1. Introduction
Sixty years have passed since Kare Berg first described Lipoprotein(a) [Lp(a)] in humans, initially as the presence or absence of the lipoprotein antigen [Citation1]. Since then, we have learned what Lp(a) is, the genetic control affecting apolipoprotein(a) [apo(a)] and Lp(a) plasma levels, how to measure Lp(a) levels, and the causal association between Lp(a) and atherosclerotic cardiovascular disease (ASCVD) and calcific aortic valve stenosis [Citation2–4]. Elevated Lp(a) levels, a predominantly genetic disorder, are consequent on a combination of heritable factors that contribute to >90% of plasma concentrations [Citation5]. Approximately 20% of the general population have elevated levels of Lp(a) considered as ≥50 mg/dL (~125 nmol/L) [Citation2]. On the other hand, until recently there were no specific drugs to reduce Lp(a) levels. New emerging RNA-based therapies targeting apo(a) production achieve reductions in Lp(a) up to 90%; however, they have not yet been approved and there is no evidence that reducing Lp(a) decreases ASCVD outcomes [Citation3,Citation6]. These facts have been considered by different international clinical practice guidelines and statements incorporating Lp(a) testing in the assessment and management of cardiovascular risk emphasizing the importance of its detection and screening [Citation2,Citation7–9].
1.1. What is Lp(a)?
Lp(a) consists of a low-density lipoprotein (LDL) particle containing apoB-100 that is covalently bound by a disulfide bond to apo(a) [Citation2]. Plasma levels are 70% to 90% determined by the LPA gene locus encoding apo(a) on chromosome 6q26–27 [Citation5]. Apolipoprotein(a) is highly heterogeneous, formed by loop structures known as Kringle IV and V, and the number of KIV2 repeats determines the size of the protein and plasma levels of Lp(a). The higher the number of KIV2, the higher the size of the protein and the lower the plasma levels of Lp(a) [Citation2].
1.2. What is the risk of having high Lp(a)?
Epidemiological studies, mendelian randomization, and genome-wide association studies have confirmed that Lp(a) levels increase independently the risk of ASCVD and calcific aortic valve disease in primary and secondary prevention [Citation10]. The ASCVD risk associated with Lp(a) increases continuously, in a linear relationship from a threshold of 30 mg/dL (~70 nmol/L). However, a level of Lp(a) more than 50 mg/dL (~125 nmol/L) corresponding to the 80th percentile of European population, is the most common threshold used in clinical practice [Citation2,Citation7]. Different studies have evaluated ASCVD risk using Lp(a) measured in mass units (mg/dL) or molar units (nmol/L) showing both the continuous and linear risk in white and black people. Guidelines recommend to not use a conversion factor to convert Lp(a) levels from mg/dL to nmol/L or vice versa due to differences in antibodies and calibrators used in available assays. Regarding the size of Lp(a), studies have shown an increase in CV risk when the number of KIV-2 repeats is low, which is associated with smaller apo(a) and high levels of Lp(a) [Citation2].
1.3. How to measure and screen for high Lp(a)?
Assays for plasma Lp(a) levels measurement have improved in the last years with the development of different isoform-independent molar assays providing a significant advantage compared to those Lp(a) mass assays since there is no influence of particle size [Citation2,Citation7–9]. In the general population, Lp(a) levels are highly skewed toward low levels, with a high inter-individual and ethnic variability, ranging from <0.1 mg/dL to more than 200 mg/dL (~476 nmol/L) and in general remain with little variations throughout life [Citation2].
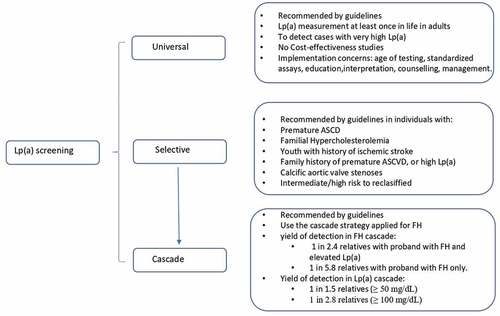
Being high Lp(a) a very common heritable disorder, associated to high ASCVD risk, different strategies have been suggested by guidelines to detect patients with high Lp(a), including universal or population screening, selective testing and cascade testing () [Citation2,Citation7–9].
