ABSTRACT
Objectives
This umbrella review aims to quality assess published meta-analyses, conduct a de-novo meta-analysis of the available randomized control trials (RCTs), and test the hypothesis that there is a long-term difference in mortality between OSR and EVAR.
Methods
A systematic search was conducted in MEDLINE and EMBASE’s bibliographic databases (June 2022). Data were extracted using standardized extraction forms. The methodological quality of publications was assessed using the ROBIS tool. Data were analyzed with ‘one-stage’ and ‘two-stage’ approaches.
Results
According to two-stage analysis, EVAR has significantly favorable mortality for up to four years (increasing evidence). Subsequently, until the longest available time period, there is no difference between EVAR and OSR; all the results are statistically non-significant.
In one stage analysis, the Cox model demonstrated a non-significant (weak evidence) hazard ratio of 1.03 (95% confidence interval [CI]: 0.94–1.12) in favor of OSR. The best-fitting parametric model (generalized gamma), leads to an hazard ratio of 0.97 (95% CI: 0.93–1.01) in favor of EVAR, with the results approaching significance (weak evidence).
Conclusion
The results of this umbrella systematic review and meta-analysis failed to demonstrate any difference in long-term mortality following planned EVAR, compared with OSR of infrarenal AAA.
1. Introduction
An abdominal aortic aneurysm (AAA) is defined as an abdominal aorta with a diameter of 3.0 cm or above [Citation1]. The United Kingdom (UK) National Screening Programme has reported an AAA prevalence of 1.3% among males older than 65 years. According to the screening program results from 2013 onwards, the prevalence of AAA is declining [Citation2]. Similar findings have been reported by the Swedish Screening Programme, with a prevalence of 1.7% [Citation3] in the same demographic group [Citation4]. A higher prevalence of 3.3% was reported in the Danish Screening Programme [Citation5], and 5.1% was reported in the United States of America (US) [Citation6] (although screening in the US was limited to those who smoke tobacco, which is an additional independent risk factor). The prevalence is much lower among females, younger people, and populations of Asian/African/Hispanic heritage [Citation1].
AAA can cause several complications; however, the most significant is when the aorta ruptures, causing acute life-threatening bleeding. The overall mortality associated with rupture is in excess of 80%. In patients reaching hospital, in-hospital mortality is 53%–66%, falling to 42% in those undergoing emergency repair [Citation7]. Prophylactic repair of intact AAAs is offered to patients to avoid this high risk of death.
Two treatment modalities are currently available for AAA: endovascular aneurysm repair (EVAR) and open surgical repair (OSR) [Citation8]. Based on data from the UK National Vascular Registry early (in-hospital) mortality is lower following EVAR vs. OSR (0.4% vs. 2.3%, respectively) [Citation9]. However, concerns have been raised over reported higher long-term mortality following EVAR [Citation8]. This fact has led to considerable clinical uncertainty in decision-making, with some physicians preferentially offering OSR to younger and lower risk patients who would benefit from improved long-term survival at the cost of a potential increase in in-hospital mortality and morbidity [Citation10]. Others believe that improvements in case selection, planning, procedural strategies, device technology, surveillance and the long-term management of grafts and their complications have addressed the deficiencies seen in EVAR 1, yielding similar long-term results. Extremely high variation in EVAR utilization has resulted. For example, in the UK, the use of EVAR to treat screen-detected AAA ranged between 20% and 97% across the regional programs [Citation11], and there is also significant variation in EVAR use globally [Citation12].
Several meta-analyses have been published comparing survival rates, complication rates and reinterventions between OSR and EVAR [Citation13–20]. A Cochrane meta-analysis of RCTs found a short-term benefit for EVAR but no significant difference in all-cause mortality between OSR and EVAR [Citation13]. A recent evidence review by the UK National Institute for Health and Care Excellence (NICE) guidelines group recognized the potential short-term benefits of EVAR but was sufficiently concerned about long-term mortality (which also impacted economic modeling) that it recommended against the routine use of EVAR in prophylactic AAA repair [Citation16]. A recent long-term RCT from the United States reported that EVAR led to improved survival (although the results were statistically non-significant) [Citation21]. However, these studies used different metrics for the effect measure, reported different results and had different conclusions, which confuses rather than clarifies the long-term survival difference between OSR and EVAR.
