ABSTRACT
Introduction
Statins are the cornerstone for atherosclerotic cardiovascular disease risk reduction with recognized efficacy in primary and secondary prevention. Despite this, they remain underutilized due to concerns regarding adverse effects. Statin-associated muscle symptoms (SAMS) are the most frequent cause of medication intolerance and discontinuation with a prevalence estimated at 10%, regardless of causality, with the consequence of increased risk of adverse cardiovascular outcomes.
Areas covered
This clinical perspective reviews recent developments in mechanisms underlying the pathogenesis of statin myopathy, the role of the nocebo effect in perception of statin intolerance, and explores diverse components endorsed by international societies in establishing a statin intolerance syndrome. Non-statin drug alternatives that reduce low-density lipoprotein-cholesterol are also discussed, with emphasis on therapies with established effects on cardiovascular outcomes.
Expert opinion
Ultimately, a patient-centered clinical approach to managing SAMS is proposed to optimize statin tolerability, achieve guideline-recommended therapeutic goals and improve cardiovascular outcomes.
1. Introduction
Atherosclerotic cardiovascular disease (ASCVD) and its major sequelae including myocardial infarction and stroke, are the leading causes of mortality and morbidity worldwide [Citation1,Citation2]. Evidence from meta-analyses of randomized controlled trials, prospective cohort studies, and Mendelian randomization studies have unequivocally established that using safe agents to lower low-density lipoprotein-cholesterol (LDL-C), the predominant subpopulation of apolipoprotein B (apoB)-containing lipoproteins, reduces risk of ASCVD events in proportion to the absolute reduction in LDL-C [Citation3,Citation4]. Statins, or 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors, are the cornerstone drugs for cardiovascular disease risk reduction with recognized efficacy in primary and secondary prevention, in both sexes, and across all ages [Citation5,Citation6]. Beyond cholesterol-lowering, they are associated with multiple beneficial pleiotropic properties including anti-inflammatory, immunomodulatory, and anti-oxidative effects, as well as increased plaque stabilization [Citation7]. Despite these benefits, statin use and treatment optimization have been undermined in clinical practice with more than 80% of high-risk patients failing to achieve guideline-recommended LDL-C targets [Citation8–10] due to poor adherence and/or high discontinuation rates [Citation11].
Although statins are generally well-tolerated and among the most commonly prescribed drugs, adverse effects remain the main cause of non-adherence of therapy [Citation12,Citation13]. Statin intolerance refers to a clinical ‘’umbrella term’’ of various signs and symptoms experienced by patients, manifesting on a continuum and pertaining to multiple organ systems [Citation14,Citation15]. Statin-associated muscle symptoms (SAMS) are the most common form of statin intolerance, also cited as the most frequent reason for medication modification and discontinuation [Citation16–18], in turn resulting in increased risk of adverse cardiovascular outcomes. Although, the term SAMS is used to describe muscle symptoms that occur in the presence of statin use, it does not necessarily imply causation by the statin. Symptoms are typically bilateral, symmetrical, and confined to skeletal muscle [Citation12], usually without creatine kinase (CK) elevation and without objective neuromuscular findings. Some of the frequently reported symptoms are muscle aches or soreness, cramps, weakness and/or fatigue. Very rarely, myopathy or rhabdomyolysis, accompanied by objective signs of weakness and/or CK elevation may occur at a rate of 1 in 10,000 patients per year [Citation17,Citation19,Citation20], simvastatin being the most commonly implicated.
There are several scores that have been developed to predict the likelihood that a patient’s muscle symptoms are due to statin use. These include Statin-Associated Muscle Symptom Clinical Index (SAMS-CI) [Citation17,Citation21], an updated version of this index developed by the Canadian Consensus Working Group [Citation22] or the American College of Cardiology Statin Intolerance tool (https://www.acc.org/statinintoleranceapp) [Citation23], among others. Each have their own strengths and limitations, which can also vary based on the difficulty in describing the clinical presentation of SAMS. A recent systematic review by Meza-Contreras et al. [Citation24] found substantial variability in the definition of statin intolerance among experts. The use of symptom assessment scores, such as SAMS-CI, was only recommended by 6 (23%) articles.
The lack of consensus for the definition of statin-induced muscle events, the broad circumstances associated with subjective myalgia and the commonness of non-muscle symptoms associated with statins all act to hinder the precise estimation of the true incidence of either SAMS or statin intolerance in general. Recent position statements include one from the National Lipid Association (NLA) [Citation14]. Because patients considered to have a high susceptibility to statin toxicity are generally excluded from clinical trials, reported adverse event rates from controlled trials may underestimate the true rate of these adverse effects in an unselected patient population. As such, SAMS is estimated to occur in approximately 10% (range 5% to 20%) of statin-treated patients in the general population [Citation14,Citation15,Citation25], regardless of causality, with a prevalence generally higher in observational and cohort studies (5–30%) as compared to randomized clinical trials (1–5%) [Citation26,Citation27]. In the largest meta-analysis of more than 4 million patients aimed to estimate the overall prevalence of statin intolerance worldwide (112 randomized controlled trials, 64 cohort studies), the latter was determined at 9.1% (8.1–10%) [Citation28]. Lower numbers were estimated when the prevalence was diagnosed using different recognized international definitions, such as the NLA (7.0%), International Lipid Expert Panel (ILEP, 6.7%), and European Atherosclerosis Society (EAS, 5.9%) criteria. As expected, this prevalence was significantly lower in RCTs [4.9% (4.0–6.0%) compared with cohort studies 17% (14–19%)]. This highlights the need for careful assessment of patients with potential symptoms related to statin intolerance.
2. Overview of the clinical need
2.1. Recent developments in mechanisms and biology of SAMS
The mechanisms that underlie the pathogenesis of SAMS in most cases are poorly understood and constitute a highly heterogenous clinical spectrum with numerous complex etiologies. Muscle-related adverse events can be manifestations of mitochondrial dysfunction, changes in enzymes or protein metabolism, autoimmune phenomena, changes in cell membrane dynamics or a variety of genetic backgrounds [Citation29]. Several pathophysiological hypotheses have been proposed, but none have been unequivocally proven as underlying causes of SAMS [Citation30]. describes some of the most prominent mechanisms, focusing on their corresponding pathophysiology, with clinical recommendations based on level of evidence available, ranging from validated systematic reviews and meta-analysis to animal and laboratory studies.
Table 1. Proposed mechanisms of SAMS.
Several of these mechanisms merit emphasis (). These include alterations in gene expression predisposing to statin-induced myopathy, with genetic causes of SAMS being extensively investigated in candidate gene studies, genome-wide association studies and multi-omic networks [Citation31]. Several single nucleotide polymorphisms have been identified to play a role in statin pharmacokinetics, contributing to the pathogenesis of SAMS and accounting for interindividual variability in statin tolerance. Key variants are found in SLCO1B1, responsible for hepatic uptake of statins, cytochrome P450 genes (CYPs), efflux ATP-binding cassette (ABC) transporters, and the carnitine palmitoyl transferase (CPT) enzyme. In rare cases, statins can trigger statin-associated autoimmune myopathy (SAAM), associated with auto-antibodies that recognize HMG-CoA reductase (HMGCR), the pharmacologic target of statins and the expression of which is up-regulated in response to statin therapy. Rarely, these drugs may also aggravate inherent neuromuscular disorders such as myasthenia gravis, polymyositis, or motor neuron diseases. In these cases, discontinuation of statin and initiation of immunosuppressive therapies are recommended. Furthermore, there is increasing evidence that mitochondrial dysfunction plays an essential role in SAMS, given that most cellular processes depend on its energy through oxidative phosphorylation. Beta-oxidation, production of reactive oxygen species and calcium homeostasis also require mitochondrial function. Imbalances in these metabolic processes can thus affect normal skeletal muscle function, leading to myalgias [Citation71]. Other mechanisms, such as HMGCR pathway-mediated effects, can perturb the mevalonate pathway and decrease intermediates of cholesterol synthesis, leading to reduction in protein prenylation or CoQ10. Similarly, results of some studies have suggested that statins may induce myopathy through impaired calcium homeostasis and signaling, atrogin-1 upregulation, or reduced vitamin D levels.
