ABSTRACT
Introduction
Congenital heart disease (CHD) is the most common cardiac disorder in pregnancy in the western world (around 80%). Due to improvements in surgical interventions more women with CHD are surviving to adulthood and choosing to become pregnant.
Areas covered
Preconception counseling, antenatal management of CHDs and strategies to prevent maternal and fetal complications.
Preconception counseling should start early, before the transition to adult care and be offered to both men and women. It should include the choice of contraception, lifestyle modifications, pre-pregnancy optimization of cardiac state, the chance of the child inheriting a similar cardiac lesion, the risks to the mother, and long-term prognosis. Pregnancy induces marked physiological changes in the cardiovascular system that may precipitate cardiac complications. Risk stratification is based on the underlying cardiac disease and data from studies including CARPREG, ZAHARA, and ROPAC.
Expert opinion
Women with left to right shunts, regurgitant lesions, and most corrected CHDs are at lower risk and can be managed in secondary care. Complex CHD, including systemic right ventricle need expert counseling in a tertiary center. Those with severe stenotic lesions, pulmonary artery hypertension, and Eisenmenger’s syndrome should avoid pregnancy, be given effective contraception and managed in a tertiary center if pregnancy does happen.
1. Introduction
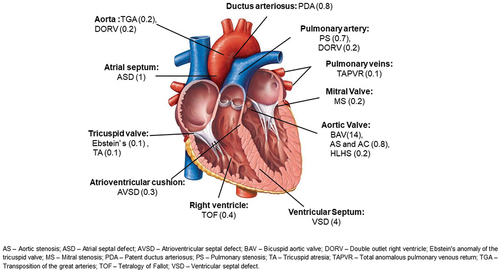
Cardiac disease remains the single largest indirect cause of maternal mortality in the developed world. CHD is a birth defect that affects the function of the heart. It occurs in 1% of the population and, during pregnancy in western countries, it is the most frequent cardiac disease accounting for 75–82% of cases [Citation1,Citation2] (). The etiology of CHD is multifactorial, due to both genetic predisposition and environmental causes. Typically, the term CHD excludes cardiomyopathies and rhythm and conduction system problems, although these can be present at birth but have a distinct and, most of the time, later presentation. As the management of CHD has improved, predominantly through better surgical approaches, most patients are surviving to adulthood and are considering pregnancy [Citation3]. For women, preconception counseling involves considering the impact of pregnancy on their condition both in the short and long term and the impact of their underlying condition on the fetus, which is mediated through their cardiac function, medication, and any heritable risk that their condition carries. Increasingly, more men are attending pre-conception counseling to determine the heritable risk of their condition and their personal prognosis. In this review, we focus on the antenatal management of CHD and strategies to prevent maternal and fetal complications.
2. Pre-conception counseling
Pre-conception counseling should be offered to everyone with preexisting heart disease, and, in the case of CHD, this should start before the transition from the pediatric to adult cardiac service. The question of a future pregnancy should be discussed with both males and females, initially considering their short- and long-term prognosis and then the chance of inheritance of their condition [Citation4]. Women who are advised against pregnancy due to their cardiac disease, should be counseled early and have a full discussion about their options, which include surrogacy and adoption. Women with CHD who have a single gene defect or a genetic syndrome such as Di George’s Syndrome can consider pre-implantation genetic diagnosis (). There is a 50% risk of inheritance in children whose parents suffer from autosomal dominant conditions like Marfan syndrome (MFS), long QT syndrome, and familial hypertrophic cardiomyopathy [Citation5]. Appropriate genetic counseling tailored to individual needs should be offered by expert counselors working with multidisciplinary teams (MDT) for women with CHD, congenital arrythmia, cardiomyopathies, or aortic pathology (). Finally, for women, how pregnancy might impact their condition and how their condition might affect the pregnancy should be considered. Each patient will have a variable prognosis during pregnancy depending on the underlying cardiac defect and other comorbidities [Citation6,Citation7]. General considerations, including the importance of optimal weight, physical exercise, smoking cessation, effects of alcohol and drugs, folic acid for supplementation pre-pregnancy, cervical screening, choice of contraception, medication history, and importance of vaccination (covid and influenza) should be discussed. Medication should be reviewed to optimize cardiac function and/or avoid fetal exposure to known teratogens, for example, angiotensin converting enzyme (ACE) inhibitors, angiotensin receptor blockers, amiodarone, warfarin, and spironolactone. Some cardiac lesions such as severe symptomatic aortic (AS) or mitral stenosis (MS), MFS with aortic dilatation of more than 45 mm should be corrected pre-pregnancy.
Table 1. Gene defects causing CHD (the ones that are known till now, the list with keep changing as new gene linkages are found).
Table 2. Risk of CHD in offspring of mothers with CHD according to different diseases.
Women needing valve replacement should be counseled about the risks and benefits of autografts/homografts, bioprosthetic and metallic heart valves. Bioprosthetic valves are associated with structural deterioration requiring re-operation in around 8–10 years, however design improvements are prolonging their lifespan and the risk of replacement has further decreased with the advent of transcatheter approaches. Currently, the stented biological valves are the most commonly used bioprosthetic valves. The autograft (pulmonary valve used for aortic valve replacement as used in Ross Procedure) and homograft can also be used for valve replacement. However, the use of homografts has declined due to availability issues. Also, their durability is found to be inferior compared to bioprosthetic and metallic valves [Citation8]. They are still preferred in patients with aortic valve endocarditis needing surgery [Citation9]. The main reason to avoid metallic valves is the need for lifelong anticoagulation, which is particularly complicated during pregnancy when the risk of valve thrombosis rises to 3.7–9.4% requiring close monitoring of anticoagulation [Citation8]. Women taking warfarin (more than 5 mg/day) or other vitamin K antagonists are at increased risk of embryopathy especially in the first trimester [Citation10–12], and often opt to switch to low molecular weight heparin (LMWH). Indeed, both the American and European guidelines suggest that the desire for pregnancy should be considered when deciding between a metallic and tissue valve [Citation13,Citation14].
If there is a history of subfertility and the patient is considering further investigation of the underlying cause or assisted reproduction techniques (ART), it is important to discuss the investigations and the risks that they pose. For example, diagnostic laparoscopy and the associated pneumoperitoneum or hysteroscopy (needing fluid distension of uterine cavity) can cause life threatening compromise to a woman with a univentricular circulation or pulmonary hypertension. It is therefore important to liaise with the gynecologist who will be undertaking this care and give clear guidance regarding the cardiac tolerance of the patient. Ovarian hyperstimulation syndrome associated with ART, is pro thrombotic and associated with marked fluid shifts that could cause further cardiac complications. A single embryo transfer should be recommended after ART, especially in women with cardiac disease, as a multiple pregnancy is associated with greater cardiovascular changes and increased risks of fetal and maternal morbidity.
