inhibitor monotherapy using clopidogrel asserts similar (or even better) ischemic protection and is associated with a reduction in bleeding. Hopefully, future clinical trials evaluating P2Y12-inhibitor monotherapy will consider extended follow-up beyond 6 or 12 months to assess the efficacy and safety of P2Y12-inhibitor monotherapy in the chronic maintenance period.
Article highlights
P2Y12-inhibitor monotherapy following 1–3 months of DAPT has emerged as an alternative to 6–12 months of DAPT following PCI, but there are important differences between clopidogrel, prasugrel, and ticagrelor as agents of choice for P2Y12-inhibitor monotherapy.
Ticagrelor has a reliable effect on platelet reactivity and has been predominantly used in clinical trials evaluating P2Y12-inhibitor monotherapy.
Prasugrel could serve as a useful substitute for those not tolerating ticagrelor, but prasugrel has so far been vastly underrepresented in clinical trials evaluating P2Y12-inhibitor monotherapy.
Clopidogrel might be less suitable as agent of choice for P2Y12-inhibitor monotherapy in patients undergoing PCI for ACS, especially if patients do not undergo CYP2C19 genotyping prior to aspirin withdrawal
Future research will need to address the minimal duration of DAPT before switching to P2Y12-inhibitor monotherapy and what the optimal antithrombotic therapy is beyond 12 months.
Declaration of Interest
JM ten Berg has received research grants from AstraZeneca and ZonMw and personal fees from AstraZeneca, Accu-Metrics, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Eli Lilly, Ferrer, Idorsia, Pfizer and The Medicines Company. MAM Beijk has received a research grant from Actelion (Johnson & Johnson). Y Appelman has received a research grant from the Dutch Heart Foundation. WJ Kikkert has received a research grant from AstraZeneca. JPS Henriques has received research grants from Abbott Vascular, AstraZeneca, B. Braun, Getinge, Ferrer, Infraredx and ZonMw. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Cao D, Chandiramani R, Chiarito M, et al. Evolution of antithrombotic therapy in patients undergoing percutaneous coronary intervention: a 40-year journey. Eur Heart J. 2021 Jan 21;42(4):339–351.
- Knuuti J, Wijns W, Saraste A, et al. ESC guidelines for the diagnosis and management of chronic coronary syndromes: the task force for the diagnosis and management of chronic coronary syndromes of the European Society of Cardiology (ESC). Eur Heart J. 2019;41(3):407–477.
- Collet J-P, Thiele H, Barbato E, et al. ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: the task force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2020;41(37):3495–3497.
- Ibanez B, James S, Agewall S, et al. ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2017;39(2):119–177.
- Piccolo R, Oliva A, Avvedimento M, et al. Mortality after bleeding versus myocardial infarction in coronary artery disease: a systematic review and meta-analysis. EuroIntervention. 2021 Sep 20;17(7):550–560.
- Giustino G, Mehran R, Dangas GD, et al. Characterization of the average daily ischemic and bleeding risk after primary PCI for STEMI. J Am Coll Cardiol. 2017 Oct 10;70(15):1846–1857.
- Steg PG, Bhatt DL, Simon T, et al. Ticagrelor in patients with stable coronary disease and diabetes. N Engl J Med. 2019;381(14):1309–1320.
- Mauri L, Kereiakes DJ, Yeh RW, et al. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N Engl J Med. 2014 Dec 4;371(23):2155–2166.
- Bonaca MP, Bhatt DL, Cohen M, et al. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med. 2015;372(19):1791–1800.
- Valgimigli M, Borghesi M, Tebaldi M, et al. Should duration of dual antiplatelet therapy depend on the type and/or potency of implanted stent? A pre-specified analysis from the prolonging dual antiplatelet treatment after grading stent-induced intimal hyperplasia studY (PRODIGY). Eur Heart J. 2013 Mar;34(12):909–919.
- Vranckx P, Valgimigli M, Juni P, et al. Ticagrelor plus aspirin for 1 month, followed by ticagrelor monotherapy for 23 months vs aspirin plus clopidogrel or ticagrelor for 12 months, followed by aspirin monotherapy for 12 months after implantation of a drug-eluting stent: a multicentre, open-label, randomised superiority trial. Lancet. 2018 Sep 15;392(10151):940–949. London, England.
- Hahn JY, Song YB, Oh JH, et al. Effect of P2Y12 inhibitor monotherapy vs dual antiplatelet therapy on cardiovascular events in patients undergoing percutaneous coronary intervention: the SMART-CHOICE randomized clinical trial. Jama. 2019 Jun 25;321(24):2428–2437.
