ABSTRACT
Objectives
Women with Turner syndrome (TS) have an increased risk of aortic disease, reducing life-expectancy. This study aimed to systematically review the prevalence of thoracic aortic dilatation, aortic dimensions and growth, and the incidence of aortic dissection.
Methods
A systematic literature search was conducted up to July 2022. Observational studies with an adult TS population were included, and studies including children aged <15 years old or specific TS populations were excluded.
Results
In total 21 studies were included. The pooled prevalence of ascending aortic dilatation was 23% (95% CI 19–26) at a mean pooled age of 29 years (95% CI 26–32), while the incidence of aortic dissection was 164 per 100.000 patient-years (95% CI 95–284). Three reporting studies showed aortic growth over time to be limited. Risk factors for aortic dilation or dissection were older age, bicuspid aortic valve, aortic coarctation, and hypertension.
Conclusion
In adult TS women, ascending aortic dilatation is common and the hazard of aortic dissection increased compared to the general population, whereas aortic growth is limited. Conventional risk markers do not explain all aortic dissection cases; therefore, new imaging parameters and blood biomarkers are needed to improve prediction, allowing for patient-tailored follow-up and surgical decision-making.
1. Introduction
Turner syndrome (TS) affects women with either an absent or incomplete X-chromosome. The incidence of TS among live-born females is approximately 1:2500 [Citation1,Citation2]. Some typical features include short stature, gonadal dysgenesis, and cardiovascular disease [Citation3]. Common congenital cardiac defects include bicuspid aortic valve (BAV), aortic coarctation (COA), abnormal pulmonary venous return, aortic dilatation, and dissection [Citation4–6]. Alarming case reports published on aortic dissection in women with TS at the end of the 20th century [Citation7–10] led to more awareness and research on aortic disease in this patient group. As a result, the guidelines now recommend lifelong close monitoring of women with TS to prevent aortic complications [Citation11]. However, there might be a bias in the published literature, especially on the topic of aortic dissection. Most studies concern case reports or retrospective series, highlighting the patients with an event, thus the exact burden of aortic disease in women with TS is unclear.
Several studies point out an increased prevalence of aortic dilatation and incidence of aortic dissection in Turner women compared to the general population [Citation12–14]. Current European guidelines [Citation15] report an incidence rate of 40 dissections per 100.000 person years, based on a single study [Citation16]. Recent guidelines advise preventive surgery in Turner patients when the aortic size index (ASI) exceeds 25.0 mm/m2 and have associated risk factors for aortic dissection, which is mainly based on two studies: a study of 20 patients with dissections [Citation16] and a 3-year follow-up study of 166 TS patients with a total of 3 dissections [Citation17]. Reviews of the literature on aortic disease in TS women only investigated pregnant TS women [Citation18] or included case reports solely [Citation7].
Since aortic complications greatly impact not only the life expectancy of women with TS [Citation19] but will also cause fear and anxiety in these women [Citation20,Citation21], a complete overview of aortic disease in this specific population is warranted. The aim of this study was to systematically review published observational studies in adult TS women investigating aortic dilatation, dimension, growth, and/or the incidence of aortic dissection at three levels in the aorta: the aortic root, ascending aorta, and the descending aorta. Thereby, we sought to generate normative data for future research, improve patient care through better information provision, and evaluate the impact of current treatment guidelines.
2. Methods
A systematic review and meta-analysis were performed following PRISMA guidelines [Citation22] and registered to PROSPERO (ID number CRD42021231389).
2.1. Literature search
On 20 July 2022, a systematic literature search was conducted by the Erasmus MC Medical Library. Embase, Medline, Web of Science Core Collection, Cochrane Central Register of Controlled Trials, and the top 100 hits on Google Scholar were searched for eligible articles using derivatives or synonyms of ‘Turner syndrome,’ and derivatives or synonyms of ‘Aortic diameter,’ ‘Aortic dilatation,’ or ‘Aortic dissection’ as keywords, excluding irrelevant publication types, such as editorials, conference abstracts, or letters. Full search strategies, and the number of yielded articles per strategy can be found in Appendix A.
2.2. Study selection
Studies were selected using the following inclusion criteria: 1) observational studies and 2) studies reporting on thoracic aortic dimensions, aortic dilatation, or the incidence of aortic dissections in adult TS women. Exclusion criteria were as follows: 1) case report studies, conference abstracts, and reviews, 2) studies including children who have not reached final height (aged <15 years old), and 3) studies with a selected TS population: studies solely including specific karyotypes, excluding TS women with congenital heart disease or structural heart disease or studies with only pregnant TS women. In the studies investigating aortic dimensions, studies including women <15 years old were excluded, since aortic dimensions are correlated with body height and women are assumed to reach their final height around the age of 15. As lifetime risks were obtained for the studies focusing on the incidence of aortic dissection, an age at baseline of <15 years was not an exclusion criterion for these studies. However, follow-up should at least reach until adulthood for the incidence of aortic dissection.
In case of overlapping study populations, for each outcome the study with the largest study population or most person years (in case of aortic growth and dissection incidence) was included. In pooling of the patient characteristics, the study of which the most endpoints were extracted was used. The authors were contacted if the full text of the article was not available. Two reviewers (JD and JB) independently screened the articles using Endnote X9, for the predetermined inclusion and exclusion criteria. Disagreements during screening were resolved by consulting a third party (FM).
2.3. Endpoints
The primary outcomes of our systematic review were as follows: 1) the prevalence of dilation, absolute, and indexed diameters and growth over time at the aortic root (AR), ascending (AA), and descending aorta (DA), and 2) the incidence rate of thoracic aortic dissection in adult TS women. Secondary endpoints included a description of aortic dissection cases of the included studies and risk factor analyses for the primary outcomes.
