ABSTRACT
Introduction
Due to the improved survival in individuals with congenital heart disease (CHD), considering their reproductive health has become more important. Currently, this topic is still underexplored.
Areas covered
We discuss fertility, sexuality, assisted reproductive technology (ART), and contraception in adults with CHD.
Expert opinion
Timely counseling regarding fertility, sexuality, pregnancy, and contraception is necessary, preferably during teenage years. Due to a lack of data, whether or not to perform ART in adults with CHD is almost always based on expert opinion and follow-up in an expert center is recommended. Future research is necessary to fill the gaps in knowledge on the risks and frequency of complications of ART in adults with CHD, but also to be able to differentiate the relative risks in the different types of CHD. Only then will we be able to counsel adults with CHD correctly and not unjustly deprive someone of a chance of pregnancy.
1. Introduction
Congenital heart disease (CHD) affects around 1% of all live births worldwide and is the most frequently diagnosed congenital disorder [Citation1,Citation2]. Bicuspid aortic valve, ventricular septal defect, and atrial septal defect are the most common subtypes of CHD and are relatively mild compared with more complex abnormalities such as hypoplastic left heart syndrome and pulmonary atresia [Citation3]. The survival rates vary between the different subtypes of CHD and substantially across the world [Citation3,Citation4]. Due to improvements in diagnosis, surgical and medical treatments, more people with CHD are reaching adulthood and considering pregnancy [Citation5]. The European Society of Cardiology (ESC) guideline and the American Heart Association (AHA) guideline provide information on the management and organization of care for adults with CHD and give a clear overview of the risks and treatments of the different types of CHD [Citation6,Citation7]. However, due to the improved survival in individuals with CHD, considering their reproductive health has become more important, and this topic is still underexplored. In this review, we will discuss multiple aspects of reproductive health in both women and, when applicable, in men, including fertility, sexual health, assisted reproductive technology and contraception.
2. Fertility, menstrual disorders, and sexual health
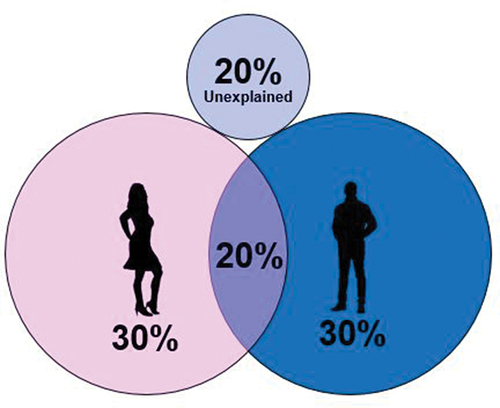
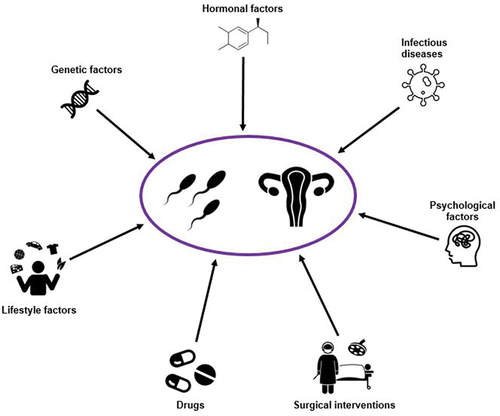
In biology, ‘fertility’ is defined by fecundity or the ability to reproduce [Citation8]. This should not be confused with the fertility rate, which is defined as the average number of children per woman. On the other hand, ‘infertility’ or ‘impaired fertility’ is defined as failure to achieve a pregnancy after 12 months or more of regular and unprotected sexual intercourse [Citation9]. Infertility can be attributed to the male or female, or to be unexplained () and can be caused by several factors, as presented in . In the male reproductive system, infertility is most commonly caused by abnormal sperm function and quality or problems with semen ejection [Citation10]. In women, infertility is most commonly caused by abnormalities of ovulation or structural problems in the uterus or fallopian tubes [Citation11]. Infertility can also be caused in both men and women by problems in the endocrine system causing imbalances of reproductive hormones, but also several environmental and lifestyle factors can influence fertility, such as smoking, alcohol consumption, and obesity [Citation12]. The proportion of couples affected by infertility is progressively increasing, and it is estimated that currently 15–18% of the couples around the world suffer from infertility [Citation13]. However, these numbers may be underestimated as they only include data from couples actively seeking help for fertility issues.
2.1. Fertility issues in adults with congenital heart disease
Knowledge about fertility in adults with CHD is limited, but the available data suggest that fertility is reduced in patients with CHD, mainly due to reports of reduced fertility in patients with complex CHD, such as after Fontan correction [Citation14,Citation15]. Serum Anti-Müllerian hormone (AMH), a product of ovarian granulosa cells that reflects the ovarian reserve [Citation16], are decreased in women with a Fontan circulation [Citation17]. Additionally, divided by age, serum AMH levels are lower in women with complex CHD compared with the AMH levels in healthy controls ≥35 years of age, although this difference is not found among women under 35 years of age [Citation17]. This suggests that a more pronounced decline in ovarian reserve occurs over time in women with complex CHD than in healthy controls. Patients with a Fontan circulation have a high central venous pressure and limited ability to increase cardiac output. It is suggested that exposure to chronic hypoxemia and reduced blood flow may influence ovarian function with subsequent menstrual disorders and infertility. There are no data available on semen quality in men with complex CHD, but if a similar process of hypoxemia also affects male fertility, then semen quality could also be affected. Additionally, when women with Fontan circulation become pregnant, maintaining the pregnancy is another challenge and high miscarriage rates (30%) are reported [Citation18]. In patients with simple or moderate CHD, fertility does not appear to be affected [Citation19,Citation20], although both men and women with CHD have lower birth rates compared to those without CHD, independent of the CHD severity. However, there is no evidence that people with CHD attend fertility clinics more frequently [Citation19,Citation20]. The majority of patients with CHD have specific worries and fears about sexuality (section 2.3), heredity, and pregnancy, which could explain the lower birth rates in this patient group [Citation21].
2.2. Menstrual disorders
Menstrual disorders are more common in women with CHD compared to women without CHD and are associated with the presence of cyanosis, the number of surgical interventions, and the severity of CHD [Citation22–24]. Due to an increased prevalence of primary amenorrhea, the average age at menarche in girls with CHD is slightly higher compared to the general population [Citation23]. The occurrence of secondary amenorrhea and oligomenorrhea also appear to be greater. An irregular menstrual cycle can make it difficult to determine the fertile period and so contribute to fertility issues.