This study aimed to review the existing available data and robustly evaluate comparative survival following EVAR and OSR for intact AAA. The first objective was a quality appraisal and identification of factors that caused differences in the results of previous meta-analyses. The second objective was to perform an individual patient data (IPD) meta-analysis reconstructed from published Kaplan – Meier curves that compared the long-term overall survival of patients after confirmed unruptured (elective) infrarenal AAA repair with EVAR vs. OSR.
2. Methods
2.1. Literature search
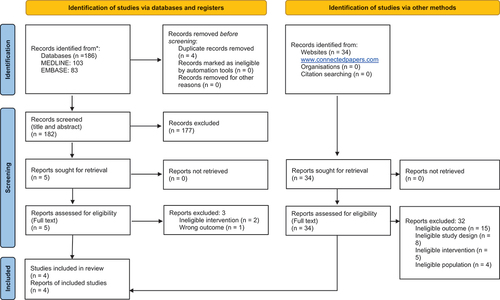
This systematic review was conducted in line with the guidance of the Centre for Reviews and Dissemination [Citation22] and reported in line with the PRISMA [Citation23,Citation24]. The inclusion and exclusion criteria for the review are presented in .
Table 1. Inclusion/Exclusion criteria for systematic screening of identified studies.
2.2. Data sources and search strategy
Searches were conducted in June 2022 in MEDLINE via PubMed and EMBASE via Dialog. The search strings used per database are reported in Supplementary Tables S1 and S2. Two independent reviewers (DB and AI) assessed the quality of all the included meta-analyses, and the third reviewer (VV) resolved any discordances. All the identified meta-analyses were extracted in standardized data tables.
2.3. Methodological quality
The methodological quality of the meta-analyses was assessed using the ROBIS tool 2 [Citation25]. Three independent and blinded parallel reviewers were assigned to screen each study’s title/abstract and full text and extractions. All the studies were screened by all the reviewers (DB, AI and VV), and VV supervised the review processes and made the final decision when disagreement occurred.
2.4. Analysis and data synthesis
For the IPD meta-analyses, we selected RCTs from the identified meta-analyses in that met the PICO inclusion criteria. Derivation of the time-to-event IPD from the Kaplan – Meier (KM) plots from selected RCTs was performed using WebPlotDigitizer and Guyot et al.‘s algorithm [Citation26]. The data were analyzed in one-stage and two-stage approaches. In the one-stage approach, IPD derived from KM curves were transformed into the format for survival analysis using a modified algorithm by Guyot et al. [Citation26]. Standard semi-parametric (a Cox proportional hazards [PH] model) and parametric survival analyses, including the Weibull PH, accelerated failure time, exponential, log-normal, log-logistic, gamma, generalized gamma and Gompertz models, were conducted using the hazard ratio (HR) as a metric for the effect. The models were tested against the Akaike information criterion (AIC) and the Bayesian information criterion (BIC). Data were analyzed using the R package ‘Survival.’
The two-stage approach for each study entailed preparing binary data (event/no event) in 2 × 2 tables for each year in the follow-up period. Meta-analyses were conducted per year, and pooled results from all RCTs are graphically presented for total follow-up time using risk ratio and odds ratio as effect measures. Data were analyzed using the R package ‘Meta,’ which automated the estimation of summary statistics for the included studies. In the next step, the weighted average was calculated across all included studies. The Mantel – Haenszel method was used for pooling, and both the DerSimonian and Laird random effects method [Citation27] and the fixed-effect model were used in all meta-analyses. The Mantel – Haenszel method was used instead of the inverse variance and Peto methods because it is considered a more robust pooling method [Citation28]. Forest plots were used to display the results, and drapery plots were used as complementary figures [Citation29]. Heterogeneity was formally assessed using the Chi2 and I2 statistics.
In both analytical methods, p-values were generated and analyzed according to a 5% threshold. Additionally, they were interpreted as a qualifier of the strength of the evidence [Citation30,Citation31]. P-values between 0.0001 and 0.001 were interpreted as strong evidence against the null hypothesis, p-values between 0.1 and 1 were interpreted as weak evidence against the null hypothesis, and between those two extremes, p-values were interpreted as increasing evidence against the null hypothesis as the p-value decreased [Citation30].
