ABSTRACT
Introduction
Stroke is one of the leading causes of mortality and morbidity globally. Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia. It is set to reach epidemic proportions. AF is associated with a five-fold increase in risk of stroke. Strokes caused by AF more often are fatal or result in severe disability. Even though the incidence of stroke has been significantly reduced by oral anticoagulation, AF is thought to account for a significant proportion of cryptogenic strokes where no etiology is identified.
Areas covered
This article reviews the literature related to AF and stroke, pathophysiological insights, diagnosis of AF in stroke patients, and its management (Graphical Abstract).
Expert opinion
The pathophysiology of thrombogenesis that links AF and stroke is not well understood and is an area of active research to identify new therapeutic targets to prevent AF and stroke. As the nature of AF and stroke is multifaceted, an integrated care approach to managing AF and stroke is increasingly essential.
Graphical Abstract
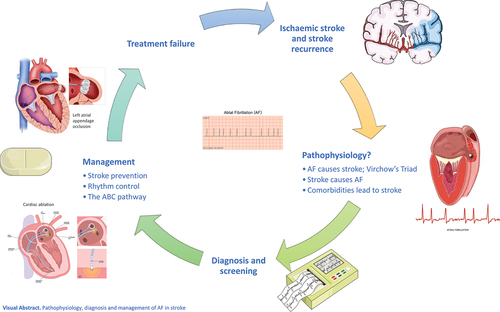
1. Introduction
Stroke is one of the leading causes of mortality, morbidity, and long-term disability worldwide [Citation1]. It is the second highest cause of death globally after ischemic heart disease [Citation1]. Atrial fibrillation (AF) is the most common cardiac arrhythmia in adults increasing the risk of stroke five-fold and represents a growing epidemic and public health burden [Citation2]. Of note, AF has an estimated prevalence of 1% to 4% in Australia, Europe, and the United States [Citation3–9], with a lower prevalence (0.49% to 1.9%) in Asian countries [Citation10]. Its prevalence increases markedly with age and cardiovascular comorbidities, reaching up to 17% among adults aged 80 or older [Citation11,Citation12].
The lifetime risk of developing AF is approximately one in four [Citation13,Citation14]. With an aging population and improved management of cardiovascular diseases, the estimated worldwide prevalence of AF during the next 30 years is projected to increase by 66% reaching 62.5 million cases [Citation15]. In the United States per se, the prevalence of AF is projected to rise from 5.1 million in 2000 to 12.1 to 15.9 million by 2050 [Citation16]. The estimated prevalence of AF in the European Union in 2010 was 8.8 million individuals over 55 years and is projected to double to 17.9 million by 2060 [Citation17].
AF is the most important cause of cardioembolism, representing 35% of patients with non-lacunar strokes and 15% to 24% of all ischemic strokes [Citation18,Citation19]. AF-related ischemic stroke is almost twice as likely to be fatal, usually more severe, or recurs more frequently than non-AF stroke [Citation20,Citation21]. Oral anticoagulation (OAC), comprised of vitamin-K antagonists (VKA) and non-vitamin K antagonist oral anticoagulants (NOAC), is the treatment of choice, significantly reducing the risk of stroke or systemic embolism, as well as mortality [Citation22–26].
Due to patients’ and physicians’ deeper awareness of AF, the increasing use of several AF screening modalities, as well as improved control of modifiable stroke risk factors, there has been a trend toward proportionally higher incidence of cardioembolic strokes, compared to other stroke subtypes [Citation27–30].
Despite an extensive diagnostic work-up during the acute or chronic phase of ischemic stroke, the cause of ischemic stroke remains unexplained for 20% of patients, termed cryptogenic stroke [Citation31,Citation32]. A subgroup of patients with cryptogenic stroke, in which despite an extensive diagnostic work-up, no potential cause is recognized, have what is described as embolic stroke of undetermined source (ESUS) [Citation33]. Although occult AF and atrial cardiomyopathy may be a potential source of embolism in these patients, low-degree atherosclerotic stenosis, patent foramen ovale, left ventricular disease and others, may serve as potential embolic sources, which frequently overlap [Citation34]. Due to its dynamic nature, identifying occult AF may be challenging in clinical practice, although it may be present in a significant proportion of patients presenting with cryptogenic stroke [Citation35–37].
2. Pathophysiology of AF in stroke and insights into stroke risk
Despite the clear mechanistic association between AF and systemic thromboembolism, AF may also represent a marker of cardiovascular burden in the continuously aging contemporary population [Citation38]. Moreover, stroke may affect the autonomic nervous system [Citation39], which in turn is thought to play a role in triggering cardiac arrhythmia, most commonly, AF [Citation40]. However, there is a paucity of data to explain the clinically important difference between the brief new-onset AF following a stroke and the long-standing AF, in terms of future stroke recurrence [Citation41,Citation42].
The pathophysiology of thrombogenesis in AF is multifaceted and complex. The pathogenic mechanisms of thrombus formation in the left atrium and left atrial appendage are incompletely understood and best framed by Virchow’s triad [Citation43]. Evidence that the processes described in Virchow’s triad to explain thrombogenesis in vascular disease, namely (i) vessel wall abnormalities, (ii) abnormal blood flow, and (iii) hypercoagulability from abnormal hemostasis, platelet function, and fibrinolysis, are active in AF is well documented [Citation44–46]. Moreover, several studies suggested the presence of these alterations among patients with AF-related strokes, fostering their association to thrombus formation and subsequently to systemic embolism (). However, the precise interplay between those pathophysiological elements, AF, and ischemic stroke is not fully understood. Improved insights into those components and how they lead to stroke in AF patients will facilitate improved stratification and understanding of stroke risk and prognosis, and the development of management therapies and new targets for treatment strategies in the future.
Table 1. Pathophysiology of thromboembolism in AF and stroke: vessel wall abnormalities and abnormal blood flow.
Table 2. Pathophysiology of thromboembolism in AF and stroke: abnormal blood constituents.
2.1. Abnormal atrial wall structural changes
As to the first limb of Virchow’s, abnormal changes in the structure and anatomy of the left atrial wall may lead to the development of atrial fibrillation and contribute to promoting a prothrombotic environment () [Citation47–57].
Masawa et al. [Citation47] described a ‘rough endocardium’ attributable to a wrinkled appearance of the left atrial endocardium due to edema and fibrous thickening in autopsy patients with AF and cerebral embolism. Almost all patients identified with ‘rough endocardium,’ had changes of mural microthrombi on light microscopy. These changes in AF patients may suggest endocardial injury and represent the established knowledge that AF is associated with structural remodeling of the left atrium, the hallmark of which is atrial fibrosis. The pathogenesis of atrial fibrosis is highly complex and thought to involve numerous mechanisms on a cellular and neurohormonal level, which are not fully understood. There is an important interplay of the previously neglected innate immunity pathways, through the local activation of inflammasome [Citation58], a process that leads to cardiac inflammation and myocyte loss, leading to abnormal activation and proliferation of cardiac fibroblasts and differentiation into myofibroblasts, followed by the excessive synthesis and deposition of extracellular matrix (ECM) proteins [Citation59,Citation60]. The abnormal ECM homeostasis is reflected in the upregulation of matrix metalloproteinases (MMPs) and downregulation of tissue inhibitors of metalloproteinases (TIMPs) [Citation59,Citation61]. The increased expression of collagen forms a proarrhythmogenic substrate for the initiation and perpetuation of AF. Abnormal levels or ratios of MMPs and TIMPs as markers of atrial remodeling and endothelial dysfunction have been demonstrated in AF with some contradictory results [Citation61,Citation62]. Although elevated MMP-2 has been associated with stroke, MMPs and TIMPs have not been generally studied in patients with stroke associated with AF.
Elevated Von Willebrand factor (vWF) as a marker of endothelial damage and dysfunction is associated with an increased stroke risk in AF patients [Citation48,Citation49]. CRP marking the role of inflammation in AF has been shown to be positively correlated to stroke risk [Citation63]. Whether atrial fibrosis is the causative link to AF or merely a marker of underlying disease, and whether atrial fibrosis leads to ischemic stroke is unclear, since the nature of the relationship between atrial fibrosis, AF, and stroke has not been fully elucidated [Citation64].
Nevertheless, there is growing evidence of the association between left atrial fibrosis (as detected in cardiac magnetic resonance imaging) and stroke in patients with AF, and that in this group of patients, atrial fibrosis is a predictor of cerebrovascular events [Citation50,Citation51] or atrial thrombosis [Citation52]. In particular, a greater amount or severity of left atrial fibrosis correlates with an increased risk of stroke or thrombus [Citation50–52]. Compared to patients with ischemic stroke from other causes, patients with cryptogenic stroke were found to have a higher percentage of left atrial fibrosis similar to that found in patients with cardioembolic stroke [Citation53]. Recent studies have found that even in the absence of AF, patients with ESUS had high atrial fibrosis, supporting the notion that atrial substrate changes may precede the development of AF and atrial fibrosis may be associated with cardioembolism independently of the presence of AF [Citation54,Citation55]. In patient-derived simulated models for the evaluation of the arrhythmogenic potential of fibrotic substrate in post-stroke-ESUS patients and in pre-ablation AF patients, similar fibrosis levels were found in inducible ESUS models as in AF models suggesting similar substrate properties in the two groups of patients [Citation56]. Changes in atrial wall including atrial fibrosis, which may precede the clinical identification of AF and be linked to ischemic stroke are collectively described as atrial cardiopathy [Citation65,Citation66]. Its clinical correlation and the effect of oral anticoagulation in these patients are currently being investigated in the AtRial Cardiopathy and Antithrombotic Drugs In prevention After cryptogenic stroke (ARCADIA) randomized trial [Citation67].
2.2. Abnormal blood flow
Abnormal blood flow or abnormal blood stasis in the left atrium promotes an environment for thrombogenesis () [Citation68–81]. Left atrial enlargement, which may represent the burden of atrial wall changes in patients with AF, is a common finding in patients with prolonged AF and promotes blood stasis [Citation82,Citation83]. Reduced left atrial appendage flow suggests aberrant blood stasis and may be associated with an increased risk of thrombus formation and subsequent thromboembolism [Citation68]. Several studies suggested that left atrial enlargement may be associated with an increased risk of stroke [Citation69,Citation70] and may serve as a potential marker of recurrent cardioembolic or cryptogenic stroke in ischemic stroke patients [Citation71].
Abnormal blood stasis may be reflected and visualized in the form of spontaneous echo contrast (SEC), an active smoke-like signal seen in the left atrium on transesophageal echocardiogram [Citation72]. The identification of SEC and increased density of SEC have been associated with increased risk of thromboembolic events and stroke in patients with AF [Citation68,Citation72,Citation73,Citation75,Citation84]. SEC may be predictive of subsequent stroke and poor long-term outcome following stroke [Citation85] [Citation76]. The identification of both SEC and left atrial enlargement has been associated with death from stroke [Citation86]. SEC has been associated with left atrial thrombus as an important risk factor in suspected cardioembolic stroke that is independent of AF [Citation77].
2.3. Platelet and coagulation abnormalities
The third limb of Virchow’s triad, abnormal blood constituents, specifically proteins involved in the coagulation cascade and platelet aggregation, is well supported in AF () [Citation48,Citation49,Citation87–129]. However, no single plasma marker has been shown to reliably predict stroke in AF. In AF, enhanced platelet activation, by a prevalent increase of β-thromboglobulin, platelet fragment 4, P-selectin, and platelet microparticles, is well described [Citation87,Citation130,Citation131]. However, the importance and clinical correlation of these factors in patients with AF and ischemic stroke is unclear [Citation87–90,Citation126].
Mean platelet volume (MPV) has been associated with increased platelet reactivity and aggregation in patients with myocardial infarction [Citation132] and those with previous ischemic stroke or transient ischemic attack (TIA) [Citation133]. Among patients with AF, several studies suggest that higher levels of MPV significantly increased risk of ischemic stroke [Citation91–96]. Similarly, platelet distribution width (PDW) was associated with an increased risk of stroke in patients with AF [Citation134]. Although these studies suggest that MPV and PDW may serve as potential new and cost-effective biomarkers for prediction of stroke risk in AF patients [Citation93,Citation97], their validity and clinical relevance remains unclear.
Several studies suggested a potential association of von Willebrand factor (vWF) and D-dimer among patients with AF and ischemic stroke [Citation90,Citation98–102]. Among AF patients, vWF increase was associated with higher risk of ischemic stroke and adverse cardiovascular events [Citation48,Citation49,Citation103–111,Citation135,Citation136], while both D-dimer and vWF have been associated with ischemic stroke severity and prognosis [Citation104,Citation112]. Still, the evidence related to the clinical significance of D-dimer levels in patients with AF is conflicting [Citation106,Citation113–121]. Other coagulation markers such as fibrinogen [Citation98], thrombin-antithrombin complexes [Citation122–124], prothrombin fragments 1 + 2 [Citation99,Citation123,Citation124], plasminogen activator inhibitor-1 [Citation125], and antithrombin III [Citation119] have been associated with higher risk of stroke in AF patients, yet their clinical significance remains unclear.
3. Diagnosis and screening
Atrial fibrillation is a dynamic arrhythmia with a broad spectrum of clinical and electrophysiological findings. Although AF diagnosis is based on surface ECGs [Citation137], the increasing use of several screening modalities, especially among patients with cryptogenic stroke, significantly increased our ability to identify atrial fibrillation during follow-up.