- Watanabe H, Domei T, Morimoto T, et al., Effect of 1-month dual antiplatelet therapy followed by clopidogrel vs 12-month dual antiplatelet therapy on cardiovascular and bleeding events in patients receiving PCI: the STOPDAPT-2 randomized clinical trial. Jama. 2019;321(24):2414–2427.
- Watanabe H, Morimoto T, Natsuaki M, et al., Comparison of clopidogrel monotherapy after 1 to 2 months of dual antiplatelet therapy with 12 months of dual antiplatelet Therapy in patients with acute coronary syndrome: the STOPDAPT-2 ACS randomized clinical trial. JAMA Cardiol. 2022;7(4):407.
- Kim B-K, Hong S-J, Cho Y-H, et al., Effect of ticagrelor monotherapy vs ticagrelor with aspirin on major bleeding and cardiovascular events in patients with acute coronary syndrome: the TICO randomized clinical trial. Jama. 2020;323(23):2407–2416.
- Mehran R, Baber U, Sharma SK, et al. Ticagrelor with or without aspirin in high-risk patients after PCI. N Engl J Med. 2019 Sep 26;381(21):2032–2042.
- McClure JD, Ramsay JC, Berry C. Pooled analysis of bleeding, major adverse cardiovascular events, and all-cause mortality in clinical trials of time-constrained dual-antiplatelet therapy after percutaneous coronary intervention. J Am Heart Assoc. 2020 Aug 18;9(16):e017109.
- Giacoppo D, Matsuda Y, Fovino LN, et al. Short dual antiplatelet therapy followed by P2Y12 inhibitor monotherapy vs. prolonged dual antiplatelet therapy after percutaneous coronary intervention with second-generation drug-eluting stents: a systematic review and meta-analysis of randomized clinical trials. Eur Heart J. 2020;42(4):308–319.
- Capodanno D, Mehran R, Valgimigli M, et al. Aspirin-free strategies in cardiovascular disease and cardioembolic stroke prevention. Nat Rev Cardiol. 2018 Aug;15(8):480–496.
- Verheugt FWA, Damman P, Damen SAJ, et al. P2Y12 blocker monotherapy after percutaneous coronary intervention. Neth Heart J. 2021 Nov;29(11):566–576.
- Ahmad S, Storey RF. Development and clinical use of prasugrel and ticagrelor. Curr Pharm Des. 2012;18(33):5240–5260.
- Furie B, Furie BC. Mechanisms of thrombus formation. N Engl J Med. 2008 Aug 28;359(9):938–949.
- Patrono C, García Rodríguez LA, Landolfi R, et al. Low-dose aspirin for the prevention of atherothrombosis. N Engl J Med. 2005 Dec 1;353(22):2373–2383.
- Norgard NB, Abu-Fadel M. Comparison of prasugrel and clopidogrel in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Vasc Health Risk Manag. 2009;5:873–882.
- Sibbing D, Aradi D, Alexopoulos D, et al. Updated expert consensus statement on platelet function and genetic testing for guiding P2Y(12) receptor inhibitor treatment in percutaneous coronary intervention. JACC Cardiovasc Interv. 2019 Aug 26;12(16):1521–1537.
- Li S, Choi J-L, Guo LZ, et al. Correlation between the CYP2C19 phenotype status and the results of three different platelet function tests in cardiovascular disease patients receiving antiplatelet therapy: an emphasis on newly introduced platelet function analyzer-200 P2Y test. Ann Lab Med. 2016;36(1):42–48.
- Aradi D, Kirtane A, Bonello L, et al. Bleeding and stent thrombosis on P2Y12-inhibitors: collaborative analysis on the role of platelet reactivity for risk stratification after percutaneous coronary intervention. Eur Heart J. 2015 Jul 14;36(27):1762–1771.
- Thomas MR, Storey RF. Genetics of response to antiplatelet therapy. Prog Mol Biol Transl Sci. 2014;124:123–153.
- Simon T, Verstuyft C, Mary-Krause M, et al. Genetic determinants of response to clopidogrel and cardiovascular events. N Engl J Med. 2009 Jan 22;360(4):363–375.
- Lee CH, Franchi F, Angiolillo DJ. Clopidogrel drug interactions: a review of the evidence and clinical implications. Expert Opin Drug Metab Toxicol. 2020 Nov;16(11):1079–1096.
- Serbin MA, Guzauskas GF, Veenstra DL. Clopidogrel-proton pump inhibitor drug-drug interaction and risk of adverse clinical outcomes among PCI-treated ACS patients: a meta-analysis. J Manag Care Spec Pharm. 2016 Aug;22(8):939–947.