1.3.1. Universal screening
In the last years, different guidelines and consensus statements have recommended testing Lp(a) at least once in adult’s lifetime as part of, or preferably in the initial lipid screening to identify individuals with high CV risk [Citation2,Citation9]. At what age to start testing Lp(a) remains to be answered. The LPA gene is fully expressed by 2 years old, with adult Lp(a) concentrations tend to be achieved by age of 5 years; however, a variation in Lp(a) levels from childhood to adolescence has been observed [Citation11]. Moreover, the implementation of this type of screening strategy requires the use of globally standardized assays, the participation and education of health professionals in the interpretation of results, genetic counseling, and management in case of high Lp(a). Also, it is necessary studies demonstrating that this strategy is cost-effectiveness.
1.3.2. Selective testing
This strategy is recommended by most followed international guidelines, and consists in Lp(a) testing in all subjects with premature ASCVD, familial hypercholesterolemia (FH), youth with history of ischemic stroke, family history of premature ASCVD, individuals with intermediate/high risk, relative with Lp(a) >180 mg/dL (~430 nmol/L), and calcific aortic valve stenosis [Citation2,Citation7–9,Citation12].
1.3.3. Cascade screening
Cascade screening refers to the testing for a condition in first-degree relatives of a proband known to have a genetic condition. Therefore, it requires first having a diagnosed individual, probably from the selective approach. There is good evidence that cascade screening is the best and most cost-effective approach to detect individuals with FH [Citation11].
1.4. Is there any controversy regarding testing Lp(a) with this strategy?
The answer should be that there are fewer controversies than agreements. High Lp(a) is more common than FH, with a co-dominant autosomal heritability, and a high correlation between parents and offspring’s levels; therefore, the use of cascade screening like in FH is recommended by guidelines to detect asymptomatic cases with high Lp(a) [Citation2,Citation7–9]. Although, there are few studies conducted to evaluate the yield of detection of high Lp(a) using cascade screening, they have demonstrated the great efficacy of testing Lp(a) in FH and in isolated high Lp(a) [Citation13–15].
In the SAFEHEART study, Lp(a) measurement during genetic cascade screening of FH in 2927 relatives of 755 probands with genetically diagnosed FH, showed a high yield of detection of elevated Lp(a) defined as ≥50 mg/dL (~125 nmol/L). The systematic screening in relatives from probands with FH and high Lp(a) had a higher yield than the opportunistic approach when probands had only FH and no elevated Lp(a). The systematic approach identified 1 case of high Lp(a) every 2.4 screened, while the opportunistic approach identified 1 case of high Lp(a) every 5.8, similar to the general population [Citation13]. Identification of one or both conditions in relatives has implications for ASCVD risk. Hazard ratio (HR) of CV event or death was higher in relatives with elevated Lp(a) and FH (HR: 4.40), followed by isolated Lp(a) (HR: 3.17), and finally FH alone (HR: 2.47).
Similar results have been obtained in the FH Western Australia program including some children, adding that the probability of detection of elevates Lp(a) was higher from probands with Lp(a) >100 mg/dL (~2.38 nmol/L) [Citation14]. Therefore, the incorporation of Lp(a) measurement once into cascade screening for FH is highly effective in identifying new cases of high Lp(a) especially when probands have very high Lp(a) levels. This approach will permit to identify individuals at high ASCVD risk with high Lp(a) with or without FH.
There is only one study that evaluated the effectiveness and prevalence of high Lp(a) in relatives from probands with Lp(a) >100 mg/dL (~238 nmol/L) using the cascade testing in a tertiary center in Australia [Citation15]. One new case of Lp(a) ≥50 mg/dL (~125 nmol/L) and 1 new case with Lp(a) ≥100 mg/dL (~238 nmol/L) were detected for every 1.5 and 2.8 relatives tested, respectively. Like in previous report in FH, the yield of detection was higher from probands with higher Lp(a) levels.
There are some issues that need further investigation in the cascade screening for Lp(a) like cost-effectiveness studies of this approach considering that in most places cascade screening for other genetic disorders like FH are available, only a single determination of plasma Lp(a) is required, do not require genetic testing for Lp(a), and the cost has been changing as new methods have been developed. Also, there are scarce data on the detection of high Lp(a) and its implication in CV risk in children.
2. Discussion
Elevated Lp(a) is very common in the general population. One in five individuals have a plasma Lp(a) level ≥50 mg/dL, the most common threshold used in clinical practice. Its causal association with ASCVD and aortic valve stenosis makes its early detection necessary as stated by different guidelines and consensus statements. Different strategies to detect elevated Lp(a) have been recommended, universal, selective and cascade testing, each with its pros and cons. In recent years, the improvement in Lp(a) measurement methods, the reduction of costs and/or reimbursement, and previous experience using cascade screening in FH make Lp(a) testing in relatives of a proband known to have isolated elevated Lp(a) and/or FH a good option to detect asymptomatic individuals. Although cost-effectiveness studies of different screening approaches are lacking, yield of detection for Lp(a) ≥50 mg/dL is higher when testing is performed during FH cascade screening, and also when proband has isolated very high Lp(a) levels.