3. Results
A total of 216 articles were identified through a systematic search of all databases and gray literature. After excluding duplicates, the search yielded 212 records screened by title and abstract, and 7 records were screened in full text for eligibility. Four articles met all the inclusion criteria. presents the PRISMA diagram. All the excluded articles are reported in Supplementary Tables S4 and S5, including the reasons for exclusion. The included studies are reported in . This systematic review identified four meta-analyses, all reporting the results of three RCTs: DREAM (the Netherlands) [Citation32], EVAR 1 (UK) [Citation8] and OVER (US) [Citation21], which included the AAAs of 2,484 patients. One meta-analysis also included an additional RCT, ACE (France), for shorter follow-up periods [Citation33]. However, the ACE trial does not meet the inclusion criteria due to the inclusion of aortoiliac aneurysm and the short follow-up.
Table 2. Studies included in the systematic review.
3.1. Results of published meta-analyses
The results of the published meta-analyses are depicted in . Seven different periods were used to conduct a meta-analysis of EVAR vs. OSR all-cause mortality. The only significant result was reported by Antoniou et al. [Citation34] during the perioperative period of index surgery up to six months; increasing evidence of a reduction in all-cause mortality for EVAR compared with OSR (HR 0.62, p = 0.01) was demonstrated.
Table 3. Results of included meta-analyses of all-cause mortality from randomized control trials.
Weak evidence (HR 1.01, p = 0.72) for all-cause mortality slightly in favor of OSR was reported by Bulder et al. for the combined period from index surgery up to three years. However, Bulder et al. [Citation18] included the ACE trial [Citation33] that, apart from an infrarenal AAA, included patients with a good prognosis and aortoiliac aneurysms, which may not be representative of infrarenal AAA patients.
Furthermore, Antoniou et al. [Citation34] reported weak evidence marginally in favor of OSR for all-cause mortality from six months to four years (HR 1.02 p = 0.87), and no difference was reported from zero to five years by Bulder et al. [Citation18] (HR 1.00 p = 0.91). Both these meta-analyses included the same studies. Antoniou et al. [Citation34] excluded results from zero to six months and reported only the period of six months to four years.
Regarding mid-term results, for the period of four to eight years, two meta-analyses reported the same results, with slightly different HRs of 1.09 and 1.13, in favor of OSR (both results are non-significant, weak evidence). Therefore, Antoniou et al. [Citation34] and Giannopoulos et al. [Citation35] reported different results with the same conclusion. However, for the stated period, different study data were included. Antoniou et al. [Citation34] did not include the DREAM trial and did not report the HR in the primary RCT study for the target periods. In addition, these two meta-analyses reported longer-term results for eight years with HRs of 1.05 [Citation34] and 1.07 [Citation35].
Reporting overall results for the period of zero to ten years, Bulder et al. [Citation18] reported a HR of 1.04 (non-significant, weak evidence) in favor of OSR, while Chen et al. [Citation19] reported an odds ratio of 0.90 in favor of EVAR (non-significant, weak evidence). Besides using different effect measure metrics, Chen et al. [Citation19] and Bulder et al. [Citation18] both included the ACE trial in their results but did not include long-term results from the OVER trial. Finally, Antoniou et al. [Citation34] and Giannopoulos et al. [Citation35] reported HRs of 1.02 and 1.04 (non-significant, weak evidence), respectively, for zero to fifteen years. However, Giannopoulos et al.‘s [Citation35] meta-analysis lacked long-term results from the OVER trial.
3.2. Methods of the published meta-analyses
As depicted in , despite including the same RCTs, the methods varied across the meta-analyses, with both random and fixed effect models utilized and results reported as HRs, odds ratios and risk ratios. Not all the RCTs reported HRs for all the periods selected for the meta-analyses, meaning that important RCTs were excluded from some analyses. The methods for pooling outcomes were not transparently reported, apart from by Antoniou et al. 2020 [Citation34], who reported using the inverse variance method. Follow-up periods for the meta-analyses were selected arbitrarily and without justification. Having different follow-up periods, different metrics for effect measures and potentially different methods for pooling results led to differences in the reported results, despite the fact that the same three RCTs (DREAM, EVAR 1 and OVER) were included in all the meta-analyses.