Pre-pregnancy risk assessment using the modified WHO classification (mWHO) is used most widely [Citation15,Citation16] () and can be personalized with the information from other large studies like the CARPREG [Citation2,Citation17], ZAHARA [Citation18,Citation19] and ROPAC [Citation6] (). Baseline investigations including a full blood count, liver and renal function, an electrocardiogram, and an echocardiogram should be performed to establish the cardiac status in early pregnancy. An inter pregnancy interval of at least 18 months is recommended for women classified as mWHO class II-IV. Advice regarding effective and safe contraception should be given (see below).
Table 3. Modified WHO (m WHO) risk stratification classification.
Table 4. Predictors of adverse maternal cardiovascular events during pregnancy.
In general, women with a left to right shunt like atrial septal defect (ASD) or ventricular septal defect (VSD) and regurgitant valve lesions, tolerate pregnancy well and have a good prognosis. Also, women with tetralogy of Fallot, Morbus Ebstein, corrected coarctation and transposition of the great arteries have good outcomes, although more events are seen when compared to normal pregnancy, especially arrhythmias and preterm birth. Women who underwent Fontan correction for univentricular heart do need dedicated attention by specialists as they not only have fertility issues, they also face a high risk of miscarriage and complications during pregnancy. At the far end of the spectrum, patients with pulmonary artery hypertension (PAH), severe symptomatic AS, cyanotic heart disease with oxygen saturation less than 85%, severe left ventricular (LV) dysfunction, and severe mitral stenosis (MS) have worse outcomes, and so pregnancy is contraindicated.
3. Haemodynamic changes during pregnancy
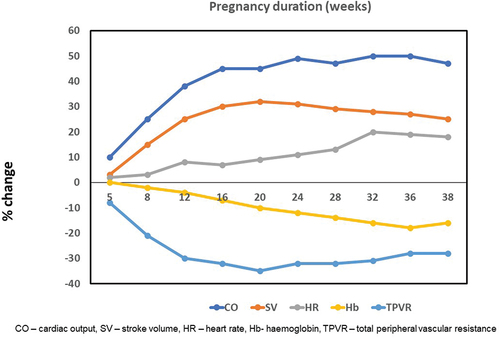
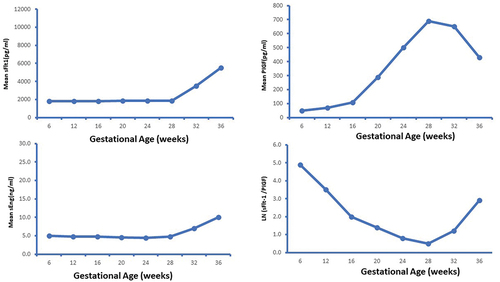
Pregnancy is associated with various hemodynamic changes to meet the demands of the growing fetus. The initial change is a 30–40% decrease in peripheral vascular resistance (PVR), which leads to an increase of 40–50% in the cardiac output and a two-third increase in plasma volume during pregnancy. The cardiac output increases because of an increase in stroke volume during the first half of pregnancy and a 10–20% increase in the heart rate [Citation20–22]. Cardiac output peaks between 20 and 26 weeks and then remains stable for the remainder of pregnancy. Coincidentally, PVR declines to a nadir at the same time and then starts to reverse (). This is critical for the ongoing increase in uterine blood flow essential for fetal growth and development (). The reversal in the initial fall in PVR is possibly driven by the placental release of sFlt-1 (soluble fms-like tyrosine kinase-1) and sEng (soluble endoglin) [Citation23] ().
Figure 3. Changes in blood flow in pelvic blood vessels during pregnancy compared to non-pregnant state.
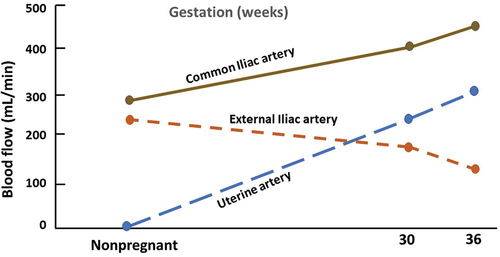
Figure 4. Graphs depicting release of anti-angiogenic factor soluble fms-like tyrosine kinase-1 (sFlt1) and soluble endoglin (eEng) and proangiogenic placental growth factor (PlGF)23.
Due to these hemodynamic changes, there is an increase in pressure gradients across stenotic lesions and patients particularly with MS and AS have worsening symptoms [Citation24]. Chronic regurgitant lesions like mitral or aortic regurgitation are generally well tolerated in pregnancy [Citation25]. In contrast, acute valvular regurgitant lesions, due to infective endocarditis for example, can cause severe heart failure [Citation26]. Left to right shunts (ASD, VSD, and patent ductus arteriosus (PDA)) do not increase in pregnancy, given the fall in systemic vascular resistance (SVR). On the other hand, the right to left shunts increase leading to worsening cyanosis. These patients are at high risk of maternal morbidity and poor fetal outcome (including risk of preterm delivery, fetal growth restriction (FGR), and neonatal death) due to a fall in the oxygen saturation [Citation7].
3.1. Other physiological changes in pregnancy
Pregnancy is known to be a hypercoagulable state and increases risk of VTE. Care should be taken when changing anticoagulant regimens in women with prosthetic heart valve as this is a high-risk time for thrombosis [Citation27–29]. Strict monitoring of Antifactor Xa (peak and trough) levels is recommended during pregnancy and particularly during any change in agent.
There are changes in the media of large vessels, especially the aorta, which increases the risk of dilatation and dissection during pregnancy especially in those with MFS or other HTAD [Citation30,Citation31]. The estimated incidence of aortic dissection during pregnancy is 0.05–1.39 per 100,000 person-years, but the maternal mortality rates associated with it is high, around 21–53% [Citation32].
There is an increase in glomerular filtration rate and liver metabolism during pregnancy. This causes an increased clearance of some drugs. The risk of teratogenesis for the developing fetus is maximum during week 6 to week 12 of pregnancy when organogenesis takes place. Hence, any drug should be used with caution during this stage of pregnancy [Citation33].
Labor causes significant hemodynamic changes including an increase in blood pressure (BP), cardiac output and heart rate. Immediately post-delivery there is an autotransfusion of up to 500 ml of blood from the utero-placental circulation into the maternal circulation, which increases the risk of volume overload and heart failure (HF).
3.2. Risk stratification
For risk estimation, a thorough history including other co-morbidities, physical examination: including BP, heart rate, auscultation; blood tests: including full blood count (FBC), ferritin, tests of liver and renal function, B-type natriuretic peptide (BNP); investigations: including electrocardiography (ECG), exercise tolerance test, echocardiography, observations including oxygen saturation and uptake, and functional class assessment (using NYHA classification) should be performed. In patients with aortic disease, complete aortic imaging using computed tomography (CT), or magnetic resonance imaging (MRI) is essential before pre-pregnancy counseling. Current ESC/EACTS guidelines recommend surgery in patients with MFS who have an aortic root more than 45 mm and risk factors like family history of dissection, or size increase of 2 mm/year [Citation15]. Standard treatment for such patients is either total root replacement (TRR) or valve-sparing root replacement (VSRR). However, even after successful surgery for root dilatation, complications can occur and these patients remain high risk.