An important proportion of patients with cryptogenic stroke might have undiagnosed asymptomatic AF [Citation37]. It is suggested that stroke patients should undergo at least 24 to 72 hours ECG ambulatory monitoring to identify potential AF episodes [Citation137,Citation138]. In patients with ESUS, a 72 hours Holter monitor is used to further investigate the presence of AF, and although no consensus has been reached regarding prolonged monitoring, several non-invasive or implantable monitors can considerably increase the detection of AF following a stroke [Citation36,Citation139,Citation140]. Indeed, clinical guidelines have issued recommendations for more prolonged monitoring following cryptogenic stroke [Citation137,Citation141], including a Class IIa recommendation from the European Society of Cardiology (ESC) for either longer term ECG monitoring or an insertable cardiac monitor [Citation137]. As it is thought that the proportion of strokes associated with AF may be higher than estimated, by sequentially combining the various methods of cardiac monitoring using a tiered approach, it might be possible to detect new AF in almost one-quarter of patients with stroke or TIA [Citation142].
Although systematic screening of the population for AF is not generally recommended largely due to considerations concerning cost-effectiveness and net-benefit, some guidelines suggest screening in groups at high-risk of stroke or in those aged 75 and over [Citation137,Citation143]. Otherwise, opportunistic screening for AF by pulse check and 12-lead ECG has been recommended for patients aged 65 and over, or those in high-risk groups such as hypertensive patients [Citation137]. With the unfolding of new technologies, new portable and wearable devices, such as patch sensors, smartphones, smartwatches, wristbands, and rings, could develop into useful tools for screening for AF and lead to increased detection of AF [Citation144–146].
3.1. Device-detected and subclinical AF
Modern cardiac implantable electronic devices (CIED), which include dual chamber permanent pacemakers, cardiac resynchronization therapy devices, implantable cardioverter-defibrillators, and implantable loop recorders (ILR), are capable of monitoring and recording atrial tachyarrhythmias. Stored episodes have been found to be well correlated with AF, particularly when they have an atrial rate of more than 250 complexes per minute or a duration exceeding 5 minutes [Citation147]. Such atrial asymptomatic tachyarrhythmias, in patients who have no previous history of clinical or documented AF, are known as atrial high-rate episodes (AHRE), a term often used interchangeably with ‘subclinical AF.’ Several studies have shown that AHRE are associated with an increased risk of stroke and thromboembolism () [Citation148–160], while patients with AHRE are at increased risk of developing clinical AF [Citation148,Citation151,Citation160].
Table 3. Subclinical AF, AHRE, and stroke risk.
Current data suggest that AHRE increase the risk of stroke or systemic embolism when they last for at least 30 seconds in patients with CIED [Citation161–163]. Among patients with previous cryptogenic stroke who undergo continuous monitoring with ILR, AHRE lasting greater than 2 minutes significantly increased the risk of future stroke or systemic embolism [Citation161,Citation164,Citation165]. However, it is still unclear, especially in primary stroke prevention, whether the potential benefits of OAC will not be outweighed by the risks of bleeding [Citation166,Citation167]. AF burden greater than 5 minutes is associated with an increased risk of both clinical AF and stroke [Citation168]. A high burden of AHRE of 5.5 hours or more, occurring within 30 days of stroke, doubled the risk of thromboembolism compared to a lower burden of AHRE [Citation150]. The risk was found to be highest in the initial period of 10 days after the AHRE and rapidly diminished after longer periods [Citation154,Citation169].
Several studies have found the lack of any temporal relationship between the detected AHRE and stroke, with few patients having AHRE within the month before their stroke or patients were not in AF at the time of the stroke [Citation152,Citation156,Citation170,Citation171], raising the inference that the pathogenesis of stroke and thromboembolism may involve mechanisms other than AF [Citation156]. Rather, AF may represent the marker of other mechanisms or conditions leading to stroke instead of a risk factor for stroke [Citation172,Citation173]. However, the studies were open to several biases including the lack of any adjudication of strokes to cardioembolic or non-cardioembolic strokes [Citation174].
The apparent lack of a temporal association between subclinical AF and stroke adds to the controversy surrounding the burden or length of duration of subclinical AF that would justify commencing anticoagulant therapy [Citation175], until we have the results of a number of randomized controlled trials evaluating the implications. The ESC guidelines [Citation137] suggest that anticoagulant therapy may be considered in selected patients with longer durations of AHRE of at least 24 hours who are at high risk of stroke if a net clinical benefit can be expected [Citation137].
3.2. Prediction of incident AF in cryptogenic stroke
Although various scores have been proposed to predict the onset of new AF, including the Framingham Heart Study score [Citation176] and CHARGE-AF score [Citation177], they have not been widely used in practice. The scores were designed to allow early identification of patients at risk who would benefit from timely targeted intervention and prevention. Similarly, risk scores have been proposed for post-stroke patients, particularly cryptogenic stroke patients. As clinical and radiological methods lack sensitivity for identifying patients suspected of having had a cardioembolic stroke, attempts at devising an algorithm to guide management and investigation, selection of patients for longer-term monitoring, and stroke prevention have been made in a number of observational studies.
These published scores include the STAF [Citation178], LADS [Citation179], NDAF [Citation180], Intermountain AF [Citation181], HAVOC [Citation182], CHA2DS2-VASc [Citation183], C2HEST[Citation184], AS5F [Citation185], CHASE-LESS [Citation186], AF-ESUS [Citation187], Decryptoring [Citation188], Brown ESUS-AF [Citation189], Graz AF Risk [Citation190] and SAFE [Citation191] scores for predicting AF, and iPAB [Citation192] and Fujii [Citation193] scores for predicting paroxysmal AF in acute ischemic stroke patients. As the risk scores identified performed variably in their discriminative ability and the utility of these scores to predict newly detected AF in clinical practice remains uncertain [Citation194], none have been generally adopted in current clinical practice.
A tailored approach to patient selection for longer-term cardiac monitoring or comprehensive predictive models that adopt multimodal biomarkers for predicting newly detected AF after cryptogenic stroke may be more discriminating [Citation195]. The markers can range from clinical, ECG, and blood-based biomarkers [Citation195,Citation196] to echocardiographic and brain imaging biomarkers [Citation188–193]. Clinical variables associated with greater likelihood of newly detected AF following cryptogenic stroke include older age, female sex, hypertension, heart failure, ischemic heart disease, diabetes, treatment with statin, being a non-smoker, higher National Institutes of Health Stroke Scale or modified Rankin Scale scores, and IV thrombolysis treatment [Citation195,Citation196].
ECG markers linked with higher likelihood of AF detection include frequent premature atrial contractions, left ventricular hypertrophy, atrioventricular block, as well as more prolonged PR interval, P-wave duration, P-wave dispersion, P-wave index, and QTc interval [Citation195,Citation197,Citation198]. Associated blood biomarkers include NT-proBNP [Citation188,Citation190,Citation192,Citation193,Citation195,Citation199] and high-density lipoproteins [Citation195]. Echocardiographic and radiological biomarkers linked with AF detection after cryptogenic stroke include left atrial enlargement [Citation189–191,Citation193], decreased left atrial strain [Citation188,Citation200], reduced left ventricular ejection fraction [Citation198], prior cortical or cerebellar infarction [Citation37,Citation190], cortical topography [Citation191], intracranial large vessel occlusion [Citation191], and multi-territory brain infarction [Citation190].
AF as the suspected cause of cryptogenic stroke may never be found. However, commencing OAC for stroke thromboprophylaxis for suspected cardioembolic stroke in such cases is not recommended. Two randomized controlled trials, the Rivaroxaban Versus Aspirin in Secondary Prevention of Stroke and Prevention of Systemic Embolism in Patients with Recent Embolic Stroke of Undetermined Source (NAVIGATE ESUS) and the Dabigatran Etexilate for Secondary Stroke Prevention in Patients with Embolic Stroke of Undetermined Source (RE-SPECT ESUS), evaluated OAC for the prevention of recurrent stroke following ESUS. In the NAVIGATE ESUS trial, rivaroxaban was found to be non-superior to aspirin for prevention of recurrent stroke after an initial ESUS but was associated with a higher risk of bleeding [Citation201]. The RE-SPECT ESUS trial made similar findings regarding the non-superiority of dabigatran compared to aspirin, though non-major bleeding events but not major bleeding events were greater [Citation202]. The results might be explained by the heterogeneity of the ESUS population involved in the studies where patients were even included who had large artery atherosclerosis resulting in less than 50% occlusion or aortic atherosclerosis [Citation203].
4. Management
4.1. Stroke prevention
4.1.1. Stroke risk stratification
Stroke risk is not homogenous but dependent on various risk factors, which have been incorporated into clinical stroke risk stratification algorithms, all of which have only modest predictive value for identifying patients at high risk of stroke. However, being based on clinical risk scores, they are appealing for their simplicity and convenience for use in daily clinical practice and decision-making. The most commonly adopted stratification scores are CHADS2 and CHA2DS2-VASc [Citation204], which have been widely validated [Citation205,Citation206]. Low-risk patients, those having a CHA2DS2-VASc score of 0 in men or 1 in women, with a rate of stroke of less than 1% per year do not require any antithrombotic therapy. Any score above that due to the presence of at least one stroke risk factor triggers the requirement to consider OAC with either a VKA or NOAC. Clinical risk scores do not remain static over time but are dynamic and likely to change with additional comorbidities and age.
Prior to commencing OAC, an evaluation of bleeding risk using a validated bleeding risk score such as the HAS-BLED score is recommended with the goal of reducing modifiable bleeding risk factors (such as uncontrolled hypertension, labile INR, excessive use of alcohol, or concomitant drugs predisposing to bleeding). Patients with a high HAS-BLED score will benefit from more frequent review. A high HAS-BLED score by itself is rarely a cause to avoid OAC [Citation207].
As more predictors of stroke risk become known, they may be reflected in risk stratification schemes to make scores more discriminating. For example, echocardiographic markers (such as spontaneous echo contrast on transesophageal echocardiography and left ventricular systolic dysfunction on transthoracic echocardiography) and blood biomarkers may be relevant, as well as electrocardiographic markers of atrial cardiomyopathy such as abnormal p-wave axis [Citation208,Citation209]. Potential new markers of stroke risk are being recognized with time. White matter changes and chronic intracranial arterial calcification are being recognized as risk factors for stroke [Citation210,Citation211]. Mitral annular calcification was found to be an independent predictor of cardioembolic stroke in elderly patients with AF [Citation212].
4.1.2. Appropriate antithrombotic therapy
The cornerstone of management of AF is stroke prevention. In patients with AF, the use of OAC, such as a VKA like warfarin or a NOAC, is recommended, reducing the risk of stroke by approximately 60% and lowers all-cause mortality [Citation213]. Multicenter Phase III randomized controlled trials (RCTs) have confirmed that NOACs are as efficacious as warfarin, providing a safety profile for patients with AF [Citation22–25]. In these trials, individual NOACs provided comparable results in terms of efficacy and safety compared to warfarin. Despite the signs of potential superiority of one NOAC compared to others in relation to their effectiveness and safety [Citation214] including in AF patients with chronic kidney disease [Citation215], the lack of head-to-head comparison in RCTs and the diverse patients’ characteristics in RCTs and observational studies do not allow for safe conclusions.
Nevertheless, the risk of bleeding remains an impediment to the use of and adherence to OAC. There exists developing evidence that activated coagulation factor XI (factor XIa) may provide a target for a next-generation NOAC with advantages over conventional factor X inhibitors in terms of lower risk of major bleeding. A recent randomized Phase II dose-finding study of asundexian, a direct inhibitor of factor XIa, observed significantly lower rates of bleeding with asundexian compared to apixaban in patients with AF [Citation216]. The trial paves the way for larger studies exploring the efficacy, safety, and incidence of major bleeding events in factor XIa inhibition in patients with AF at risk of stroke.
Even in the presence of stable coronary artery disease, OAC monotherapy is effective in preventing both stroke or systemic embolism and new coronary artery events. Recently, rivaroxaban monotherapy was found to be noninferior to a combination of rivaroxaban and antiplatelet treatment in patients with AF and stable coronary artery disease, for the composite outcome of stroke, systemic embolism, myocardial infarction, unstable angina requiring revascularization, or death from any cause (HR: 0.72; 95% CI, 0.55 to 0.95; P < 0.001 for noninferiority), while it was associated with a significantly lower risk of major bleeding (HR: 0.59, 95% CI, 0.39 to 0.89; P = 0.01 for superiority) [Citation217]. This was also confirmed in meta-analysis including both randomized and observational studies, where OAC monotherapy was as effective as OAC combined with antiplatelet, but significantly associated with a reduced risk of bleeding [Citation218]. The position is less clear where other stable vascular diseases (such as carotid artery or peripheral artery disease) co-exist with AF. Although It is thought that OAC monotherapy should suffice, actual practice may differ between clinicians [Citation219].