2.3. Sexual health
Sexual functioning is impaired in adults with CHD, independent of CHD severity [Citation21,Citation25,Citation26]. Women with CHD, especially younger women, score worse on sexual functioning scales compared with normative data (i.e. unfavorable sexual functioning), including desire, arousal, lubrication, orgasm, satisfaction, and pain. Concerns about their sex life (24%), fears of being infertile (26%) and fears of passing on their heart disease (65%) are common in women with CHD [Citation21]. Erectile dysfunction is present in 10–38% of males with CHD, which is more than twice as high as in males without CHD. The use of cardiac medication, such as beta-blockers or ACE inhibitors, is associated with erectile dysfunction, but the data are inconsistent [Citation21,Citation25,Citation27,Citation28]. Fears related to cardiac events before or during sexual intercourse are more common in men with CHD compared to unaffected men (10%) [Citation21,Citation26].
3. Assisted reproductive technology in adults with congenital heart disease
Assisted Reproductive Technology (ART), also called medically assisted reproduction, is defined as all clinical and laboratory procedures with the objective of establishing a pregnancy, including in vitro handling of both oocytes and sperm, or embryos [Citation29]. Over the last 30 years, the use of ART has risen steeply, which can be explained by couples choosing to delay pregnancy until later life, and the greater acceptance and dissemination of these technologies [Citation30,Citation31]. Various studies have shown an increased risk of adverse outcomes in pregnancies conceived with ART, and it is still unclear if this risk arises from the fertility treatments themselves or the preexisting risk profile of the infertile population [Citation31–33]. Moreover, the fertility treatments themselves can also be complicated by, for example, ovarian hyperstimulation syndrome (OHSS), bleeding, thromboembolic events, or anesthesia-related risks, resulting in acute and/or major hemodynamic shifts [Citation34,Citation35]. In adults with congenital heart disease, these hemodynamic shifts and other risks can lead to life-threatening situations as they may be less capable to adapt due to a compromised cardiac function.
Before considering ART in patients with CHD, it is recommended to first determine the impact of a pregnancy on their heart disease and vice versa. Owing to data regarding pregnancy outcomes in women with heart disease from several registries, it has become increasingly clear which specific congenital heart defects are associated with a low, moderate, or high risk of maternal morbidity or mortality during pregnancy [Citation36–39]. The modified World Health Organization (mWHO) classification is a reliable tool for the prediction of the maternal risk in women with CHD () [Citation15,Citation40]. If pregnancy is considered safe, the pros and cons and the possible complications of an ART procedure should be considered. However, data on ART complications specifically in patients with heart disease are scarce and the available studies are all small and retrospective, so the discussion is almost always based on expert opinion [Citation41–43]. The occurrence of complications during ART in women with heart disease varies from 3% to 18%, and includes OHSS, syncope, intra-abdominal bleeding, and endocarditis. The ESC guideline states that fertility treatment is contraindicated in women with mWHO class IV (as is pregnancy itself) and that caution is advised in women with mWHO class III or those who are anticoagulated [Citation15]. A detailed understanding of ART is necessary to be able to discuss the risks of the available investigations and treatments for patients with CHD who are considering ART ().
Figure 3. The Assisted Reproductive Technology (ART) cycle and associated complications. OHSS – ovarian hyperstimulation syndrome.
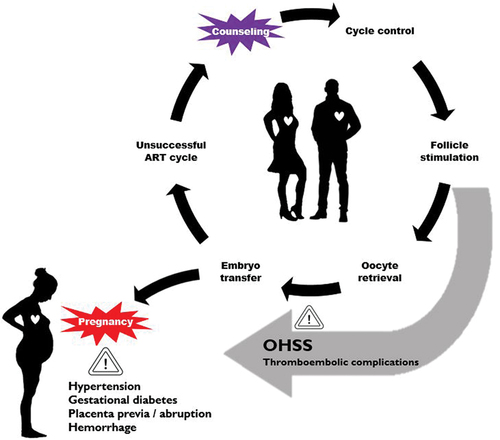
Table 1. Modified World Health Organization (mWHO) classification [Citation15].
3.1. Evaluating infertility
Couples who present to a fertility clinic will undergo preliminary blood work, pelvic ultrasound, and semen analysis. Subsequently, more detailed examinations may include assessments of tubal patency, which can be achieved using a Hystero-contrast-sonography (HyCoSy) or a hysterosalpingogram. In both, a tube is passed through the cervix and fluid is injected into the cavity of the uterus, outlining its shape and showing whether the fallopian tubes are open. Since both involve cervical manipulation, a vasovagal attack may occur. This occurs in 5% of healthy women during insertion of an IUCD and usually resolves spontaneously, but in women with CHD (particularly pulmonary hypertension or a Fontan circulation), a vasovagal event can be more serious. Consequently, procedures involving instrumentation of the cervix should only be performed with cardiovascular monitoring, anesthetic support on standby, and appropriate pain relief to minimize the risk of a vagal reaction [Citation44,Citation45]. More invasive, laparoscopic assessments do allow diagnosis and treatment at the same time (such as for tube adhesions and endometriosis), but require increased intra-abdominal pressure, which can impact cardiac output by reducing the venous return and elevating cardiac afterload [Citation46]. Further, the raised intra-abdominal pressure can compromise respiratory function, causing CO2 retention and increasing pulmonary vascular resistance. Positional changes can exacerbate these factors and lead to significant hypoxemia, hypotension, and hemodynamic instability, which are all poorly tolerated by those with CHD. If necessary, laparoscopic surgery can be performed with minimal abdominal inflation under regional or even local anesthesia [Citation47]. Hysteroscopy is well tolerated, but close attention to fluid balance is essential. Prophylactic antibiotics are advised for most gynecological procedures, and in the presence of CHD, these can be adapted to cover infectious endocarditis [Citation48].
3.2. Menstrual cycle control and follicle stimulation
Polycystic Ovary Syndrome (PCOS) is the most common cause of anovulation or infrequent ovulation, affecting between 4% and 8% of women. Weight loss, exercise, and diet can improve endocrine profiles and rates of ovulation in women with PCOS [Citation49]. If ovulation induction is necessary, then clomiphene citrate results in a 70–85% ovulation rate and 40–70% conception rate after six cycles but has a high rate of multiple pregnancy [Citation50]. Gonadotrophins can cause multiple follicular development and increase the risk of multiple pregnancy (5–8%), but this risk can be minimized by close ultrasound monitoring of follicular development [Citation51].