3.3. Contraception
Having effective contraception is essential to allow for the optimization of the clinical state, change in medication and preparation for pregnancy. The combined oral contraceptive pill (OCP), containing both estrogen and progesterone, increases the risk of VTE and is known to increase BP and hence not recommended in patients with preexisting heart disease or hypertension. Progesterone only pills (POP) are safe and frequently used in patients with CHD, particularly the higher dose (Desogestrel 75 mg), which is more reliable as it inhibits ovulation. Long-acting reversible contraceptive methods including Levonorgestrel (LNG) based long-acting implants or intra uterine devices (IUD) are the safest and most effective contraceptives. They offer other non-contraceptive benefits like reduction of heavy menstrual bleeding, iron deficiency anemia, pelvic pain (especially in women with endometriosis), and prevention of endometrial hyperplasia. Infective endocarditis prophylaxis is not needed for insertion of these devices. However, IUD insertion may cause a vasovagal reaction, and this may need to be performed in a hospital setting, especially for Fontan and Eisenmenger syndrome patients [Citation34,Citation35]. Barrier contraceptives are unreliable but prevent sexually transmitted infections. A combination of barrier contraception and LNG IUD is considered to be ideal. For emergency contraception, copper IUD, LNG, or ulipristal acetate (UPA) are safe with no increased risk of thrombosis.
4. Antenatal care for women with CHD
Women with CHD should be cared for by a MDT, including a cardiologist, obstetrician, anesthetist, specialist nurse, and midwife. Their pregnancies are at higher risk of complications of both obstetrics: miscarriage, FGR, pre-eclampsia, preterm labor, postpartum hemorrhage (PPH), and neonatal death [Citation36] and cardiological: HF, arrythmias, aortic dissection, VTE, and maternal cardiovascular collapse and death [Citation37]. To minimize these risks, the MDT must pay close attention to both the routine and patient-specific aspects of antenatal care. The level of these risks, particularly the cardiological risks, will determine the correct level of care she needs during her pregnancy, secondary or tertiary and if secondary care is deemed to be sufficient, the next question is whether the local hospital is willing to care for her.
Routine obstetric care should be carried out for all women with CHD including full blood count, blood group and rhesus status, antibody screen, screening tests to detect hemoglobinopathies, diabetes, infections like Hepatitis B/Human Immunodeficiency virus (HIV), syphilis, rubella, and vitamin D deficiency. Routine ultrasound screening for Down’s syndrome at around 12 weeks and structural abnormalities at around 20 weeks should be carried out. Vaccination for covid and influenza should be recommended and for pertussis in later pregnancy. Risk of VTE should be assessed and a low threshold for prophylactic anticoagulation adopted for women with CHD, as they are likely to be at higher risk and the consequences of a pulmonary embolism (PE) are more profound in women with CHD.
For the detection of fetal congenital heart defects, the first trimester nuchal fold thickness can identify some major congenital heart defects. Fetal echocardiogram should be offered to all women with CHD between 18 and 22 weeks of gestation as the risk of fetal congenital heart defects is 3–5% in women with CHD against a background risk of ~ 1% in general population. Regular fetal growth scans at 28-, 32- and 36-weeks’ gestation are recommended to diagnose FGR and plan timing and mode of delivery.
From a cardiovascular point of view, the frequency of visits, echocardiogram and/or cMR assessments should be tailored to the nature and severity of the underlying cardiac lesion and clinical condition. The development of new murmurs should be investigated. Women with palpitations or known arrythmias should be investigated with an ECG or Holter monitoring. In women at particularly high risk of significant arrhythmia, the use of an implantable loop cardiac monitor (ILC) has been shown to be most effective in the identification of arrhythmias in pregnancy [Citation38]. Unexplained dyspnea, chest pain, shortness of breath, orthopnea in pregnancy should be thoroughly investigated to exclude the development of HF or PE and to diagnose pathology accurately.
A delivery plan should be formulated by the MDT between 20 and 32 weeks of gestation. Induction of labor should be considered around 40 weeks in most women with CHD, as in the low-risk population it has been shown to reduce the chance of an emergency Cesarean section (CS) and is associated with better fetal outcomes [Citation39]. Mechanical methods such as Cooks balloon, prostaglandin E1 analogue, and slow-release formulation of 10 mg prostaglandin E2 are all considered safe for induction of labor as is artificial rupture of membranes with oxytocin. Augmentation of labor post ruptured membranes should be immediate to reduce the risk of infection and should be with oxytocin to minimize the number of vaginal examinations.
5. Specific CHD’s and management during pregnancy
5.1. Left to right shunts
An ASD is the commonest form of CHD seen during pregnancy. In contrast, it is extremely rare to have an undiagnosed moderate or large VSD. Most women with repaired ASD/VSD tolerate pregnancy very well. Similarly, patients with a small PDA can be managed expectantly in pregnancy [Citation40,Citation41]. Women with repaired atrioventricular septal defects (AVSD) are at higher risk, due to the presence of residual lesions, particularly left AV-valve regurgitation. These women have a higher risk of arrhythmia and of functional deterioration during pregnancy. However, an incompletely repaired AVSD in isolation and with a small persisting defect are at low risk of pregnancy complications. Of note, the inheritance risk of AVSD is relatively high at around 10% ().
Management. Patients with a repaired ASD or VSD with no residual lesions and without pulmonary hypertension should be reviewed once/twice antenatally to make a birth plan and to establish a referral pathway if complications arise. In unrepaired ASD, there is a risk of thromboembolic complication, particularly paradoxical embolism and prophylactic anticoagulation, which should be considered in the presence of other risk factors. Atrial arrythmias are more common and persisting palpitations should be investigated.
5.2. Stenotic lesions
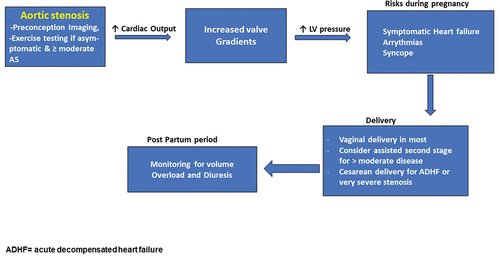
The most frequent cause of AS is a BAV. HF is reported in 25% women with severe symptomatic AS in pregnancy (mWHO Class IV). The gradient across a stenotic lesion tend to worsen during pregnancy due to the increased cardiac output and fall in PVR. The gradients increase and the symptoms worsen, specially toward the end of second trimester when the increase in cardiac output is maximum.