Although anticoagulation treatment and stroke prevention in AF might be straightforward, in the acute and early post-stroke phase, the decision to start anticoagulation may vary. In the early post-stroke period, especially in patients with AF, the risk of a recurrent event is significantly high [Citation220]. Despite this increased risk of recurrent event, anticoagulation in the acute and early phase of ischemic stroke is contraindicated, especially in patients with large ischemic strokes due to the risk of hemorrhagic transformation. Hence, the optimal time in which the risk of recurrent stroke outweighs the bleeding risk and subsequently when to start anticoagulation in these patients is still not clear. This might be answered by several RCTs, comparing the early to late initiation of NOACs in patients with AF-related ischemic strokes [Citation221–224]. One of the RCTs has published its results. In the Timing of Oral Anticoagulant Therapy in Acute Ischemic Stroke With Atrial Fibrillation: a Prospective Multicenter Registry-based Non-inferiority Randomized Controlled Clinical Trial (TIMING), a study involving 888 patients with AF, early initiation of NOAC at 4 days or less from onset of acute ischemic stroke was noninferior to delayed start at between 5 and 10 days from stroke onset [Citation225]. In TIMING, no patient suffered a symptomatic intracerebral hemorrhage in either the early or delayed groups and the early group had a numerically lower rate of recurrent stroke and death. While waiting for the results of all the RCTs, there are also observational cohort data suggesting that the relative risk of recurrent stroke and symptomatic intracerebral hemorrhage may be highest in the first 2 days after a stroke before attenuating to become constant over time. Thus, early introduction of OAC 2–3 days after a stroke was associated with considerably fewer recurrent stroke events over the ensuing weeks without excess risk of symptomatic intracerebral hemorrhage [Citation226].
The European Heart Rhythm Association of the European Society of Cardiology (EHRA-ESC) introduced the ‘1–3–6–12 days rule’ in 2013, depending on the neurologic deficit of the stroke patient [Citation227]. Nevertheless, in view of the lack of solid evidence on the time of OAC initiation, current guidelines suggest that OAC should be initiated or re-initiated as soon as possible, usually within the first 2 weeks following an acute stroke [Citation137].
Although NOACs have predictable pharmacokinetic properties with rapid onset and need for dose adjustment in special populations such as patients with chronic kidney disease, the elderly, and those with low bodyweight that need to be considered to optimize their benefit-risk profile, the pharmacokinetic modeling of NOACs has rarely been studied in a post-stroke population in contrast to the data gathered from healthy subjects [Citation228–232]. In the earlier Phase III RCTs comparing individual NOACs with warfarin, as patients who had experienced a stroke within days of randomization were excluded from those studies, the efficacy and safety of NOACs in acute stroke patients who may commonly be older, or have renal impairment associated with the effects of acute ischemic stroke, is unknown [Citation232]. Limited observational data in a small study examined the anticoagulation intensity of rivaroxaban in stroke patients in Japan, the majority of whom were enrolled soon after stroke onset and commenced rivaroxaban within a median of 5 days [Citation232]. The ongoing RCTs comparing the early to late initiation of NOACs after acute ischemic stroke in AF patients may shed light on the pharmacokinetic implications and efficacy and safety of individual NOACs and dosing regimens in an acute ischemic stroke setting.
Notwithstanding OAC therapy, there remains a residual risk of treatment failure. Observational data suggests that patients with AF who suffer a stroke while on treatment with OAC are at high risk of recurrent ischemic stroke. Furthermore, changing the type of OAC by switching between VKA and NOAC or from one NOAC to another was not associated with a decreased risk of recurrent ischemic events. Thus, the optimal approach to secondary prevention to reduce the risk of further recurrent events in this high-risk group of patients remains uncertain [Citation233–235]. Although there may be a benefit from alternative strategies, such as left atrial appendage occlusion, there is currently limited evidence on the benefits. However, The Left Atrial Appendage Occlusion Versus Novel Oral Anticoagulation for Stroke Prevention in Atrial Fibrillation Multicenter Randomised Clinical Trial (Occlusion-AF), currently recruiting, will compare left atrial appendage occlusion to NOAC treatment for secondary stroke prevention in patients with AF and a recent stroke or TIA at high risk of recurrent thromboembolic events [Citation236].
4.1.3. Left atrial appendage occlusion
In patients who are unable to tolerate OAC, left atrial appendage occlusion (LAAO) may be a potential alternative treatment modality in patients with AF, especially after an ischemic stroke. Patients who do not have a contraindication to short-term antithrombotic use may be suitable. The WATCHMAN Left Atrial Appendage System for Embolic PROTECTion in Patients With Atrial Fibrillation (PROTECT AF) and the Prospective Randomized Evaluation of the WATCHMAN LAA Closure Device in Patients with Atrial Fibrillation (AF) Versus Long Term Warfarin Therapy (PREVAIL) trials showed that LAAO was non-inferior to warfarin in stroke prevention in patients with AF [Citation237,Citation238]. In the more recent Interventional Left Atrial Appendage Closure vs. Novel Anticoagulation Agents in High-risk Patients With Atrial Fibrillation (PRAGUE-17) trial, LAAO using an Amulet or Watchman device was noninferior to NOAC in preventing major cardiovascular, neurological, and bleeding events related to AF in high-risk patients [Citation239].
During the period of endothelialization following implantation of an LAAO device, antithrombotic therapy is essential for reducing the risk of thromboembolism. The optimal post-procedural antithrombotic regime, however, is unclear, and clinical practice has tended to vary, with registries indicating majority use of dual antiplatelet therapy (DAPT) in Europe, and OAC plus antiplatelet in contemporary U.S. practice [Citation240,Citation241]. However, recent studies suggest that OAC monotherapy may potentially be considered as an alternative post-procedural antithrombotic strategy. A randomized pilot study found reduced thrombin generation following LAAO in patients treated with reduced-dose rivaroxaban rather than DAPT [Citation242]. In a meta-analysis, including mainly single-arm studies, OAC had a better efficacy and safety profile than antiplatelet therapy favoring OAC over DAPT as anti-thrombotic therapy following LAAO [Citation243]. In a recent study, post-procedural OAC without concomitant aspirin was associated with lower risk of adverse outcomes [Citation241].
4.2. Rhythm control
Rhythm control may be achieved through pharmacological and non-pharmacological means. Antiarrhythmic drugs are commonly used for restoration and maintenance of sinus rhythm. Catheter ablation is an alternative to medical therapy for rhythm control in AF. Catheter ablation of AF is usually performed through the standard approach of pulmonary vein isolation. The two most frequently used techniques for pulmonary vein isolation are radiofrequency ablation and cryoablation [Citation244]. Radiofrequency ablation is the most common method and involves the application of a radiofrequency current to heat tissue and achieve cellular necrosis. Cryoablation entails the application of cryogenic energy with a balloon to induce tissue necrosis by freezing.
Guidelines have historically recommended rhythm control for improvement of symptoms and quality of life in symptomatic patients with AF [Citation137]. Until recently, rate control was thought to be equivalent to rhythm control. The Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) trial demonstrated that rhythm control (using an antiarrhythmic drug and, if necessary, cardioversion) offered no survival benefit over rate-control [Citation245]. Similarly, the Rate Control versus Electrical Cardioversion for Persistent Atrial Fibrillation (RACE) trial demonstrated that rate control is not inferior to rhythm control for preventing cardiovascular death and morbidity in patients with recurrent persistent AF after cardioversion [Citation246].
However, the Early Treatment of Atrial Fibrillation for Stroke Prevention Trial (EAST-AFNET 4) RCT showed that, among 2789 patients with early AF diagnosed within 12 months who were randomized to either early rhythm control or usual care, a rhythm control strategy was associated with a lower risk of stroke, hospitalization for heart failure, and cardiovascular death [Citation247]. Following the landmark EAST-AFNET 4 trial, clinical practice now tends to favor adopting an early rhythm control strategy including ablation therapy early in the diagnosis of AF. Nevertheless, an ancillary analysis from the ESC-European Heart Rhythm Association (EHRA) EURObservational Research Programme (EORP) AF (ESC‐EHRA EORP‐AF) General Long-Term Registry, conducted to evaluate real-world applicability and impact of the EAST-AFNET 4 study, showed a lower rate of major adverse events, but no significant difference in the primary outcome of cardiovascular death, stroke, acute coronary syndrome, and worsening of heart failure [Citation248]. Although cardioversion seems beneficial in patients with AF, whether patients with paroxysmal AF detected after stroke benefit from cardioversion is still not clear. An observational study showed that sinus rhythm restoration, either with the use of antiarrhythmic medication or in patients with self-terminated paroxysmal AF, was associated with a 36% reduction of overall mortality and led to reduction in both stroke recurrence and MACE by 46% [Citation249]. Similarly, the Risk and Benefits of Urgent Rhythm Control of Atrial Fibrillation in Patients With Acute Stroke (RAFAS) randomized trial recently showed that post-stroke early rhythm control significantly reduced recurrent events within 1 year [Citation250]. These findings suggest that even in the early post-stroke phase, cardioversion and sinus rhythm restoration may benefit future cardiovascular outcomes.
When comparing pharmacological antiarrhythmic therapy with ablative therapy for rhythm control, no clear difference in reduction of stroke risk has been demonstrated. Although several observational studies found that catheter ablation reduces the risk of stroke compared to antiarrhythmic drug therapy in high-risk patients, no difference in stroke risk has been observed in randomized trials [Citation251]. The recent Catheter Ablation vs Anti-arrhythmic Drug Therapy for Atrial Fibrillation Trial (CABANA) showed that compared to antiarrhythmic drug therapy, catheter ablation did not significantly lower the primary composite end point of disabling stroke, death, bleeding, or cardiac arrest [Citation252]. Only randomized controlled trials have demonstrated mortality benefit from catheter ablation in patients with heart failure with left ventricular systolic dysfunction [Citation251].
Until recently, catheter ablation has been recommended for rhythm control after failed antiarrhythmic drug therapy. However, recent evidence from the randomized trials Early Aggressive Invasive Intervention for Atrial Fibrillation (EARLY-AF), Catheter Cryoablation Versus Antiarrhythmic Drug as First-Line Therapy of Paroxysmal Atrial Fibrillation (Cryo-FIRST), and STOP AF First: Cryoballoon Catheter Ablation in an Antiarrhythmic Drug Naive Paroxysmal Atrial Fibrillation (STOP AF First) demonstrates that ablation is superior to antiarrhythmic drug therapy for reducing recurrence of AF [Citation253–255]. Similarly, the Atrial Fibrillation Progression Trial (ATTEST) suggests radiofrequency ablation is superior to antiarrhythmic drugs for delaying the progression of paroxysmal AF to persistent AF [Citation256].
4.3. Integrated care in AF and in stroke: Atrial fibrillation Better Care (ABC) pathway
Although stroke is relatively infrequent in anticoagulated patients, even in large and well-conducted anticoagulation RCTs, the annual rate of ischemic stroke in AF patients despite OAC was 1–2% [Citation257–261], while observational studies suggest that this might be higher reaching 5% [Citation262,Citation263]. Also, mortality associated with AF doubled between 1990 and 2010 [Citation264]. Due to its multifactorial background and the coexistence of several cardiovascular risk factors in patients with AF, a more holistic or integrated care approach in AF management has been promoted in recent years [Citation265–267].
The Atrial fibrillation Better Care (ABC) holistic pathway (the ABC pathway) is an example of such approach and is increasingly recommended by International guidelines [Citation137,Citation265,Citation268–270]. The three pillars of the ABC pathway are ‘A’ – Anticoagulation/Avoid stroke, ‘B’ – Better symptom control (with patient centered, symptom directed decisions on rate or rhythm control), and ‘C’ – Cardiovascular risk factors and Comorbidities management, including lifestyle changes. It has consistently been shown that the ABC pathway is associated with improved clinical outcomes and a significant reduction in adverse outcomes [Citation271–277].
In accordance with this notion, a recent position paper of the European Society of Cardiology Council of Stroke proposed an integrated care approach for optimization of ‘general’ stroke management and associated cardiovascular disease in the form of a post-stroke ABC pathway [Citation219]. Along the lines of the AF ABC pathway, the post-stroke ABC pathway includes three pillars of care: ‘A’ – Appropriate Antithrombotic therapy, ‘B’ – Better functional and psychological status, and ‘C’ – Cardiovascular risk factors and Comorbidity optimization (including lifestyle changes) [Citation219]. In the context of AF, NOACs are preferred over VKAs due to their favorable safety profile, while patients should undergo a multidisciplinary evaluation to recognize post-stroke depression and dementia, together with the optimization of cardiovascular comorbidities and risk factors [Citation219].
5. Expert opinion
More research is needed into the paradigm of interaction between AF, thrombogenesis and stroke, and the pathways that lead to abnormal development of the atrial substrate that favors the generation of arrhythmia. The hope is that the knowledge gained will help to identify novel markers of stroke risk for refining current models of risk stratification and new molecular targets for treatment and stroke prevention. The renin-angiotensin-aldosterone system may play an important role in the development of atrial fibrosis, as has already been shown in experimental canine models, where angiotensin-converting enzyme inhibition suppressed atrial fibrosis [Citation278,Citation279], while sacubitril/valsartan was associated with reduction in atrial fibrosis in mice [Citation280]. Recently, the new coronavirus 2019 pandemic brought up the importance of angiotensin-converting enzyme 2 (ACE 2), which, apart from serving as the virus’ functional cell receptor [Citation281], among others, may provide important information on pathophysiology of several cardiovascular diseases including AF and atrial fibrosis [Citation282]. These data may provide further knowledge on the evaluation and treatment of patients with AF or atrial fibrosis [Citation283–285].
Accumulating evidence and the expanding research interest associated with the use of artificial intelligence (AI) and machine learning (ML) may provide future perspectives for risk stratification and stroke prevention in patients with AF. As multimorbidity risk factors and AF predispose to stroke in a dynamic way, AI technics may help in the optimization of the preventive and treating pathways. Research continues to take advantage of AI and ML techniques for identifying and recognizing imaging and electrocardiographic markers of stroke risk. In silico models are proving highly useful to simulate computational models to predict outcomes, providing a way to examine the effect of several interventions based on artificial models. For example, to assess the pathophysiological link between atrial fibrillation and stroke, potential pro-arrhythmic substrate properties of fibrosis have been assessed through patient- and magnetic resonance imaging-derived inducible in silico models to computationally predict the presence of triggers, and re-entrant drivers, needed for perpetuation of AF [Citation56].