2.4. Data collection
From each study, study characteristics, patient characteristics, and variables regarding outcome measures were extracted (Appendix B). The data were extracted by one reviewer and checked by a second reviewer in Microsoft Excel 2016. If data were unclear, authors were contacted. Aortic diameters obtained by cardiac tomography (CT) and magnetic resonance imaging (MRI) were preferred over ultrasound if measured with different modalities in the included study. Regarding risk factor analyses for the primary outcomes, estimates of the regression analyses were described.
Risk of bias of included studies was assessed using the Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies of the National Heart, Lung, and Blood Institute [Citation23]. Quality assessment was done independently by two reviewers (JD and JB). Studies scoring below 50% were rated as qualitatively ‘poor,’ between 50% and 75% ‘fair,’ and above 75% ‘good.’
2.5. Statistical analysis
The data were analyzed using the Metafor package [Citation24] in statistical and computing program R (R foundation for Statistical Computing, Vienna, Austria, Version 4.2.1). Patient characteristics were described as reported in the individual study and pooled using an inverse-variance weighted approach. Studies reporting on aortic dissection incidence alone were not included in the pooling of patient characteristics, yet the characteristics of aortic dissection cases were described. Normally distributed numerical data were presented as mean ± standard deviation (SD) and non-normally distributed numerical data as median and range or interquartile range (IQR). Categorical data were presented as proportion (%). For the primary outcomes, the range of the included studies was shown and pooled estimates were calculated. Pooled categorical data were shown as percentages, numerical data as mean and dissection incidence as cases per 100.000 patient-years, all with accompanying 95% confidence interval (CI).
In case of more than one reporting study on one of the main study outcomes, a meta-analysis was performed using random-effects models based on the DerSimonian and Laird method [Citation25]. With regard to pooling of numerical data, the median was considered as mean and the SD was calculated from the range or IQR [Citation26]. When an article mentioned only the absolute diameter of the ascending aorta, the ASI with corresponding SD was calculated by multiplying it with the mean body surface area (BSA) and vice versa if possible. In case of zero cases in dichotomous outcomes, 0.5 cases were assumed in the meta-analysis.
Publication bias was examined with the use of the regression test for funnel plot asymmetry and trim-and-fill analysis for every outcome for which we found 10 or more reporting studies. A P-value of less than 0.05 was considered statistically significant. I2 was used to assess heterogeneity between studies.
Sensitivity analyses were performed on the patient characteristics and the incidence of aortic dissection. To study whether the patient populations were comparable for each endpoint, pooling of patient characteristics for selected studies was performed for studies reporting on 1) aortic dilatation defined as ASI > 20.0 mm/m2 in the ascending aorta and 2) aortic growth in the ascending aorta. Furthermore, for the incidence of aortic dissection, a meta-analysis was performed excluding studies published before 2010.
3. Results
3.1. Search results and study characteristics
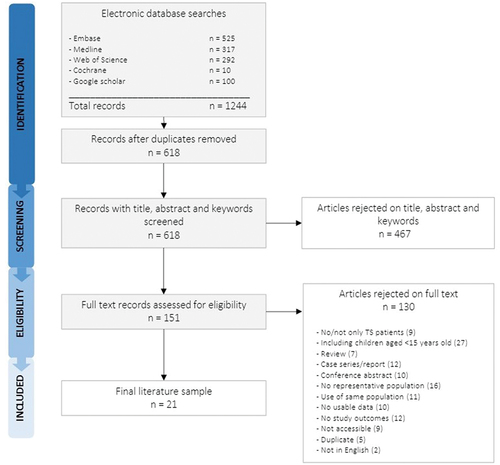
The flow chart of the study selection is shown in . The search yielded a total of 618 records, of which 21 were eligible for this systematic review [Citation14,Citation17,Citation27–45]. The study characteristics, extracted outcomes, and quality assessment are presented in Appendix C. In Appendix D, the scoring of the quality assessment is depicted and in Appendix E the extracted outcomes per study. Six out of 21 studies were cross-sectional [Citation29,Citation31,Citation34,Citation36,Citation38,Citation39], 14 studies were cohort studies [Citation14,Citation17,Citation27,Citation28,Citation32,Citation33,Citation35,Citation37,Citation40–45], and one study was a retrospective epidemiological study investigating the incidence of aortic dissection [Citation30].
3.2. Patient characteristics
The patient characteristics of the included studies and pooled estimates are presented in . Pooled mean age was 30 years (95% CI 28–32). Furthermore, the pooled prevalence of commonly reported features was 51% (95% CI 42–59) for monosomy, 25% (95% CI 20–30) for BAV, 8.3% (95% CI 5.1–12) for CoA, and 27% (95% CI 18–35) for hypertension (). Six studies were not included in the pooling of patient characteristics due to overlapping study populations (n = 2) [Citation32,Citation40] or since only the aortic dissection incidence was extracted (n = 4) [Citation27,Citation28,Citation30,Citation37]. In the studies reporting on aortic dilatation in the ascending aorta [Citation14,Citation17,Citation34,Citation35,Citation38,Citation42,Citation43], the patient characteristics did not differ substantially from the total study population (Appendix F). For the studies reporting on ascending aortic growth [Citation14,Citation40,Citation42], the pooled percentage of hypertension seemed lower (16.6% 95% CI 3.82–29.4) than in the total population (Appendix F), mainly driven by the low proportion of hypertension (4.6%) in the study by Donadille et al. [Citation42].
Table 1. Patient characteristics at baseline of the included studies.
3.3. Aortic dilatation
Aortic dilatation at the level of the aortic root (AR), ascending aorta (AA) or descending aorta (DA) was described by 12/21 included studies. In Appendix G, the prevalence of aortic dilatation at the three levels and its definitions are depicted. For aortic dilatation at the level of the AR defined by an ASI > 20 mm/m2 a pooled prevalence of 44.8% (95% CI 34.7–54.9, I2: 47.5%) was found in two reporting studies [Citation38,Citation42]. AR dilatation with other definitions were reported by three studies: 15.8% (19/120) [Citation29], 42.1% (16/38) [Citation31] and 18.9% (15/79) [Citation32]. In Yetman et al., the definition of aortic dilatation included both the AR and the AA, and a prevalence of 29.1% (68/234) was described [Citation44].