3.3. Quality assessment of the included meta-analyses
The quality of all the meta-analyses was assessed using the ROBIS tool, and the results are reported in Supplementary File (Robis MS Access file). Giannopoulos et al. [Citation35] achieved the highest quality, while Chen et al. [Citation19] had critically low quality.
All identified weaknesses in published meta-analyses would require re-analysis using a more advanced approach based on individual patient data (IPD) from clinical trials, taking into account all health outcomes metrics and using multiple methods to confirm the same conclusions. In summary, the following issues were identified: (1) Different meta-analyses yield varying results, even when the same trials were included. The results of different meta-analyses should be similar or only differ slightly due to differences in metrics or methods, so further research would be necessary. (2) The quality of the meta-analyses varies greatly, as detailed in the supplementary material. (3) The outcome metrics used in the meta-analyses differ, including safety ratios, risk ratios, and odds ratios. (4) The methods used to pool data in the meta-analyses vary or are not reported adequately. When reported, the simplest method, that is, the inverse variance method, was used. (5) The follow-up periods in the meta-analyses vary, leading to limited comparability. (6) Due to these factors, published meta-analyses have caused significant differences in results, leading partly to confusion, rather than providing clear guidance for healthcare practitioners and decision-makers. To address these issues, we conducted a new meta-analysis by reconstructing the IPD from studies and analyzing the data using multiple methods for each year of the follow-up period.
3.3.1. Two-stage meta-analysis approach results
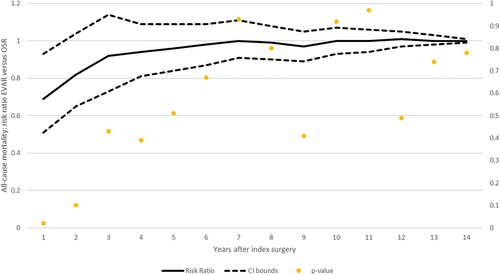
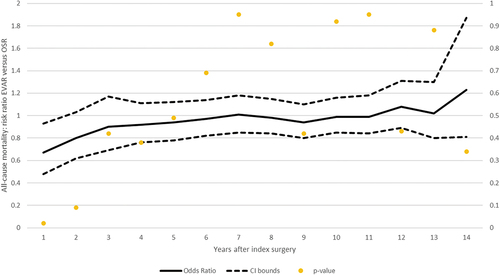
The results of our analysis using risk ratios and odds ratios as effect measure metrics are presented in , respectively. A series of meta-analyses was conducted for three main trials – DREAM, EVAR-1 and OVER – every year from index surgeries until the longest available follow-up (14 years). Annual risk ratios are connected in the meta-analytic curve, and the results are presented with associated p-values on the secondary y-axis. As depicted in the graph, EVAR has significantly favorable mortality for up to four years (increasing evidence). Subsequently, there are no significant (weak evidence) differences in the risk of all-cause mortality. From the mid-term period (after five years) until the longest available time period, there is no difference between EVAR and OSR; all the results are statistically non-significant. Forest plots for both effect measures (risk ratios and odds ratios) per year are reported in Supplementary Figures S1–S52 and Supplementary Table S3.
3.4. One-stage meta-analysis approach results
The results of the pooled analysis of IPD data reconstructed from published graphs are reported in . The results of all the standard semi-parametric and parametric tests are presented in . The Cox model demonstrated a non-significant (weak evidence) HR of 1.03 (95% confidence interval [CI]: 0.94–1.12) in favor of OSR. According to the AIC and BIC tests (generalized gamma), the best-fitting parametric model leads to an HR of 0.97 (95% CI: 0.93–1.01) in favor of EVAR, with the results approaching significance (weak evidence).
Figure 4. One stage approach: all-cause mortality EVAR versus OSR.