Although many cases of cryptogenic stroke are suspected to be caused by AF, these patients will never get the chance to reduce their further thromboembolic risk if they will not undergo an extensive search for AF. More research is much needed in this area, along with more refined RCT evidence for the efficacy of NOACs in stroke prevention in ESUS. In the meantime, advancements in information technology and the increasing use of smartwear and smartphones by the general public will provide more opportunities for detecting arrhythmias. As demonstrated in the Apple Heart Study (Assessment of Wristwatch-Based Photoplethysmography (PPG) to Identify Cardiac Arrhythmias) [Citation145], the Mobile Health Technology for Atrial Fibrillation Screening Using Photoplethysmography-Based Smart Devices (The HUAWEI Heart Study) [Citation144] and the Fitbit Heart Study (Detection of Atrial Fibrillation in a Large Population Using Wearable Devices) [Citation146], continuous monitoring with PPG-based smartwear could be feasible for screening and early detection of AF in large populations. In the Apple Heart Study, notifications of an irregular pulse had an 84% positive predictive value for concurrent AF, while in the HUAWEI Heart Study, 91.6% of PPG-positive signals were confirmed as AF. In the Fitbit Heart Study, the PPG software algorithm for Fitbit devices resulted in a positive predictive value of 98.2%.
As mortality associated with AF remains high and there is still a five-fold increased risk of having a stroke in patients with AF, a holistic integrated care approach to managing AF and stroke prevention will continue to be essential, with greater development of multidisciplinary inputs. Care may involve more sophisticated and structured lifestyle programs such as weight reduction, diet, and physical exercise programs to address the pro-arrhythmic consequences of obesity. More integrated involvement of sleep disorder units and specialists in treatment of conditions like obstructive sleep apnea will be important. With the advent of more sophisticated smart technology, mobile health apps are likely to feature, which will help enhance patients’ knowledge of their own conditions, and encourage greater involvement in their clinical care[Citation286]. Nevertheless, there is a recognition that OAC therapy is still underused in practice in the contemporary high-risk population of stroke survivors with AF, especially individuals of older age or those affected by socioeconomic deprivation. Thus, there is a need to identify barriers to OAC and develop strategies to improve prescription of OAC treatment [Citation287].
Article highlights
Atrial fibrillation (AF) is a common cause of ischemic stroke, and AF-related stroke is associated with higher severity and mortality than non-AF stroke.
A better understanding of the interplay between pathophysiological mechanisms of thrombogenesis, AF, and stroke will help to identify new targets for treatment and stroke prevention in patients with AF.
Although AF is diagnosed on surface ECGs, the increasing use of several screening modalities and predictive models that adopt multimodal biomarkers will enhance our ability to detect new AF during follow-up for acute ischemic stroke.
Although oral anticoagulation following ischemic stroke in AF patients is crucial for prevention of recurrent strokes, the optimal time for starting or restarting anticoagulation remains to be determined by several RCTs comparing early to late initiation of oral anticoagulants.
Adopting an early rhythm control strategy including ablation early in the diagnosis of AF after stroke may lower the risk of recurrent stroke.
A holistic integrated care approach associated with reduction in adverse outcomes, such as the Atrial fibrillation Better Care (ABC) pathway, will continue to be essential.
Declaration of interests
GYH Lip is a consultant and speaker for BMS/Pfizer, Boehringer Ingelheim, Daiichi-Sankyo, and Anthem. No fees have been personally received. D Sagris has received research support from the European Society of Cardiology Council on Stroke. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- World Health Organisation. Global health estimates: life expectancy and leading causes of death and disability. Cited 8 Dec 2022. Available from: https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates.
- Lane DA, Skjøth F, Lip GYH, et al. Temporal trends in incidence, prevalence, and mortality of atrial fibrillation in primary care. J Am Heart Assoc. 2017 Apr 28;6(5):e005155.
- Rahman F, Kwan GF, Benjamin EJ. Global epidemiology of atrial fibrillation. Nat Rev Cardiol. 2014 Nov;11(11):639–654.
- Majeed A, Moser K, Carroll K. Trends in the prevalence and management of atrial fibrillation in general practice in England and Wales, 1994-1998: analysis of data from the general practice research database. Heart. 2001 Sep;86(3):284–288.
- Sturm JW, Davis SM, O’Sullivan JG, et al. The Avoid Stroke as Soon as Possible (ASAP) general practice stroke audit. Med J Aust. 2002 Apr 1;176(7):312–316.
- DeWilde S, Carey IM, Emmas C, et al. Trends in the prevalence of diagnosed atrial fibrillation, its treatment with anticoagulation and predictors of such treatment in UK primary care. Heart. 2006 Aug;92(8):1064–1070.
- Naccarelli GV, Varker H, Lin J, et al. Increasing prevalence of atrial fibrillation and flutter in the United States. Am J Cardiol. 2009 Dec 1;104(11):1534–1539.
- NHS Digital (2022). The Information Centre for health and social care: Quality and Outcomes Framework 2009-10. England:NHS Digital;2010 Oct 20. Available from https://digital.nhs.uk/data-and-information/publications/statistical/quality-and-outcomes-framework-achievement-prevalence-and-exceptions-data/quality-and-outcomes-framework-2009-10.
- Friberg L, Bergfeldt L. Atrial fibrillation prevalence revisited. J Intern Med. 2013 Nov;274(5):461–468.
- Zulkifly H, Lip GYH, Lane DA. Epidemiology of atrial fibrillation. Int J Clin Pract. 2018 Mar;72(3):e13070.
- Zoni-Berisso M, Filippi A, Landolina M, et al. Frequency, patient characteristics, treatment strategies, and resource usage of atrial fibrillation (from the Italian Survey of Atrial Fibrillation Management [ISAF] study). Am J Cardiol. 2013 Mar 1;111(5):705–711.
- Gómez-Doblas JJ, Muñiz J, Martin JJ, et al. Prevalence of atrial fibrillation in Spain. OFRECE study results. Rev Esp Cardiol (Engl Ed). 2014 Apr;67(4):259–269.
- Lloyd-Jones DM, Wang TJ, Leip EP, et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation. 2004 Aug 31;110(9):1042–1046.
- Heeringa J, van der Kuip DA, Hofman A, et al. Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. Eur Heart J. 2006 Apr;27(8):949–953.
- Lippi G, Sanchis-Gomar F, Cervellin G. Global epidemiology of atrial fibrillation: an increasing epidemic and public health challenge. Int J Stroke. 2021 Feb;16(2):217–221.
- Miyasaka Y, Barnes ME, Gersh BJ, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006 Jul 11;114(2):119–125.
- Krijthe BP, Kunst A, Benjamin EJ, et al. Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur Heart J. 2013 Sep;34(35):2746–2751.
- Kammersgaard LP, Olsen TS. Cardiovascular risk factors and 5-year mortality in the Copenhagen Stroke Study. Cerebrovasc Dis. 2006;21(3):187–193.
- Marini C, De Santis F, Sacco S, et al. Contribution of atrial fibrillation to incidence and outcome of ischemic stroke: results from a population-based study. Stroke. 2005 Jun;36(6):1115–1119.
- Lin HJ, Wolf PA, Kelly-Hayes M, et al. Stroke severity in atrial fibrillation. The Framingham Study. Stroke. 1996 Oct;27(10):1760–1764.
- Jørgensen HS, Nakayama H, Reith J, et al. Acute stroke with atrial fibrillation. The Copenhagen Stroke Study. Stroke. 1996 Oct;27(10):1765–1769.
- Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011 Sep 8;365(10):883–891.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009 Sep 17;361(12):1139–1151.
- Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013 Nov 28;369(22):2093–2104.
- Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011 Sep 15;365(11):981–992.
- SPAF. Stroke Prevention in Atrial Fibrillation study. Final results. Circulation. 1991 Aug;84(2):527–539.
- Pistoia F, Sacco S, Tiseo C, et al. The epidemiology of atrial fibrillation and stroke. Cardiol Clin. 2016 May;34(2):255–268.
- Bogiatzi C, Hackam DG, McLeod AI, et al. Secular trends in ischemic stroke subtypes and stroke risk factors. Stroke. 2014 Nov;45(11):3208–3213.
- Marnane M, Duggan CA, Sheehan OC, et al. Stroke subtype classification to mechanism-specific and undetermined categories by TOAST, A-S-C-O, and causative classification system: direct comparison in the North Dublin population stroke study. Stroke. 2010 Aug;41(8):1579–1586.
- Palm F, Urbanek C, Wolf J, et al. Etiology, risk factors and sex differences in ischemic stroke in the Ludwigshafen Stroke Study, a population-based stroke registry. Cerebrovasc Dis. 2012;33(1):69–75.
- Hart RG, Catanese L, Perera KS, et al. Embolic stroke of undetermined source: a systematic review and clinical update. Stroke. 2017 Apr;48(4):867–872.
- Ntaios G, Papavasileiou V, Milionis H, et al. Embolic strokes of undetermined source in the Athens stroke registry: a descriptive analysis. Stroke. 2015 Jan;46(1):176–181.
- Hart RG, Diener HC, Coutts SB, et al. Embolic strokes of undetermined source: the case for a new clinical construct. Lancet Neurol. 2014 Apr;13(4):429–438.
- Ntaios G, Perlepe K, Lambrou D, et al. Prevalence and overlap of potential embolic sources in patients with embolic stroke of undetermined source. J Am Heart Assoc. 2019 Aug 6;8(15):e012858.
- Tayal AH, Tian M, Kelly KM, et al. Atrial fibrillation detected by mobile cardiac outpatient telemetry in cryptogenic TIA or stroke. Neurology. 2008 Nov 18;71(21):1696–1701.
- Gladstone DJ, Spring M, Dorian P, et al. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med. 2014 Jun 26;370(26):2467–2477.
- Favilla CG, Ingala E, Jara J, et al. Predictors of finding occult atrial fibrillation after cryptogenic stroke. Stroke. 2015 May;46(5):1210–1215.
- Akar JG, Marieb MA. Atrial fibrillation and thrombogenesis: innocent bystander or guilty accomplice? JACC Clin Electrophysiol. 2015 Jun;1(3):218–219.
- Xiong L, Leung H, Chen XY, et al. Preliminary findings of the effects of autonomic dysfunction on functional outcome after acute ischemic stroke. Clin Neurol Neurosurg. 2012 May;114(4):316–320.
- Chen PS, Chen LS, Fishbein MC, et al. Role of the autonomic nervous system in atrial fibrillation: pathophysiology and therapy. Circ Res. 2014 Apr 25;114(9):1500–1515.
- Heijman J, Voigt N, Nattel S, et al. Cellular and molecular electrophysiology of atrial fibrillation initiation, maintenance, and progression. Circ Res. 2014 Apr 25;114(9):1483–1499.
- Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991 Aug;22(8):983–988.
- Virchow R. Handbuch der speciellen Pathologie und Therapie. Erlangen: Enke; 1854. German.
- Lip GY. Does atrial fibrillation confer a hypercoagulable state? Lancet. 1995 Nov 18;346(8986):1313–1314.
- Choudhury A, Lip GY. Atrial fibrillation and the hypercoagulable state: from basic science to clinical practice. Pathophysiol Haemost Thromb. 2003;33(5–6):282–289.
- Watson T, Shantsila E, Lip GY. Mechanisms of thrombogenesis in atrial fibrillation: Virchow’s triad revisited. Lancet. 2009 Jan 10;373(9658):155–166.
- Masawa N, Yoshida Y, Yamada T, et al. Diagnosis of cardiac thrombosis in patients with atrial fibrillation in the absence of macroscopically visible thrombi. Virchows Arch A Pathol Anat Histopathol. 1993;422(1):67–71.
- Conway DS, Pearce LA, Chin BS, et al. .Prognostic value of plasma von Willebrand factor and soluble P-selectin as indices of endothelial damage and platelet activation in 994 patients with nonvalvular atrial fibrillation. Circulation 2003. Jul 1;107(25):3141–3145.
- Krishnamoorthy S, Khoo CW, Lim HS, et al. Prognostic role of plasma von Willebrand factor and soluble E-selectin levels for future cardiovascular events in a ‘real-world’ community cohort of patients with atrial fibrillation. Eur J Clin Invest. 2013 Oct;43(10):1032–1038.
- Daccarett M, Badger TJ, Akoum N, et al. Association of left atrial fibrosis detected by delayed-enhancement magnetic resonance imaging and the risk of stroke in patients with atrial fibrillation. J Am Coll Cardiol. 2011 Feb 15;57(7):831–838.
- King JB, Azadani PN, Suksaranjit P, et al. Left atrial fibrosis and risk of cerebrovascular and cardiovascular events in patients with atrial fibrillation. J Am Coll Cardiol. 2017 Sep 12;70(11):1311–1321.
- Akoum N, Fernandez G, Wilson B, et al. Association of atrial fibrosis quantified using LGE-MRI with atrial appendage thrombus and spontaneous contrast on transesophageal echocardiography in patients with atrial fibrillation. J Cardiovasc Electrophysiol. 2013 Oct;24(10):1104–1109.