A meta-analysis was performed for the seven studies using an ASI > 20 mm/m2 as threshold for aortic dilatation in the AA [Citation14,Citation17,Citation34,Citation35,Citation38,Citation42,Citation43], which showed a pooled prevalence of 22.7% (95% CI 19.0–26.4) () in TS women with a pooled mean age of 29.2 years (95% CI 26.3–32.0) (Appendix F). AA dilatation according to other definitions was reported in two studies: 36.7% (29/79) [Citation32] and 10.6% (21/198) [Citation41] (Appendix G).
Figure 2. Forest plot of prevalence of aortic dilatation defined as an ASI > 20 mm/m2 in the ascending aorta. Heterogeneity as assessed by I2 was 57.5%. CI = confidence interval, RE = random effects.
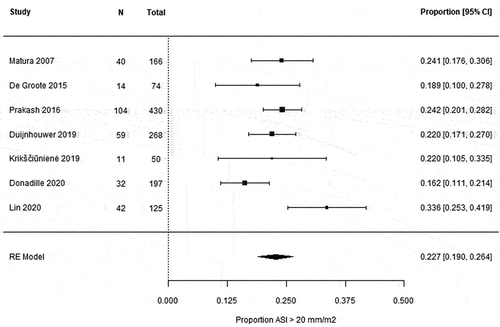
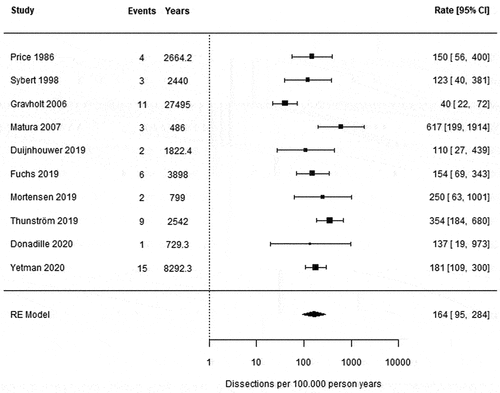
Figure 3. Forest plot of the incidence of aortic dissections per 100.000 person-years. Heterogeneity as assessed by I2 was 73.1%. CI = confidence interval, RE = random effects.
Regarding aortic dilatation at the level of the DA, two studies reported a prevalence [Citation32,Citation38] (Appendix G). In Mortensen et al., a prevalence of 34.1% (27/79) was found for DA dilatation as compared with mean values in their control population [Citation32], and in Krikščiūnienė et al., a prevalence of zero (0/50) was found as defined ASI > 20 mm/m2 in the DA [Citation38].
3.4. Aortic size and growth
shows the results of the meta-analysis for absolute and indexed aortic diameter and aortic growth at the three predefined levels in the aorta. In the three studies reporting an annual growth rate, the pooled baseline age was 30.7 years (95% CI 24.1–37.3) (Appendix F), and the follow-up durations were 8.8 years (range 1.6–12.6), 5.1 years (IQR: 2.8–7.5) and 3.0 years (range 2.4–3.6), respectively [Citation40,Citation42,Citation45]. The annual aortic growth varied between 0.0 mm and 0.32 mm per year in the reporting studies depending on the level in the aorta (). In 3/21 studies, absolute Turner-specific Z-scores were calculated with different calculation methods [Citation14,Citation37,Citation39], hampering pooling of these values.
Table 2. Pooled estimates of absolute and indexed aortic diameters, and aortic growth at the level of the aortic root, ascending, and descending aorta.
3.5. Aortic dissection
shows a forest plot of the meta-analysis of the number of dissections per 100.000 person years for each study. In a total of 10 studies, 56 aortic dissection cases were reported, resulting in a pooled incidence of 164 dissections per 100.000 person years (95% CI 95–284). When excluding the four studies conducted before the previous decade, a pooled incidence of 200 dissections per 100.000 person years (95% CI 143–280) (Appendix H) was observed, which was not substantially different from the pooled incidence in the total population.
In all individual dissection cases and the averages of the groups are described. The age at the time of dissection ranged from 18 to 66 years. In the cases in which a karyotype was defined, 79% of the cases (15/19) had a monosomy 45,X. Type A aortic dissection was observed in 70% (16/23) of known cases and type B dissection in the remaining reported cases (7/23). The reported absolute aortic diameters at last follow-up before or at the time of dissection ranged from 28 to 66 mm and the indexed diameters ranged from 15 to 39.2 mm/m2. The ASI of six-eighths patients of whom the ASI was reported exceeded 25 mm/m2. The 2/8 patients with ASI < 25 mm/m2 presented with type B aortic dissections. In 54% (21/39) of the known cases BAV was present, in 36% (14/38) COA was present and hypertension in 56% (14/25) of the reported cases. As specific data on aortic dissection in epidemiological studies were scarce, all types of thoracic aortic dissections were extracted, including dissections due to trauma or surgery, and aortic ruptures were interpreted as dissections. Two cases of aortic dissection related to blunt trauma were reported [Citation28,Citation44].
Table 3. Description of aortic dissection cases in included studies.
3.6. Risk factor analyses
3.6.1. Aortic dilatation
In 9/21 included studies (excluding overlapping studies) a risk factor analysis was performed for one of the primary endpoints [Citation14,Citation17,Citation29,Citation31,Citation33,Citation41–43,Citation45]. For aortic root and/or ascending aortic dilatation, Ostberg et al. identified a significant positive association with age and BAV [Citation29], in Duijnhouwer et al. age, BAV, karyotype 45,X, weight, and hypertension were independently associated [Citation14], and in Meccanici et al., age, systolic blood pressure, and BAV were associated with a higher prevalence of aortic dilatation [Citation45]. Furthermore, Donadille et al. performed a regression analysis for aortic dilatation-free survival, reporting age and BAV as significant risk factors, while past growth hormone treatment, the presence of CoA and monosomy 45,X were not [Citation42].