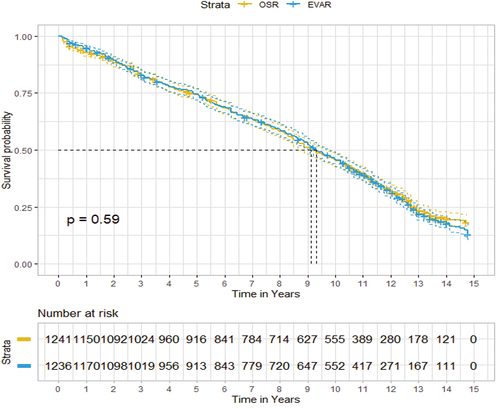
Table 4. One-stage meta-analysis approach resultswith patients from all randomized control trials (DREAM, OVER, and EVAR-1).
4. Discussion
This meta-analysis has demonstrated that in the early period after intervention (up to three years), EVAR is associated with improved patient survival. Following this there was no differences in survival between EVAR and OSR in the mid-term and importantly there is no evidence of any difference after long-term follow-up.
Overall, the previous meta-analyses, published before 2019, point to EVAR being associated with lower mortality in the short- and mid-term periods up to eight years and higher mortality in the most extended follow-up period [Citation13,Citation16]. However, the inclusion of the long-term data from the OVER trial results [Citation19] from 2019 led to a mortality risk ratio of 1.02 (0.94–1.09), demonstrating that there is no significant difference in favor of OSR for the most extended follow-up period as well.
Importantly, confusion has arisen from meta-analyses conducted after the OVER trial results were published, that reported results confirming no difference in the longest follow-up period, contrary to previous meta-analyses (including a Cochrane meta-analysis [Citation13]). Antoniou et al. [Citation34], Giannopoulos et al. [Citation35] and Bulder et al. [Citation18] reported lower mortality for EVAR in the mid-term periods up to 10 years. Chen et al. [Citation19] confirmed previous findings that EVAR has better survival for the same period. The primary source of variability was the use of different metrics for effect measures. Specifically, Antoniou et al. [Citation34], Giannopoulos et al. [Citation35] and Bulder et al. [Citation18] used HR metrics, while previous Cochrane analysis [Citation13] and Chen et al. [Citation19] used odds/risk ratio metrics. Although the HR has clear advantages with time-to-event data, such as mortality/survival, the present analysis is in line with Cochrane [Citation13], using odds ratios, because HRs are not consistently reported in all RCTs for target follow-up periods used in meta-analyses. In particular, as HRs are not reported for all periods or in a time-dependent fashion, there is a need to exclude some studies or make strong assumptions to compute the results of meta-analyses. However, these assumptions can have a substantial impact on the results and evidently cause more confusion than enlightenment. The second source of confusion comes from the arbitrary selection of follow-up periods, which are sometimes from index surgery up to a certain period of time (e.g. 10 years) and sometimes for specific periods (e.g. 4 to 10 years). For these reasons, year-by-year meta-analyses were performed, in which the meta-analytic curve based on odds/risk ratio clearly demonstrated mortality trends over time in both direction and magnitude. In addition, an attempt to reconstruct survival curves and analyze them with standard survival analysis and using HR as effect measure confirmed the same conclusions.
Although surgical experience and EVAR technology have evolved, it was impossible to explicitly explore the learning curve in the meta-analyses. The randomized controlled clinical trials (RCT) recruited patients 15–20 years ago, and current techniques and patient management could potentially result in improved EVAR outcomes. In addition, EVAR devices with poor outcomes were removed from the market due to poor performance. Increased reintervention and aneurysm rupture have been attributed to these devices. Long-term data, including these devices, are likely to favor OSR. Undoubtedly, technological and technical advances are ongoing in EVAR. While there have been advances in OSR techniques and critical care, it would be anticipated that advances in the less ‘mature’ treatment would show more striking improvements now and going forward. The improved EVAR mortality in the OVER trial, compared to EVAR 1 and DREAM, may be explained by the fact that the study was performed more recently, benefitting from improvements in the EVAR learning curve. In addition, overall survival after AAA repair is accepted to be 65%–70% at five years and less than 50% at eight years. AAA repair does not need to be durable for 15 years for most patients<15%.