Our summation of the evidence leads us to conclude that the etiology of SAMS is likely multifactorial. It represents a constellation of distinct phenomena with separate mechanisms and overlapping phenotypes. The importance lies in ruling out serious, modifiable, and actionable entities that could represent treatable medical conditions, as well as detecting potential SAMS contributors such as vitamin D deficiency, medication interactions, excessive alcohol use, or hypothyroidism among others. These should ideally be evaluated and corrected where possible, even prior to statin use, and re-considered if SAMS occurs.
2.2. Nocebo effect
Given the unexpectedly high rate of muscle-related symptoms ascribed to statins from observational studies compared to RCTs, a nocebo or drucebo effect has been proposed [Citation13,Citation72]. This effect, in which the expectation of harm results in side effects that may be unrelated to the pharmacological effects of the drug, is thought to contribute substantially to the incidence of SAMS in non-randomized studies [Citation73]. This is supported by observations of increased reports of adverse effects with the use of open-label statins as compared to blinded treatments, as shown in GAUSS-3 [Citation74] and ASCOT-LLA [Citation75] studies. Furthermore, a systematic review of clinical trials that included both open-label and blinded phases estimated that between 38% and 78% of SAMS-related statin intolerance could be attributed to expectation alone [Citation72].
Nevertheless, a 2022 meta-analysis from the Cholesterol Treatment Trialists’ Collaboration analyzing data from 19 double-blind trials with 123,940 participants followed for 4.3 years provided evidence of a small, causal contribution of statins to muscle symptoms [Citation27]. The authors determined that at the end of the study period, SAMS was nearly as common in clinical trial participants receiving statins (27.1%) as in those receiving an indistinguishable placebo (26.6%) with only 3% relative increase in the former group (RR 1.03; 95% CI 1.01–1.06). After the first year of therapy, statin therapy produced a 7% relative increase in SAMS (RR 1.07; 95% CI 1.04–1.10) but no significant rise in subsequent years (0.99; 0.96–1.02). The relative 7% increase in muscle pain or weakness during year 1 corresponded to approximately 1 in 15 muscle-related reports [(1.07–1.00)/1.07] which were attributable to statin therapy. As such, the authors concluded that more than 90% (14/15) of SAMS reports were not due to statins, suggesting that the probability of muscle symptoms being caused by statin is low (<10%).
In light of this, The NLA Scientific Statement on Statin Intolerance [Citation15] proposes that it is reasonable to consider the nocebo effect as a possible cause of SAMS, however it does not make such symptoms less clinically relevant, and ASCVD risk related to elevated atherogenic lipoproteins should be addressed (Class IIa Recommendation, Level of evidence A). As such, the importance lies in treating the patient regardless of a nocebo or drucebo effect and take into account their discomfort and preferences.
2.3. N-of-1 trials
To further support the view that a significant proportion of statin intolerance can be attributed to the nocebo/drucebo effect, the ‘n-of-1’ approach has been implemented in several trials. Using this design, individual patients in a trial cross-over between active treatment and placebo in a blinded fashion. These trials showed similar rates of side effects between statin and placebo groups. In a proof-of-concept study, Joy et al. [Citation76] first utilized the n-of-1 design to assess statin tolerability. Eight patients with prior statin-related myalgia underwent three pairs of double-blind statin or placebo challenges with washout periods between treatments. None of the participants had significant differences in myalgia or other pain measures during statin therapy as compared with placebo, and most resumed statins after reviewing their results. This study’s novel use of the n-of-1 approach for SAMS assessment served as the basis for subsequent trials [Citation77].
Findings from two recent, large series of n-of-1 trials, the Self-Assessment Method for Statin Side-effects or Nocebo (SAMSON) [Citation78] and Statin Web-based Investigation of Side Effects (StatinWISE) [Citation79], showed similar results. Patients were provided with treatment sequences of 1 to 2 months of atorvastatin 20 mg or placebo, with no subsequent statistical difference between reported symptom burden attributed to statins or placebo. Of note, SAMSON utilized a study design whereby participants alternated randomly between three 1-month periods of ‘no treatment,’ placebo tablets, and statin-containing tablets, reporting their symptoms each day [Citation78]. Interestingly, the intensity of symptoms was similar when taking placebo tablets or statins but was markedly lower in the period of ‘no treatment,’ with no pill ingestion. This allowed for a true assessment of the ‘nocebo effect’ demonstrating that symptoms resulted from the process of pill-taking, rather than from some pharmacologic property of the statin [Citation13].
A common feature in all trials was that when presented with trial results, at least half of patients reported intending to restart treatment with statins. As such, conducting these n-of-1 experiments highlights the importance of blinding when assessing for adverse effects and suggests that the n-of-1 approach could be employed in clinical practice in patients presenting with SAMS [Citation79]. This further confirms the utility of personalized n-of-1 trials to help enable patient decision-making on statin resumption and enhance medication adherence which is essential for cardiovascular risk reduction. However, there are practical concerns that remain with this approach since consent is required, diverse placebo pills may be warranted, and the protracted duration for the cross-over trials may be inappropriate in high-risk patients. Cost and time should also be taking in consideration. Additionally, and in spite of the high likelihood of demonstrating a nocebo effect, there is not 100% resumption of statin use, with rates being not dissimilar from rates reported from more traditional, non-blinded approaches. Finally, there are limited comprehensive reports about the effect of such trials on the relationship between the practicing physician and the patient receiving results suggesting a nocebo effect, but who nevertheless does not accept a retrial of statin therapy. As previously mentioned, however, the strength of this relationship is essential in all forms of chronic disease management, and validating the patient’s concerns remains of utmost importance.
3. Goal-inhibiting statin intolerance: emphasizing pragmatism to optimize cardiovascular risk reduction
Numerous societies world-wide have attempted to define ‘statin intolerance’ for diverse purposes ranging from regulatory purposes to clinical purposes [Citation15,Citation19,Citation22,Citation80–87]. Statin therapy for CV risk reduction is essential. The ultimate and overriding clinical challenge is the facilitation of long-term CV risk reduction. Persistence and adherence to statin is considered foundational. But it is sometimes necessary to augment this through judicious use of non-statin agents also having proven safety and CV outcome benefits. It was for this reason that the Canadian Consensus Working Group proposed a re-focusing of statin intolerance definitions by emphasizing a syndrome that identified circumstances that were not optimal for lowering lipid-related CV risk using maximally tolerated statins alone [Citation22,Citation84]. Accordingly, the term ‘goal-inhibiting statin intolerance’ syndrome was coined to provide this emphasis. It is defined as:
Significant symptoms and/or biomarker abnormalities that
Prevent long-term use of and adherence to national guideline-indicated use of statins as
Documented by challenge/de-challenge/re-challenge, when appropriate, using at least two statins, including atorvastatin and rosuvastatin, that are
Not due to avoidable drug–drug interactions, co-morbidities contraindicating or requiring cautious statin use (e.g. chronic kidney disease, or muscular dystrophies) or untreated risk factors for intolerance (e.g. untreated hypothyroidism) and that lead to
Failure to maintain therapeutic goals as defined by national guidelines.