If in vitro fertilization (IVF) is thought to be necessary, natural cycle IVF should be considered as it avoids the risk of OHSS, and results in a single embryo transfer, minimizing the risk of multiple pregnancy. If superovulation is thought to be necessary, then an antagonist-based protocol reduces the risk of OHSS, with a similar pregnancy rate and opting for a single embryo transfer (and freezing any additional embryos) reduces the risk of OHSS and multiple pregnancy [Citation52]. Superovulation results in dramatic changes in circulating reproductive hormone levels that can have quite profound effects on the cardiovascular system, which have been well described by Manau et al. () [Citation53]. During the menstrual cycle before the ART attempt, a gonadotrophin-releasing hormone (GnRH) analogue is given to prevent early ovulation during ovarian stimulation, which is achieved with gonadotrophins [Citation54]. Circulating estradiol levels are low with the GnRH agonist treatment, which coincides with an increase in the peripheral resistance and blood pressure. With ovarian stimulation, estradiol levels rise and peripheral resistance and blood pressure fall significantly in association with an increase in the cardiac output [Citation53]. These changes in estradiol levels and associated hemodynamic changes are not likely to cause major cardiac problems in women with heart disease in whom pregnancy is considered safe. However, caution is advised in women with CHD classified as mWHO class III and IV, as stated by the ESC guidelines [Citation15]. More concerning is the effect of superovulation on the clotting system, described in section 3.6.
Figure 4. Changes in estrogen level and cardiac output before and during ART, and during pregnancy. Modified from Manau et al [Citation53].
![Figure 4. Changes in estrogen level and cardiac output before and during ART, and during pregnancy. Modified from Manau et al [Citation53].](/cms/asset/17ac16d8-f5db-4398-aebf-b252f3d7281c/ierk_a_2223979_f0004_oc.jpg)
3.3. Follicle monitoring, ovulation induction, and oocyte retrieval
The next step of the ART cycle is to closely monitor the ovarian response to superovulation, usually by vaginal ultrasound and serum estradiol measurements. Once the ideal follicle size is reached, human chorionic gonadotrophin (hCG) is administered followed by oocyte retrieval [Citation54,Citation55]. Oocyte retrieval is a low-risk procedure (<1% complications) in the general population, but in women with some forms of heart disease (pulmonary hypertension and women with Fontan circulation) it can be a life-threatening [Citation15,Citation56] with a significant risk of bleeding in women on anticoagulant therapy. Endocarditis prophylaxis is not routinely recommended during oocyte retrieval [Citation57].
3.4. Embryo transfer and pre-implantation genetic diagnosis (PGD)
Single embryo transfer is strongly advised above multiple embryo transfer in women with complex CHD, because multiple pregnancy is associated with greater hemodynamic changes and a higher risk of maternal and fetal complications [Citation58,Citation59]. These hemodynamic changes, detailed in another article in this issue, can lead to serious maternal cardiac complications, such as heart failure (7%) and arrhythmias (3%) [Citation60]. In multiple pregnancies, the cardiac output is 20% higher than in singleton pregnancies, which results in higher risks of maternal cardiac complications, especially in patients with more complex CHD, valvular stenosis, and an already compromised cardiac function [Citation58].
Pre-implantation genetic diagnosis (PGD) is an essential part of looking after couples where one of the couple is affected by an inherited cardiac disease [Citation61]. Men or women with a known genetic mutation causing their cardiac disease can create embryos through IVF, which can then be tested using PGD. Embryos are typically biopsied on day 3 of life, when they are at the 8-cell stage. One or two cells are removed, and the embryo is then cryopreserved to await the test results. Embryos without the mutation can then be subsequently transferred in a natural cycle or an artificial cycle.
Currently, genetic testing has an important role in the care of patients with CHD and needs to be considered in preconception counseling in those where it is relevant. Indeed, avoiding recurrence of the heart disease in the child is of paramount importance to people with CHD [Citation62,Citation63].
3.5. Ovarian hyperstimulation syndrome (OHSS)
In its most severe form, OHSS is associated with ascites, pleural and pericardial effusions, and intravascular hypovolemia with hypotension. The associated intravascular volume depletion increases the risk of thrombosis, which often involves the arterial system. In women with heart disease, these changes may be poorly tolerated and even life threatening, particularly in those with ventricular dysfunction, left ventricular outflow tract obstruction, Fontan circulation, or pulmonary hypertension. Mild OHSS complicates 33% and moderate-to-severe OHSS complicates 3–8% of the cycles [Citation64]. Similar rates are described in women with CHD undergoing ART [Citation41–43]. Risk factors for OHSS include age (<30 years), low body weight, PCOS, increased antral follicle count (AFC), and high levels of AMH. High-risk women should be prescribed gonadotrophins according to their AMH levels and AFC [Citation65,Citation66], aiming to use the lowest effective gonadotrophin dose. In women with CHD undergoing ART, careful cycle planning and monitoring is mandatory. If there is early ultrasound evidence of an excessive ovarian response, the cycle can be canceled or egg collection performed and the resulting embryos frozen for later transfer. If the risk of OHSS is thought to be high despite these steps, agents that inhibit vascular endothelial growth factor signaling, such as cabergoline, have been used successfully to prevent OHSS.
3.6. Thromboembolic complications
As mentioned, superovulation increases the risk of thromboembolic events, as the levels of von Willebrand factor, Factor VII, and Factor V rise and anticoagulant and fibrinolytic activity fall as a result of the higher estradiol levels [Citation54,Citation67,Citation68]. The incidence of arterial (ATE) and venous (VTE) thromboembolic events in women undergoing ART is around 0.1% for each cycle, a 10-fold increase on the background age-related risk [Citation54,Citation68]. The majority (98%) occur after ovulation induction [Citation69] and in association with the development of OHSS and pregnancy. The timing of ATEs and VTEs vary with ATE occurring in the first 2 weeks after embryo transfer, concurrent with OHSS development, in contrast, VTE is typically seen between 1 week after embryo transfer and up to the end of the first trimester of pregnancy, so possibly after clinical resolution of OHSS [Citation68,Citation69]. In naturally conceived pregnancies, the highest incidence of thromboembolic events is in the postpartum period [Citation70]. Patients with CHD are at increased risk of thromboembolic events, with differences in risk according to CHD type and complexity [Citation6]. The highest risks are found in patients with transposition of the great arteries, Fontan circulation, and cyanotic CHD [Citation71]. Valve thrombosis is the most feared complication in patients with metallic prosthetic valves and is associated with significant morbidity and mortality [Citation72]. Interruption of anticoagulant therapy in patients with a metallic prosthetic valve in aortic position is lower risk than compared to patients with a mechanical valve in mitral position, based on the 2–3 times higher risk of mitral prosthetic valve thrombosis compared to aortic prosthetic valve thrombosis [Citation73]. Therefore, the possibility and duration of interrupting anticoagulant therapy depends on the underlying condition and type of prosthetic valve, the approach should be discussed by a multidisciplinary team.