Our meta-analysis has several limitations. Although using the most robust approach to reconstruct IPD is not sufficient to replace access to original prospectively collected data. Therefore, inaccuracies in the results are possible. For that reason, the data were analyzed with several methods to confirm the conclusions, and it should be expected that accuracy is better than with the traditional approach relying on reported averages for different time points. Even when reported in RCTs, it is often not explicitly stated whether the complete population in the studies was treated according to strict instruction for use (IFU) criteria or whether some may have been treated under more ‘relaxed’ criteria. For example, EVAR 1 had considerable variation as to the anatomy considered ‘suitable,’ and the randomized data include a mixture of on-IFU, ‘relaxed IFU,’ and ‘off-IFU’ anatomies [Citation8]. Separating those groups would require a review of every baseline CT scan from the trial and a comparison with the IFU of the device used. This is important, as non-adherence to IFU is a reliable predictor of graft-related adverse events. It is plausible that even RCTs may have some level of bias in favor of OSR. Therefore, much EVAR failure is down to selecting patients with poor anatomy, particularly seal zones, and implanting devices off IFU. This may lead to good short-term outcomes but poor longer-term results. This meta-analysis was unable to properly assess the impact of new technology outcomes due to a lack of relevant RCTs. Also, the current meta-analysis is not able to properly address the question of the evolution of new EVAR devices in the same or extended indication due to the lack of sufficient details from current RCTs. EVAR devices with poor outcomes were removed from the market due to poor performance. Increased reintervention and increased aneurysm rupture have been attributed to these devices. The long-term data, including these devices, likely favor OSR. Over and above, the even more complicated factor, in conducted long-term RCTs and real-world use, is that a device with the same name can have different IFU conditions on different dates, due to changes in approval circumstances. Thus, not only would we need to look at the scans and devices for all cases, but we would also have to look at the IFU conditions of the device on that date. In addition, this meta-analysis focused on all-cause survival only, without considering other patient-relevant outcomes, such as quality of life. Ideally, further research would be much more inclusive of all patients receiving EVAR and OSR to enable more rapid conclusions (particularly in those younger patients who may not be offered EVAR), so that a potentially promising treatment option is not denied to them. Conversely, these are precisely the groups that need the benefit of long-term follow-up.
We expect the screening and prevention of AAA to further improve in the next years. New techniques will be developed for both OSR and EVAR, and there will be advances, particularly in EVAR technology. In the next ten years, one would expect EVAR to become utilized more, although current The National Institute for Health and Care Excellence (NICE) guidelines may
modify this in the UK and elsewhere. One would anticipate that in the next 5–10 years, the prevalence of AAA will continue to fall, screening will further reduce the number of ruptures, and EVAR will be more likely to become the first choice.
Novel advanced predictive analytic tools may offer better baseline risk stratification of patients and improved decision-making in selecting appropriate candidates for one of the two surgical options, leading to overall AAA survival improvement. Such tools can be used before surgery for prospective optimization of intervention benefit – harm balance and cost-effectiveness profiles.
Therefore, this study should be enhanced using decision-analytic modeling to explicitly assess the benefit – harm balance and cost-effectiveness to properly inform clinical and reimbursement decision-making in a complex real-world clinical environment [Citation36]. Future evidence synthesis should therefore aim to combine the results from this meta-analysis with other patient-relevant outcomes, such as health-related quality of life and patient preferences, and with society/national insurance costs.
5. Conclusion
According to this meta-analysis of all-cause mortality following elective EVAR compared with conventional OSR of infrarenal AAA, found no evidence of a difference in mid-term and long-term mortality.
It is plausible that RCTs may have some level of external bias in favor of OSR due to patient selection, which is not always strictly on IFU for EVAR. In addition, EVAR devices with poor outcomes were removed from the market due to poor performance but are still captured in our analysis as part of the final results, which is likely to favor OSR in long-term results. These limitations should be taken into account when interpreting results.
Further benefit – harm, cost-effectiveness analyses, and technology appraisal of the new generation devices are needed to properly inform clinical decision-making by combining results from our meta-analysis and other relevant inputs.
Declaration of interest
VM Veličković has previously received grant funding from ABHI thought Biomath Models. S Neequaye has received fees for consulting services and speaker fees from Terumo Aortic. A Ignjatović previously received grant funding from ABHI thought Biomath Models. D Bogdanović previously received grant funding from ABHI thought Biomath Models.