Given enough statin almost anyone might incur side effects. The terms ‘partial’ and ‘complete statin intolerance’ were coined to convey, within approved dose ranges, that many patients can tolerate some statins at some dosages (partial intolerance) whereas a rare individual might not tolerate any statin at any dose (complete intolerance). With the diversity of available, generic statins, evaluation of all options would not be practical and so emphasis was placed on using at least the two most effective agents, rosuvastatin, and atorvastatin, both available in a wide dosage range and both having sufficiently long half-lives to be useful in alternate day or other intermittent dosing strategies. The overriding operational issue, however, is whether by adhering to this approach, cholesterol lowering is optimized to reduce CV risk and, if not, to go beyond statin therapy to overcome this therapeutic deficiency.
The diverse definitions of statin intolerance generally explicitly include or imply the tenets outlined above, including the understanding that use of statin is indicated in the first place, presumably based on the prevailing, relevant national guidelines (). Similarly, achievement of therapeutic goals as defined either by national guidelines or as agreed upon through physician-patient decisions are conceptually very similar. The former, may hold more sway than individualistic therapeutic goals when access to expensive non-statin drugs is required through third-party payers. One firm distinction among definitions is the stipulation that at least two statins are trialed, one at the lowest approved dose compared with the alternative explicit recommendation to assess the two most efficacious statins, atorvastatin, and rosuvastatin (see ). Although the latter may be required by some third-party payers to enable access to expensive non-statin medications, there are multiple statins available that may be better tolerated by patients or, when utilized systematically, may also identify statin intolerant patients who require augmented therapy [Citation88].
Table 2. Components and concepts endorsed by international societies pertaining to establishment of a statin intolerance syndrome. SAMS = statin-associated muscle symptoms, CK = creatine kinase, R = rosuvastatin, a = atorvastatin, S = simvastatin, Pr = pravastatin, L = lovastatin, F = Fluvastatin, Pi = pitavastatin.
4. Statin-associated muscle symptoms management strategies
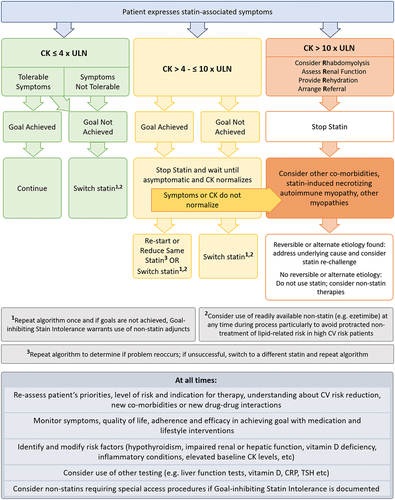
A multifaceted approach is necessary for the management of statin intolerance (). This includes first and foremost a trusting patient-clinical relationship in order to forge an individualized approach and optimize outcomes in SAMS. Central to this are detailed patient-centered and effective communication strategies, including shared decision-making, to help mitigate SAMS and improve medication adherence. This also comprises assessment of illness perceptions, perceived need for the treatment, background beliefs, affordability, and concerns about medication adverse effects [Citation14]. Validating and acknowledging patient-reported SAMS remains essential, while also emphasizing the increased cardiovascular risk that may manifest without the use of statin treatment.
Identifying and modifying potential risk factors that may aggravate or cause SAMS should also be considered. Then, and in parallel to pharmacological optimization, employing healthy strategies such as optimizing lifestyle interventions should be attempted [Citation14]. As such, while SAMS management is centered on statin dosing strategies to improve tolerability, a foundational emphasis on dietary and lifestyle interventions is recommended for all patients to improve cardiovascular risk profile and potentially enable use of lower statin doses. Subsequently, as detailed in the next section (), management strategies in statin optimization consist of utilizing the same statin but at a lower dose, trying a different statin, and/or non-statins therapy.
4.1. Non-statin therapies in statin intolerance
Several available non-statin drug alternatives reduce LDL-C in patients with goal-inhibiting statin intolerance [Citation89–91]. While they are biochemically efficacious and well-tolerated, only some reduce adverse cardiovascular outcomes and/or have undergone randomized controlled trials (RCTs) in statin intolerant patients (). There are several comprehensive reviews of these therapies [Citation89–91], including in important patient subgroups such as those with diabetes [Citation92]. As such, we will discuss these non-statin alternatives only briefly here.
Table 3. Selected non-statin alternative therapies.
4.1.1. Ezetimibe
Ezetimibe is an oral inhibitor of intestinal cholesterol transport that reduces LDL-C by an incremental 15–25% either as monotherapy or in combination with statins [Citation89]. Ezetimibe incrementally reduces ASCVD risk by 7% in statin-treated patients and 15% in those with diabetes [Citation93]. It also permits a statin dosage decrease with no long-term loss of efficacy for both LDL-C and ASCVD outcomes [Citation94]. Despite no double-blind RCTs in statin intolerant patients, ezetimibe is frequently used in statin intolerant patients due to its favorable cost, tolerability, and acceptance in practice guidelines.
4.1.2. PCSK9 inhibitors: monoclonal antibodies
By binding circulating proprotein convertase subtilisin kexin type 9 (PCSK9), two commercially available monoclonal antibodies – alirocumab and evolocumab – reduce LDL-C concentration by 55–70% over baseline statin treatment [Citation89]. Both agents reduce ASCVD events in high-risk clinical populations [Citation95,Citation96] and are recommended in clinical practice guidelines. Both agents have been evaluated in double-blind randomized clinical trials (RCTs) in patients with statin intolerance [Citation74,Citation97] and show superior LDL-C lowering efficacy and tolerability compared with statin alone or ezetimibe [Citation98]. However, use of these agents in clinical practice is limited by accessibility and cost, with restrictions from both regulators and third-party payers. Other potential barriers include parenteral administration, although these agents are remarkably effective and well tolerated. Nevertheless, persistence with these treatments has been reported as excellent in various patient cohorts [Citation99–101]. Practically, statin intolerance is not currently a recognized indication for prescribing these medications but in many jurisdictions, access to them may be contingent upon demonstration of many of the elements defining goal-inhibiting statin intolerance.
4.1.3. PCSK9 inhibitors: RNA interference
Inclisiran is a small interfering RNA (siRNA) that inhibits the cellular production of PCSK9 that reduces LDL-C by 44–55% over baseline therapy [Citation102]. A single subcutaneous injection reduces both circulating PCSK9 and LDL-C for up to 6 months [Citation102]. Pooled data from three RCTs of 3660 patients with hypercholesterolemia or ASCVD showed that inclisiran reduced ASCVD events risk by 24% [Citation103], although these results are hypothesis generating and need to be confirmed in proper RCTs. A four-year study reported an overall LDL-C reduction by inclisiran of 44% with good tolerability [Citation104]. However, there are no double-blind RCTs of inclisiran in statin intolerant patients. Two large RCTs of ASCVD outcomes with inclisiran – namely ORION-4 (NCT03705234) and VICTORION-2P (NCT05030428) – are underway and should be completed in 2025 and 2027, respectively. The drug appears to be well tolerated and effective but it is costly and requires parenteral administration in a health-care setting by a health-care provider, which may present as barriers to an already overburdened health-care system. Inclisiran is approved in the United Kingdom and North America for patients with familial hypercholesterolemia with varying approval conditions for statin intolerance.