3.7. Obstetric and perinatal complications in ART pregnancies
Pregnancies conceived by ART should be managed as high-risk pregnancies, as even ART singleton pregnancies are associated with higher risks of adverse obstetric and perinatal outcomes [Citation31]. Women with an ART conceived pregnancy have a 30% higher risk to develop pregnancy-induced hypertension compared with women with pregnancies conceived naturally [Citation31]. In women with CHD, the prevalence of hypertensive disorders in pregnancy (HDP) is roughly comparable or slightly increased with the prevalence in the general population (10%) [Citation74–76]. However, women with heart disease and HDP have higher incidences of adverse maternal and perinatal outcomes than those without HDP, such as heart failure (18.5% vs 10.6%), preterm birth (27.4% vs 16.9%), small for gestational age (14.6% vs 9.7%), and maternal- and perinatal death (1.4% vs 0.6% and 3.1% vs 1.7%, respectively) [Citation74]. Caution is needed in women with CHD who develop HDP, not only during pregnancy but also in the postpartum period because most of the reported maternal deaths due to HDP occurred after delivery. Other complications that are more prevalent in ART singleton pregnancies are gestational diabetes, placenta previa, placental abruption, and antepartum and postpartum hemorrhage [Citation31]. In multiple pregnancies conceived by ART compared with multiple pregnancies conceived naturally, there is a higher risk of premature rupture of membranes (15%), pregnancy-induced hypertension (6%), gestational diabetes (42%), preterm birth (83%), low birth weight (47%), and congenital malformation (30%). As stated before, single embryo transfer is advised above multiple embryo transfer in women with CHD (section 3.4).
3.8. Oocyte donation and surrogate mother
Donor oocyte ART cycles account for 12% of all ART cycles. The main reasons women opt for oocyte donation are poor oocyte quality, premature ovarian failure, or advanced maternal age [Citation77]. In oocyte donation pregnancies, the risk of developing preeclampsia and the risk of preterm birth and low birthweight is higher compared to these risks in pregnancies conceived by conventional IVF/ICSI or spontaneous conception [Citation78]. Preeclampsia in women with heart disease is associated with a high rate of heart failure (30%) and maternal death (3.5%), so awareness of this complication and frequent follow-up is necessary [Citation74]. Women with CHD may look for a surrogate mother to avoid the impact of a potential pregnancy on their heart disease and vice versa, but with the use of their own oocytes. This may be possible in women in which pregnancy is contraindicated (mWHO class IV). However, ART is necessary for oocyte retrieval with additional complications as mentioned above and should be considered carefully. Moreover, the life expectancy of adults with complex CHD must be taken into account. Although adults with CHD have optimistic expectations for their own life spans (±70 years) and the greatest overall survival benefit in the past few decades is among those with the most complex defects, the median age at death for adults with complex CHD is nowadays 50 years but for some it is dramatically worse [Citation79–81]. Advice should be individualized and an in-depth discussion undertaken so that the couple have a clear understanding of the risks involved in any proposed treatment and also taking into account the perspective of the child. This hopefully minimize the risk that the couple will seek treatment in another, non-expert center or outside the country, where proper care may not be provided. However, there are differences in the legal status of surrogacy and oocyte donation in different countries, and local availability should be considered [Citation82].
3.9. Male factor
In around 30–40% of the infertile couples, the cause of infertility is attributable to a male factor () [Citation10]. Sperm retrieval from the epididymis or testicles may be necessary because of azoospermia. Although effective, this procedure is as any other surgical procedure not without complications. The risk of bleeding and hematoma formation needs to be considered, especially in men on anticoagulation, as well as the risk of infection and anesthesia. Percutaneous aspiration or biopsy is preferred, as it can be performed under local anesthesia. International guidelines recommend that non-cardiac surgery and interventional procedures in patients with CHD should be performed in a center with CHD expertise [Citation6,Citation7].
4. Contraception
Contraception should be discussed in teenagers and young adults with CHD to avoid unplanned pregnancy because of the increased risk of maternal morbidity and mortality, but also to avoid complications of specific contraceptive methods. Barrier methods have a relative high failure rate and are not considered sufficient alone but are valuable in combination with other methods as they offer protection from sexually transmitted diseases [Citation83,Citation84]. Estrogens are pro-thrombotic via the increased hepatic production of coagulation factors, which makes contraception containing estrogens (combination pills, patches, or rings) unsuitable for women with CHD, as these women already have an increased risk for thromboembolic events [Citation6,Citation83,Citation85]. In addition, in women with mild hypertension (>140/90 mm Hg), estrogen-containing contraceptives are relatively contraindicated, and absolutely contraindicated in women with uncontrolled hypertension (>160/100 mm Hg). In women with CHD, progesterone-only contraceptive methods are considered safe, as they have no effect on the coagulation cascade and blood pressure [Citation83,Citation85]. The potential side effects of progestogen-only contraception, such as irregular bleeding patterns and continuous spotting, should be discussed to improve compliance. In women with CHD who are on anticoagulation, long-acting reversible contraceptives, especially subdermal implants or Levonogestrel-IUD, are the methods of choice. Importantly, in patients with preload-dependent conditions, such as pulmonary arterial hypertension, severe valvular stenosis, or a Fontan circulation, it is recommended to insert and remove an IUD with cardiovascular monitoring and appropriate pain relief, as pain can elicit a vagal reaction [Citation86]. Taking this into account, subdermal implants may be more suitable for women with preload-dependent conditions, as these are easily inserted under local anesthesia [Citation83]. Antibiotic prophylaxis is not recommended during insertion of an intrauterine device as stated by the ESC guidelines for infective endocarditis [Citation57].