- Fonseca AC, Alves P, Inácio N, et al. Patients with undetermined stroke have increased atrial fibrosis: a cardiac magnetic resonance imaging study. Stroke. 2018 Mar;49(3):734–737.
- Tandon K, Tirschwell D, Longstreth WT Jr., et al. Embolic stroke of undetermined source correlates to atrial fibrosis without atrial fibrillation. Neurology. 2019 Jul 23;93(4):e381–e387.
- Kühnlein P, Mahnkopf C, Majersik JJ, et al. Atrial fibrosis in embolic stroke of undetermined source: a multicenter study. Eur J Neurol. 2021 Nov;28(11):3634–3639.
- Bifulco SF, Scott GD, Sarairah S, et al. Computational modeling identifies embolic stroke of undetermined source patients with potential arrhythmic substrate. Elife. 2021 May 4;10:e64213. doi: 10.7554/eLife.64213
- Ehrlich JR, Kaluzny M, Baumann S, et al. Biomarkers of structural remodelling and endothelial dysfunction for prediction of cardiovascular events or death in patients with atrial fibrillation. Clin Res Cardiol. 2011 Nov;100(11):1029–1036.
- Li N, Brundel B. Inflammasomes and proteostasis novel molecular mechanisms associated with atrial fibrillation. Circ Res. 2020 Jun 19;127(1):73–90.
- Li CY, Zhang JR, Hu WN, et al. Atrial fibrosis underlying atrial fibrillation (Review). Int J Mol Med. 2021 Mar;47(3):9. doi:10.3892/ijmm.2020.4842.
- Noubiap JJ, Sanders P, Nattel S, et al. Biomarkers in atrial fibrillation: pathogenesis and clinical implications. Card Electrophysiol Clin. 2021 Mar;13(1):221–233.
- Liu Y, Xu B, Wu N, et al. Association of MMPs and TIMPs with the occurrence of atrial fibrillation: a systematic review and meta-analysis. Can J Cardiol. 2016 Jun;32(6):803–813.
- Marín F, Roldán V, Climent V, et al. Is thrombogenesis in atrial fibrillation related to matrix metalloproteinase-1 and its inhibitor, TIMP-1? Stroke. 2003 May;34(5):1181–1186.
- Lip GY, Patel JV, Hughes E, et al. High-sensitivity C-reactive protein and soluble CD40 ligand as indices of inflammation and platelet activation in 880 patients with nonvalvular atrial fibrillation: relationship to stroke risk factors, stroke risk stratification schema, and prognosis. Stroke. 2007 Apr;38(4):1229–1237.
- Shen MJ, Arora R, Jalife J. Atrial Myopathy. JACC Basic Transl Sci. 2019 Sep;4(5):640–654.
- Kamel H, Bartz TM, Elkind MSV, et al. Atrial cardiopathy and the risk of ischemic stroke in the CHS (Cardiovascular Health Study). Stroke. 2018 Apr;49(4):980–986.
- Edwards JD, Healey JS, Fang J, et al. Atrial cardiopathy in the absence of atrial fibrillation increases risk of ischemic stroke, incident atrial fibrillation, and mortality and improves stroke risk prediction. J Am Heart Assoc. 2020;9(11):e013227. DOI:10.1161/JAHA.119.013227.
- Kamel H, Longstreth WT Jr., Tirschwell DL, et al. The AtRial Cardiopathy and Antithrombotic Drugs In prevention After cryptogenic stroke randomized trial: rationale and methods. Int J Stroke. 2019 Feb;14(2):207–214.
- Kamp O, Verhorst PM, Welling RC, et al. Importance of left atrial appendage flow as a predictor of thromboembolic events in patients with atrial fibrillation. Eur Heart J. 1999 Jul;20(13):979–985.
- Predictors of thromboembolism in atrial fibrillation: II. Echocardiographic features of patients at risk. The Stroke Prevention in Atrial Fibrillation Investigators. Ann Intern Med. 1992 Jan 1;116(1):6–12.
- Hamatani Y, Ogawa H, Takabayashi K, et al. Left atrial enlargement is an independent predictor of stroke and systemic embolism in patients with non-valvular atrial fibrillation. Sci Rep. 2016 Aug 3;6(1):31042.
- Yaghi S, Moon YP, Mora-McLaughlin C, et al. Left atrial enlargement and stroke recurrence: the Northern Manhattan Stroke Study. Stroke. 2015 Jun;46(6):1488–1493.
- Chimowitz MI, DeGeorgia MA, Poole RM, et al. Left atrial spontaneous echo contrast is highly associated with previous stroke in patients with atrial fibrillation or mitral stenosis. Stroke. 1993 Jul;24(7):1015–1019.
- Briley DP, Giraud GD, Beamer NB, et al. Spontaneous echo contrast and hemorheologic abnormalities in cerebrovascular disease. Stroke. 1994 Aug;25(8):1564–1569.
- Zabalgoitia M, Halperin JL, Pearce LA, et al. Transesophageal echocardiographic correlates of clinical risk of thromboembolism in nonvalvular atrial fibrillation. Stroke Prevention in Atrial Fibrillation III Investigators. J Am Coll Cardiol. 1998 Jun;31(7):1622–1626.
- Zhou ZQ, Hu DY, Chen J, et al. An epidemiological survey of atrial fibrillation in China. Zhonghua Nei Ke Za Zhi. 2004 Jul;43(7):491–494.
- Yoo J, Song D, Baek JH, et al. Poor outcome of stroke patients with atrial fibrillation in the presence of coexisting spontaneous echo contrast. Stroke. 2016 Jul;47(7):1920–1922.
- Kumagai T, Matsuura Y, Yamamoto T, et al. Risk factors for left atrial thrombus from transesophageal echocardiography findings in ischemic stroke patients. Fukushima J Med Sci. 2014;60(2):154–158.
- Kochi K, Kanehiro K, Mukada K, et al. Relationship between left atrial spontaneous echo contrast and the features of middle cerebral artery territory in nonvalvular atrial fibrillation. Heart Vessels. 1999;14(3):149–153.
- Okura H, Inoue H, Tomon M, et al. Transesophageal echocardiographic detection of cardiac sources of embolism in elderly patients with ischemic stroke. Intern Med. 1999 Oct;38(10):766–772.
- Zhao Y, Ji L, Liu J, et al. Intensity of left atrial spontaneous echo contrast as a correlate for stroke risk stratification in patients with nonvalvular atrial fibrillation. Sci Rep. 2016 Jun 9;6(1):27650.
- Ohya Y, Osaki M, Fujimoto S, et al. Usefulness of transesophageal echocardiography for predicting covert paroxysmal atrial fibrillation in patients with embolic stroke of undetermined source. Cerebrovasc Dis Extra. 2019;9(3):98–106.
- Sanfilippo AJ, Abascal VM, Sheehan M, et al. Atrial enlargement as a consequence of atrial fibrillation. A prospective echocardiographic study. Circulation. 1990 Sep;82(3):792–797.
- Goette A, Kalman JM, Aguinaga L, et al. EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: definition, characterization, and clinical implication. Europace. 2016 Oct;18(10):1455–1490.
- Transesophageal echocardiographic correlates of thromboembolism in high-risk patients with nonvalvular atrial fibrillation. The Stroke Prevention in Atrial Fibrillation Investigators Committee on Echocardiography. Ann Intern Med. 1998 Apr 15;128(8):639–647.
- Leung DY, Black IW, Cranney GB, et al. Prognostic implications of left atrial spontaneous echo contrast in nonvalvular atrial fibrillation. J Am Coll Cardiol. 1994 Sep;24(3):755–762.
- O’Brien PJ, Thiemann DR, McNamara RL, et al. Usefulness of transesophageal echocardiography in predicting mortality and morbidity in stroke patients without clinically known cardiac sources of embolus. Am J Cardiol. 1998 May 1;81(9):1144–1151.
- Feinberg WM, Pearce LA, Hart RG, et al. Markers of thrombin and platelet activity in patients with atrial fibrillation: correlation with stroke among 1531 participants in the Stroke Prevention in Atrial Fibrillation III study. Stroke. 1999 Dec;30(12):2547–2553.
- Woo E, Huang CY, Chan V, et al. Beta-thromboglobulin in cerebral infarction. J Neurol Neurosurg Psychiatry. 1988 Apr;51(4):557–562 .
- Nagao T, Hamamoto M, Kanda A, et al. Platelet activation is not involved in acceleration of the coagulation system in acute cardioembolic stroke with nonvalvular atrial fibrillation. Stroke. 1995 Aug;26(8):1365–1368.
- Gustafsson C, Blombäck M, Britton M, et al. Coagulation factors and the increased risk of stroke in nonvalvular atrial fibrillation. Stroke. 1990 Jan;21(1):47–51.
- Ha SI, Choi DH, Ki YJ, et al. Stroke prediction using mean platelet volume in patients with atrial fibrillation. Platelets. 2011;22(6):408–414.
- Choi SW, Kim BB, Choi DH, et al. Stroke or left atrial thrombus prediction using antithrombin III and mean platelet volume in patients with nonvalvular atrial fibrillation. Clin Cardiol. 2017 Nov;40(11):1013–1019.
- Zheng M, Chen S, Zhu Y, et al. Mean platelet volume: a new predictor of ischaemic stroke risk in patients with nonvalvular atrial fibrillation. BMC Cardiovasc Disord. 2020 May 20;20(1):241.
- Turfan M, Erdogan E, Ertas G, et al. Usefulness of mean platelet volume for predicting stroke risk in atrial fibrillation patients. Blood Coagul Fibrinolysis. 2013 Jan;24(1):55–58.
- Gul SS, Gozke E. Mean platelet volume in patients with acute ischemic stroke with nonvalvular atrial fibrillation. Clin Lab. 2018 Oct 31;64(11). doi: 10.7754/Clin.Lab.2018.180543
- Zhu N, Shu H, Jiang W, et al. Mean platelet volume and mean platelet volume/platelet count ratio in nonvalvular atrial fibrillation stroke and large artery atherosclerosis stroke. Medicine (Baltimore). 2020 Jul 10;99(28):e21044.
- Lyu QS, Liu B, Huang C, et al. The association between platelet distribution width and stroke in atrial fibrillation patients. Ann Clin Lab Sci. 2019 Jan;49(1):143–147.
- Kahn SR, Solymoss S, Flegel KM. Nonvalvular atrial fibrillation: evidence for a prothrombotic state. Cmaj. 1997 Sep 15;157(6):673–681.
- Turgut N, Akdemir O, Turgut B, et al. Hypercoagulopathy in stroke patients with nonvalvular atrial fibrillation: hematologic and cardiologic investigations. Clin Appl Thromb Hemost. 2006 Jan;12(1):15–20.
- Paulin BK, Cedric KK, Tamomh AG, et al. Assessment of cardiac biomarkers (troponin, B-type natriuretic peptide, and D-dimer) in patients with non-valvular atrial fibrillation and stroke. Int J Health Sci (Qassim). 2019 Nov-Dec;13(6):3–12.
- Harpaz D, Bajpai R, Ng GJL, et al. Blood biomarkers to detect new-onset atrial fibrillation and cardioembolism in ischemic stroke patients. Heart Rhythm. 2021 Jun;18(6):855–861.
- Yip HK, Lai SL, Lan MY, et al. Time course of platelet activation and von Willebrand factor in patients with non-valvular atrial fibrillation after ischemic stroke. Circ J. 2007 Mar;71(3):321–326.
- Lip GY, Lane D, Van Walraven C, et al. Additive role of plasma von Willebrand factor levels to clinical factors for risk stratification of patients with atrial fibrillation. Stroke. 2006 Sep;37(9):2294–2300.
- Sato M, Suzuki A, Nagata K, et al. Increased von Willebrand factor in acute stroke patients with atrial fibrillation. J Stroke Cerebrovasc Dis. 2006 Jan-Feb;15(1):1–7.
- Pinto A, Tuttolomondo A, Casuccio A, et al. Immuno-inflammatory predictors of stroke at follow-up in patients with chronic non-valvular atrial fibrillation (NVAF). Clin Sci (Lond). 2009 May;116(10):781–789.
- Roldán V, Marín F, Muiña B, et al. Plasma von Willebrand factor levels are an independent risk factor for adverse events including mortality and major bleeding in anticoagulated atrial fibrillation patients. J Am Coll Cardiol. 2011 Jun 21;57(25):2496–2504.
- Freynhofer MK, Gruber SC, Bruno V, et al. Prognostic value of plasma von Willebrand factor and its cleaving protease ADAMTS13 in patients with atrial fibrillation. Int J Cardiol. 2013 Sep 20;168(1):317–325.
- García-Fernández A, Roldán V, Rivera-Caravaca JM, et al. Does von Willebrand factor improve the predictive ability of current risk stratification scores in patients with atrial fibrillation? Sci Rep. 2017 Jan 30;7(1):41565.
- Ancedy Y, Berthelot E, Lang S, et al. Is von Willebrand factor associated with stroke and death at mid-term in patients with non-valvular atrial fibrillation? Arch Cardiovasc Dis. 2018 May;111(5):357–369.
- Roldán V, Rivera-Caravaca JM, Shantsila A, et al. Enhancing the ‘real world’ prediction of cardiovascular events and major bleeding with the CHA(2)DS(2)-VASc and HAS-BLED scores using multiple biomarkers. Ann Med. 2018 Feb;50(1):26–34.
- Wysokinski WE, Melduni RM, Ammash NM, et al. Von Willebrand factor and ADAMTS13 as predictors of adverse outcomes in patients with nonvalvular atrial fibrillation. CJC Open. 2021 Mar;3(3):318–326.