4.1.4. Bempedoic acid
Bempedoic acid is an oral inhibitor of ATP-citrate lyase (ACLY) [Citation105] that reduces LDL-C by up to 20% over background statin therapy and up to 30% as monotherapy [Citation106]. The purported mechanism of action targeting liver-espressed ACLY has been proposed to explain why muscle is spared. A pooled analysis of four phase 3 studies in hypercholesterolemic patients who could not take statins showed that bempedoic acid lowered LDL-C by 26.5% vs placebo, and the bempedoic acid plus ezetimibe lowered LDL-C by 39.2%, both with very good tolerability [Citation107]. Positive results of the CLEAR OUTCOMES RCT in patients with ASCVD or at high-risk with statin intolerance showed a significant 13% reduction in the primary outcome of major cardiovascular events, with similar significant reductions in other key clinical outcomes [Citation108]. As a result, an important position paper on bempedoic acid has recently been published [Citation109]. Although the drug is clearly well tolerated, there are divergent results in real-world analysis that suggest higher rates of intolerance and drug discontinuation than seen in RCTs [Citation110]. Bempedoic acid has been approved in the US and Europe. Because it is orally administered and less costly than PCSK9 inhibitors, bempedoic acid may find an easier path to approval for patients with statin intolerance, although bempedoic acid is still quite costly, at ~80% of the cost of PCSK9 inhibitors.
4.1.5. Other available agents
Older lipid-lowering agents can be considered for statin intolerant patients. Older drugs such as bile acid sequestrants, fibrates, and niacin have the advantage of archival outcome studies from the pre-statin era suggesting cardiovascular benefit when given as monotherapy. However, different tolerability concerns are raised for bile acid sequestrants and for niacin, while for fibrates, their potential benefits among patients with normal triglyceride levels is questionable. Bile acid sequestrants, of which colesevelam is most widely available, have such advantages as lack of systemic absorption and thus no exposure to muscle, plus a long track record in the clinic, but their disadvantages include no contemporary ASCVD outcome data plus unrelated tolerability concerns, principally gastrointestinal adverse effects [Citation89]. Niacin use and availability have declined following negative ASCVD outcome RCTs in addition to tolerability concerns, which are mainly skin flushing, headaches, dysglycemia, and hyperuricemia [Citation89]. Fibrates do not lower LDL-C or apo B and RCTs of ASCVD outcomes have been neutral [Citation111]. Thus, the principal limitations of these traditional lipid-lowering therapies are both lack of clinical efficacy in the modern context plus their own non-SAMS tolerability issues.
Newer available agents that reduce LDL-C include lomitapide, an oral inhibitor of microsomal triglyceride transfer protein that reduces LDL-C by 50% but which has no ASCVD outcomes data. Lomitapide is associated with development of fatty liver and is approved only for the orphan indication of homozygous familial hypercholesterolemia [Citation112]. Evinacumab, a monoclonal antibody against angiopoietin like protein 3 (ANGPTL3) that is given by monthly intravenous infusion, is similarly approved only for homozygous familial hypercholesterolemia [Citation113]. It reduces LDL-C by 50% and its use in two patients with homozygous familial hypercholesterolemia was associated with regression of atherosclerotic plaque on noninvasive coronary imaging [Citation114]. However, evinacumab will likely never be approved for common statin intolerance due to its extremely high cost.
4.1.6. Drugs in development
Drugs in development for LDL-C reduction include obicetrapib, an oral inhibitor of cholesterol ester transfer protein [Citation115], and agents that inhibit PCSK9 by various alternative mechanisms [Citation116–118], including genome editing [Citation119]. However, these drugs would all likely face barriers to eventual approval for statin intolerance, including high cost, limited accessibility and lack of evidence of efficacy with respect to ASCVD outcomes reduction. Other agents in development targeting triglycerides and Lipoprotein(a) [Citation89] are even more remote from LDL lowering and are not relevant in statin intolerance.
4.1.7. Nutraceuticals
Nutraceuticals, including red yeast rice, soy derivatives, and phytosterols show mild LDL-C reduction effects [Citation90]. These are generally well-tolerated and inherently attractive to patients [Citation120]. However, these will never replace pharmaceutical grade, evidence-based lipid lowering treatments since these agents lack: 1) consistent therapeutic response, 2) consistent manufacturing process and standardization and 3) peer-reviewed safety data. Postulated mechanisms of action include reducing cholesterol absorption and raising sterol excretion. One study showed that red yeast rice lowered risk of ASCVD outcomes [Citation121]. The combination of red yeast extract, policosanols, berberine, folic acid, astaxanthin, and coenzyme Q10 reduced LDL-C by 22% in high-risk, high dose statin-intolerant patients compared to low-dose statin therapy [Citation122]. The role for nutraceuticals in statin intolerance remains to be clarified. Moreover, LDL-C lowering compared to even low-dose rosuvastatin therapy has recently been shown to be minimal in the SPORT trial that included assessment of some nutraceuticals previously shown to have some LDL-C-lowering properties [Citation123].
5. Conclusions
SAMS remain the most commonly encountered cause of goal-inhibiting statin intolerance with implications for statin adherence and increased risk of adverse CV outcomes. Health care providers must find appropriate strategies to mitigate and discontinuation of therapy. Management strategies involve a patient-centered approach, modulation of underlying risk factors and consideration of other co-morbidities, pharmacological optimization and emphasis on benefits and safety of statin therapy. Alternative treatment options include combination of different dose statins with other lipid-lowering regiments, or use of non-statin adjuncts with clinically proved reduction in adverse ASCVD events. The ultimate objective is lipid goal achievement which is fundamental for cardiovascular risk reduction.
6. Expert opinion: clinical approach to the SAMS patient
A recent systematic review of only statin intolerance management approaches published within the past few years emphasizes high variability in recommendations, most with little supporting evidence and lacking in participation of patients in shared decision-making [Citation24]. Part of the variability relates to heterogeneity in definitions although we posit that there are many common, conceptual elements () in management of statin intolerance. Moreover, the criticism that evidence of shared decision-making with patients is absent is perhaps overstated given that a component of prevention of statin intolerance is appropriate education designed to prevent ‘intolerance’ in the first place. This includes two-way dialogue regarding the reasons for recommending CV risk reduction, the expected side effects of statins, and the high incidence of nocebo effects. Furthermore, all of this is considered as perceived through the lens of patient expectations and priorities for foundational, non-statin recommendations such as lifestyle and behavioral components of therapy as well as the potential use of non-statin medications that might be required to achieve the goals with or without statins.
Importantly, the overview does identify at least six elements represented in many existing management approaches: having a clear definition of statin intolerance, excluding other causes, ensuring that CV risk is indeed sufficiently established to warrant statin therapy, assessing biomarkers (particularly CK since muscle symptoms are of commonest and greatest concern), and when present, allowing washout and sufficient time for normalization of biomarkers and symptoms before statin re-challenge. These features have emerged to ensure pragmatic and safe CV risk reduction. Furthermore, many of these features are intertwined. For example, co-morbidities and drug–drug interactions would be assessed both before prescribing any statin and again when intolerance is suspected after initiation of therapy. Two-way dialogue may need to be re-visited when real or perceived issues occur during therapy.