In choosing the most appropriate contraceptive method, multiple factors should be considered and the input of a cardiologist and obstetrician is recommended. In addition to considering the patient’s risk of cardiovascular complications during pregnancy and the relative or absolute contraindications of the different types of contraception, it is important to consider the patient’s personal preferences. In patients who do not desire a pregnancy or who have completed their families, permanent contraception, such as tubal ligation or vasectomy, can be considered. However, the age of the patient, social situation, and chance of regret should be discussed. For patients who prefer a non-hormonal contraceptive method, the copper-IUD, is an option but is associated with increased pain and menstrual flow. Alternatives, such as barrier methods, have a high failure rate, and as stated above, the consequences of pregnancy must be taken into account when deciding on the contraceptive method to be used.
5. Conclusion
In conclusion, there are indications that fertility is lower in both men and women with complex CHD, however in patients with mild and moderate CHD, fertility appears normal. Sexual functioning is worse in adults with CHD and discussing sexuality by the physician definitely needs more attention. Due to the increasing life-expectancy of adults with CHD, the complex question of pregnancy and contraception will be asked more frequently, at the moment we lack the necessary information to give accurate advice particularly about the impact of ART and its associated complications. This is particularly true of fertility treatments that are associated with complications such as OHSS and thromboembolic complications, which can be life-threatening, especially in adults with complex CHD. A detailed understanding of ART is necessary to be able to discuss the risks of the available investigations and treatments for patients with CHD who are considering ART. Due to the heterogeneity of CHD, an individual approach is necessary, and it is recommended to discuss every adult with CHD considering ART with a multidisciplinary team, consisting of at least a congenital cardiologist, high-risk obstetrician, fertility specialist, and anesthetist. Due to the lack of data, it seems justified to centralize care for adults with CHD undergoing ART to tertiary, expert centers. A surrogate mother with the use of a patient’s own oocyte is an option for women with a high risk of maternal morbidity or mortality during pregnancy due to their underlying cardiac disease (mWHO III-IV), however ART for oocyte retrieval can still cause life-threatening situations in these women. Lastly, it is important to have a timely discussion about contraceptive options with teenage CHD patients, not only to prevent pregnancy but also to choose an appropriate contraceptive method with the least cardiovascular risk (which may exclude estrogen-containing contraceptives). In short, timely counseling on sexuality, contraception, pregnancy, and ART is crucial in adults with CHD.
6. Expert opinion
Reproductive health has been an underexplored topic in patients with CHD. As the life-expectancy of adults with CHD has increased due to improvements in the treatment of complex CHD, more adults with CHD are reaching childbearing age, and issues regarding the safety of fertility treatments in these patient group will become increasingly important and frequent. Available data about the risks of ART in adults with CHD are limited, so the discussion is based on expert opinion. It is recommended that every woman with CHD considering fertility treatment should be discussed by a multidisciplinary pregnancy heart team to consider possible complications of both ART and pregnancy. Additional testing, such as echocardiography and exercise test, should be undertaken as necessary to form a clear picture of the severity of the CHD and the cardiac condition of the patient prior to any treatment. Because of the heterogeneity of CHD, the advice should be individualized to the woman’s needs and complexity of her CHD. The mWHO classification, used to predict the risk for maternal morbidity and mortality in pregnancy in women with heart disease, may also be applicable in women with heart disease who are eligible for ART.
The diagnostic procedures to investigate the cause of infertility, such as a hysterosalpingogram, are not without risks. Cardiovascular monitoring is required and appropriate pain relief is recommended to minimize the risk of a vagal reaction. Women with CHD undergoing ART should be closely monitored, especially those with moderate or complex CHD, in an expert center. The most common complications of ART are OHSS and multiple pregnancy. In women with CHD, the key principle is to use the ART with the least likelihood of these complications. OHSS is a feared complication, and even the mild form of OHSS can be life-threatening in women with CHD. Careful follicle monitoring is essential and, in the case of excessive response to follicle stimulation, cycle cancellation or freezing the embryos is recommended. Single embryo transfer is recommended to minimize the risk of multiple pregnancies. Antibiotic prophylaxis is not recommended during insertion of an intra-uterine device or during oocyte or sperm retrieval as stated by the ESC guidelines for infective endocarditis. However, in high-risk patients (e.g. with a prosthetic valve or earlier endocarditis) it can be considered, given the incidence of endocarditis since the introduction of these new guideline has been increased, and the incidence of serious side-effects of antibiotic prophylaxis is relatively low [Citation57,Citation83].
As stated before, more research regarding ART in adults with CHD is needed. Observational studies can give a good picture of the incidence and causes of fertility issues in adults with CHD. It is crucial to have more data on the risks of ART and frequency of complications in the different types of CHD at a minimum to be able to accurately advise women with CHD about the risks and optimally to determine which drugs, hormones, and ART approaches should be avoided in adults with CHD. Surrogacy is an option in women with complex CHD in whom pregnancy is contraindicated (mWHO IV), but the risk of ART to retrieve oocytes and the individual’s life-expectancy needs to be considered when deciding whether or not to use ART. As the question of fertility is asked more often by people with CHD, we need more data to give accurate answers and to use ART safely in this high risk group of patients.
Article highlights
Due to the improved survival of adults with CHD, considering their reproductive health has become more important.
Sexual functioning is impaired in adults with CHD and menstrual disorders are more common.
Fertility seems reduced in adults with complex CHD, such as those with a Fontan circulation.
Patients with CHD considering ART should be discussed in a multidisciplinary team, consisting of at least a congenital cardiologist, high-risk obstetrician, fertility specialist, and anesthetist.
ART is associated with OHSS, thromboembolic complications, and multiple pregnancies.
Careful follicle monitoring is necessary, and in the case of an excessive response, consider canceling the cycle or freezing the embryos.
Timely counseling of teenagers with CHD about sexuality, contraception, fertility, and pregnancy is crucial.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Bouma BJ, Mulder BJM. Changing landscape of congenital heart disease. Circ Res. 2017;120(6):908–922. doi: 10.1161/CIRCRESAHA.116.309302
- van der Linde D, Konings EE, Slager MA, et al. Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J Am Coll Cardiol. 2011;58(21):2241–2247. doi: 10.1016/j.jacc.2011.08.025
- Wu W, He J, Shao X. Incidence and mortality trend of congenital heart disease at the global, regional, and national level, 1990-2017. Medicine (Baltimore). 2020;99(23):e20593. doi: 10.1097/MD.0000000000020593
- Collaborators GBDCo D, Abate D, Abate KH. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2018;392(10159):1736–1788. doi: 10.1016/S0140-6736(18)32203-7
- Moons P, Bovijn L, Budts W, et al. Temporal trends in survival to adulthood among patients born with congenital heart disease from 1970 to 1992 in Belgium. Circulation. 2010;122(22):2264–2272. doi: 10.1161/CIRCULATIONAHA.110.946343
- Baumgartner H, De Backer J. The ESC clinical practice guidelines for the management of adult congenital heart disease 2020. Eur Heart J. 2020;41(43):4153–4154. doi: 10.1093/eurheartj/ehaa701
- Stout KK, Daniels CJ, Aboulhosn JA, et al. 2018 AHA/ACC guideline for the management of adults with congenital heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;73(12):1494–1563. doi: 10.1016/j.jacc.2018.08.1028
- Skakkebaek NE, Lindahl-Jacobsen R, Levine H, et al. Environmental factors in declining human fertility. Nat Rev Endocrinol. 2022;18(3):139–157. doi: 10.1038/s41574-021-00598-8
- (WHO) WHO. International Classification of Diseases, 11th Revision (ICD-11). Geneva: WHO; 2018.