Management. Regular monthly/bimonthly follow up is recommended in severe cases of AS with regular echocardiograms. Patients with severe AS need surgical intervention pre pregnancy if they are symptomatic, have LV dysfunction or develop symptoms during exercise testing. An aortic balloon valvuloplasty can be considered during pregnancy in women with severe AS and worsening symptoms [Citation42–44]. A CS should be considered in women with severe symptomatic AS or acute HF ().
5.3. Ebstein’s anomaly (mWHO Class II or III)
Patients with mild disease and little/no ventricular dysfunction tolerate pregnancy well. Patients with preexisting right or left heart dysfunction may experience overt HF symptoms or arrythmias. Symptomatic patients with cyanosis or HF should be counseled against pregnancy or recommended termination of pregnancy if pregnant. Fetal complications include FGR, prematurity, and neonatal death.
Management. Patients who are at low risk (mWHO class II) can be seen by a cardiologist in each trimester. Those in mWHO class III need monthly/bimonthly MDT follow up. Supraventricular tachycardia (SVT) can complicate pregnancy and may need vagal maneuvers or, if resistant, chemical or electrical cardioversion. Severe tricuspid regurgitation (TR) with HF can mostly be managed medically during pregnancy. However, women with interatrial shunts are at an increased risk of paradoxical emboli and if cyanosed, this may increase worsening maternal and fetal outcomes.
5.4. Fontan’s circulation
Pregnant women with a Fontan’s circulation are classified as high risk to very high-risk (mWHO class III or IV). Maternal complications include tachyarrhythmias, atrial arrythmia (needing electrical cardioversion), risk of deterioration of ventricular function, worsening aortic valve (AV) regurgitation and functional deterioration including HF. They have a high risk of miscarriage (40%), preterm delivery, FGR, and neonatal death [Citation45–47]. Pregnancy is contraindicated in women with Fontan circulation who have oxygen saturation <85%, ventricular dysfunction, moderate to severe AV regurgitation, refractory arrythmias or protein losing enteropathy.
Management. These women should be monitored in a tertiary unit with an MDT. Strenuous exercise should be limited and bed rest with oxygen supplementation considered with medical treatment of arrythmia especially during third trimester. Early diagnosis of HF, arrythmias, VTE, and worsening cyanosis is key [Citation48,Citation49]. Therapeutic anticoagulation should be considered as they are at high risk of VTE in pregnancy.
5.5. Aortopathy
Various hereditary diseases affect the aorta including HTAD, MFS, Loeys-Dietz syndrome, vascular Ehler-Danlos syndrome, and Turner syndrome, and are associated with an increased risk of aortic dissection in pregnancy. Hypertension, increasing gestation, and advanced maternal age also increase the risk of aortic dilatation and dissection. Data from GenTAC registry on MFS showed a significantly higher aortic dissection rate in pregnancy and post-partum (5.4 per 100 person‐years vs 0.6 per 100 patient‐years in non-pregnant population). Pregnancy is contraindicated in women with MFS and an aortic root more than 4.5 cm and BAV with aortic root more than 5 cm. These patients should undergo corrective surgery pre-pregnancy [Citation50]. Even after corrective surgery, these patients are at high risk for aortic dissection in pregnancy and pregnancy should be very carefully considered in the pre-conception clinic [Citation51].
Management. Women with aortopathy should have increased right to left shunt serial aortic root monitoring (depending on diagnosis and severity of dilatation) by an echocardiogram every 4–12 weeks in pregnancy and up to 6 months postpartum. Prophylactic beta blockers are often prescribed, but with little data to support their use. Serial fetal growth scans should be performed for those taking beta-blockers. Stanford type A dissection or rapid aortic dilatation is an emergency, requiring urgent surgical intervention. Depending on the gestational age, delivery may occur prior to surgery [Citation52,Citation53]. In contrast, an uncomplicated type B aortic dissection is treated conservatively with tight BP control.
5.6. Post operative Tetralogy of Fallot (TOF; mWHO class II)
Repaired TOF patients most of the time do well during pregnancy. Cardiac complications, including HF and arrythmias, have been reported in around 7–10% of pregnancies, predominantly in association with severe pulmonary regurgitation (PR) and right ventricular (RV) dysfunction [Citation54]. Arrhythmias are typically atrial and increased with atrial dilatation. Pregnancy may be complicated by FGR and preterm delivery, up to 35% and 18%, respectively [Citation55]. However, these reports are often small, perhaps giving a misleading picture as the large ROPAC series (n = 421) reported low rates of HF (5%) and ventricular arrhythmia (1.7%) with good pregnancy outcomes, preterm birth (16.3%) and low birth weight (9.7%) [Citation54]. RV failure in pregnancy should be treated with diuretics, bed rest, and LMWH prophylaxis [Citation56]. The chance of occurrence of CHD in the offspring is 50% in the context of Di George’s syndrome, but 2–3% otherwise [Citation57].
Management is as outlined above, but with close attention on women with impaired RV function and moderate or severe PR, who are more likely to experience cardiac and obstetric complications.
5.7. Transposition of great arteries (TGA, mWHO Class III for atrial switch and II for arterial switch)
Women with an atrial switch (Senning and Mustard) with a systemic RV are at increased risk of arrythmias, HF, and thromboembolic events during pregnancy. Their risk of arrythmia is higher than for patients with TOF/ASD. Rates of complications vary, with Drenthen et al. reporting lower rates of cardiac complications, HF (11%) and arrhythmia (16%), and moderate rates of pregnancy complications, preterm birth (33%) and small for gestation age (SGA) (19%) in a large literature review including over 170 pregnancies [Citation58]. The ROPAC prospective experience of pregnancy in women with TGA and a systemic RV reported similarly low rates of complications in 163 women, HF (9.8%) and arrhythmia (6.7%), and moderate rates of pregnancy complications, preterm birth (21%) and SGA (17.8%) [Citation58]. In contrast, in 41 pregnancies with an arterial switch correction of TGA, ROPAC reported much better rates of cardiac complications, HF (2.4%) and arrhythmia (2.4%), but similar rates of pregnancy complications, preterm birth (17.1%) and SGA (14.6%) [Citation59].
Management is as outlined but with a particular focus on patients with moderate to severe systemic RV dysfunction or moderate-severe TR who are at higher risk of HF and arrhythmia and a worse pregnancy outcome [Citation58].
5.8. Pulmonary Hypertension (PH) and Eisenmenger’s syndrome
PH is defined when the pulmonary artery pressure is 25 mm Hg or over at right heart catheterization. Pregnancy is contraindicated in women with PH, as there is a 16–30% risk of maternal mortality [Citation60]. Causes of mortality in these women include pulmonary hypertensive crisis, right HF, or pulmonary thrombosis. However, the ROPAC data found in a series of 151 women with a range of etiologies and severity of PH that mortality was lower at 5.9%, with HF in 27%, preterm birth occurred in 21.7% and low birth weight in 19.1% [Citation60]. Similarly, in a large series from China (n = 729), which included more than 400 women with PH, mortality was 5.7% in women with moderate to severe PH, HF (38%), arrythmias needing treatment (6%), preterm delivery (43%), and low birth weight (43%) [Citation61].