- Matsumoto M, Sakaguchi M, Okazaki S, et al. Relationship between plasma (D)-dimer level and cerebral infarction volume in patients with nonvalvular atrial fibrillation. Cerebrovasc Dis. 2013;35(1):64–72.
- Vene N, Mavri A, Kosmelj K, et al. High D-dimer levels predict cardiovascular events in patients with chronic atrial fibrillation during oral anticoagulant therapy. Thromb Haemost. 2003 Dec;90(6):1163–1172.
- Nozawa T, Inoue H, Hirai T, et al. D-dimer level influences thromboembolic events in patients with atrial fibrillation. Int J Cardiol. 2006 Apr 28;109(1):59–65.
- Sadanaga T, Kohsaka S, Ogawa S. D-dimer levels in combination with clinical risk factors can effectively predict subsequent thromboembolic events in patients with atrial fibrillation during oral anticoagulant therapy. Cardiology. 2010;117(1):31–36.
- Sadanaga T, Sadanaga M, Ogawa S. Evidence that D-dimer levels predict subsequent thromboembolic and cardiovascular events in patients with atrial fibrillation during oral anticoagulant therapy. J Am Coll Cardiol. 2010 May 18;55(20):2225–2231.
- Christersson C, Wallentin L, Andersson U, et al. D-dimer and risk of thromboembolic and bleeding events in patients with atrial fibrillation–observations from the ARISTOTLE trial. J Thromb Haemost. 2014 Sep;12(9):1401–1412.
- Siegbahn A, Oldgren J, Andersson U, et al. D-dimer and factor VIIa in atrial fibrillation - prognostic values for cardiovascular events and effects of anticoagulation therapy. A RE-LY substudy. Thromb Haemost. 2016 May 2;115(5):921–930.
- Kneihsl M, Gattringer T, Bisping E, et al. Blood biomarkers of heart failure and hypercoagulation to identify atrial fibrillation-related stroke. Stroke. 2019 Aug;50(8):2223–2226.
- Krarup LH, Sandset EC, Sandset PM, et al. D-dimer levels and stroke progression in patients with acute ischemic stroke and atrial fibrillation. Acta Neurol Scand. 2011 Jul;124(1):40–44.
- You LR, Tang M. The association of high D-dimer level with high risk of ischemic stroke in nonvalvular atrial fibrillation patients: a retrospective study. Medicine (Baltimore). 2018 Oct;97(43):e12622.
- Heppell RM, Berkin KE, McLenachan JM, et al. Haemostatic and haemodynamic abnormalities associated with left atrial thrombosis in non-rheumatic atrial fibrillation. Heart. 1997 May;77(5):407–411.
- Soncini M, Casazza F, Mattioli R, et al. Hypercoagulability and chronic atrial fibrillation: the role of markers of thrombin generation. Minerva Med. 1997 Dec;88(12):501–505.
- Topcuoglu MA, Haydari D, Ozturk S, et al. Plasma levels of coagulation and fibrinolysis markers in acute ischemic stroke patients with lone atrial fibrillation. Neurol Sci. 2000 Aug;21(4):235–240.
- Ząbczyk M, Majewski J, Lelakowski J. Thromboembolic events are associated with prolonged clot lysis time in patients with permanent atrial fibrillation. Pol Arch Med Wewn. 2011 Nov;121(11):400–407.
- Shah AB, Beamer N, Coull BM. Enhanced in vivo platelet activation in subtypes of ischemic stroke. Stroke. 1985 Jul-Aug;16(4):643–647.
- Bayar N, Arslan S, Cagirci G, et al. Usefulness of mean platelet volume for predicting stroke risk in paroxysmal atrial fibrillation patients. Blood Coagul Fibrinolysis. 2015 Sep;26(6):669–672.
- Tarnowski D, Poitz DM, Plichta L, et al. Comparison of diverse platelet activation markers as indicators for left atrial thrombus in atrial fibrillation. Platelets. 2018 Jan;29(1):41–47.
- Heeringa J, Conway DS, van der Kuip DA, et al. A longitudinal population-based study of prothrombotic factors in elderly subjects with atrial fibrillation: the Rotterdam Study 1990-1999. J Thromb Haemost. 2006 Sep;4(9):1944–1949.
- Lip GY, Lip PL, Zarifis J, et al. Fibrin D-dimer and beta-thromboglobulin as markers of thrombogenesis and platelet activation in atrial fibrillation. Effects of introducing ultra-low-dose warfarin and aspirin. Circulation. 1996 Aug 1;94(3):425–431.
- Choudhury A, Chung I, Blann AD, et al. Platelet surface CD62P and CD63, mean platelet volume, and soluble/platelet P-selectin as indexes of platelet function in atrial fibrillation: a comparison of “healthy control subjects” and “disease control subjects” in sinus rhythm. J Am Coll Cardiol. 2007 May 15;49(19):1957–1964.
- Chu SG, Becker RC, Berger PB, et al. Mean platelet volume as a predictor of cardiovascular risk: a systematic review and meta-analysis. J Thromb Haemost. 2010 Jan;8(1):148–156.
- Bath P, Algert C, Chapman N, et al. Association of mean platelet volume with risk of stroke among 3134 individuals with history of cerebrovascular disease. Stroke. 2004 Mar;35(3):622–626.
- de Jonge P, Rosmalen JG, Kema IP, et al. Psychophysiological biomarkers explaining the association between depression and prognosis in coronary artery patients: a critical review of the literature. Neurosci Biobehav Rev. 2010 Sep;35(1):84–90.
- Zhong C, Xin M, He L, et al. Prognostic value of von Willebrand factor in patients with atrial fibrillation: a meta-analysis. Medicine (Baltimore). 2018 Jul;97(27):e11269.
- Ye YZ, Chang YF, Wang BZ, et al. Prognostic value of von Willebrand factor for patients with atrial fibrillation: a meta-analysis of prospective cohort studies. Postgrad Med J. 2020 May;96(1135):267–276.
- Hindricks G, Potpara T, Dagres N, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2021;42(5):373–498.
- European Stroke Organisation (ESO) Executive Committee; ESO Writing Committee. Guidelines for management of ischaemic stroke and transient ischaemic attack 2008. Cerebrovasc Dis. 2008;25(5):457–507
- Sanna T, Diener HC, Passman RS, et al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med. 2014 Jun 26;370(26):2478–2486.
- Sagris D, Harrison SL, Buckley BJR, et al. Long-term cardiac monitoring after embolic stroke of undetermined source: search longer, look harder. Am J Med. 2022 Sep;135(9):e311–e317.
- Royal College of Physicians Intercollegiate Stroke Working Party. National clinical guideline for stroke. Fifth Edition 2016. Cited 8 Dec 2022. Available from: https://www.rcplondon.ac.uk/guidelines-policy/stroke-guidelines.
- Sposato LA, Cipriano LE, Saposnik G, et al. Diagnosis of atrial fibrillation after stroke and transient ischaemic attack: a systematic review and meta-analysis. Lancet Neurol. 2015 Apr;14(4):377–387.
- Mairesse GH, Moran P, Van Gelder IC, et al. Screening for atrial fibrillation: a European Heart Rhythm Association (EHRA) consensus document endorsed by the Heart Rhythm Society (HRS), Asia Pacific Heart Rhythm Society (APHRS), and Sociedad Latinoamericana de Estimulación Cardíaca y Electrofisiolog. EP Europace. 2017;19(10):1589–1623
- Guo Y, Wang H, Zhang H, et al. Mobile photoplethysmographic technology to detect atrial fibrillation. J Am Coll Cardiol. 2019 Nov 12;74(19):2365–2375.
- Perez MV, Mahaffey KW, Hedlin H, et al. Large-scale assessment of a smartwatch to identify atrial fibrillation. N Engl J Med. 2019 Nov 14;381(20):1909–1917.
- Lubitz SA, Faranesh AZ, Selvaggi C, et al. Detection of atrial fibrillation in a large population using wearable devices: the Fitbit Heart Study. Circulation. 2022 Nov 8;146(19):1415–1424.
- Pollak WM, Simmons JD, Interian A Jr., et al. Clinical utility of intraatrial pacemaker stored electrograms to diagnose atrial fibrillation and flutter. Pacing Clin Electrophysiol. 2001 Apr;24(4 Pt 1):424–429.
- Glotzer TV, Hellkamp AS, Zimmerman J, et al. Atrial high rate episodes detected by pacemaker diagnostics predict death and stroke: report of the Atrial Diagnostics Ancillary Study of the MOde Selection Trial (MOST). Circulation. 2003 Apr 1;107(12):1614–1619.
- Capucci A, Santini M, Padeletti L, et al. Monitored atrial fibrillation duration predicts arterial embolic events in patients suffering from bradycardia and atrial fibrillation implanted with antitachycardia pacemakers. J Am Coll Cardiol. 2005 Nov 15;46(10):1913–1920.
- Glotzer TV, Daoud EG, Wyse DG, et al. The relationship between daily atrial tachyarrhythmia burden from implantable device diagnostics and stroke risk: the TRENDS study. Circ Arrhythm Electrophysiol. 2009 Oct;2(5):474–480.
- Healey JS, Connolly SJ, Gold MR, et al. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med. 2012 Jan 12;366(2):120–129.
- Shanmugam N, Boerdlein A, Proff J, et al. Detection of atrial high-rate events by continuous home monitoring: clinical significance in the heart failure-cardiac resynchronization therapy population. Europace. 2012 Feb;14(2):230–237.
- Boriani G, Glotzer TV, Santini M, et al. Device-detected atrial fibrillation and risk for stroke: an analysis of >10,000 patients from the SOS AF project (Stroke preventiOn Strategies based on Atrial Fibrillation information from implanted devices). Eur Heart J. 2014 Feb;35(8):508–516.
- Turakhia MP, Ziegler PD, Schmitt SK, et al. Atrial fibrillation burden and short-term risk of stroke: case-crossover analysis of continuously recorded heart rhythm from cardiac electronic implanted devices. Circ Arrhythm Electrophysiol. 2015 Oct;8(5):1040–1047.
- Witt CT, Kronborg MB, Nohr EA, et al. Early detection of atrial high rate episodes predicts atrial fibrillation and thromboembolic events in patients with cardiac resynchronization therapy. Heart Rhythm. 2015 Dec;12(12):2368–2375.
- Daoud EG, Glotzer TV, Wyse DG, et al. Temporal relationship of atrial tachyarrhythmias, cerebrovascular events, and systemic emboli based on stored device data: a subgroup analysis of TRENDS. Heart Rhythm. 2011 Sep;8(9):1416–1423.
- Botto GL, Padeletti L, Santini M, et al. Presence and duration of atrial fibrillation detected by continuous monitoring: crucial implications for the risk of thromboembolic events. J Cardiovasc Electrophysiol. 2009 Mar;20(3):241–248.
- Kawakami H, Nagai T, Saito M, et al. Clinical significance of atrial high-rate episodes for thromboembolic events in Japanese population. Heart Asia. 2017;9(2):e010954.
- Sandgren E, Rorsman C, Edvardsson N, et al. Stroke incidence and anticoagulation treatment in patients with pacemaker-detected silent atrial fibrillation. PLoS One. 2018;13(9):e0203661.
- Park YJ, Kim JS, Park KM, et al. Subclinical atrial fibrillation burden and adverse clinical outcomes in patients with permanent pacemakers. Stroke. 2021 Apr;52(4):1299–1308.
- Sagris D, Georgiopoulos G, Pateras K, et al. Atrial high-rate episode duration thresholds and thromboembolic risk: a systematic review and meta-analysis. J Am Heart Assoc. 2021 Nov 16;10(22):e022487.
- Jons C, Jacobsen UG, Joergensen RM, et al. The incidence and prognostic significance of new-onset atrial fibrillation in patients with acute myocardial infarction and left ventricular systolic dysfunction: a CARISMA substudy. Heart Rhythm. 2011 Mar;8(3):342–348.
- Nakano M, Kondo Y, Nakano M, et al. Impact of atrial high-rate episodes on the risk of future stroke. J Cardiol. 2019 Aug;74(2):144–149.
- Christensen LM, Krieger DW, Højberg S, et al. Paroxysmal atrial fibrillation occurs often in cryptogenic ischaemic stroke. Final results from the SURPRISE study. Eur J Neurol. 2014 Jun;21(6):884–889.
- Israel C, Kitsiou A, Kalyani M, et al. Detection of atrial fibrillation in patients with embolic stroke of undetermined source by prolonged monitoring with implantable loop recorders. Thromb Haemost. 2017 Oct 5;117(10):1962–1969.
- Schnabel RB, Haeusler KG, Healey JS, et al. Searching for atrial fibrillation poststroke: a white paper of the AF-SCREEN International Collaboration. Circulation. 2019 Nov 26;140(22):1834–1850.
- Martin DT, Bersohn MM, Waldo AL, et al. Randomized trial of atrial arrhythmia monitoring to guide anticoagulation in patients with implanted defibrillator and cardiac resynchronization devices. Eur Heart J. 2015 Jul 7;36(26):1660–1668.
- Yang SY, Huang M, Wang AL, et al. Atrial fibrillation burden and the risk of stroke: a systematic review and dose-response meta-analysis. World J Clin Cases. 2022 Jan 21;10(3):939–953.
- Singer DE, Ziegler PD, Koehler JL, et al. Temporal association between episodes of atrial fibrillation and risk of ischemic stroke. JAMA Cardiol. 2021 Dec 1;6(12):1364–1369.