- Winters BR, Walsh TJ. The epidemiology of male infertility. Urol Clin North Am. 2014;41(1):195–204. doi: 10.1016/j.ucl.2013.08.006
- Abrao MS, Muzii L, Marana R. Anatomical causes of female infertility and their management. Int J Gynaecol Obstet. 2013;123 Suppl 2:S18–24. doi: 10.1016/j.ijgo.2013.09.008
- Homan GF, Davies M, Norman R. The impact of lifestyle factors on reproductive performance in the general population and those undergoing infertility treatment: a review. Hum Reprod Update. 2007;13(3):209–223. doi: 10.1093/humupd/dml056
- Mascarenhas MN, Flaxman SR, Boerma T, et al. National, regional, and global trends in infertility prevalence since 1990: a systematic analysis of 277 health surveys. PLOS Med. 2012;9(12):e1001356. doi: 10.1371/journal.pmed.1001356
- Drenthen W, Pieper PG, Roos-Hesselink JW, et al. Pregnancy and delivery in women after Fontan palliation. Heart. 2006;92(9):1290–1294. doi: 10.1136/hrt.2005.085407
- Regitz-Zagrosek V, Roos-Hesselink JW, Bauersachs J, et al. 2018 ESC Guidelines for the management of cardiovascular diseases during pregnancy. Eur Heart J. 2018;39(34):3165–3241. doi: 10.1093/eurheartj/ehy340
- Dewailly D, Andersen CY, Balen A, et al. The physiology and clinical utility of anti-Mullerian hormone in women. Hum Reprod Update. 2014;20(3):370–385. doi: 10.1093/humupd/dmt062
- Matsushita KA, Izumi M, Miyake C, et al. Reduced ovarian function in women with complex congenital heart disease. Int J Cardiol Congenit Heart Dis. 2022;7(100317):100317. doi: 10.1016/j.ijcchd.2021.100317
- Gouton M, Nizard J, Patel M, et al. Maternal and fetal outcomes of pregnancy with Fontan circulation: a multicentric observational study. Int J Cardiol. 2015;187:84–89. doi: 10.1016/j.ijcard.2015.03.344
- Chien SJ, Lin YJ, Lo MH, et al. Congenital heart diseases impair female fertility. Front Pediatr. 2021;9:687276. doi: 10.3389/fped.2021.687276
- Udholm LF, Arendt LH, Knudsen UB, et al. Congenital heart disease and fertility: a Danish nationwide cohort study including both men and women. J Am Heart Assoc. 2023;12(2):e027409. doi: 10.1161/JAHA.122.027409
- Opic P, Roos-Hesselink JW, Cuypers JA, et al. Sexual functioning is impaired in adults with congenital heart disease. Int J Cardiol. 2013;168(4):3872–3877. doi: 10.1016/j.ijcard.2013.06.029
- Canobbio MM, Rapkin AJ, Perloff JK, et al. Menstrual patterns in women with congenital heart disease. Pediatr Cardiol. 1995;16(1):12–15. doi: 10.1007/BF02310327
- Drenthen W, Hoendermis ES, Moons P, et al. Menstrual cycle and its disorders in women with congenital heart disease. Congenit Heart Dis. 2008;3(4):277–283. doi: 10.1111/j.1747-0803.2008.00202.x
- Vigl M, Kaemmerer M, Niggemeyer E, et al. Sexuality and reproductive health in women with congenital heart disease. Am J Cardiol. 2010;105(4):538–541. doi: 10.1016/j.amjcard.2009.10.025
- Cook SC, Arnott LM, Nicholson LM, et al. Erectile dysfunction in men with congenital heart disease. Am J Cardiol. 2008;102(12):1728–1730. doi: 10.1016/j.amjcard.2008.08.017
- Vigl M, Hager A, Bauer U, et al. Sexuality and subjective wellbeing in male patients with congenital heart disease. Heart. 2009;95(14):1179–1183. doi: 10.1136/hrt.2008.156695
- Shiri R, Koskimaki J, Hakkinen J, et al. Cardiovascular drug use and the incidence of erectile dysfunction. Int J Impot Res. 2007;19(2):208–212. doi: 10.1038/sj.ijir.3901516
- Fischer AJ, Grundlach C, Helm PC, et al. Erectile dysfunction in men with adult congenital heart disease: a prevalent but neglected issue. Korean Circ J. 2022;52(3):233–242. doi: 10.4070/kcj.2021.0184
- Zegers-Hochschild F, Adamson GD, de Mouzon J, et al. International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary of ART terminology, 2009. Fertil Steril. 2009;92(5):1520–1524. doi: 10.1016/j.fertnstert.2009.09.009
- Kushnir VA, Barad DH, Albertini DF, et al. Systematic review of worldwide trends in assisted reproductive technology 2004-2013. Reprod Biol Endocrinol. 2017;15(1):6. doi: 10.1186/s12958-016-0225-2
- Qin J, Liu X, Sheng X, et al. Assisted reproductive technology and the risk of pregnancy-related complications and adverse pregnancy outcomes in singleton pregnancies: a meta-analysis of cohort studies. Fertil Steril. 2016;105(1):73-85 e1–6. doi:10.1016/j.fertnstert.2015.09.007
- Helmerhorst FM, Perquin DA, Donker D, et al. Perinatal outcome of singletons and twins after assisted conception: a systematic review of controlled studies. BMJ. 2004;328(7434):261. doi: 10.1136/bmj.37957.560278.EE
- Pandey S, Shetty A, Hamilton M, et al. Obstetric and perinatal outcomes in singleton pregnancies resulting from IVF/ICSI: a systematic review and meta-analysis. Hum Reprod Update. 2012;18(5):485–503. doi: 10.1093/humupd/dms018
- El-Shawarby S, Margara R, Trew G, et al. A review of complications following transvaginal oocyte retrieval for in-vitro fertilization. Hum Fertil (Camb). 2004;7(2):127–133. doi: 10.1080/14647270410001699081
- Fujitake E, Jaspal R, Monasta L, et al. Acute cardiovascular changes in women undergoing in vitro fertilisation (IVF), a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2020;248:245–251. doi: 10.1016/j.ejogrb.2020.01.033