Management. Although pregnancy is generally not advised, when it does occur, intensive monitoring is required with serial echocardiograms and frequent MDT review. Bed rest may be advised in severe symptomatic cases. VTE prophylaxis should be prescribed, and HF treated with medications including diuretics. Oral sildenafil is safe in pregnancy. Eisenmenger’s syndrome presents more challenges during pregnancy due to cyanosis and the potential for paradoxical embolism. Women with oxygen saturation of less than 85% have poor fetal outcomes. The cyanosis increases during pregnancy due to decrease in SVR and consequent increased right to left shunt [Citation4,Citation62]. Maternal mortality is estimated to be around 20–50% in these women and termination should be discussed early in the pregnancy. A termination of pregnancy is high risk and should be carried out in specialized centers with dedicated cardiologists, obstetricians, and obstetric anesthetists. Care should be taken in the post-partum period when most complications occur [Citation15].
5.9. Coarctation of aorta (CoA)
After CoA repair, pregnancy is often well tolerated. The risk is further increased in patients with a BAV and aortic dilatation. These women are at an increased risk of pre-eclampsia.
Management. Aspirin for pre-eclampsia prophylaxis (150 mg once daily from 12 weeks gestation) is recommended. BP should be monitored closely. Percutaneous intervention for residual CoA may be needed in cases of refractory hypertension or maternal or fetal compromise.
5.10. Metallic valve
Women with a metallic heart valve are one of the highest risk groups during pregnancy [Citation63]. The most frequent cause of death is valve thrombosis and the most high-risk times for this complication are when changing from one form of anticoagulation to another. This transition occurs in early pregnancy when warfarin or other vitamin K antagonist (VKA) is changed to LMWH to reduce the risk of VKA-induced embryopathy [Citation64]. There is some evidence that suggests that those taking a warfarin dose of less than 5 mg have a lower risk of embryopathy and can be maintained on warfarin until 36 weeks [Citation65]. The safest option is to admit the patient during any transition, maintaining anticoagulation with the first agent until effective anticoagulation is achieved with the second agent. Often, this is reversed again after 12 weeks of gestation, when the risk of VKA-induced embryopathy is reduced, and anticoagulation maintained with VKA until 36 weeks when again, VKA is replaced by LMWH until delivery. In lower risk women, therapeutic LMWH should be stopped 24 hours before regional anesthesia [Citation66,Citation67]. In higher risk women, LMWH should be replaced by UFH, which should be stopped 6 hours before regional anesthesia. At each point of transition, there is a risk of inadequate anticoagulation and valve thrombosis. Target INR levels for VKA antagonists are 2.5 (range 2.0–3.0) but slightly higher for those with a metallic mitral valve with INR target of 3.0 (range 2.5–3.5) and on LMWH an anti-factor Xa of 0.6–1.0 units/ml and again higher for those with a metallic mitral valve of 0.6–1.2 units/ml [Citation7]. Monitoring for the LMWH is critical, with effective anticoagulation only provided when the anti-factor Xa level is within the therapeutic range as defined above for both peak (3–4 hours post injection) and trough (immediately before injection) [Citation68].
6. Labour and mode of delivery
6.1. Mode of delivery
Vaginal delivery is considered safe for most cases of CHD and has the advantage of less blood loss, lower risk of infection, and VTE [Citation40]. CS is performed, mainly for obstetric indications. CS is recommended for women on oral anticoagulation in preterm labor, severe PAH including Eisenmenger’s syndrome, intractable HF, or have an aggressive aortic pathology, meaning an aortic diameter of 4.0–4.5 cm (moderate indication), greater than 4.5cms (strong indication), or growing at a rate of 2–3 mm/year. In women having a vaginal delivery, an assisted second stage may be recommended. Assisted delivery should be offered in the context of severe left ventricular outflow tract obstruction, commonly related to AS, sub-valvular stenosis, or hypertrophic cardiomyopathy, to women with exercise induced arrythmia, or women with an aortic dilatation of between 4.0 and 4.5cms. Less severe lesions with compromised heart function and/or reserve may be allowed a period of active pushing, the duration is arbitrary and should be judged by the MDT on a case-by-case basis [Citation69]. The labor and delivery plan should be made by an MDT and documented between 20 and 32 weeks ().
Table 5. Delivery planning in women with CHD.
6.2. Anaesthesia
Regional epidural anesthesia is preferred over general anesthesia for CS unless contra-indicated by recent anticoagulation or previous spinal surgery or clinically indicated in the context of PH in some centers. During vaginal delivery, again epidural is recommended to minimize cardiovascular stress, alternatives include patient-controlled remifentanil, particularly in the context of recent LMWH administration.
6.3. Infective endocarditis prophylaxis
It should be considered by the MDT and is advised for all high-risk cases, including patients with a metallic heart valve or history of infectious endocarditis [Citation26].
6.4. Monitoring
Continuous fetal monitoring is recommended in labor. Maternal BP, heart rate, oxygen saturation, and continuous ECG monitoring are indicated throughout labor. An arterial line may be considered in high-risk patients.
6.5. Fluid balance
Fluid balance needs to be carefully monitored. In broad terms, right-sided heart lesions are more dependent on an adequate filling pressure to maintain cardiac output, while excessive fluid is more likely to be complicated by pulmonary edema in the context of a left-sided lesion. Immediately post-delivery, auto transfusion of blood from the placenta into the maternal circulation, may cause a transient volume overload and contribute to the increase in HF identified in this period [Citation70].
6.6. Cardiac arrest
In the event of a cardiac arrest after 24 weeks of gestation, a peri-mortem CS should be performed as soon as possible and preferably within 4 min of the event to facilitate maternal resuscitation.
7. Postpartum management
Active management of third stage is recommended for all women with CHD as it reduces blood loss by 40%. A slow intravenous infusion of two units of oxytocin over 10 min is preferred to avoid risks of hypotension and tachycardia associated with bolus oxytocin administration [Citation15,Citation71]. Prostaglandin E1 analogue, misoprostol (1000 mcg) is considered safe for the treatment of PPH, whereas ergometrine and prostaglandin F analogues should be avoided.
The risk of VTE is highest during the postpartum period and prophylaxis with LMWH and compression stockings is recommended. Breastfeeding is encouraged in women with CHD although there is a small increased risk of bacteremia secondary to mastitis. However, in the context of HF, breast feeding is discouraged as it may put additional strain on the heart. Drugs like metoprolol, nadolol (beta blockers), enalapril, captopril (ACE Inhibitors), amlodipine (and most calcium channel blockers), furosemide and warfarin are considered safe during breastfeeding. Women with complex CHD should be followed jointly by their cardiologist and obstetrician, 6–12 weeks post-delivery [Citation71,Citation72].