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
All authors were involved in the conception and design of the study;
DB, AI, and VV executed screening, identifications, and extractions of the data for systematic literature review and meta-analysis;
VV executed analyses and VV, JS, US were involved in the interpretation of the data;
VV produced the first version of the paper, with all authors revising it critically for intellectual content.
All authors approved the final version of manuscript to be published and all authors agree to be accountable for all aspects of the work.
Additional information
Funding
References
- Wanhainen A, Verzini F, Van Herzeele I, et al. Editor’s Choice - European Society for Vascular Surgery (ESVS) 2019 clinical practice guidelines on the management of abdominal aorto-iliac artery aneurysms. Eur J Vasc Endovasc Surg. 2019;57(1):8–93. DOI:10.1016/j.ejvs.2018.09.020
- AAA standards report 2020 to 2021. (Ed.^(eds) (NHS England, Office for Health Improvement and Disparities, 2022)
- Jacomelli J, Summers L, Stevenson A, et al. Impact of the first 5 years of a national abdominal aortic aneurysm screening programme. Br J Surg. 2016;103(9):1125–1131.
- Svensjö S, Björck M, Gürtelschmid M, et al. Low prevalence of abdominal aortic aneurysm among 65-year-old Swedish men indicates a change in the epidemiology of the disease. Circulation. 2011;124(10):1118–1123.
- Grøndal N, Søgaard R, Lindholt JS. Baseline prevalence of abdominal aortic aneurysm, peripheral arterial disease and hypertension in men aged 65-74 years from a population screening study (VIVA trial). Br J Surg. 2015;102(8):902–906.
- Lee ES, Pickett E, Hedayati N, et al. Implementation of an aortic screening program in clinical practice: implications for the Screen for Abdominal Aortic Aneurysms Very Efficiently (SAAAVE) Act. J Vasc Surg. 2009;49(5):1107–1111.
- Karthikesalingam A, Holt PJ, Vidal-Diez A, et al. Mortality from ruptured abdominal aortic aneurysms: clinical lessons from a comparison of outcomes in England and the USA. Lancet. 2014;383(9921):963–969. DOI:10.1016/S0140-6736(14)60109-4
- Patel R, Sweeting MJ, Powell JT, et al. Endovascular versus open repair of abdominal aortic aneurysm in 15-years’ follow-up of the UK endovascular aneurysm repair trial 1 (EVAR trial 1): a randomised controlled trial. Lancet. 2016;388(10058):2366–2374.
- Waton S JA, Birmpili P, Li Q, et al. National vascular registry: 2020 Annual report. (Ed.^(eds) (The Royal College of Surgeons of England, London, 2020)
- Boyle JR, Mao J, Beck AW, et al. Editor’s choice - variation in intact abdominal aortic aneurysm repair outcomes by country: analysis of international consortium of vascular registries 2010 - 2016. Eur J Vasc Endovasc Surg. 2021;62(1):16–24. DOI:10.1016/j.ejvs.2021.03.034
- Meecham L, Jacomelli J, Davis M, et al. Outcomes in men from the NHS abdominal aortic aneurysm screening programme with a large aneurysm referred for intervention. Eur J Vasc Endovasc Surg. 2021;61(2):192–199.
- Beck AW, Sedrakyan A, Mao J, et al. Variations in abdominal aortic aneurysm care: a report from the international consortium of vascular registries. Circulation. 2016;134(24):1948–1958. DOI:10.1161/CIRCULATIONAHA.116.024870
- Paravastu SC, Jayarajasingam R, Cottam R, et al. Endovascular repair of abdominal aortic aneurysm. Cochrane Database Syst Rev. 2014;1:Cd004178.
- Bahia SS, Holt PJ, Jackson D, et al. Systematic review and meta-analysis of long-term survival after elective infrarenal abdominal aortic aneurysm repair 1969-2011: 5 year survival remains poor despite advances in medical care and treatment strategies. Eur J Vasc Endovasc Surg. 2015;50(3):320–330. DOI:10.1016/j.ejvs.2015.05.004
- Khashram M, Williman JA, Hider PN, et al. Systematic review and meta-analysis of factors influencing survival following abdominal aortic aneurysm repair. Eur J Vasc Endovascular Surg. 2016;51(2):203–215.