- Drenthen W, Boersma E, Balci A, et al. Predictors of pregnancy complications in women with congenital heart disease. Eur Heart J. 2010;31(17):2124–2132. doi: 10.1093/eurheartj/ehq200
- Gnanaraj JAP, Surendran A, Susikar A, et al. Pregnancy outcomes in women with heart disease: the madras medical college pregnancy and cardiac (M-PAC) Registry from India. Eur Heart J. 2023;44(17):1530–1540. doi: 10.1093/eurheartj/ehad003
- Roos-Hesselink J, Baris L, Johnson M, et al. Pregnancy outcomes in women with cardiovascular disease: evolving trends over 10 years in the ESC Registry of Pregnancy and Cardiac disease (ROPAC). Eur Heart J. 2019;40(47):3848–3855. doi: 10.1093/eurheartj/ehz136
- Silversides CK, Grewal J, Mason J, et al. Pregnancy outcomes in women with heart disease: the CARPREG II Study. J Am Coll Cardiol. 2018;71(21):2419–2430. doi: 10.1016/j.jacc.2018.02.076
- Lu CW, Shih JC, Chen SY, et al. Comparison of 3 risk estimation methods for predicting cardiac outcomes in pregnant women with congenital heart disease. Circ J. 2015;79(7):1609–1617. doi: 10.1253/circj.CJ-14-1368
- Dayan N, Laskin CA, Spitzer K, et al. Pregnancy complications in women with heart disease conceiving with fertility therapy. J Am Coll Cardiol. 2014;64(17):1862–1864. doi: 10.1016/j.jacc.2014.07.977
- Quien MM, Hausvater A, Maxwell SM, et al. Assisted reproductive technology outcomes in women with heart disease. Front Cardiovasc Med. 2022;9:842556. doi: 10.3389/fcvm.2022.842556
- Skorupskaite K, Joy E, Balen A, et al. Assisted reproduction in patients with cardiac disease: a retrospective review. Eur J Obstet Gynecol Reprod Biol. 2022;276:199–203. doi: 10.1016/j.ejogrb.2022.07.020
- Costello MF, Horrowitz S, Steigrad S, et al. Transcervical intrauterine topical local anesthetic at hysterosalpingography: a prospective, randomized, double-blind, placebo-controlled trial. Fertil Steril. 2002;78(5):1116–1122. doi: 10.1016/S0015-0282(02)03362-9
- Gemzell-Danielsson K, Mansour D, Fiala C, et al. Management of pain associated with the insertion of intrauterine contraceptives. Hum Reprod Update. 2013;19(4):419–427. doi: 10.1093/humupd/dmt022
- Joris JL, Noirot DP, Legrand MJ, et al. Hemodynamic changes during laparoscopic cholecystectomy. Anesth Analg. 1993;76(5):1067–1071. doi: 10.1213/00000539-199305000-00027
- Snabes MC, Poindexter AN 3rd. Laparoscopic tubal sterilization under local anesthesia in women with cyanotic heart disease. Obstet Gynecol. 1991;78(3 Pt 1):437–440.
- Van Eyk N, van Schalkwyk JN. 275-antibiotic prophylaxis in gynaecologic procedures. J Obstet Gynaecol Can. 2018;40(10):e723–e33. doi: 10.1016/j.jogc.2018.07.007
- Ujvari D, Hulchiy M, Calaby A, et al. Lifestyle intervention up-regulates gene and protein levels of molecules involved in insulin signaling in the endometrium of overweight/obese women with polycystic ovary syndrome. Hum Reprod. 2014;29(7):1526–1535. doi: 10.1093/humrep/deu114
- Balen AH. Ovulation induction in the management of anovulatory polycystic ovary syndrome. Mol Cell Endocrinol. 2013;373(1–2):77–82. doi: 10.1016/j.mce.2012.10.008
- Weiss NS, Nahuis M, Bayram N, et al. Gonadotrophins for ovulation induction in women with polycystic ovarian syndrome. Cochrane Database Of Systematic Reviews. 2015;2015(9):CD010290. doi: 10.1002/14651858.CD010290.pub2
- Lin H, Li Y, Li L, et al. Is a GnRH antagonist protocol better in PCOS patients? A meta-analysis of RCTs. PLoS ONE. 2014;9(3):e91796. doi: 10.1371/journal.pone.0091796
- Manau D, Balasch J, Arroyo V, et al. Circulatory dysfunction in asymptomatic in vitro fertilization patients. Relationship with hyperestrogenemia and activity of endogenous vasodilators. J Clin Endocrinol Metab. 1998;83(5):1489–1493. doi: 10.1210/jc.83.5.1489
- Chan WS, Dixon ME. The “ART” of thromboembolism: a review of assisted reproductive technology and thromboembolic complications. Thromb Res. 2008;121(6):713–726. doi: 10.1016/j.thromres.2007.05.023
- Farquhar C, Marjoribanks J. Assisted reproductive technology: an overview of Cochrane Reviews. Cochrane Database Syst Rev. 2018;8(8):CD010537. doi:10.1002/14651858.CD010537.pub5
- Levi-Setti PE, Cirillo F, Scolaro V, et al. Appraisal of clinical complications after 23,827 oocyte retrievals in a large assisted reproductive technology program. Fertil Steril. 2018;109(6):1038–43 e1. doi: 10.1016/j.fertnstert.2018.02.002
- Habib G, Lancellotti P, Antunes MJ, et al. ESC Guidelines for the management of infective endocarditis: the task force for the management of infective endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J. 2015;36(44):3075–3128. doi: 10.1093/eurheartj/ehv319
- Kametas NA, McAuliffe F, Krampl E, et al. Maternal cardiac function in twin pregnancy. Obstet Gynecol. 2003;102(4):806–815. doi: 10.1097/00006250-200310000-00024
- Ombelet W, Martens G, De Sutter P, et al. Perinatal outcome of 12,021 singleton and 3108 twin births after non-IVF-assisted reproduction: a cohort study. Hum Reprod. 2006;21(4):1025–1032. doi: 10.1093/humrep/dei419