The choice of statin and its dosing will be considered in the context of severity of symptoms or biomarker abnormalities. Additionally, the choice of statin will be guided with a view toward prevailing regulations for access to non-statin drugs should statins not suffice to achieve therapeutic goals (i.e. demonstration of inability to tolerate at least two statins versus whether those two statins should both be highly efficacious ones such as atorvastatin and rosuvastatin; use of the lowest available dose versus doses likely to achieve goal, and so on).
The algorithm provided in is influenced by the goal-inhibiting statin intolerance syndrome definition described above and is particularly intended to define when specialist involvement is truly warranted. It is a simplified version of many available algorithms for management of SAMS. As memory aids, we emphasize the ‘4 R’s’ when there is a concern with rhabdomyolysis (R = rhabdomyolysis, R = renal function, R = rehydration, R = referral). The useful acronym of ‘SLAP’ has also been proposed (S = Switch, L = Lower dose, A = Alternate dosing, P = Polypharmacy) and conveniently embodies many tenets of addressing SAMS that we have discussed above [Citation92].
6.1. Future directions
SAMS is a clinical entity with a variable incidence and presentation that can present a true, clinical challenge in both its diagnosis and treatment. SAMS may decrease patients’ adherence with medication, lead to discontinuation or dose reduction with subsequent attenuated LDL-C lowering, and consequently increased risk for adverse ASCVD outcomes. Appropriate treatment strategies are therefore needed, with the ultimate goal being reduction of atherogenic lipoprotein species, particularly among patients benefiting from intensive LDL-lowering and in which discontinuation due to adverse effects leaves them vulnerable to ASCVD events. Currently, management is primarily based on dose changes or substitutions with alternate statins, although introduction of new non-statin LLT are expanding treatment options.
Future strategies aiming to move beyond statin-intolerance should favor non-statin therapies proven in outcome trials to reduce cardiovascular endpoints. Non-statin interventions such as ezetimibe and PCSK9 inhibitors are currently at the forefront, can be used independently or in conjunction with statins, have well-established safety and efficacy profiles and critically are proven to reduce risk of ASCVD. As such, in the recent RACING trial [Citation94], among patients with diabetes and ASCVD, the use of moderate-intensity statin with ezetimibe combination therapy was found to be comparable to high-intensity statin monotherapy in terms of 3-year rate of composite cardiovascular outcomes, LDL-C reduction but with lower intolerance-related drug discontinuation or dose reduction. These findings are important since ezetimibe is well tolerated, orally administered as well as being genericized, resulting in favorable cost, although lipoprotein reduction is relatively modest as monotherapy.
Additionally, the use of PCSK9 inhibitors has revolutionized the field of lipid-lowering therapies, with both alirocumab and evolocumab demonstrating greater LDL-C reduction and tolerability compared to statins. This evidence should thus prompt third-party payers to ease restricted access to these medications that have been shown to be highly effective in reducing cardiovascular events. Ongoing and planned RCTs are further expected to provide additional information regarding risks and potential benefits of non-statin therapies for reducing cardiovascular outcomes, including those from bempedoic acid. Furthermore, the advent of genome editing may be a future therapuetic option, with the potential to provide ‘one-and-done’ therapies to offer durable and possibly lifelong treatment of dyslipidemia.
Abbreviations
ACLY, adenosine triphosphate (ATP) citrate lyase; ANGPTL3, angiopoietin like 3 protein; ASCVD, atherosclerotic cardiovascular disease; CV, cardiovascular; LDL-C, low-density lipoprotein cholesterol; MTP, microsomal triglyceride transfer protein; NPC1L1, Niemann-Pick C1-like 1 protein PCSK9, proprotein convertase subtilisin kexin type 9; RCTs, randomized controlled trials
Article highlights
Statin-associated muscle symptoms (SAMS) are a common cause of goal-inhibiting statin intolerance.
Reduced statin adherence due to SAMS increases the risk of adverse cardiovascular outcomes.
Mechanisms underlying SAMS in most cases are poorly understood and underlie a heterogenous clinical spectrum with complex etiologies, sometimes including patient expectation of adverse effects.
Management strategies involve a patient-centered approach, control of risk factors, and pharmacological optimization with emphasis on benefits and safety of statin therapy.
Alternative treatment options include combining different dose statins with other lipid-lowering regimens, and use of non-statin adjuncts that are proven to reduce adverse CV events, such as ezetimibe, PCSK9 inhibitors and bempedoic acid.
The ultimate objective is lipid goal or target achievement, which is fundamental for cardiovascular risk reduction.
Declaration of interest
RA Hegele reports consulting fees from Akcea/Ionis, Amgen, HLS Therapeutics, Novartis, Pfizer, Regeneron, Sanofi, and Ultragenyx.
GB Mancini reports advisory board activity for Amgen, Sanofi, Esperion; Grants from Amgen, Sanofi, Astra Zeneca, and Merck.
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Leading causes of death in Canada 2022. Statistics Canada. 2022. [cited 14 Feb, 2023] Available from: https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1310039401.
- Tsao CW, Aday AW, Almarzooq ZI, et al. Heart disease and stroke statistics-2023 update: a report from the American Heart Association. Circulation. 2023;147:e93–621.
- Ference BA, Ginsberg HN, Graham I, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2017;38:2459–2472.
- Baigent C, Blackwell L, Emberson J, et al.; Cholesterol Treatment Trialists Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–1681.
- Cholesterol Treatment Trialists Collaboration, Fulcher J, O’Connell R, et al. Efficacy and safety of LDL-lowering therapy among men and women: meta-analysis of individual data from 174,000 participants in 27 randomised trials. Lancet. 2015;385:1397–1405.
- Cholesterol Treatment Trialists Collaboration. Efficacy and safety of statin therapy in older people: a meta-analysis of individual participant data from 28 randomised controlled trials. Lancet. 2019;393:407–415.
- Gorabi AM, Kiaie N, Hajighasemi S, et al. Statin-induced nitric oxide signaling: mechanisms and therapeutic implications. J Clin Med. 2019;8(12):2051. DOI:10.3390/jcm8122051
- Piepoli MF, Hoes AW, Agewall S, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J. 2016;37:2315–2381.
- Nelson AJ, Haynes K, Shambhu S, et al. High-intensity statin use among patients with atherosclerosis in the U.S. J Am Coll Cardiol. 2022;79:1802–1813.
- Cannon CP, de Lemos JA, Rosenson RS, et al. Use of lipid-lowering therapies over 2 years in GOULD, a registry of patients with atherosclerotic cardiovascular disease in the US. JAMA Cardiol. 2021;6:1–9.
- Gislason GH, Rasmussen JN, Abildstrøm SZ, et al. Long-term compliance with beta-blockers, angiotensin-converting enzyme inhibitors, and statins after acute myocardial infarction. Eur Heart J. 2006;27:1153–1158.
- Newman CB, Preiss D, Tobert JA, et al. Statin safety and associated adverse events: a scientific statement from the American Heart Association. Arterioscler Thromb Vasc Biol. 2019;39:e38–81.