3.6.2. Aortic size and growth
Studies investigating associations with baseline diameters found age [Citation14,Citation17,Citation43,Citation45], BSA [Citation17,Citation43], BAV [Citation14,Citation43,Citation45], CoA [Citation45], systolic blood pressure [Citation45], hypertension [Citation14,Citation43], karyotype 45,X [Citation14], weight [Citation14] and past growth hormone treatment [Citation14] as independently associated factors with greater aortic diameters. In two studies, linear mixed models were used to incorporate aortic diameters at multiple time points [Citation33,Citation45]. These studies showed that age [Citation33,Citation45], BAV [Citation33,Citation45], CoA [Citation33,Citation45], systolic blood pressure [Citation45], diastolic blood pressure [Citation33] were independently associated with greater diameters at any level in the aorta, whereas antihypertensive treatment was associated with smaller aortic dimensions at the level of the aortic root [Citation33]. In Mortensen et al., CoA was associated with greater diameters and growth in the descending aorta [Citation33], also described in the analysis by Meccanici et al. [Citation45].
3.6.3. Aortic dissection
The study by Thünstrom et al. describes an univariable risk factor analysis for the incidence of aortic dissection, which found aortic dilatation, treatment for hypertension, BAV, or CoA associated with increased risk, whereas the following variables were not: age at baseline, karyotype, number of typical TS features, previous growth hormone treatment, ongoing estrogen replacement treatment, and statin use [Citation41].
3.7. Publication bias
Publication bias was assessed for two endpoints reaching the minimum of ten included studies: 1) the indexed ascending aortic diameter and 2) the aortic dissection incidence (Appendix I). The funnel plots did not show evidence of publication bias.
4. Discussion
In this systematic review and meta-analysis of aortic dilatation and dissection in adult women with Turner syndrome (TS), we found a pooled prevalence of 23% (95% CI 19–26) for ascending aorta dilatation at a pooled mean age of 29 years (95% CI 26–32), while the incidence of aortic dissection was estimated at 164 per 100.000 patient-years (95% CI 95–284). The aortic growth rate was reported in three studies only and was highest in the proximal parts of the aorta, yet still low in absolute terms. The main risk factors identified for aortic dilatation or dissection in the included studies were older age, bicuspid aortic valve (BAV), aortic coarctation (CoA), and hypertension.
4.1. Aortic dilatation
Ascending aortic dilatation in TS is common (23%) and largely dependent on its definition, the population under study, and the imaging modality used. Taking into account the study population characteristics and aortic dimensions in the included studies, we can conclude that larger dimensions were observed in the studies with relatively older patients and higher proportions of common risk factors BAV, CoA, and hypertension. Dilatation in the descending aorta is not frequently reported in women with Turner syndrome, as most research is focused on the ascending aorta. However, when compared to matched females in the normal population, descending aorta dimensions were also larger [Citation32]. Most of the aortic dissection cases occurred in the proximal part of the thoracic aorta, although distal dissections were not uncommon (30%, 7/23). In the study by Yetman et al., vascular dissections in pulmonary and cerebral vessels were also observed [Citation44]. These findings reflect a vasculopathy in the entire thoracic aorta and beyond [Citation46]. No other study has reported vascular abnormalities beyond the aorta, so this might be important to include in future studies.
Aortic dilation is prevalent among TS women, yet longitudinal data on progression of aortic dilatation is scarce. In the included studies that reported on aortic dilatation progression, the aortic growth over time was significant, however limited in absolute terms and not different from normal growth [Citation40,Citation42,Citation45]. During 8-year follow-up of 91 TS women by Mortensen et al., aortic growth did not differ from a matched cohort [Citation40]. Nonetheless, the observed growth rates in TS women appear highly skewed. It seems that some TS women can be reassured, while there is a group that is indeed at risk of developing aortic dilatation or aortic dissection, who might not be identified by measuring the aortic diameter alone. Promising new imaging markers in TS include aortic stiffness [Citation46–48], aortic distensibility [Citation49], and pulse wave velocity [Citation46,Citation50]. These relatively new techniques require more research to assess their added value as prognostic factors.
4.2. Aortic dissection
Regarding aortic dissection, we found a pooled incidence of 164 per 100.000 patient-years (95% CI 95–284) in TS women, markedly higher than the incidence in the general population ranging from 4.6 to 7.2 cases per 100.000 patient-years [Citation51–53]. Our hypothesis was that in earlier published studies the incidence of aortic dissection might have been overestimated, due to selection of a more severe phenotype in previous time cohorts and increased awareness in later time cohorts. However, this was not confirmed by our sensitivity analysis excluding studies published before 2010. Even though most TS women are carefully monitored by multidisciplinary expert centers, aortic dissection remains associated with mortality in women with TS, greatly affecting their life-expectancy [Citation52]. Interestingly, in the study by Yetman et al., a lack of surveillance by a cardiologist was associated with a higher incidence of vascular dissection in TS women [Citation44]. This highlights the importance of regular follow-up by specialized health-care providers.
In women with TS, aortic dissections can already occur as early as the second decade of life, with ages ranging from 18 to 66 years in reporting studies, compared to a mean age around 68 years in women presenting with aortic dissection in the general population [Citation53]. Apart from a younger age, women with TS also tend to dissect at smaller diameters when compared to the general population [Citation54]. In order to prevent these potentially lethal aortic events, TS women with certain risk factors and/or aortic dilatation should be checked regularly. The most common modifiable and unmodifiable risk factors are described in the following section.