- Ramlakhan KP, Roos-Hesselink JW. Promising perspectives on pregnancy in women with congenital heart disease. Eur Heart J. 2021;42(41):4261–4263. doi: 10.1093/eurheartj/ehab692
- Flinter FA. Preimplantation genetic diagnosis. BMJ. 2001;322(7293):1008–1009. doi: 10.1136/bmj.322.7293.1008
- De Backer J, Bondue A, Budts W, et al. Genetic counselling and testing in adults with congenital heart disease: a consensus document of the ESC Working group of grown-up congenital heart disease, the ESC Working Group on Aorta and Peripheral Vascular Disease and the European Society of Human Genetics. Eur J Prev Cardiol. 2020;27(13):1423–1435. doi: 10.1177/2047487319854552
- Zaidi S, Brueckner M. Genetics and genomics of congenital heart disease. Circ Res. 2017;120(6):923–940. doi: 10.1161/CIRCRESAHA.116.309140
- Delvigne A, Rozenberg S. Epidemiology and prevention of ovarian hyperstimulation syndrome (OHSS): a review. Hum Reprod Update. 2002;8(6):559–577. doi: 10.1093/humupd/8.6.559
- Humaidan P, Quartarolo J, Papanikolaou EG. Preventing ovarian hyperstimulation syndrome: guidance for the clinician. Fertil Steril. 2010;94(2):389–400. doi: 10.1016/j.fertnstert.2010.03.028
- Humaidan P, Nelson SM, Devroey P, et al. Ovarian hyperstimulation syndrome: review and new classification criteria for reporting in clinical trials. Hum Reprod. 2016;31(9):1997–2004. doi: 10.1093/humrep/dew149
- Nelson SM, Greer IA. The potential role of heparin in assisted conception. Hum Reprod Update. 2008;14(6):623–645. doi: 10.1093/humupd/dmn031
- Sennstrom M, Rova K, Hellgren M, et al. Thromboembolism and in vitro fertilization - a systematic review. Acta Obstet Gynecol Scand. 2017;96(9):1045–1052. doi: 10.1111/aogs.13147
- Chan WS. The ‘ART’ of thrombosis: a review of arterial and venous thrombosis in assisted reproductive technology. Curr Opin Obstet Gynecol. 2009;21(3):207–218. doi: 10.1097/GCO.0b013e328329c2b8
- Henriksson P, Westerlund E, Wallen H, et al. Incidence of pulmonary and venous thromboembolism in pregnancies after in vitro fertilisation: cross sectional study. BMJ. 2013;346(jan15 3):e8632. doi: 10.1136/bmj.e8632
- Karsenty C, Zhao A, Marijon E, et al. Risk of thromboembolic complications in adult congenital heart disease: a literature review. Arch Cardiovasc Dis. 2018;111(10):613–620. doi: 10.1016/j.acvd.2018.04.003
- Dangas GD, Weitz JI, Giustino G, et al. Prosthetic Heart Valve Thrombosis. J Am Coll Cardiol. 2016;68(24):2670–2689. doi: 10.1016/j.jacc.2016.09.958
- Roudaut R, Serri K, Lafitte S. Thrombosis of prosthetic heart valves: diagnosis and therapeutic considerations. Heart. 2007;93(1):137–142. doi: 10.1136/hrt.2005.071183
- Ramlakhan KP, Malhame I, Marelli A, et al. Hypertensive disorders of pregnant women with heart disease: the ESC EORP ROPAC Registry. Eur Heart J. 2022;43(38):3749–3761. doi:10.1093/eurheartj/ehac308
- Hutcheon JA, Lisonkova S, Joseph KS. Epidemiology of pre-eclampsia and the other hypertensive disorders of pregnancy. Best Pract Res Clin Obstet Gynaecol. 2011;25(4):391–403. doi: 10.1016/j.bpobgyn.2011.01.006
- Hayward RM, Foster E, Tseng ZH. Maternal and fetal outcomes of admission for delivery in women with congenital heart disease. JAMA Cardiol. 2017;2(6):664–671. doi: 10.1001/jamacardio.2017.0283
- Tulay P, Atilan O. Oocyte donors’ awareness on donation procedure and risks: a call for developing guidelines for health tourism in oocyte donation programmes. J Turk Ger Gynecol Assoc. 2019;20(4):236–242. doi: 10.4274/jtgga.galenos.2018.2018.0110
- Storgaard M, Loft A, Bergh C, et al. Obstetric and neonatal complications in pregnancies conceived after oocyte donation: a systematic review and meta-analysis. BJOG: Int J Obstet Gy. 2017;124(4):561–572. doi: 10.1111/1471-0528.14257
- Reid GJ, Webb GD, Barzel M, et al. Estimates of life expectancy by adolescents and young adults with congenital heart disease. J Am Coll Cardiol. 2006;48(2):349–355. doi: 10.1016/j.jacc.2006.03.041
- Marelli AJ, Mackie AS, Ionescu-Ittu R, et al. Congenital heart disease in the general population: changing prevalence and age distribution. Circulation. 2007;115(2):163–172. doi: 10.1161/CIRCULATIONAHA.106.627224
- Oliver JM, Gallego P, Gonzalez AE, et al. Risk factors for excess mortality in adults with congenital heart diseases. Eur Heart J. 2017;38(16):1233–1241. doi: 10.1093/eurheartj/ehw590
- Brandao P, Garrido N. Commercial surrogacy: an overview gestacao de substituicao comercial: uma visao global. Rev Bras Ginecol Obstet. 2022;44(12):1141–1158. doi: 10.1055/s-0042-1759774
- Roos-Hesselink JW, Cornette J, Sliwa K, et al. Contraception and cardiovascular disease. Eur Heart J. 2015;36(27):1728-34, 34a–34b. doi: 10.1093/eurheartj/ehv141
- Trussell J. Contraceptive failure in the United States. Contraception. 2011;83(5):397–404. doi: 10.1016/j.contraception.2011.01.021
- Lindley KJ, Teal SB. Contraception in women with cardiovascular disease. JAMA. 2022;328(6):577–578. doi: 10.1001/jama.2022.11541
- Silversides CK, Sermer M, Siu SC. Choosing the best contraceptive method for the adult with congenital heart disease. Curr Cardiol Rep. 2009;11(4):298–305. doi: 10.1007/s11886-009-0043-7