8. Expert opinion
Pregnancy in women with CHD is associated with increased risks to the mother and the fetus mostly related to the associated physiological changes and hypercoagulable state. People with heart disease should receive pre-conception counseling around the time of puberty, when transitioning to adult care and when actively considering a pregnancy [Citation6]. For men and women, the advice should include general lifestyle modification, options for contraception, and the risk of passing on CHD to the child. For women, more detailed advice should be given about contraception options, the use of medication with known teratogenic side effects, the optimization of cardiac status, and the risk to the mother and fetus of their specific CHD. The mWHO classification is most widely used for risk stratification [Citation7]. Fetal complications include risk of miscarriage, preterm delivery, FGR, and neonatal death [Citation37]. An individualized management plan to look after these women should be made by the MDT between 20 and 32 weeks of gestation. High-risk patients should be managed in a tertiary unit with a monthly/bimonthly follow up. Lower risk women can be looked after in secondary care with input from MDT. Women in mWHO Class IV should be counseled against pregnancy and have a detailed discussion of available options including adoption and surrogacy. If pregnancy occurs, the choice of termination should be offered early in pregnancy [Citation15].
At the first visit during pregnancy, a good history including comorbidities, detailed examination, and investigations including ECG, Holter, echocardiogram, or cMRI (where necessary) should be performed. Drugs like ACE inhibitors, Angiotensin receptor blockers (ARBs), Statins, Spironolactone, Amiodarone, and Atenolol should be stopped and replaced, if necessary, with drugs that are safe during pregnancy. In women on warfarin or other VKA, particularly those with a metallic heart valve, any change in anticoagulation needs very close monitoring.
Vaginal delivery is preferred in most cases with CS performed mostly for obstetric indications [Citation69]. Elective assisted delivery should be considered in women with severe left ventricular outflow tract obstruction (AS, sub-valvular stenosis, or hypertrophic cardiomyopathy), women with exercise induced arrhythmia, or women with an aortic dilatation of between 4.0 and 4.5 cm. Elective CS is recommended for women on oral anticoagulation in labor, severe PAH including Eisenmenger’s syndrome, intractable HF, or aggressive aortic pathology. Adequate pain relief with regional anesthesia may help attenuate the hemodynamic changes during labor and delivery. Active management of third stage with slow infusion of oxytocin should be considered. The risk of VTE increases further in postpartum period and anticoagulation should be adjusted accordingly.
Article highlights
Preconception counseling for both men and women with CHD during transition from pediatric to adult cardiac service.
Effective contraception to allow optimization of cardiac state and to change teratogenic drugs.
Pregnancy is contraindicated in women classified as mWHO Class IV and if it occurs, women should be offered the option of termination as soon as possible.
Women in mWHO Class III or IV should be managed by a multidisciplinary team in a tertiary center.
Risk of aortic dissection is elevated in pregnancy. Women with Heritable Thoracic Aortic Disease (HTAD), bicuspid aortic valve (BAV), or Turner syndrome should have serial echocardiograms to monitor aortic root dimension during pregnancy and in the postpartum period.
Vaginal delivery is preferred in most cases.
Venous thromboembolism (VTE) prophylaxis post-delivery is adjusted according to obstetric guidelines as the postpartum period is the time for highest risk of VTE.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Curtis SL, Marsden-Williams J, Sullivan C, et al. Current trends in the management of heart disease in pregnancy. Int J Cardiol. 2009;133(1):62–69. doi: 10.1016/j.ijcard.2007.11.084
- Siu SC, Sermer M, Colman JM, et al. Cardiac Disease in Pregnancy (CARPREG) investigators. Prospective multicenter study of pregnancy outcomes in women with heart disease. Circulation. 2001;104(5):515–521. doi: 10.1161/hc3001.093437
- American College of Cardiology Cardiovascular Disease in Women Committee and the Cardio-Obstetrics Work Group, Lindley KJ, Bairey Merz CN, et al. Management of women with congenital or inherited cardiovascular disease from pre-conception through pregnancy and postpartum: JACC focus seminar 2/5. J Am Coll Cardiol. 2021;77(14):1778–1798.
- Bruneau BG. The developmental genetics of congenital heart disease. Nature. 2008;451(7181):943–948. doi: 10.1038/nature06801
- Goland S, Elkayam U. Pregnancy and Marfan syndrome. Ann Cardiothorac Surg. 2017;6(6):642–653. doi: 10.21037/acs.2017.10.07
- Roos-Hesselink J, Baris L, Johnson M, et al. Pregnancy outcomes in women with cardiovascular disease: evolving trends over 10 years in the ESC Registry of Pregnancy and Cardiac disease (ROPAC). Eur Heart J. 2019;40(47):3848–3855. doi: 10.1093/eurheartj/ehz136
- Van Hagen IM, Roos-Hesselink JW. Pregnancy in congenital heart disease: risk prediction and counselling. Heart. 2020;106(23):1853–1861. doi: 10.1136/heartjnl-2019-314702
- El-Hamamsy I, Zaki M, Stevens LM, et al. Rate of progression and functional significance of aortic root calcification after homograft vs freestyle aortic root replacement. Circulation. 2009;120(11_suppl_1):S269–75. doi: 10.1161/CIRCULATIONAHA.108.843748
- Musci M, Weng Y, Hubler M, et al. Homograft aortic root replacement in native or prosthetic active infective endocarditis: twenty-year single-center experience. J Thorac Cardiovasc Surg. 2010;139(3):665–673. doi: 10.1016/j.jtcvs.2009.07.026
- Elkayam U, Bitar F. Valvular heart disease and pregnancy: part II: prosthetic valves. J Am Coll Cardiol. 2005;46(3):403–410. doi: 10.1016/j.jacc.2005.02.087
- Ginsberg JS, Chan WS, Bates SM, et al. Anticoagulation of pregnant women with mechanical heart valves. Arch Intern Med. 2003;163(6):694–698. doi: 10.1001/archinte.163.6.694
- McLintock C. Anticoagulant therapy in pregnant women with mechanical prosthetic heart valves: no easy option. Thromb Res. 2011;127(3):S56–S60. doi: 10.1016/S0049-3848(11)70016-0
- Vahanian A, Beyersdorf F, et al. ESC/EACTS Scientific Document Group, 2021 ESC/EACTS Guidelines for the management of valvular heart disease: Developed by the Task Force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Rev Esp Cardiol (Engl Ed). 2022;75(6):524.