5. 4.3 (Un)modifiable risk factors
Several congenital heart defects tend to be associated with aortic dilatation, growth, or dissection. BAV seems to be mainly associated with diameters in the aortic root and ascending aorta, whereas CoA has a more pronounced effect in the descending aorta [Citation14,Citation33,Citation43,Citation45]. BAV might be associated with aortic dilatation through abnormal flow patterns and a genetic component [Citation55,Citation56]. In the reported studies, dysfunction of BAV was not independently associated with greater aortic diameters [Citation32,Citation45]. Probably the combination of BAV-related hemodynamics and associated aortic wall disruption in addition to the intrinsic wall abnormality due to the genetic defect in TS causes the increased risk of aortic complications [Citation47,Citation48].
CoA, and in particular CoA repair was frequently reported as a comorbid condition in the studies on aortic dissection. Complications in the descending aorta can be associated with CoA repair. In an international multicenter series (n=19), stent treatment for CoA was associated with aortic dissection in TS patients [Citation57], and development of local aneurysms after correction for CoA has also been reported in an adult TS population [Citation58].
Lastly, elongation of the aortic arch is stated in guidelines as a risk factor for aortic disease in women with TS [Citation15], mostly based on the study by Matura et al. [Citation17]. However, based on our review, evidence is not strong: in the longitudinal study by Mortensen et al. elongation of the arch was not associated with greater aortic dimensions [Citation33]. The exact influence of aortic arch morphology on the aortic dilatation risk in women with TS requires further investigation, using a clear definition for arch abnormalities.
The risk of aortic disease in women with TS cannot be fully explained by the associated congenital anomalies: hypertension also plays a key role [Citation14,Citation33,Citation39,Citation41,Citation45]. Hypertension is common in TS: in this study, a pooled prevalence of 27% (95% CI 18–35) was found corresponding with other literature in TS women reporting a prevalence ranging from 13% to 58% [Citation59–61]. In some studies, the systolic blood pressure was mostly associated with aortic dimensions [Citation39,Citation45], whereas others found an association for diastolic blood pressure [Citation33]. In a general aging population, diastolic blood pressure was also associated with aortic growth [Citation62].
For women with TS, assisted reproductive technology with oocyte donation may provide an opportunity to become successfully pregnant. Although still rare, aortic dissection does occur more often during pregnancy [Citation63]. A recently published review summarized 14 cases of aortic dissection associated with pregnancy in women with TS, often after oocyte donation [Citation18]. If women with TS wish to become pregnant after an elaborate preconception consultation, rigid attention to blood pressure is necessary in addition to monitoring aortic diameters during pregnancy. Postpartum is also a period of high risk warranting close follow-up.
5.1. Management
In the end, the goal is to provide optimal guidance for women with TS throughout their lifespan and reduce the risk for aortic events. In addition, preventive surgery might result in better outcomes. Guidelines state that aortic surgery should be considered when the ASI exceeds 25 mm/m2 [Citation15,Citation64], which seems a suitable threshold based on the indexed diameters mentioned in this review for the aortic dissection cases. Still, there are TS patients who dissect when the ASI is below 20 mm/m2 when other risk factors are present [Citation65]. Accordingly, when a TS patient presents with an ASI ranging between 20 and 25 mm/m2 and accompanying risk factors such as BAV, CoA, or hypertension, preventive surgery might be indicated. The ASI remains a valuable marker for risk assessment, yet the indication for prophylactic surgery should always be carefully considered in all TS women not only based on the ASI.
Noninvasive drug treatment with beta-blockers or angiotensin receptor blockers is described to halt progression of aortic dilatation in other syndromes and may therefore be an appropriate option in women with TS [Citation15,Citation64]. In Marfan syndrome patients, a meta-analysis of clinical trials depicted that angiotensin receptor blockers were associated with declined aortic growth at the level of the aortic root [Citation66]. Antihypertensive treatment was associated with smaller aortic root dimensions in women with TS in one reporting study [Citation33]. To date, no such drug trials have been performed in TS women. Furthermore, estrogen replacement therapy could be protective of aortic dilatation, as it is known to decrease the stiffness of the large arteries [Citation67]. However, no independent associations between estrogen replacement therapy and aortic dimensions or dissection incidence have been reported [Citation33,Citation41,Citation45]. The effect of early initiation of estrogen supplements even before dilatation is present, could be valuable and warrants further study.
5.2. Recommendations
Future research should reach beyond the aortic diameter, as this metric does not seem to fully capture the risk of aortic dissection in TS women. Novel imaging markers, such as the aortic stiffness [Citation46–48], seem promising in detecting aortic pathology in women with TS possibly even before aortic dilatation is present. Preferably, structural aortic markers such as the aortic diameter and functional characteristics should be measured longitudinally in prospective cohorts already from an early age. Besides imaging markers, blood biomarkers could also be an early marker of thoracic aortic disease progression in TS women, requiring further study [Citation68].
Although TS women dissect at a smaller diameter than women in the general population, we believe caution should be made in the indication for preventive aortic surgery, as women with TS tend to have high complication rates after surgery [Citation69]. Noninvasive treatment with beta-blockers or angiotensin receptor blockers might be slowing down aortic growth [Citation66], and if proven effective in TS, it should be used more aggressively as treatment option in a larger proportion of the TS population. Unfortunately, it remains difficult to study the exact effect of medication on aortic dilatation in TS women, as the study population is typically small and there are often multiple risk factors at hand, which bias the results. A large prospective registry with before-after measurements might provide a more pragmatic study design than a randomized clinical trial.
In addition to surgical and medical treatment, lifestyle could also play a crucial role in women with TS and influence aortic disease progression over time. As hypertension is a modifiable risk factor and highly prevalent, it is important to focus on this during follow-up and act upon. Dietary interventions or guided physical activity could be valuable in improving overall health and cardiovascular status. The influence of lifestyle and exercise on aortic dilatation remains to be elucidated, but positive effects are currently found in mice studies [Citation70].