- Brambatti M, Connolly SJ, Gold MR, et al. Temporal relationship between subclinical atrial fibrillation and embolic events. Circulation. 2014 May 27;129(21):2094–2099.
- Li YG, Miyazawa K, Pastori D, et al. Atrial high-rate episodes and thromboembolism in patients without atrial fibrillation: the West Birmingham Atrial Fibrillation Project. Int J Cardiol. 2019 Oct 1;292:126–130. doi: 10.1016/j.ijcard.2019.04.055.
- Lip GY, Fauchier L, Freedman SB, et al. Atrial fibrillation. Nat Rev Dis Primers. 2016 Mar 31;2(1):16016.
- Boriani G, Vitolo M, Imberti JF, et al. What do we do about atrial high rate episodes? Eur Heart J Suppl. 2020 Dec;22(Suppl O):O42–O52.
- Mahajan R, Perera T, Elliott AD, et al. Subclinical device-detected atrial fibrillation and stroke risk: a systematic review and meta-analysis. Eur Heart J. 2018 Apr 21;39(16):1407–1415.
- DeCicco AE, Finkel JB, Greenspon AJ, et al. Clinical significance of atrial fibrillation detected by cardiac implantable electronic devices. Heart Rhythm. 2014 Apr;11(4):719–724.
- Schnabel RB, Sullivan LM, Levy D, et al. Development of a risk score for atrial fibrillation (Framingham Heart Study): a community-based cohort study. Lancet. 2009 Feb 28;373(9665):739–745.
- Alonso A, Krijthe BP, Aspelund T, et al. Simple risk model predicts incidence of atrial fibrillation in a racially and geographically diverse population: the CHARGE-AF consortium. J Am Heart Assoc. 2013 Mar 18;2(2):e000102.
- Suissa L, Bertora D, Lachaud S, et al. Score for the targeting of atrial fibrillation (STAF): a new approach to the detection of atrial fibrillation in the secondary prevention of ischemic stroke. Stroke. 2009 Aug;40(8):2866–2868.
- Malik S, Hicks WJ, Schultz L, et al. Development of a scoring system for atrial fibrillation in acute stroke and transient ischemic attack patients: the LADS scoring system. J Neurol Sci. 2011 Feb 15;301(1–2):27–30.
- Bugnicourt JM, Flament M, Guillaumont MP, et al. Predictors of newly diagnosed atrial fibrillation in cryptogenic stroke: a cohort study. Eur J Neurol. 2013 Oct;20(10):1352–1359.
- Brunner KJ, Bunch TJ, Mullin CM, et al. Clinical predictors of risk for atrial fibrillation: implications for diagnosis and monitoring. Mayo Clin Proc. 2014 Nov;89(11):1498–1505.
- Kwong C, Ling AY, Crawford MH, et al. A clinical score for predicting atrial fibrillation in patients with cryptogenic stroke or transient ischemic attack. Cardiology. 2017;138(3):133–140.
- Bisson A, Bodin A, Clementy N, et al. Prediction of incident atrial fibrillation according to gender in patients with ischemic stroke from a nationwide cohort. Am J Cardiol. 2018 Feb 15;121(4):437–444.
- Li YG, Bisson A, Bodin A, et al. C(2) HEST score and prediction of incident atrial fibrillation in poststroke patients: a French nationwide study. J Am Heart Assoc. 2019 Jul 2;8(13):e012546.
- Uphaus T, Weber-Krüger M, Grond M, et al. Development and validation of a score to detect paroxysmal atrial fibrillation after stroke. Neurology. 2019 Jan 8;92(2):e115–e124.
- Hsieh CY, Lee CH, Sung SF. Development of a novel score to predict newly diagnosed atrial fibrillation after ischemic stroke: the CHASE-LESS score. Atherosclerosis. 2020 Feb;295:1–7.
- Ntaios G, Perlepe K, Lambrou D, et al. Identification of patients with embolic stroke of undetermined source and low risk of new incident atrial fibrillation: the AF-ESUS score. Int J Stroke. 2021 Jan;16(1):29–38.
- Vera A, Cecconi A, Ximénez-Carrillo Á, et al. A comprehensive model to predict atrial fibrillation in cryptogenic stroke: the decryptoring score. J Stroke Cerebrovasc Dis. 2022 Jan;31(1):106161.
- Ricci B, Chang AD, Hemendinger M, et al. A simple score that predicts paroxysmal atrial fibrillation on outpatient cardiac monitoring after embolic stroke of unknown source. J Stroke Cerebrovasc Dis. 2018 Jun;27(6):1692–1696.
- Kneihsl M, Bisping E, Scherr D, et al. Predicting atrial fibrillation after cryptogenic stroke via a clinical risk score-a prospective observational study. Eur J Neurol. 2022 Jan;29(1):149–157.
- Amaya Pascasio L, Quesada López M, García-Torrecillas JM, et al. Development of a score to predict the paroxysmal atrial fibrillation in stroke patients: the screening for atrial fibrillation scale. Front Neurol. 2022;13:900582.
- Yoshioka K, Watanabe K, Zeniya S, et al. A score for predicting paroxysmal atrial fibrillation in acute stroke patients: iPAB score. J Stroke Cerebrovasc Dis. 2015 Oct;24(10):2263–2269.
- Fujii S, Shibazaki K, Kimura K, et al. A simple score for predicting paroxysmal atrial fibrillation in acute ischemic stroke. J Neurol Sci. 2013 May 15;328(1–2):83–86.
- Kishore AK, Hossain MJ, Cameron A, et al. Use of risk scores for predicting new atrial fibrillation after ischemic stroke or transient ischemic attack—A systematic review. Int J Stroke. 2022;17(6):608–617.
- Cameron A, Cheng HK, Lee RP, et al. Biomarkers for atrial fibrillation detection after stroke: systematic review and meta-analysis. Neurology. 2021 Nov 2;97(18):e1775–e1789.
- Bahit MC, Sacco RL, Easton JD, et al. Predictors of atrial fibrillation development in patients with embolic stroke of undetermined source: an analysis of the RE-SPECT ESUS trial. Circulation. 2021;144(22):1738–1746.
- Suzuki S, Sagara K, Otsuka T, et al. Usefulness of frequent supraventricular extrasystoles and a high CHADS2 score to predict first-time appearance of atrial fibrillation. Am J Cardiol. 2013 Jun 1;111(11):1602–1607.
- Miller DJ, Khan MA, Schultz LR, et al. Outpatient cardiac telemetry detects a high rate of atrial fibrillation in cryptogenic stroke. J Neurol Sci. 2013 Jan 15;324(1–2):57–61.
- Wasser K, Weber-Krüger M, Gröschel S, et al. Brain natriuretic peptide and discovery of atrial fibrillation after stroke: a subanalysis of the Find-AF(RANDOMISED) Trial. Stroke. 2020 Feb;51(2):395–401.
- Pagola J, González-Alujas T, Flores A, et al. Left atria strain is a surrogate marker for detection of atrial fibrillation in cryptogenic strokes. Stroke. 2014;45(8):e164–e166.
- Hart RG, Sharma M, Mundl H, et al. Rivaroxaban for stroke prevention after embolic stroke of undetermined source. N Engl J Med. 2018 Jun 7;378(23):2191–2201.
- Diener HC, Sacco RL, Easton JD, et al. Dabigatran for prevention of stroke after embolic stroke of undetermined source [Artikel]. N Engl J Med. 2019;380(20):1906–1917.
- Paciaroni M, Kamel H. Do the results of RE-SPECT ESUS call for a revision of the embolic stroke of undetermined source definition? Stroke. 2019;50(4):1032–1033.
- Lip GY, Nieuwlaat R, Pisters R, et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010 Feb;137(2):263–272.
- Friberg L, Rosenqvist M, Lip GYH. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182 678 patients with atrial fibrillation: the Swedish Atrial Fibrillation cohort study. Eur Heart J. 2012;33(12):1500–1510.
- Olesen JB, Lip GY, Hansen ML, et al. Validation of risk stratification schemes for predicting stroke and thromboembolism in patients with atrial fibrillation: nationwide cohort study. Bmj. 2011 Jan 31;342(jan31 1):d124.
- Lip G, Freedman B, De Caterina R, et al. Stroke prevention in atrial fibrillation: past, present and future. Comparing the guidelines and practical decision-making. Thromb Haemost. 2017 Jun 28;117(7):1230–1239.
- Maheshwari A, Norby FL, Soliman EZ, et al. Abnormal P-wave axis and ischemic stroke: the ARIC study (Atherosclerosis Risk In Communities). Stroke. 2017 Aug;48(8):2060–2065.
- Maheshwari A, Norby FL, Roetker NS, et al. Refining prediction of atrial fibrillation-related stroke using the P(2)-CHA(2)DS(2)-VASc score. Circulation. 2019 Jan 8;139(2):180–191.
- Ntaios G, Lip GY, Lambrou D, et al. Leukoaraiosis and stroke recurrence risk in patients with and without atrial fibrillation. Neurology. 2015 Mar 24;84(12):1213–1219.
- Wu X, Bos D, Ren L, et al. Intracranial arterial calcification relates to long-term risk of recurrent stroke and post-stroke mortality. Front Neurol. 2020;11:559158.
- Oksuz F, Yarlioglues M, Duran M, et al. Mitral annular calcification and its severity predict high risk for cardio-embolic stroke in elderly patients with first diagnosed atrial fibrillation. Acta Cardiol. 2021 Feb;76(1):56–62.
- Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007 Jun 19;146(12):857–867.
- Li G, Lip GYH, Holbrook A, et al. Direct comparative effectiveness and safety between non-vitamin K antagonist oral anticoagulants for stroke prevention in nonvalvular atrial fibrillation: a systematic review and meta-analysis of observational studies. Eur J Epidemiol. 2019 Feb;34(2):173–190.
- Andò G, Capranzano P. Non-vitamin K antagonist oral anticoagulants in atrial fibrillation patients with chronic kidney disease: a systematic review and network meta-analysis. Int J Cardiol. 2016;231:162–169.
- Piccini JP, Caso V, Connolly SJ, et al. Safety of the oral factor XIa inhibitor asundexian compared with apixaban in patients with atrial fibrillation (PACIFIC-AF): a multicentre, randomised, double-blind, double-dummy, dose-finding phase 2 study. Lancet. 2022;399(10333):1383–1390.
- Yasuda S, Kaikita K, Akao M, et al. Antithrombotic therapy for atrial fibrillation with stable coronary disease. N Engl J Med. 2019 Sep 19;381(12):1103–1113.
- Lee SR, Rhee TM, Kang DY, et al. Meta-analysis of oral anticoagulant monotherapy as an antithrombotic strategy in patients with stable coronary artery disease and nonvalvular atrial fibrillation. Am J Cardiol. 2019 Sep 15;124(6):879–885.
- Lip GYH, Lane DA, Lenarczyk R, et al. Integrated care for optimizing the management of stroke and associated heart disease: a position paper of the European Society of Cardiology Council on Stroke. Eur Heart J. 2022 Jul 7;43(26): 2442–2460.
- Ntaios G, Michel P. Temporal distribution and magnitude of the vulnerability period around stroke depend on stroke subtype. Cerebrovasc Dis. 2011;32(3):246–253.
- Best JG, Arram L, Ahmed N, et al. Optimal timing of anticoagulation after acute ischemic stroke with atrial fibrillation (OPTIMAS): protocol for a randomized controlled trial. Int J Stroke. 2022 Jun;17(5):583–589.
- Fischer U, Trelle S, Branca M, et al. Early versus Late initiation of direct oral Anticoagulants in post-ischaemic stroke patients with atrial fibrillatioN (ELAN): protocol for an international, multicentre, randomised-controlled, two-arm, open, assessor-blinded trial. Eur Stroke J. 2022 Dec;7(4):487–495.
- Timing of oral anticoagulant therapy in acute ischemic stroke with atrial fibrillation: a prospective multicenter registry-based non-inferiority randomized controlled clinical trial. Cited 8 Dec 2022. Available from: https://clinicaltrials.gov/ct2/show/NCT02961348.
- Optimal delay time to initiate anticoagulation after ischemic stroke in atrial fibrillation (START). Cited 8 Dec 2022. Available from: https://clinicaltrials.gov/ct2/show/NCT03021928.
- Oldgren J, Åsberg S, Hijazi Z, et al. Early versus delayed non–vitamin k antagonist oral anticoagulant therapy after acute ischemic stroke in atrial fibrillation (TIMING): a registry-based randomized controlled noninferiority study. Circulation. 2022;146(14):1056–1066.
- Abdul-Rahim AH, Fulton RL, Frank B, et al. Association of improved outcome in acute ischaemic stroke patients with atrial fibrillation who receive early antithrombotic therapy: analysis from VISTA. Eur J Neurol. 2015 Jul;22(7):1048–1055.
- Heidbuchel H, Verhamme P, Alings M, et al. EHRA practical guide on the use of new oral anticoagulants in patients with non-valvular atrial fibrillation: executive summary. Eur Heart J. 2013 Jul;34(27):2094–2106.
- Trujillo T, Dobesh PP. Clinical use of rivaroxaban: pharmacokinetic and pharmacodynamic rationale for dosing regimens in different indications. Drugs. 2014;74(14):1587–1603.
- Byon W, Garonzik S, Boyd RA, et al. Apixaban: a clinical pharmacokinetic and pharmacodynamic review. Clin Pharmacokinet. 2019;58(10):1265–1279.