- Penson PE, Bruckert E, Marais D, et al. Step-by-step diagnosis and management of the nocebo/drucebo effect in statin-associated muscle symptoms patients: a position paper from the International Lipid Expert Panel (ILEP). J Cachexia Sarcopenia Muscle. 2022;13:1596–1622.
- Warden BA, Guyton JR, Kovacs AC, et al. Assessment and management of statin-associated muscle symptoms (SAMS): a clinical perspective from the National lipid association. J Clin Lipidol. 2023;17:19–39.
- Cheeley MK, Saseen JJ, Agarwala A, et al. NLA scientific statement on statin intolerance: a new definition and key considerations for ASCVD risk reduction in the statin intolerant patient. J Clin Lipidol. 2022;16:361–375.
- Ward NC, Watts GF, Eckel RH. Statin toxicity. Circ Res. 2019;124:328–350.
- Rosenson RS, Baker SK, Jacobson TA, et al. The national lipid association’s muscle safety expert panel. An assessment by the statin muscle safety task force: 2014 update. J Clin Lipidol. 2014;8:S58–71.
- Cohen JD, Brinton EA, Ito MK, et al. Understanding Statin Use in America and Gaps in Patient Education (USAGE): an internet-based survey of 10,138 current and former statin users. J Clin Lipidol. 2012;6:208–215.
- Stroes ES, Thompson PD, Corsini A, et al. Statin-associated muscle symptoms: impact on statin therapy-European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur Heart J. 2015;36:1012–1022.
- Mendes P, Robles PG, Mathur S. Statin-induced rhabdomyolysis: a comprehensive review of case reports. Physiother Can. 2014;66:124–132.
- Rosenson RS, Miller K, Bayliss M, et al. The Statin-Associated Muscle Symptom Clinical Index (SAMS-CI): revision for clinical use, content validation, and inter-rater reliability. Cardiovasc Drugs Ther. 2017;31:179–186.
- Mancini GB, Baker S, Bergeron J, et al. Diagnosis, prevention, and management of statin adverse effects and intolerance: Canadian consensus working group update. Can J Cardiol. 2016;32:S35–65.
- Taylor BA, Sanchez RJ, Jacobson TA, et al. Application of the statin-associated muscle symptoms-clinical index to a randomized trial on statin myopathy. J Am Coll Cardiol. 2017;70:1680–1681.
- Meza-Contreras A, Wenczenovicz C, Ruiz-Arellanos K, et al. Statin intolerance management: a systematic review. Endocrine. 2023;79:430–436.
- Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73:e285–350.
- Collins R, Reith C, Emberson J, et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet. 2016;388:2532–2561.
- Blazing M, Braunwald E, de Lemos J, et al. Effect of statin therapy on muscle symptoms: an individual participant data meta-analysis of large-scale, randomised, double-blind trials. Lancet. 2022;400:832–845.
- Bytyci I, Penson PE, Mikhailidis DP, et al. Prevalence of statin intolerance: a meta-analysis. Eur Heart J. 2022;43:3213–3223.
- Gluba-Brzozka A, Franczyk B, Toth PP, et al. Molecular mechanisms of statin intolerance. Arch Med Sci. 2016;12:645–658.
- Vinci P, Panizon E, Tosoni LM, et al. Statin-associated myopathy: emphasis on mechanisms and targeted therapy. Int J Mol Sci. 2021;22(21):22. DOI:10.3390/ijms222111687
- Brunham LR, Baker S, Mammen A, et al. Role of genetics in the prediction of statin-associated muscle symptoms and optimization of statin use and adherence. Cardiovasc Res. 2018;114:1073–1081.
- Mombelli G. Statin muscle toxicity and genetic risk factors. Int J Genomics Med. 2013;01(02):01.
- Ghatak A, Faheem O, Thompson PD. The genetics of statin-induced myopathy. Atherosclerosis. 2010;210:337–343.
- Marez D, Legrand M, Sabbagh N, et al. Polymorphism of the cytochrome P450 CYP2D6 gene in a European population: characterization of 48 mutations and 53 alleles, their frequencies and evolution. Pharmacogenetics. 1997;7:193–202.
- Johansson I, Lundqvist E, Bertilsson L, et al. Inherited amplification of an active gene in the cytochrome P450 CYP2D locus as a cause of ultrarapid metabolism of debrisoquine. Proc Natl Acad Sci U S A. 1993;90:11825–11829.
- Li JH, Joy SV, Haga SB, et al. Genetically guided statin therapy on statin perceptions, adherence, and cholesterol lowering: a pilot implementation study in primary care patients. J Pers Med. 2014;4:147–162.
- Genetically guided statin therapy. ClinicalTrials.gov, 2017. [cited 14 Feb, 2023]; Available from: https://clinicaltrials.gov/ct2/show/NCT01894230.
- Singh K, Peyser B, Trujillo G, et al. Rationale and design of the SLCO1B1 genotype guided statin therapy trial. Pharmacogenomics. 2016;17:1873–1880.
- Mammen AL. Statin-associated autoimmune myopathy. N Engl J Med. 2016;374:664–669.
- Nazir S, Lohani S, Tachamo N, et al. Statin-associated autoimmune myopathy: a systematic review of 100 cases. J Clin Rheumatol. 2017;23:149–154.
- Thomas JE, Lee N, Thompson PD. Statins provoking MELAS syndrome. A case report. Eur Neurol. 2007;57:232–235.
- Tsivgoulis G, Spengos K, Karandreas N, et al. Presymptomatic neuromuscular disorders disclosed following statin treatment. Arch Intern Med. 2006;166:1519–1524.
- Al-Jubouri MA, Briston PG, Sinclair D, et al. Myxoedema revealed by simvastatin induced myopathy. BMJ. 1994;308:588.
- Scalvini T, Marocolo D, Cerudelli B, et al. Pravastatin-associated myopathy. Report of a case. Recenti Prog Med. 1995;86:198–200.
- Voermans NC, Lammens M, Wevers RA, et al. Statin-disclosed acid maltase deficiency. J Intern Med. 2005;258:196–197.
- Thirupathi A, de Souza CT. Multi-regulatory network of ROS: the interconnection of ROS, PGC-1 alpha, and AMPK-SIRT1 during exercise. J Physiol Biochem. 2017;73:487–494.
- Schick BA, Laaksonen R, Frohlich JJ, et al. Decreased skeletal muscle mitochondrial DNA in patients treated with high-dose simvastatin. Clin Pharmacol Ther. 2007;81:650–653.
- Gambelli S, Dotti MT, Malandrini A, et al. Mitochondrial alterations in muscle biopsies of patients on statin therapy. J Submicrosc Cytol Pathol. 2004;36:85–89.
- Kavalipati N, Shah J, Ramakrishan A, et al. Pleiotropic effects of statins. Indian J Endocrinol Metab. 2015;19:554–562.
- Moßhammer D, Schaeffeler E, Schwab M, et al. Mechanisms and assessment of statin-related muscular adverse effects. Br J Clin Pharmacol. 2014;78:454–466.
- Vaklavas C, Chatzizisis YS, Ziakas A, et al. Molecular basis of statin-associated myopathy. Atherosclerosis. 2009;202:18–28.
- Vladutiu GD, Simmons Z, Isackson PJ, et al. Genetic risk factors associated with lipid-lowering drug-induced myopathies. Muscle Nerve. 2006;34:153–162.