Lastly, optimal guidance for TS women is not only based on their physical status. It is important to maintain regular follow-up visits at multi-disciplinary care units, providing clinical as well as psychological guidance. Quality of life has shown to be impaired in girls and women with TS when compared to healthy controls, with more perceived stress, fatigue, depression, and lack of self-esteem [Citation20,Citation21,Citation71]. Communication of therisk of aortic complications might decrease anxiety and psychological stress and improve knowledge of their disease status. When risk prediction is improved based on future studies, TS women with low risk could be reassured, whereas women with increased risk could be regularly monitored and receive appropriate treatment.
5.3. Limitations
This review has a few limitations worth mentioning. Although we aimed to include unselected TS cohorts, the study populations included in the systematic review were still heterogeneous. Different imaging modalities were used in the detection of aortic dilatation and aortic diameter measurements, which may have caused heterogeneity [Citation72]. Furthermore, children aged younger than 15 years old were excluded in the current study, although aortic dilatation has also been reported in younger cohorts. In order to compare aortic dimensions, we believe the exclusion of children was necessary. Finally, the limitations inherent of meta-analysis on data from observational studies need to be acknowledged [Citation73].
6. Conclusion
The review shows that aortic dilatation is found in a quarter of women with TS and observed mainly at the level of the ascending aorta. In the few reported studies on aortic growth over time, progression seems limited and not different from the normal population. Still, the incidence of aortic dissections is around 27 times higher than in the general population and occurs in both the ascending and the descending aorta. In a large phenotypic spectrum of aortic diseases, especially TS women with aortic dilatation presenting with risk factors, such as BAV, CoA, and hypertension are at high risk of developing dissection. However, not all TS patients with aortic dilatation or aortic dissection seem to present with these risk factors, warranting further investigation into pathophysiological processes in this heterogeneous disease. International collaboration is essential in conducting these relevant studies, ideally through a large prospective registry.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
A reviewer on this manuscript has disclosed that their study was one of the manuscripts included in this review. The remaining peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Author contributions
Study design: F Meccanici, J Roos-Hesselink. Data collection: F Meccanici, J de Bruijn, J Dommisse. Data analysis: F Meccanici, J de Bruijn, J Dommisse. Data interpretation: all authors. Manuscript draft: F Meccanici. Critical revision, editing, and approval of the final manuscript: all authors.
Supplemental Material
Download MS Word (251.5 KB)Acknowledgments
The authors would like to thank Dr. Sabrina T.G. Meertens-Gunput from the Erasmus University Medical Centre Medical Library for conducting the systematic literature search.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14779072.2023.2172403
Additional information
Funding
References
- Nielsen J, Wohlert M. Chromosome abnormalities found among 34910 newborn children: results from a 13-year incidence study in Århus, Denmark. Hum Genet. 1991 May;87(1):81–83.
- Nielsen J, Sillesen I. Incidence of chromosome aberrations among 11148 newborn children. Humangenetik. 1975 Oct 20;30(1):1–12.
- Sybert VP, McCauley E. Turner’s syndrome. N Engl J Med. 2004 Sep 16;351(12):1227–1238.
- Ho VB, Bakalov VK, Cooley M, et al. Major vascular anomalies in Turner syndrome: prevalence and magnetic resonance angiographic features. Circulation. 2004 Sep 21;110(12):1694–1700.
- Marin A, Weir-McCall JR, Webb DJ, et al. Imaging of cardiovascular risk in patients with Turner’s syndrome. Clin Radiol. 2015 Aug;70(8):803–814.
- van den Hoven AT, Chelu RG, Duijnhouwer AL, et al. Partial anomalous pulmonary venous return in Turner syndrome. Eur J Radiol. 2017;95:141–146.
- Carlson M, Silberbach M. Dissection of the aorta in Turner syndrome: two cases and review of 85 cases in the literature. J Med Genet. 2007 Dec;44(12):745–749.
- Goldberg SM, Pizzarello RA, Goldman MA, et al. Aortic dilatation resulting in chronic aortic regurgitation and complicated by aortic dissection in a patient with Turner’s syndrome. Clin Cardiol. 1984 Apr;7(4):233–235.
- Shiroma K, Ebine K, Tamura S, et al. A case of Turner’s syndrome associated with partial anomalous pulmonary venous return complicated by dissecting aortic aneurysm and aortic regurgitation. J Cardiovasc Surg (Torino). 1997 Jun;38(3):257–259.
- Slater DN, Grundman MJ, Mitchell L. Turner’s syndrome associated with bicuspid aortic stenosis and dissecting aortic aneurysm. Postgrad Med J. 1982 Jul;58(681):436–438.
- Erbel R, Aboyans V, Boileau C, et al. 2014 ESC guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The task force for the diagnosis and treatment of Aortic diseases of the European society of cardiology (ESC). Eur Heart J. 2014 Nov 1;35(41):2873–2926.
- Bondy CA. Aortic dissection in Turner syndrome. Curr Opin Cardiol. 2008 Nov;23(6):519–526.
- Wong SC, Cheung M, Zacharin M. Aortic dilatation and dissection in Turner syndrome: what we know, what we are unclear about and what we should do in clinical practice? Int J Adolesc Med Health. 2014;26(4):469–488.
- Duijnhouwer AL, Bons LR, Timmers H, et al. Aortic dilatation and outcome in women with Turner syndrome. Heart. 2019 May;105(9):693–700.
- Baumgartner H, De Backer J, Babu-Narayan SV, et al. 2020 ESC Guidelines for the management of adult congenital heart disease. Eur Heart J. 2021 Feb 11;42(6):563–645.
- Carlson M, Airhart N, Lopez L, et al. Moderate aortic enlargement and bicuspid aortic valve are associated with aortic dissection in Turner syndrome: report of the international turner syndrome aortic dissection registry. Circulation. 2012 Oct 30;126(18):2220–2226.
- Matura LA, Ho VB, Rosing DR, et al. Aortic dilatation and dissection in Turner syndrome. Circulation. 2007 Oct 9;116(15):1663–1670.