- Otto CM, Nishimura RA, bonow ro, et al. 2020 acc/aha guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation. 2021;143(5):e72–e227. doi: 10.1161/CIR.0000000000000923
- Regitz-Zagrosek V, Roos-Hesselink JW, Bauersachs J, et al. 2018 ESC guidelines for the management of cardiovascular diseases during pregnancy: the task force for the management of cardiovascular diseases during pregnancy of the European society of cardiology (ESC). Eur Heart J. 2018;39(34):3165–3241. doi: 10.1093/eurheartj/ehy340
- Pijuan-Domenech A, Galian L, Goya M, et al. Cardiac complications during pregnancy are better predicted with the modified who risk score. Int J Cardiol. 2015;195:149–154. doi: 10.1016/j.ijcard.2015.05.076
- Silversides CK, Grewal J, Mason J, et al. Pregnancy outcomes in women with heart disease: the CARPREG II study. J Am Coll Cardiol. 2018;71(21):2419–2430. doi: 10.1016/j.jacc.2018.02.076
- Drenthen W, Boersma E, Balci A, et al. Predictors of pregnancy complications in women with congenital heart disease. Eur Heart J. 2010;31(17):2124–2132. doi: 10.1093/eurheartj/ehq200
- Lu CW, Shih JC, Chen SY, et al. Comparison of 3 Risk estimation methods for predicting cardiac outcomes in pregnant women with congenital heart disease. Circ J. 2015;79(7):1609–1617. doi: 10.1253/circj.CJ-14-1368
- Meah VL, Cockcroft JR, Backx K, et al. Cardiac output and related haemodynamics during pregnancy: a series of meta-analyses. Heart. 2016;102(7):518–526. doi: 10.1136/heartjnl-2015-308476
- Boardman H, Ormerod O, Leeson P. Haemodynamic changes in pregnancy: what can we learn from combined datasets? Heart. 2016;102(7):490–491. doi: 10.1136/heartjnl-2015-309166
- Ruys TP, Cornette J, Roos-Hesselink JW. Pregnancy and delivery in cardiac disease. J Cardiol. 2013;61(2):107–112. doi: 10.1016/j.jjcc.2012.11.001
- Kim MY, Buyon JP, Guerra MM, et al. Angiogenic factor imbalance early in pregnancy predicts adverse outcomes in patients with lupus and antiphospholipid antibodies: results of the PROMISSE study. Am J Obstet Gynecol. 2016;214(1):.e108.1–.e108.14. doi: 10.1016/j.ajog.2015.09.066
- Lewey J, Andrade L, Levine LD. Valvular heart disease in pregnancy. Cardiol Clin. 2021;39(1):151–161. doi: 10.1016/j.ccl.2020.09.010
- Anthony J, Osman A, Sani MU. Valvular heart disease in pregnancy. Cardiovasc J Afr. 2016;27(2):111–118. doi: 10.5830/CVJA-2016-052
- Shapero KS, Nauriyal V, Megli C, et al. Management of infective endocarditis in pregnancy by a multidisciplinary team: a case series. Ther Adv Infect Dis. 2022;9:20499361221080644. doi: 10.1177/20499361221080644
- Greer IA. Thrombosis in pregnancy: maternal and fetal issues. Lancet. 1999;353(9160):1258–1265. doi: 10.1016/S0140-6736(98)10265-9
- Kujovich JL. Hormones and pregnancy: thromboembolic risks for women. Br J Haematol. 2004;126(4):443–454. doi: 10.1111/j.1365-2141.2004.05041.x
- Heit JA, Kobbervig CE, James AH, et al. Trends in the incidence of venous thromboembolism during pregnancy or postpartum: a 30-year population-based study. Ann Intern Med. 2005;143(10):697–06. doi: 10.7326/0003-4819-143-10-200511150-00006
- Curry RA, Gelson E, Swan L, et al. Marfan syndrome and pregnancy: Maternal and neonatal outcomes. BJOG: Int J Obstet Gy. 2014;121(5):610–617. doi: 10.1111/1471-0528.12515
- Carlson M, Airhart N, Lopez L, et al. Moderate aortic enlargement and bicuspid aortic valve are associated with aortic dissection in turner syndrome: Report of the international Turner syndrome aortic dissection registry. Circulation. 2012;126(18):2220–2226. doi: 10.1161/CIRCULATIONAHA.111.088633
- Nienaber CA, Fattori R, Mehta RH, et al. Gender related differences in acute aortic dissection. Circulation. 2004;109(24):3014–3021. doi: 10.1161/01.CIR.0000130644.78677.2C
- Pieper PG. Use of medication for cardiovascular disease during pregnancy. Nat Rev Cardiol. 2015;12(12):718–729. doi: 10.1038/nrcardio.2015.172
- Roos-Hesselink JW, Cornette J, Sliwa K, et al. Contraception and cardiovascular disease. Eur Heart J. 2015;36(27):1728–1734. doi: 10.1093/eurheartj/ehv141
- Lidegaard O, Lokkegaard E, Svendsen AL, et al. Hormonal contraception and risk of venous thromboembolism: National follow-up study. BMJ. 2009;339(aug13 2):b2890. doi: 10.1136/bmj.b2890
- Martinez-Portilla RJ, Poon LC, Benitez-Quintanilla L, et al. Incidence of pre-eclampsia and other perinatal complications among pregnant women with congenital heart disease: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2021 Oct;58(4):519–528.
- Lammers AE, Diller GP, Lober R, et al. Maternal and neonatal complications in women with congenital heart disease: a nationwide analysis. Eur Heart J. 2021;42(41):4252–4260. doi: 10.1093/eurheartj/ehab571
- Sliwa K, Azibani F, Johnson MR, et al. Effectiveness of implanted cardiac rhythm recorders with electrocardiographic monitoring for detecting arrhythmias in pregnant women with symptomatic arrhythmia and/or structural heart disease: a randomized clinical trial. JAMA Cardiol. 2020;5(4):458–463. doi: 10.1001/jamacardio.2019.5963
- Kirby A, Curtis E, Hlohovsky S, et al. Pregnancy outcomes and risk evaluation in a contemporary adult congenital heart disease cohort. Heart Lung Circ. 2021;30(9):1364–1372. doi: 10.1016/j.hlc.2021.03.005
- Yap SC, Drenthen W, Meijboom FJ, et al. Comparison of pregnancy outcomes in women with repaired versus unrepaired atrial septal defect. BJOG. 2009;116(12):1593–1601. doi: 10.1111/j.1471-0528.2009.02301.x
- Mendelson MA. Pregnancy in women with left-to-right cardiac shunts: Any risk? Int J Cardiol Congenit Heart Dis. 2021;5:100209. doi: 10.1016/j.ijcchd.2021.100209
- Silversides CK, Colman JM, Sermer M, et al. Early and intermediate-term outcomes of pregnancy with congenital aortic stenosis. Am J Cardiol. 2003;91(11):1386–1389. doi: 10.1016/S0002-9149(03)00340-0
- Elkayam U, Bitar F. Valvular heart disease and pregnancy part I: native valves. J Am Coll Cardiol. 2005;46(2):223–230. doi: 10.1016/j.jacc.2005.02.085
- Samiei N, Amirsardari M, Rezaei Y, et al. Echocardiographic evaluation of hemodynamic changes in left-sided heart valves in pregnant women with valvular heart disease. Am J Cardiol. 2016;118(7):1046–1052. doi: 10.1016/j.amjcard.2016.07.005
- Zentner D, Kotevski A, King I, et al. Fertility and pregnancy in the Fontan population. Int J Cardiol. 2016;208:97–01. doi: 10.1016/j.ijcard.2016.01.180
- Canobbio MM, Mair DD, van der Velde M, et al. Pregnancy outcomes after the Fontan repair. J Am Coll Cardiol. 1996;28(3):763–767. doi: 10.1016/0735-1097(96)00234-3
- Lao TT, Sermer M, Colman JM. Pregnancy after the Fontan procedure for tricuspid atresia. A case report. J Reprod Med. 1996;41(4):287–290.