- Qu H, Guo M, Chai H, et al. Effects of coenzyme Q10 on statin-induced myopathy: an updated meta-analysis of randomized controlled trials. J Am Heart Assoc. 2018;7:e009835.
- Mohaupt MG, Karas RH, Babiychuk EB, et al. Association between statin-associated myopathy and skeletal muscle damage. CMAJ. 2009;181:E11–8.
- Taylor BA, Lorson L, White CM, et al. A randomized trial of coenzyme Q10 in patients with confirmed statin myopathy. Atherosclerosis. 2015;238:329–335.
- Derosa G, D’Angelo A, Maffioli P. Coenzyme q10 liquid supplementation in dyslipidemic subjects with statin-related clinical symptoms: a double-blind, randomized, placebo-controlled study. Drug Des Devel Ther. 2019;13:3647–3655.
- Bookstaver DA, Burkhalter NA, Hatzigeorgiou C. Effect of coenzyme Q10 supplementation on statin-induced myalgias. Am J Cardiol. 2012;110:526–529.
- Banach M, Serban C, Sahebkar A, et al. Effects of coenzyme Q10 on statin-induced myopathy: a meta-analysis of randomized controlled trials. Mayo Clin Proc. 2015;90:24–34.
- Sirvent P, Mercier J, Lacampagne A. New insights into mechanisms of statin-associated myotoxicity. Curr Opin Pharmacol. 2008;8:333–338.
- Abd TT, Jacobson TA. Statin-induced myopathy: a review and update. Expert Opin Drug Saf. 2011;10:373–387.
- Gomes MD, Lecker SH, Jagoe RT, et al. Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc Natl Acad Sci U S A. 2001;98:14440–14445.
- Buettner C, Lecker SH. Molecular basis for statin-induced muscle toxicity: implications and possibilities. Pharmacogenomics. 2008;9:1133–1142.
- Mazidi M, Rezaie P, Vatanparast H, et al. Effect of statins on serum vitamin D concentrations: a systematic review and meta-analysis. Eur J Clin Invest. 2017;47:93–101.
- Dawson-Hughes B. Vitamin D and muscle function. J Steroid Biochem Mol Biol. 2017;173:313–316.
- Michalska-Kasiczak M, Sahebkar A, Mikhailidis DP, et al. Analysis of vitamin D levels in patients with and without statin-associated myalgia - a systematic review and meta-analysis of 7 studies with 2420 patients. Int J Cardiol. 2015;178:111–116.
- Bartlomiejczyk M, Penson P, Banach M. Vitamin D and SAMS. In: Thompson P, and Taylor B, editors. Statin-associated muscle symptoms. Totowa, New Jersey: Humana Press; 2020. p. 121–128.
- Hlatky MA, Gonzalez PE, Manson JE, et al. Statin-Associated muscle symptoms among new statin users randomly assigned to vitamin D or placebo. JAMA Cardiol. 2023;8:74–80.
- Reston JT, Buelt A, Donahue MP, et al. Interventions to improve statin tolerance and adherence in patients at risk for cardiovascular disease: a systematic review for the 2020 U.S. Department of veterans affairs and U.S. department of defense guidelines for management of dyslipidemia. Ann Intern Med. 2020;173:806–812.
- Glueck CJ, Lee K, Prince M, et al. Low serum vitamin D, statin associated muscle symptoms, vitamin D supplementation. Atherosclerosis. 2017;256:125–127.
- Noyes AM, Thompson PD. The effects of statins on exercise and physical activity. J Clin Lipidol. 2017;11:1134–1144.
- Allard NAE, Timmers S. The role of the mitochondria in SAMS. In: Thompson P Taylor B, editors. Statin-associated muscle symptoms. Cham: Springer International Publishing; 2020. p. 105–112.
- Penson PE, Mancini GBJ, Toth PP, et al. Introducing the ‘Drucebo’ effect in statin therapy: a systematic review of studies comparing reported rates of statin-associated muscle symptoms, under blinded and open-label conditions. J Cachexia Sarcopenia Muscle. 2018;9:1023–1033.
- Tobert JA, Newman CB. The nocebo effect in the context of statin intolerance. J Clin Lipidol. 2016;10:739–747.
- Nissen SE, Stroes E, Dent-Acosta RE, et al. Efficacy and tolerability of evolocumab vs ezetimibe in patients with muscle-related statin intolerance: the GAUSS-3 randomized clinical trial. JAMA. 2016;315:1580–1590.
- Gupta A, Thompson D, Whitehouse A, et al. Adverse events associated with unblinded, but not with blinded, statin therapy in the Anglo-Scandinavian Cardiac Outcomes Trial—Lipid-Lowering Arm (ASCOT-LLA): a randomised double-blind placebo-controlled trial and its non-randomised non-blind extension phase. Lancet. 2017;389:2473–2481.
- Joy TR, Monjed A, Zou GY, et al. N-of-1 (single-patient) trials for statin-related myalgia. Ann Intern Med. 2014;160:301–310.
- Krishnamurthy A, Bradley C, Ascunce R, et al. SAMSON and the nocebo effect: management of statin intolerance. Curr Cardiol Rep. 2022;24:1101–1108.
- Howard JP, Wood FA, Finegold JA, et al. Side effect patterns in a crossover trial of statin, placebo, and no treatment. J Am Coll Cardiol. 2021;78:1210–1222.
- Herrett E, Williamson E, Brack K, et al. Statin treatment and muscle symptoms: series of randomised, placebo controlled n-of-1 trials. BMJ. 2021;372:n135.
- Jacobson TA. NLA task force on statin safety–2014 update. J Clin Lipidol. 2014;8:S1–4.
- Statin intolerance pathway. National Health Service England. 2022. [cited 14 Feb, 2023]; Available from: https://www.england.nhs.uk/aac/publication/statin-intolerance-pathway/
- Mancini GB, Baker S, Bergeron J, et al. Diagnosis, prevention, and management of statin adverse effects and intolerance: proceedings of a Canadian working group consensus conference. Can J Cardiol. 2011;27:635–662.
- Mancini GB, Tashakkor AY, Baker S, et al. Diagnosis, prevention, and management of statin adverse effects and intolerance: Canadian working group consensus update. Can J Cardiol. 2013;29:1553–1568.
- Mancini GBJ, Bergeron J, Fitchett D, et al. The Canadian consensus working group’s approach to identifying and managing statin-associated muscle and other symptoms. In: Thompson P, and Taylor B, editors. Statin-associated muscle symptoms. Cham: Springer International Publishing; 2020. p. 137–150.
- Chien SC, Chen PS, Huang YH, et al. 2019 Taiwan society of lipids and atherosclerosis expert consensus statement on statin intolerance. J Formos Med Assoc. 2019;118:1385–1392.
- Sposito AC, Faria Neto JR, Carvalho LS, et al. Statin-associated muscle symptoms: position paper from the Luso-Latin American Consortium. Curr Med Res Opin. 2017;33:239–251.
- Banach M, Rizzo M, Toth PP, et al. Statin intolerance - an attempt at a unified definition position paper from an international lipid expert panel. Arch Med Sci. 2015;11:1–23.
- Bruckert E, Hayem G, Dejager S, et al. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients–the PRIMO study. Cardiovasc Drugs Ther. 2005;19:403–414.