- Hynes JS, Kuller JA, Goldstein SA, et al. Increased risk of aortic dissection associated with pregnancy in women with turner syndrome: a systematic review. Obstet Gynecol Surv. 2020 Sep;75(9):566–575.
- Schoemaker MJ, Swerdlow AJ, Higgins CD, et al. Mortality in women with turner syndrome in Great Britain: a national cohort study. J Clin Endocrinol Metab. 2008 Dec;93(12):4735–4742.
- van den Hoven AT, Bons LR, Dykgraaf RHM, et al. A value-based healthcare approach: health-related quality of life and psychosocial functioning in women with Turner syndrome. Clin Endocrinol (Oxf). 2020 May;92(5):434–442.
- Liedmeier A, Jendryczko D, van der Grinten HC, et al. Psychosocial well-being and quality of life in women with Turner syndrome. Psychoneuroendocrinology. 2020;113:104548.
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009 Aug 18;151(4):264–269.
- National Heart, Lung, and BIood Institute. Quality assessment tool for observational cohort and cross-sectional studies. United States Department of Health & Human Services [updated 2014; cited 2022 Sep 13]. Available from: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools.
- Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36(3):1–48.
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986 Sep;7(3):177–188.
- Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014 Dec;19(14):135.
- Price WH, Clayton JF, Collyer S, et al. Mortality ratios, life expectancy, and causes of death in patients with Turner’s syndrome. J Epidemiol Community Health. 1986 Jun;40(2):97–102.
- Sybert VP. Cardiovascular malformations and complications in Turner syndrome. Pediatrics. 1998 Jan;101(1):E11.
- Ostberg JE, Brookes JA, McCarthy C, et al. A comparison of echocardiography and magnetic resonance imaging in cardiovascular screening of adults with Turner syndrome. J Clin Endocrinol Metab. 2004 Dec;89(12):5966–5971.
- Gravholt CH, Landin-Wilhelmsen K, Stochholm K, et al. Clinical and epidemiological description of aortic dissection in Turner’s syndrome. Cardiol Young. 2006 Oct;16(5):430–436.
- Elsheikh M, Casadei B, Conway GS, et al. Hypertension is a major risk factor for aortic root dilatation in women with Turner’s syndrome. Clin Endocrinol (Oxf). 2001 Jan;54(1):69–73.
- Mortensen KH, Hjerrild BE, Stochholm K, et al. Dilation of the ascending aorta in Turner syndrome - a prospective cardiovascular magnetic resonance study. J Cardiovasc Magn Reson. 2011 Apr 28;13(1):24.
- Mortensen KH, Erlandsen M, Andersen NH, et al. Prediction of aortic dilation in Turner syndrome–the use of serial cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2013 Jun 6;15(1):47.
- De Groote K, Devos D, Van Herck K, et al. Abnormal aortic arch morphology in Turner syndrome patients is a risk factor for hypertension. Heart Vessels. 2015 Sep;30(5):618–625.
- Prakash SK, Bondy CA, Maslen CL, et al. Autosomal and X chromosome structural variants are associated with congenital heart defects in Turner syndrome: the NHLBI GenTAC registry. Am J Med Genet A. 2016 Dec;170(12):3157–3164.
- Calanchini M, Moolla A, Tomlinson JW, et al. Liver biochemical abnormalities in Turner syndrome: a comprehensive characterization of an adult population. Clin Endocrinol (Oxf). 2018 Nov;89(5):667–676.
- Fuchs MM, Attenhofer Jost C, Babovic-Vuksanovic D, et al. Long-term outcomes in patients with turner syndrome: a 68-year follow-up. J Am Heart Assoc. 2019 Jun 4;8(11):e011501.
- Krikščiūnienė R, Navickaitė I, Ereminienė E, et al. Relationship between echocardiographic and magnetic resonance-derived measurements of the thoracic aorta in turner syndrome patients. Int J Endocrinol. 2019;2019:9258726.
- Lee YJ, Kim SM, Lee YA, et al. Relationship between systolic hypertension assessed by 24-hour ambulatory blood pressure monitoring and aortic diameters in young women with Turner syndrome. Clin Endocrinol (Oxf). 2019 Jul;91(1):156–162.
- Mortensen KH, Wen J, Erlandsen M, et al. Aortic growth rates are not increased in Turner syndrome-a prospective CMR study. Eur Heart J Cardiovasc Imaging. 2019 Oct 1;20(10):1164–1170.
- Thunström S, Krantz E, Thunström E, et al. Incidence of aortic dissection in turner syndrome. Circulation. 2019 Jun 11;139(24):2802–2804.
- Donadille B, Tuffet S, Cholet C, et al. Prevalence and progression of aortic dilatation in adult patients with Turner syndrome: a cohort study. Eur J Endocrinol. 2020 Oct;183(4):463–470.
- Lin A, Rajagopalan A, Nguyen HH, et al. Dilatation of the ascending aorta in turner syndrome: influence of bicuspid aortic valve morphology and body composition. Heart Lung Circ. 2021 Jan;30(1):e29–e36.
- Yetman AT, Bisselou KSM, Sanmann JN, et al. Vascular dissection in women with Turner syndrome. Int J Cardiol. 2021 Feb;15(325):127–131.
- Meccanici F, Schotte MH, Snoeren M, et al. Aortic dilation and growth in women with Turner syndrome. Heart. 2022;109(2):102–110. Doi:10.1136/heartjnl-2022-320922.
- Bons LR, Van Den Hoven AT, Malik M, et al. Abnormal aortic wall properties in women with turner syndrome. Aorta (Stamford). 2020 Oct;8(5):121–131.
- De Groote K, Devos D, Van Herck K, et al. Increased aortic stiffness in prepubertal girls with Turner syndrome. J Cardiol. 2017 Jan;69(1):201–207.