- Small DS, Farid NA, Payne CD, et al. Effects of the proton pump inhibitor lansoprazole on the pharmacokinetics and pharmacodynamics of prasugrel and clopidogrel. J Clin Pharmacol. 2008 Apr;48(4):475–484.
- Sibbing D, Morath T, Stegherr J, et al. Impact of proton pump inhibitors on the antiplatelet effects of clopidogrel. Thromb Haemost. 2009 Apr;101(4):714–719.
- Gilard M, Arnaud B, Cornily JC, et al. Influence of omeprazole on the antiplatelet action of clopidogrel associated with aspirin: the randomized, double-blind OCLA (Omeprazole CLopidogrel Aspirin) study. J Am Coll Cardiol. 2008 Jan 22;51(3):256–260.
- O’Donoghue ML, Braunwald E, Antman EM, et al. Pharmacodynamic effect and clinical efficacy of clopidogrel and prasugrel with or without a proton-pump inhibitor: an analysis of two randomised trials. Lancet. 2009 Sep 19;374(9694):989–997. London, England
- Shah NH, LePendu P, Bauer-Mehren A, et al. Proton pump inhibitor usage and the risk of myocardial infarction in the general population. PLoS One. 2015;10(6):e0124653.
- Li XQ, Andersson TB, Ahlström M, et al. Comparison of inhibitory effects of the proton pump-inhibiting drugs omeprazole, esomeprazole, lansoprazole, pantoprazole, and rabeprazole on human cytochrome P450 activities. Drug Metab Dispos. 2004 Aug;32(8):821–827.
- Bhatt DL, Cryer BL, Contant CF, et al. Clopidogrel with or without omeprazole in coronary artery disease. N Engl J Med. 2010 Nov 11;363(20):1909–1917.
- Bhavaraju K, Georgakis A, Jin J, et al. Antagonism of P2Y₁₂ reduces physiological thromboxane levels. Platelets. 2010;21(8):604–609.
- Armstrong PC, Dhanji AR, Tucker AT, et al. Reduction of platelet thromboxane A2 production ex vivo and in vivo by clopidogrel therapy. J Thromb Haemost. 2010 Mar;8(3):613–615.
- Trenk D, Stone GW, Gawaz M, et al. A randomized trial of prasugrel versus clopidogrel in patients with high platelet reactivity on clopidogrel after elective percutaneous coronary intervention with implantation of drug-eluting stents: results of the TRIGGER-PCI (Testing platelet reactivity in patients undergoing elective stent placement on clopidogrel to guide alternative therapy with prasugrel) study. J Am Coll Cardiol. 2012 Jun 12;59(24):2159–2164.
- Winter M-P, Schneeweiss T, Cremer R, et al. Platelet reactivity patterns in patients treated with dual antiplatelet therapy. Eur J Clin Invest. 2019;49(6):e13102–e13102.
- Franchi F, Rollini F, Rivas J, et al. Prasugrel versus ticagrelor in patients with CYP2C19 loss-of-function genotypes: results of a randomized pharmacodynamic study in a feasibility investigation of rapid genetic testing. JACC Basic Transl Sci. 2020 May;5(5):419–428.
- Armstrong PC, Leadbeater PD, Chan MV, et al. In the presence of strong P2Y12 receptor blockade, aspirin provides little additional inhibition of platelet aggregation. J Thromb Haemost. 2011 Mar;9(3):552–561.
- Vogel RF, Delewi R, Angiolillo DJ, et al. Pharmacodynamic effects of pre-hospital administered crushed prasugrel in patients with ST-segment elevation myocardial infarction. JACC Cardiovasc Interventions. 2021 Jun 28;14(12):1323–1333.
- Montalescot G, Collet JP, Ecollan P, et al. Effect of prasugrel pre-treatment strategy in patients undergoing percutaneous coronary intervention for NSTEMI: the ACCOAST-PCI study. J Am Coll Cardiol. 2014 Dec 23;64(24):2563–2571.
- Scudiero F, Canonico ME, Sanna GD, et al. Dual antiplatelet therapy with 3(rd) generation P2Y(12) inhibitors in STEMI patients: impact of body mass index on loading dose-response. Cardiovasc Drugs Ther. 2022 Feb 17. DOI:10.1007/s10557-022-07322-2.
- Ow KW, Parker WAE, Porter MM, et al. Offset of ticagrelor prior to coronary artery bypass graft surgery for acute coronary syndromes: effects on platelet function and cellular adenosine uptake. Platelets. 2020 Oct 2;31(7):945–951.
- Angiolillo DJ, Franchi F, Waksman R, et al. Effects of ticagrelor versus clopidogrel in troponin-negative patients with low-risk ACS undergoing Ad Hoc PCI. J Am Coll Cardiol. 2016 Feb 16;67(6):603–613.