- Parasrampuria DA, Truitt KE. Pharmacokinetics and pharmacodynamics of edoxaban, a non-vitamin K antagonist oral anticoagulant that inhibits clotting factor Xa. Clin Pharmacokinet. 2016;55(6):641–655.
- Ingrasciotta Y, Crisafulli S, Pizzimenti V, et al. Pharmacokinetics of new oral anticoagulants: implications for use in routine care. Expert Opin Drug Metab Toxicol. 2018 Oct;14(10):1057–1069.
- Okata T, Toyoda K, Okamoto A, et al. Anticoagulation intensity of rivaroxaban for stroke patients at a special low dosage in Japan. PLoS ONE. 2014;9(11):e113641.
- Seiffge DJ, De Marchis GM, Koga M, et al. Ischemic stroke despite oral anticoagulant therapy in patients with atrial fibrillation. Ann Neurol. 2020;87(5):677–687.
- Yaghi S, Henninger N, Giles JA, et al. Ischaemic stroke on anticoagulation therapy and early recurrence in acute cardioembolic stroke: the IAC study. J Neurol Neurosurg. 2021;92(10):1062–1067.
- Paciaroni M, Caso V, Agnelli G, et al. Recurrent ischemic stroke and bleeding in patients with atrial fibrillation who suffered an acute stroke while on treatment with nonvitamin K antagonist oral anticoagulants: the RENO-EXTEND study. Stroke. 2022;53(8):2620–2627.
- Korsholm K, Damgaard D, Valentin JB, et al. Left atrial appendage occlusion vs novel oral anticoagulation for stroke prevention in atrial fibrillation: rationale and design of the multicenter randomized occlusion-AF trial. Am Heart J. 2022 Jan;243:28–38.
- Holmes DR, Reddy VY, Turi ZG, et al. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet. 2009 Aug 15;374(9689):534–542.
- Holmes DR Jr., Kar S, Price MJ, et al. Prospective randomized evaluation of the Watchman Left Atrial Appendage Closure device in patients with atrial fibrillation versus long-term warfarin therapy: the PREVAIL trial. J Am Coll Cardiol. 2014 Jul 8;64(1):1–12.
- Osmancik P, Herman D, Neuzil P, et al. Left atrial appendage closure versus direct oral anticoagulants in high-risk patients with atrial fibrillation. J Am Coll Cardiol. 2020 Jun 30;75(25):3122–3135.
- Chew DS, Piccini JP. Postprocedural antithrombotic therapy following left atrial appendage occlusion: no longer adrift in uncertainty? Circ Cardiovasc Interv. 2020 Jul;13(7):e009534.
- Freeman JV, Higgins AY, Wang Y, et al. Antithrombotic therapy after left atrial appendage occlusion in patients with atrial fibrillation. J Am Coll Cardiol. 2022 May 10;79(18):1785–1798.
- Duthoit G, Silvain J, Marijon E, et al. Reduced rivaroxaban dose versus dual antiplatelet therapy after left atrial appendage closure: ADRIFT a randomized pilot study. Circ Cardiovasc Interv. 2020 Jul;13(7):e008481.
- Li SY, Wang J, Hui X, et al. Meta-analysis of postoperative antithrombotic therapy after left atrial appendage occlusion. J Int Med Res. 2020 Nov;48(11):300060520966478.
- Kuck K-H, Brugada J, Fürnkranz A, et al. Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med. 2016;374(23):2235–2245.
- Wyse DG, Waldo AL, DiMarco JP, et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002 Dec 5;347(23):1825–1833.
- Van Gelder IC, Hagens VE, Bosker HA, et al. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med. 2002 Dec 5;347(23):1834–1840.
- Kirchhof P, Camm AJ, Goette A, et al. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med. 2020 Oct 1;383(14):1305–1316.
- Proietti M, Vitolo M, Harrison SL, et al. Real-world applicability and impact of early rhythm control for European patients with atrial fibrillation: a report from the ESC-EHRA EORP-AF long-term general registry. Clin Res Cardiol. 2022;111(1):70–84.
- Sagris D, Korompoki E, Ntaios G, et al. Sinus rhythm restoration and improved outcomes in patients with acute ischemic stroke and in-hospital paroxysmal atrial fibrillation. Eur Stroke J. 2022;7(4):421–430.
- Park J, Shim J, Lee JM, et al. Risks and benefits of early rhythm control in patients with acute strokes and atrial fibrillation: a multicenter, prospective, randomized study (the RAFAS Trial). J Am Heart Assoc. 2022 Feb;11(3):e023391.
- Barra S, Baran J, Narayanan K, et al. Association of catheter ablation for atrial fibrillation with mortality and stroke: a systematic review and meta-analysis. Int J Cardiol. 2018 Sep 1;266:136–142. doi: 10.1016/j.ijcard.2018.03.068.
- Packer DL, Mark DB, Robb RA, et al. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation. JAMA. 2019;321(13):1261.
- Andrade JG, Wells GA, Deyell MW, et al. Cryoablation or drug therapy for initial treatment of atrial fibrillation. N Engl J Med. 2021 Jan 28;384(4):305–315.
- Kuniss M, Pavlovic N, Velagic V, et al. Cryoballoon ablation vs. antiarrhythmic drugs: first-line therapy for patients with paroxysmal atrial fibrillation. Europace. 2021 Jul 18;23(7):1033–1041.
- Wazni OM, Dandamudi G, Sood N, et al. Cryoballoon ablation as initial therapy for atrial fibrillation. N Engl J Med. 2021 Jan 28;384(4):316–324.
- Kuck KH, Lebedev DS, Mikhaylov EN, et al. Catheter ablation or medical therapy to delay progression of atrial fibrillation: the randomized controlled atrial fibrillation progression trial (ATTEST). Europace. 2021 Mar 8;23(3):362–369.
- Diener HC, Connolly SJ, Ezekowitz MD, et al. Dabigatran compared with warfarin in patients with atrial fibrillation and previous transient ischaemic attack or stroke: a subgroup analysis of the RE-LY trial. Lancet Neurol. 2010 Dec;9(12):1157–1163.
- Hankey GJ, Patel MR, Stevens SR, et al. Rivaroxaban compared with warfarin in patients with atrial fibrillation and previous stroke or transient ischaemic attack: a subgroup analysis of ROCKET AF. Lancet Neurol. 2012 Apr;11(4):315–322.
- Easton JD, Lopes RD, Bahit MC, et al. Apixaban compared with warfarin in patients with atrial fibrillation and previous stroke or transient ischaemic attack: a subgroup analysis of the ARISTOTLE trial. Lancet Neurol. 2012 Jun;11(6):503–511.
- Rost NS, Giugliano RP, Ruff CT, et al. Outcomes with edoxaban versus warfarin in patients with previous cerebrovascular events: findings from ENGAGE AF-TIMI 48 (Effective anticoagulation with factor Xa next generation in atrial fibrillation-thrombolysis in myocardial infarction 48). Stroke. 2016 Aug;47(8):2075–2082
- Almutairi AR, Zhou L, Gellad WF, et al. Effectiveness and safety of non-vitamin K antagonist oral anticoagulants for atrial fibrillation and venous thromboembolism: a systematic review and meta-analyses. Clin Ther. 2017 Jul;39(7):1456–1478.e36.
- Meinel TR, Branca M, De Marchis GM, et al. Prior anticoagulation in patients with ischemic stroke and atrial fibrillation. Ann Neurol. 2021 Jan;89(1):42–53.
- D’Anna L, Filippidis FT, Harvey K, et al. Ischemic stroke in oral anticoagulated patients with atrial fibrillation. Acta Neurol Scand. 2022 Mar;145(3):288–296.
- Chugh SS, Havmoeller R, Narayanan K, et al. Worldwide epidemiology of atrial fibrillation: a Global burden of disease 2010 study. Circulation. 2014 Feb 25;129(8):837–847.
- Lip GYH. The ABC pathway: an integrated approach to improve AF management. Nat Rev Cardiol. 2017 Nov;14(11):627–628.
- Kirchhof P. The future of atrial fibrillation management: integrated care and stratified therapy. Lancet. 2017 Oct 21;390(10105):1873–1887.
- Gallagher C, Elliott AD, Wong CX, et al. Integrated care in atrial fibrillation: a systematic review and meta-analysis. Heart. 2017 Dec;103(24):1947–1953.
- Chao TF, Joung B, Takahashi Y, et al. 2021 focused update consensus guidelines of the Asia Pacific Heart Rhythm Society on stroke prevention in atrial fibrillation: executive summary. Thromb Haemost. 2022 Jan;122(1):20–47.
- Lip GYH, Banerjee A, Boriani G, et al. Antithrombotic therapy for atrial fibrillation: CHEST guideline and expert panel report. Chest. 2018 Nov;154(5):1121–1201.
- Joung B, Lee JM, Lee KH, et al. Korean guideline of atrial fibrillation management. Korean Circ J. 2018;48(12):1033.
- Esteve-Pastor MA, Ruiz-Ortiz M, Muñiz J, et al. Impact of integrated care management on clinical outcomes in atrial fibrillation patients: a report from the FANTASIIA registry. Front Cardiovasc Med. 2022;9:856222.
- Rivera-Caravaca JM, Roldán V, Martínez-Montesinos L, et al. The Atrial Fibrillation Better Care (ABC) pathway and clinical outcomes in patients with atrial fibrillation: the prospective Murcia AF project phase II cohort. J Gen Intern Med. 2022 Apr 11. doi:10.1007/s11606-022-07567-5.
- Kotalczyk A, Guo Y, Stefil M, et al. Effects of the Atrial Fibrillation Better Care pathway on outcomes among clinically complex Chinese patients with atrial fibrillation with multimorbidity and polypharmacy: a report from the ChiOTEAF registry. J Am Heart Assoc. 2022 Apr 5;11(7):e024319.
- Wang YF, Jiang C, He L, et al. Integrated care of atrial fibrillation using the ABC (Atrial fibrillation Better Care) pathway improves clinical outcomes in Chinese population: an analysis from the Chinese Atrial Fibrillation Registry. Front Cardiovasc Med. 2021 Nov 18;8:762245.
- Proietti M, Romiti GF, Olshansky B, et al. Improved outcomes by integrated care of anticoagulated patients with atrial fibrillation using the simple ABC (Atrial Fibrillation Better Care) Pathway. Am J Med. 2018 Nov;131(11):1359–1366.e6.
- Gumprecht J, Domek M, Proietti M, et al. Compliance of atrial fibrillation treatment with the Atrial Fibrillation Better Care (ABC) pathway improves the clinical outcomes in the Middle East population: a report from the Gulf Survey of Atrial Fibrillation Events (SAFE) registry. J Clin Med. 2020 Apr 29;9(5):1286. doi: 10.3390/jcm9051286.
- Romiti GF, Pastori D, Rivera-Caravaca JM, et al. Adherence to the ‘Atrial Fibrillation Better Care’ pathway in patients with atrial fibrillation: impact on clinical outcomes-a systematic review and meta-analysis of 285,000 patients. Thromb Haemost. 2022 Mar;122(3):406–414.
- Li D, Shinagawa K, Pang L, et al. Effects of angiotensin-converting enzyme inhibition on the development of the atrial fibrillation substrate in dogs with ventricular tachypacing–induced congestive heart failure. Circulation. 2001;104(21):2608–2614.
- Sakabe M, Fujiki A, Nishida K, et al. Enalapril prevents perpetuation of atrial fibrillation by suppressing atrial fibrosis and over-expression of connexin43 in a canine model of atrial pacing-induced left ventricular dysfunction. J Cardiovasc Pharmacol. 2004 Jun;43(6):851–859.
- Suo Y, Yuan M, Li H, et al. Sacubitril/Valsartan improves left atrial and left atrial appendage function in patients with atrial fibrillation and in pressure overload-induced mice. Front Pharmacol. 2019;10:1285.
- Wallentin L, Lindbäck J, Eriksson N, et al. Angiotensin-converting enzyme 2 (ACE2) levels in relation to risk factors for COVID-19 in two large cohorts of patients with atrial fibrillation. Eur Heart J. 2020 Nov 1;41(41):4037–4046.
- Walters TE, Kalman JM, Patel SK, et al. Angiotensin converting enzyme 2 activity and human atrial fibrillation: increased plasma angiotensin converting enzyme 2 activity is associated with atrial fibrillation and more advanced left atrial structural remodelling. Europace. 2017 Aug 1;19(8):1280–1287.
- Wachtell K, Lehto M, Gerdts E, et al. Angiotensin II receptor blockade reduces new-onset atrial fibrillation and subsequent stroke compared to atenolol: the Losartan Intervention For End Point Reduction in Hypertension (LIFE) study. J Am Coll Cardiol. 2005 Mar 1;45(5):712–719.
- Yusuf S, Healey JS, Pogue J, et al. Irbesartan in patients with atrial fibrillation. N Engl J Med. 2011 Mar 10;364(10):928–938.
- Goette A, Schön N, Kirchhof P, et al. Angiotensin II-antagonist in paroxysmal atrial fibrillation (ANTIPAF) trial. Circ Arrhythm Electrophysiol. 2012 Feb;5(1):43–51.
- Guo Y, Lane DA, Wang L, et al. Mobile health technology to improve care for patients with atrial fibrillation. J Am Coll Cardiol. 2020 Apr 7;75(13):1523–1534.
- Abdul-Rahim AH, Wong J, McAlpine C, et al. Associations with anticoagulation: a cross-sectional registry-based analysis of stroke survivors with atrial fibrillation. Heart. 2014 Apr;100(7):557–562.