- Devos DG, De Groote K, Babin D, et al. Proximal aortic stiffening in Turner patients may be present before dilation can be detected: a segmental functional MRI study. J Cardiovasc Magn Reson. 2017 Feb 13;19(1):27.
- Wen J, Trolle C, Viuff MH, et al. Impaired aortic distensibility and elevated central blood pressure in Turner Syndrome: a cardiovascular magnetic resonance study. J Cardiovasc Magn Reson. 2018 Dec 13;20(1):80.
- Blunden CE, Urbina EM, Lawson SA, et al. Progression of vasculopathy in young individuals with turner syndrome. Pediatr Cardiol. 2021 Mar;42(3):481–491.
- Howard DP, Banerjee A, Fairhead JF, et al. Population-based study of incidence and outcome of acute aortic dissection and premorbid risk factor control: 10-year results from the Oxford Vascular Study. Circulation. 2013 May 21;127(20):2031–2037.
- McClure RS, Brogly SB, Lajkosz K, et al. Epidemiology and management of thoracic aortic dissections and thoracic aortic aneurysms in Ontario, Canada: a population-based study. J Thorac Cardiovasc Surg. 2018 Jun;155(6):2254–2264 e4.
- Smedberg C, Steuer J, Leander K, et al. Sex differences and temporal trends in aortic dissection: a population-based study of incidence, treatment strategies, and outcome in Swedish patients during 15 years. Eur Heart J. 2020 Jul 7;41(26):2430–2438.
- Mansour AM, Peterss S, Zafar MA, et al. Prevention of aortic dissection suggests a diameter shift to a lower aortic size threshold for intervention. Cardiology. 2018;139(3):139–146.
- Mahadevia R, Barker AJ, Schnell S, et al. Bicuspid aortic cusp fusion morphology alters aortic three-dimensional outflow patterns, wall shear stress, and expression of aortopathy. Circulation. 2014 Feb 11;129(6):673–682.
- Padang R, Bannon PG, Jeremy R, et al. The genetic and molecular basis of bicuspid aortic valve associated thoracic aortopathy: a link to phenotype heterogeneity. Ann Cardiothorac Surg. 2013 Jan;2(1):83–91.
- van den Hoven AT, Duijnhouwer AL, Eicken A, et al. Adverse outcome of coarctation stenting in patients with Turner syndrome. Catheter Cardiovasc Interv. 2017 Feb 1;89(2):280–287.
- Meijs TA, Minderhoud SCS, Muller SA, et al. Cardiovascular morbidity and mortality in adult patients with repaired aortic coarctation. J Am Heart Assoc. 2021 Nov 16;10(22):e023199.
- Hjerrild BE, Mortensen KH, Sørensen KE, et al. Thoracic aortopathy in Turner syndrome and the influence of bicuspid aortic valves and blood pressure: a CMR study. J Cardiovasc Magn Reson. 2010 Mar 11;12(1):12.
- Giordano R, Forno D, Lanfranco F, et al. Metabolic and cardiovascular outcomes in a group of adult patients with Turner’s syndrome under hormonal replacement therapy. Eur J Endocrinol. 2011 May;164(5):819–826.
- De Groote K, Demulier L, De Backer J, et al. Arterial hypertension in Turner syndrome: a review of the literature and a practical approach for diagnosis and treatment. J Hypertens. 2015 Jul;33(7):1342–1351.
- Thijssen CGE, Mutluer FO, van der Toorn JE, et al. Longitudinal changes of thoracic aortic diameters in the general population aged 55 years or older. Heart. 2022;28.
- Immer FF, Bansi AG, Immer-Bansi AS, et al. Aortic dissection in pregnancy: analysis of risk factors and outcome. Ann Thorac Surg. 2003 Jul;76(1):309–314.
- Silberbach M, Roos-Hesselink JW, Andersen NH, et al. Cardiovascular health in Turner syndrome: a scientific statement from the American Heart Association. Circ Genom Precis Med. 2018 Oct;11(10):e000048.
- Nijs J, Gelsomino S, Lucà F, et al. Unreliability of aortic size index to predict risk of aortic dissection in a patient with Turner syndrome. World J Cardiol. 2014 May 26;6(5):349–352.
- Al-Abcha A, Saleh Y, Mujer M, et al. Meta-analysis examining the usefulness of angiotensin receptor blockers for the prevention of aortic root dilation in patients with the Marfan syndrome. Am J Cardiol. 2020 Aug;1(128):101–106.
- Rajkumar C, Kingwell BA, Cameron JD, et al. Hormonal therapy increases arterial compliance in postmenopausal women. J Am Coll Cardiol. 1997 Aug;30(2):350–356.
- Balmforth D, Harky A, Adams B, et al. Is there a role for biomarkers in thoracic aortic aneurysm disease? Gen Thorac Cardiovasc Surg. 2019 Jan;67(1):12–19.
- Fuchs MM, Attenhofer Jost CH, Said SM, et al. Cardiovascular surgery in Turner syndrome - early outcome and long-term follow-up. World J Cardiol. 2020 Mar 26;12(3):97–106.
- Mas-Stachurska A, Siegert AM, Batlle M, et al. Cardiovascular benefits of moderate exercise training in marfan syndrome: insights from an animal model. J Am Heart Assoc. 2017 Sep 25;6(9).
- Amedro P, Tahhan N, Bertet H, et al. Health-related quality of life among children with Turner syndrome: controlled cross-sectional study. J Pediatr Endocrinol Metab. 2017 Aug 28;30(8):863–868.
- Bons LR, Duijnhouwer AL, Boccalini S, et al. Intermodality variation of aortic dimensions: how, where and when to measure the ascending aorta. Int J Cardiol. 2019 Feb;1(276):230–235.
- Ioannidis JP, Lau J. Pooling research results: benefits and limitations of meta-analysis. Jt Comm J Qual Improv. 1999 Sep;25(9):462–469.