- Dehghani P, Lavoie A, Lavi S, et al. Effects of ticagrelor versus clopidogrel on platelet function in fibrinolytic-treated STEMI patients undergoing early PCI. Am Heart J. 2017 Oct;192:105–112.
- Zhang H, Zhang P, Dong P, et al. Effect of ticagrelor versus prasugrel on platelet reactivity: a meta-analysis. Coron Artery Dis. 2017 Nov;28(7):597–604.
- Wen M, Li Y, Qu X, et al. Comparison of platelet reactivity between prasugrel and ticagrelor in patients with acute coronary syndrome: a meta-analysis. BMC Cardiovasc Disord. 2020 Oct 01;20(1):430.
- Alexopoulos D, Xanthopoulou I, Gkizas V, et al. Randomized assessment of ticagrelor versus prasugrel antiplatelet effects in patients with ST-segment-elevation myocardial infarction. Circ Cardiovasc Interv. 2012 Dec;5(6):797–804.
- Alexopoulos D, Galati A, Xanthopoulou I, et al. Ticagrelor versus prasugrel in acute coronary syndrome patients with high on-clopidogrel platelet reactivity following percutaneous coronary intervention: a pharmacodynamic study. J Am Coll Cardiol. 2012 Jul 17;60(3):193–199.
- Franchi F, Rollini F, Aggarwal N, et al. Pharmacodynamic comparison of prasugrel versus ticagrelor in patients with type 2 diabetes mellitus and coronary artery disease. Circulation. 2016;134(11):780–792.
- Baber U, Zafar MU, Dangas G, et al. Ticagrelor with or without aspirin after PCI: the TWILIGHT platelet substudy. J Am Coll Cardiol. 2020 Feb 18;75(6):578–586.
- Gragnano F, Zwahlen M, Vranckx P, et al. Ticagrelor monotherapy or dual antiplatelet therapy after drug‐eluting stent implantation: per‐protocol analysis of the GLOBAL LEADERS trial. J Am Heart Assoc. 2022;11(10):e024291.
- Valgimigli M, Gragnano F, Branca M, et al. P2Y12 inhibitor monotherapy or dual antiplatelet therapy after coronary revascularisation: individual patient level meta-analysis of randomised controlled trials. BMJ (Clinical research ed). 2021;373:n1332.
- Storey RF, Becker RC, Harrington RA, et al. Characterization of dyspnoea in PLATO study patients treated with ticagrelor or clopidogrel and its association with clinical outcomes. Eur Heart J. 2011 Dec;32(23):2945–2953.
- Parodi G, Storey RF. Dyspnoea management in acute coronary syndrome patients treated with ticagrelor. Eur Heart J Acute Cardiovasc Care. 2015 Dec;4(6):555–560.
- Zhang N, Xu W, Li O, et al. The risk of dyspnea in patients treated with third-generation P2Y(12) inhibitors compared with clopidogrel: a meta-analysis of randomized controlled trials. BMC Cardiovasc Disord. 2020 Mar 17;20(1):140.
- Watanabe H, Morimoto T, Ogita M, et al. Influence of CYP2C19 genotypes for the effect of 1-month dual antiplatelet therapy followed by clopidogrel monotherapy relative to 12-month dual antiplatelet therapy on clinical outcomes after percutaneous coronary intervention: a genetic substudy from the STOPDAPT-2. Cardiovasc Interv Ther. 2021 Oct;36(4):403–415.
- Claassens DMF, Vos GJA, Bergmeijer TO, et al. A genotype-guided strategy for oral P2Y(12) inhibitors in primary PCI. N Engl J Med. 2019 Oct 24;381(17):1621–1631.
- Kogame N, Guimarães PO, Modolo R, et al. Aspirin-free prasugrel monotherapy following coronary artery stenting in patients with stable cad: the aset pilot study. JACC Cardiovasc Interv. 2020 Oct 12;13(19):2251–2262.
- Chowdhury R, Khan H, Heydon E, et al. Adherence to cardiovascular therapy: a meta-analysis of prevalence and clinical consequences. Eur Heart J. 2013 Oct;34(38):2940–2948.
- Koo B-K, Kang J, Park KW, et al. Aspirin versus clopidogrel for chronic maintenance monotherapy after percutaneous coronary intervention (HOST-EXAM): an investigator-initiated, prospective, randomised, open-label, multicentre trial. Lancet. 2021 Jun 26;397(10293):2487–2496.
- van der Sangen NMR, Rozemeijer R, Chan Pin Yin DRPP, et al. Patient-tailored antithrombotic therapy following percutaneous coronary intervention. Eur Heart J. 2021 Mar 7;42(10):1038–1046.