What can we do for the moment with an individual with elevated Lp(a)? While awaiting the availability of RNA therapeutics for specifically targeting the overproduction of Lp(a) and the outcomes of the new anti-Lp(a) ongoing randomized trials to provide final evidence of ASCVD causality in a risk patient, we must strictly control LDL-C levels and other cardiovascular risk factors in the presence of elevated Lp(a) levels which are associated with higher rates of mortality and ischemic coronary events.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Berg K. A new serum type system in man - the Lp system. Acta Path Microbiol Scand. 1963;59:369–382.
- Kronenberg F, Mora S, Stroes E, et al. Lipoprotein(a) in atherosclerotic cardiovascular disease and aortic stenosis: a European Atherosclerosis society consensus statement. Eur Heart J. 2022;43(39):3925–3946. DOI:10.1093/eurheartj/ehac361.
- Mata P, Alonso R, Pérez de Isla L, et al. Dyslipidemia and aortic valve disease. Curr Opin Lipidol. 2021;32(6):349–354.
- Pérez de Isla L, Watts GF, Alonso R, et al. Lipoprotein(a), LDL-cholesterol, and hypertension: predictors of the need for aortic valve replacement in familial hypercholesterolaemia. Eur Heart J. 2021;42(22):2201–2211. DOI:10.1093/eurheartj/ehaa1066
- Boerwinkle E, Leffert CC, Lin J, et al. Apolipoprotein(a) gene accounts for greater than 90% of the variation in plasma lipoprotein(a) concentrations. J Clin Invest. 1992;90(1):52–60.
- Tsimikas S, Moriarty P, Stroes E. Emerging RNA therapies to lower blood levels of Lp(a). JACC focus seminar 2/4. J Am Coll Cardiol. 2021;7712:1576–1589. DOI:10.1016/j.jacc.2021.01.051.
- Wilson DP, Jacobson TA, Jones PH, et al. Use of lipoprotein(a) in clinical practice: a biomarker whose time has come. A scientific statement from the National Lipid Association. J Clin Lipidol. 2019;13(3):374–392. DOI:10.1016/j.jacl.2019.04.010
- Tsimikas S, Fazio S, Ferdinand K, et al. NHLBI working group recommendations to reduce Lipoprotein(a)- mediated risk of cardiovascular disease and aortic stenosis. J Am Coll Cardiol. 2018;71(2):177–192. DOI:10.1016/j.jacc.2017.11.014
- Pearson GJ, Thanassoulis G, Anderson T, et al. Canadian cardiovascular society guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in adults. Can J Cardiol. 2021;37(8):1129–1150. DOI:10.1016/j.cjca.2021.03.016
- Arsenault BJ, Kamstrup PR. Lipoprotein(a) and cardiovascular and valvular diseases: a genetic epidemiological perspective. Atherosclerosis. 2022;349:7–16.
- Loh WJ, Chan DC, Mata P, et al. Familial hypercholesterolemia and elevated lipoprotein(a): cascade testing and other implications for contextual models of care. Front Genet. 2020;13:905941.
- Alonso R, Andres E, Mata N, et al. Lipoprotein(a) levels in familial hypercholesterolemia: an important predictor of cardiovascular disease independent of the type of LDL receptor mutation. J Am Coll Cardiol. 2014;63(19):1982–1989. DOI:10.1016/j.jacc.2014.01.063
- Ellis K, Pérez de Isla L, Alonso R, et al. Value of measuring lipoprotein(a)during cascade testing for familial hypercholesterolemia. J Am Coll Cardiol. 2019;739:1029–1039. DOI:10.1016/j.jacc.2018.12.037.
- Chakraborty A, Pang J, Chan D, et al. Cascade testing for elevated lipoprotein(a) in relatives of probands with familial hypercholesterolemia and elevated lipoprotein(a). Atherosclerosis. 2022;349:219–226.
- Chakraborty A, Pang J, Chan D, et al. Cascade testing for elevated lipoprotein(a) in relatives of probands with high lipoprotein(a). Am J Prev Cardiol. 2022;10:100343. DOI:10.1016/j.ajpc.2022.100343.