- Abdominal aortic aneurysm: diagnosis and management. Evidence review K: effectiveness of endovascular aneurysm repair, open surgical repair and non-surgical management of unruptured abdominal aortic aneurysms. NICE guideline (Ed.^(eds). 2018. NICE: National Institute for Health and Care Excellence (NICE). https://www.ncbi.nlm.nih.gov/books/NBK556921/
- Tzani AI, Doulamis IP, Katsaros I, et al. Mortality after endovascular treatment of infrarenal abdominal aortic aneurysms - the newer the better? Vasa - Eur J Vasc. 2018;47(3):187–196.
- Bulder RMA, Bastiaannet E, Hamming JF, et al. Meta-analysis of long-term survival after elective endovascular or open repair of abdominal aortic aneurysm. Br J Surg. 2019;106(5):523–533.
- Chen ZG, Tan SP, Diao YP, et al. The long-term outcomes of open and endovascular repair for abdominal aortic aneurysm: a meta-analysis. Asian J Surg. 2019;42(10):899–906.
- Li B, Khan S, Salata K, et al. A systematic review and meta-analysis of the long-term outcomes of endovascular versus open repair of abdominal aortic aneurysm. J Vasc Surg. 2019;70(3):954–969.e30. DOI:10.1016/j.jvs.2019.01.076
- Lederle FA, Kyriakides TC, Stroupe KT, et al. Open versus endovascular repair of abdominal aortic aneurysm. N Engl J Med. 2019;380(22):2126–2135. DOI:10.1056/NEJMoa1715955
- Akers Jo, University of York. Systematic Reviews: Crd's Guidance for Undertaking Reviews in Health Care. York (UK): CRD University of York; 2009.
- Page MJ, McKenzie JE, Bossuyt PM, et al. Updating guidance for reporting systematic reviews: development of the PRISMA 2020 statement. J Clin Epidemiol. 2021;134:103–112.
- Page MJ, Moher D, Bossuyt PM, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160.
- Whiting P, Savović J, Higgins JPT, et al. ROBIS: a new tool to assess risk of bias in systematic reviews was developed. J Clinical Epidemiol. 2016;69:225–234.
- Guyot P, Ades AE, Ouwens MJNM, et al. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 2012;12(1):9.
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta-analysis. In: Egger M, Smith GD, Altman DG, editors. Systematic Reviews in Health Care. 2001. doi:10.1002/9780470693926.ch15.
- Hodkinson A, Kontopantelis E. Applications of simple and accessible methods for meta-analysis involving rare events: a simulation study. Stat Methods Med Res. 2021;30(7):1589–1608.
- Rücker G, Schwarzer G. Beyond the forest plot: the drapery plot. Res Synth Methods. 2021;12(1):13–19.
- Sterne JA, Davey Smith G. Sifting the evidence-what’s wrong with significance tests? BMJ. 2001;322(7280):226–231.
- Wasserstein RL, Lazar NA. The ASA statement on p-values: context, process, and purpose. Am Stat. 2016;70(2):129–133.
- De Bruin JL, Baas AF, Buth J, et al. Long-term outcome of open or endovascular repair of abdominal aortic aneurysm. N Engl J Med. 2010;362(20):1881–1889. DOI:10.1056/NEJMoa0909499
- Becquemin J-P. The ACE trial: a randomised comparison of open versus endovascular repair in good risk patients with abdominal aortic aneurysm. J Vascular Surg. 2009;50(1):222–224.
- Antoniou GA, Antoniou SA, Torella F. Editor’s choice - endovascular vs. open repair for abdominal aortic aneurysm: systematic review and meta-analysis of updated peri-operative and long term data of randomised controlled trials. Eur J Vasc Endovasc Surg. 2020;59(3):385–397.
- Giannopoulos S, Kokkinidis DG, Armstrong EJ. Long-term outcomes of endovascular vs open surgical repair for abdominal aortic aneurysms: a meta-analysis of randomised trials. Cardiovasc Revasc Med. 2020;21(10):1253–1259.
- Siebert U. When should decision-analytic modeling be used in the economic evaluation of health care? Eur J Health Econ. 2003;4(3):143–150.