- Hegele RA, Tsimikas S. Lipid-Lowering Agents. Circ Res. 2019;124:386–404. DOI:10.1161/CIRCRESAHA.118.313171
- Danilov A, Frishman WH, Aronow WS. Antihyperlipidemic Treatment options in statin resistance and intolerance. Cardiol Rev. 2022. DOI:10.1097/CRD.0000000000000498
- Lloyd-Jones DM, Morris PB, Ballantyne CM, et al. 2022 ACC expert consensus decision pathway on the role of nonstatin therapies for LDL-Cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: a report of the American College of Cardiology solution set oversight committee. J Am Coll Cardiol. 2022;80:1366–1418.
- Bosco G, Di Giacomo Barbagallo F, Spampinato S, et al. Management of statin intolerant patients in the era of novel lipid lowering therapies: a critical approach in clinical practice. J Clin Med. 2023;12(6):12. DOI:10.3390/jcm12062444
- Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387–2397.
- Kim BK, Hong SJ, Lee YJ, et al. Long-term efficacy and safety of moderate-intensity statin with ezetimibe combination therapy versus high-intensity statin monotherapy in patients with atherosclerotic cardiovascular disease (RACING): a randomised, open-label, non-inferiority trial. Lancet. 2022;400:380–390.
- Oyama K, Giugliano RP, Tang M, et al. Effect of evolocumab on acute arterial events across all vascular territories: results from the FOURIER trial. Eur Heart J. 2021;42:4821–4829.
- Sinnaeve PR, Schwartz GG, Wojdyla DM, et al. Effect of alirocumab on cardiovascular outcomes after acute coronary syndromes according to age: an ODYSSEY OUTCOMES trial analysis. Eur Heart J. 2020;41:2248–2258.
- Moriarty PM, Thompson PD, Cannon CP, et al. Efficacy and safety of alirocumab in statin-intolerant patients over 3 years: open-label treatment period of the ODYSSEY ALTERNATIVE trial. J Clin Lipidol. 2020;14:88–97 e2.
- Qian LJ, Gao Y, Zhang YM, et al. Therapeutic efficacy and safety of PCSK9-monoclonal antibodies on familial hypercholesterolemia and statin-intolerant patients: a meta-analysis of 15 randomized controlled trials. Sci Rep. 2017;7:238.
- Wong ND, Bang M, Block RC, et al. Perceptions and barriers on the use of proprotein subtilisin/kexin type 9 inhibitors in heterozygous familial hypercholesterolemia (From a survey of primary care physicians and cardiologists). Am J Cardiol. 2021;152:57–62.
- Kaufman TM, Warden BA, Minnier J, et al. Application of PCSK9 Inhibitors in Practice. Circ Res. 2019;124:32–37.
- Donald DR, Reynolds VW, Hall N, et al. Exploring rates of PCSK9 inhibitor persistence and reasons for treatment non-persistence in an integrated specialty pharmacy model. J Clin Lipidol. 2022;16:315–324.
- Brandts J, Ray KK. Small interfering RNA to proprotein convertase subtilisin/kexin type 9: transforming LDL-cholesterol-lowering strategies. Curr Opin Lipidol. 2020;31:182–186.
- Khan SA, Naz A, Qamar Masood M, et al. Meta-analysis of inclisiran for the treatment of hypercholesterolemia. Am J Cardiol. 2020;134:69–73.
- Ray KK, Troquay RPT, Visseren FLJ, et al. Long-term efficacy and safety of inclisiran in patients with high cardiovascular risk and elevated LDL cholesterol (ORION-3): results from the 4-year open-label extension of the ORION-1 trial. Lancet Diabetes Endocrinol. 2023;11:109–119.
- Burke AC, Telford DE, Huff MW. Bempedoic acid: effects on lipoprotein metabolism and atherosclerosis. Curr Opin Lipidol. 2019;30:1–9.
- Ballantyne CM, Bays HE, Louie MJ, et al. Factors associated with enhanced low-density lipoprotein cholesterol lowering with bempedoic acid. J Am Heart Assoc. 2022;11:e024531.
- Laufs U, Ballantyne CM, Banach M, et al. Efficacy and safety of bempedoic acid in patients not receiving statins in phase 3 clinical trials. J Clin Lipidol. 2022;16:286–297.
- Nissen SE, Lincoff AM, Brennan D, et al. Bempedoic acid and cardiovascular outcomes in statin-intolerant patients. N Engl J Med. 2023;388:1353–1364.
- Banach M, Penson PE, Farnier M, et al. Bempedoic acid in the management of lipid disorders and cardiovascular risk. 2023 position paper of the International Lipid Expert Panel (ILEP). Prog Cardiovasc Dis. 2023. DOI:10.1016/j.pcad.2023.03.001
- Warden BA, Cardiology BA, Purnell JQ, et al. Real-world utilization of bempedoic acid in an academic preventive cardiology practice. J Clin Lipidol. 2022;16:94–103.
- Das Pradhan A, Glynn RJ, Fruchart JC, et al. Triglyceride lowering with pemafibrate to reduce cardiovascular risk. N Engl J Med. 2022;387:1923–1934.
- Berberich AJ, Hegele RA. Lomitapide for the treatment of hypercholesterolemia. Expert Opin Pharmacother. 2017;18:1261–1268.
- Mohamed F, Botha TC, Raal FJ. Inhibition of angiopoietin-like 3 for the management of severe hypercholesterolemia. Curr Opin Lipidol. 2021;32:213–218.
- Reeskamp LF, Nurmohamed NS, Bom MJ, et al. Marked plaque regression in homozygous familial hypercholesterolemia. Atherosclerosis. 2021;327:13–17.
- Nicholls SJ, Ray KK, Nelson AJ, et al. Can we revive CETP-inhibitors for the prevention of cardiovascular disease? Curr Opin Lipidol. 2022;33:319–325.
- Tombling BJ, Zhang Y, Huang YH, et al. The emerging landscape of peptide-based inhibitors of PCSK9. Atherosclerosis. 2021;330:52–60.
- Salaheldin TA, Godugu K, Bharali DJ, et al. Novel oral nano-hepatic targeted anti-PCSK9 in hypercholesterolemia. Nanomedicine. 2022;40:102480.
- Momtazi-Borojeni AA, Jaafari MR, Afshar M, et al. PCSK9 immunization using nanoliposomes: preventive efficacy against hypercholesterolemia and atherosclerosis. Arch Med Sci. 2021;17:1365–1377.
- Whittaker MN, Musunuru K. Therapeutic application of genome editing in dyslipidemia. Curr Opin Lipidol. 2022;33:133–138.
- Banach M, Patti AM, Giglio RV, et al. The role of nutraceuticals in statin intolerant patients. J Am Coll Cardiol. 2018;72:96–118.
- Lu Z, Kou W, Du B, et al. Effect of Xuezhikang, an extract from red yeast Chinese rice, on coronary events in a Chinese population with previous myocardial infarction. Am J Cardiol. 2008;101:1689–1693.
- Marazzi G, Campolongo G, Pelliccia F, et al. Comparison of low-dose statin versus low-dose statin + armolipid plus in high-intensity statin-intolerant patients with a previous coronary event and percutaneous coronary intervention (ADHERENCE Trial). Am J Cardiol. 2017;120:893–897.
- Laffin LJ, Bruemmer D, Garcia M, et al. Comparative effects of low-dose rosuvastatin, placebo, and dietary supplements on lipids and inflammatory biomarkers. J Am Coll Cardiol. 2023;81:1–12.