- Gnanaraj JP, Anne Princy S. Surendran Anju, et al on behalf of the M-PAC investigators. Pregnancy outcomes in women with heart disease: the Madras Medical College Pregnancy and Cardiac (M-PAC) Registry from India. Eur Heart J. 2023;1–12.
- Jolien WR-H, Van der Zande JA, Johnson MR. Pregnancy outcomes in women with heart disease: how to improve? Eur Heart J. 2023;1–3.
- Pierpont ME, Basson CT, Benson DW Jr, et al. Genetic basis for congenital heart defects: Current knowledge. Circulation. 2007;115(23):3015–3038. doi: 10.1161/CIRCULATIONAHA.106.183056
- Sayama S, Takeda N, Fujii T, et al. Peripartum type B aortic dissection in patients with Marfan syndrome who underwent aortic root replacement: a case series study. BJOG. 2018 Mar;125(4):487–493.
- Song S, Lu L, Li L, et al. Antepartum acute Stanford type a aortic dissection: a case report and literature review. J Cardiothorac Surg. 2022;17(1):73. doi: 10.1186/s13019-022-01817-7
- Meccanici F, Gökalp AL, Thijssen CGE, et al. Male-female differences in acute thoracic aortic dissection: a systematic review and meta-analysis. Interact Cardiovasc Thorac Surg. 2022;34(4):616–627. doi: 10.1093/icvts/ivab270
- Baris L, Ladouceur M, Johnson MR, et al. Pregnancy in Tetralogy of Fallot data from the ESC EORP ROPAC registry. Int J Cardiol Congenit Heart Dis. 2021;2:100059. doi: 10.1016/j.ijcchd.2020.100059
- Wang K, Xin J, Wang X, et al. Pregnancy outcomes among 31 patients with tetralogy of Fallot, a retrospective study. Bmc Pregnancy Childbirth. 2019;19(1):486. doi: 10.1186/s12884-019-2630-y
- Balci A, Drenthen W, Mulder BJ, et al. Pregnancy in women with corrected tetralogy of Fallot: occurrence and predictors of adverse events. Am Heart J. 2011;161(2):307–313. doi: 10.1016/j.ahj.2010.10.027
- Fahed AC, Gelb BD, Seidman JG, et al. Genetics of congenital heart disease: the glass half empty. Circ Res. 2013;112(4):707–720. doi: 10.1161/CIRCRESAHA.112.300853
- Tutarel O, Ramlakhan KP, Baris L, et al. ROPAC (Registry of Pregnancy and Cardiac Disease) Investigators Group. Pregnancy outcomes in women after arterial switch operation for transposition of the great arteries: results from ROPAC (Registry of Pregnancy and Cardiac Disease) of the European society of cardiology EURObservational research programme. J Am Heart Assoc. 2021 5;10(1):e018176. doi: 10.1161/JAHA.120.018176
- ROPAC Investigators Group, Tutarel O, Baris L, et al. Pregnancy outcomes in women with a systemic right ventricle and transposition of the great arteries results from the ESC-EORP Registry of Pregnancy and Cardiac disease (ROPAC). Heart. 2022;108(2):117–123.
- Sliwa K, van Hagen IM, Budts W, et al. ROPAC investigators. Society of cardiology pulmonary hypertension and pregnancy outcomes: data from the Registry of Pregnancy and Cardiac Disease (ROPAC) of the European. Eur J Heart Fail. 2016;18(9):1119–1128. doi: 10.1002/ejhf.594
- Zhang Q, Zhu F, Shi G, et al. Maternal outcomes among pregnant women with congenital heart disease-associated pulmonary hypertension. Circulation. 2023;147(7):549–561. doi: 10.1161/CIRCULATIONAHA.122.057987
- Presbitero P, Somerville J, Stone S, et al. Pregnancy in cyanotic congenital heart disease. Outcome of mother and fetus. Circulation. 1994;89(6):2673–2676. doi: 10.1161/01.CIR.89.6.2673
- Economy KE, Valente AM. Mechanical heart valves in pregnancy: a sticky business. Circulation. 2015;132(2):79–81. doi: 10.1161/CIRCULATIONAHA.115.017349
- Schaefer C, Hannemann D, Meister R, et al. Vitamin K antagonists and pregnancy outcome. A multi-centre prospective study. Thromb Haemost. 2006 Jun;95(6):949–957.
- Hassounaa A, Allam H. Limited dose warfarin throughout pregnancy in patients with mechanical heart valve prosthesis: a meta-analysis. Interact Cardiovasc Thorac Surg. 2014;18(6):797–06. doi: 10.1093/icvts/ivu009
- Xu Z, Fan J, Luo X, et al. Anticoagulation regimens during pregnancy in patients with mechanical heart valves: A systematic review and meta-analysis. Can J Cardiol. 2016;32(10):1248. e1–1248.e9. doi: 10.1016/j.cjca.2015.11.005
- Stephenson ML, Serra AE, Neeper JM, et al. A randomized controlled trial of differing doses of postcesarean enoxaparin thromboprophylaxis in obese women. J Perinatol. 2016;36(2):95–99. doi: 10.1038/jp.2015.130
- Shapiro NL, Kominiarek MA, Nutescu EA, et al. Dosing and monitoring of low-molecular-weight heparin in high-risk pregnancy: single-center experience. Pharmacotherapy. 2011;31(7):678–685. doi: 10.1592/phco.31.7.678
- Ruys TPE, Roos-Hesselink JW, Pijuan-Domènech A, et al. Is a planned caesarean section in women with cardiac disease beneficial? Heart. 2015;101(7):530–536. doi: 10.1136/heartjnl-2014-306497
- San-Frutos L, Engels V, Zapardiel I, et al. Hemodynamic changes during pregnancy and postpartum: a prospective study using thoracic electrical bioimpedance. J Matern Fetal Neonatal Med. 2011;24(11):1333–1340. doi: 10.3109/14767058.2011.556203
- Davis Melinda B, Arendt K, Bello NA, et al. Team-based care of women with cardiovascular disease from pre-conception through pregnancy and postpartum. J Am Coll Cardiol. 2021;77(14):1763–1777. doi: 10.1016/j.jacc.2021.02.033
- Lima F, Nie L, Yang J, et al. Postpartum cardiovascular outcomes among women with heart disease from a nationwide study. Am J Cardiol. 2019;123(12):2006–2014. doi: 10.1016/j.amjcard.2019.03.012