1. Introduction
The 2018 Universal Definition of Myocardial Infarction (MI) lists Type 5 MI as MI associated with coronary artery bypass grafting (CABG) [Citation1]. At the time of writing the document, in the absence of evidence, an arbitrary cardiac troponin (cTn) cutoff of >10 times (x) the 99th percentile upper reference limit (URL from a healthy population) was selected as the cutoff to aid in the diagnosis of Type 5 MI. A few important nuances for consideration include: (i) the >10 × URL cutoff is only applicable in patients with normal baseline cTn concentrations; (ii) in patients with an elevated cTn concentration at baseline, the postoperative cTn must still be >10 × URL and there is also a requirement for a rise in cTn of >20%; (iii) the criteria only apply within 2 days of the procedure; and (iv) there is also a requirement for either the development of new pathological Q waves or angiographic documented new graft or coronary artery occlusion or imaging evidence to make the diagnosis. Since this publication, several large studies have assessed cTn cutoffs after cardiac surgery. It is also important to note that other cardiac biomarkers have possible utility in the cardiac surgery setting (i.e. for acute kidney injury), but for Type 5 MI the focus is principally on cTn [Citation1–3].
Over the last 16 years in the setting of cardiac surgery, several definitions with different cardiac biomarkers (i.e. CK-MB) and cutoffs have been proposed for post-CABG MI, outside of the Universal Definition framework. Until recently, the impact of the different definitions on MI incidence and the prognostic significance was unclear. To address this, authors used data from the CORONARY trial (NCT 00463294) to assess eight different definitions with CK-MB and cTn (with 7 of 8 definitions using biomarker thresholds that were arbitrarily defined) [Citation4]. Importantly, the incidence of MI varied from 0.6% to 19% using CK-MB and from 1.7% to 13% using cTn, highlighting the impact of the definition and biomarker used to diagnose Type 5 MI. Moreover, when assessing the prognostic utility of cTn alone following CABG, a cutoff of ~130 × URL was identified as a possible threshold (hazard ratio (HR) of 7.6 for death at 30 days), which is significantly higher than the 10 × URL cutoff highlighted in 2018 MI definition.
The VISION Cardiac Surgery study (NCT 01842568) further assessed the optimal cutoffs for cTn following cardiac surgery, and importantly did this for high-sensitivity cTn (hs-cTn), the recommended test for myocardial injury (with hs-cTnT and hs-cTnI both achieving regulatory approval after the CORONARY trial completed recruitment) [Citation1]. In nearly 14,000 patients who either underwent isolated CABG, aortic-valve replacement or repair (AVR), or other cardiac surgery the optimal hs-cTn thresholds that were associated with 30-day death were found to be dependent on the type of cardiac surgery and were also significantly higher than the 10 × URL threshold [Citation5]. Specifically, within 1 day after surgery the hs-cTn threshold was ~200 × URL for those with isolated CABG or AVR and ~500 × URL for other cardiac surgery. Interestingly, the ~500 × URL cutoff was also proposed in another large study to not only predict 30-day major adverse cardiac events but was also associated with repeat revascularization within 48 hours after cardiac surgery [Citation6]. To this end, other proposed cutoffs, such as >70 × URL, appear to be too low as a recent study has found minimal prognostic utility for cTn alone when using this cutoff [Citation7]. Collectively, these data provide new guidance on clinically important cTn thresholds; however, other analytically aspects related to hs-cTn also need to be addressed for Type 5 MI.
2. Change criteria for high-sensitivity cardiac troponin
For the diagnosis of acute MI, cTn concentrations have to rise or fall [Citation1]. This change in concentration has been further utilized with hs-cTn assays to quickly rule-out and rule-in MI in the emergency department (ED) setting [Citation8]. Here, important caveats include the time between samples and the hs-cTn assay used in so far that change in concentrations is assay and time dependent [Citation8]. However, the change criteria have not been thoroughly assessed in the cardiac surgery setting due to minimal hs-cTn assays being utilized and the lack of a standardized blood collection protocol. Moreover, despite this assay and time specific hs-cTn change concept being applied to specific pathways used in the ED, evidence is emerging from a multicenter study assessing six different hs-cTn assays over 1 year and over 2100 results that a change >3 ng/L for concentrations below 10 ng/L or >30% above 10 ng/L and around the 99th percentile are appropriate for all hs-cTn assays [Citation9]. However, this information may not be informative in the cardiac surgery setting where hs-cTn concentrations can be >1000 ng/L.
3. Analytical variation at high concentrations
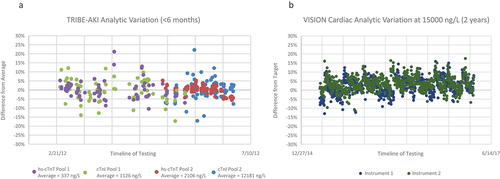
A previous assessment over 7 years assessing the impact of different hs-cTn reagent lots (52 different lots in total) on variation of hs-cTnI concentrations ≥1000 ng/L revealed an average bias of ~ 4% with 95% of differences between reagent results being <15% [Citation10]. To assess daily variation at these elevated levels, the quality control (QC) results for the TRIBE-AKI cardiac surgery cohort (NCT 00774137) and the VISION Cardiac Surgery study were plotted against the respective average or target concentration (i.e. percent deviation over time for both studies are provided in ) [Citation2,Citation3,Citation5,Citation10]. Testing for the TRIBE-AKI study was performed in a dedicated research laboratory for the Beckman cTnI assay (a non-hs-cTn assay) and the Roche hs-cTnT assay over a period of ~5 months. Two different plasma pools were tested with the percent difference range observed for Beckman cTnI (n = 155 results) being 39% (or ±19.5% from the mean) and for Roche hs-cTnT (n = 144 results) being 32% (or ±16% from the mean). In the VISION Cardiac Surgery study, the analyzed QC tested daily with the Abbott hs-cTnI assay was obtained from two different instruments in the hospital core laboratory for a period of 2 years to assess instrument variation. Here, the percent difference range on Instrument 1 (n = 695 results) was 29% (or ±14.5% from the manufacturer’s target) and for Instrument 2 (n = 695 results) was 28% (or ±14% from the manufacturer’s target). These data suggest that the >20% criterion used in the 2018 MI definition appears appropriate for non-hs-cTn assays, with a change >15% being more applicable to the hs-cTn assays at these higher concentrations as observed following cardiac surgery.
Figure 1. The percent difference between quality control (QC) results from the average for the plasma pools used in the TRIBE-AKI study for the Beckman cTnI (non-hs-cTnI assay) and Roche hs-cTnT assays (a) and from the target manufacturer QC concentration for the Abbott hs-cTnI assay on two different instruments used in the VISION cardiac surgery study (b).
4. Expert opinion
Notwithstanding the heterogeneity observed across studies related to the timing of hs-cTn measurement after surgery and differences between hs-cTn assays; the present evidence would suggest that the thresholds need to be revised upwards after cardiac surgery (i.e. possibly several hundred fold higher than the URL) [Citation4–7]. And at these higher concentrations, a relative change in hs-cTn concentration >15% would be suggestive of an acute change. Outcome data have supported the higher hs-cTn thresholds following cardiac surgery with initial data also suggesting that the >15% change being helpful for ruling in MI in patients with high hs-cTn concentrations at ED presentation [Citation4,Citation5,Citation11]. Future work on hs-cTn in the cardiac surgery setting should be performed on validated assays in this patient population, congruent with what is recommended in the noncardiac surgery setting [Citation12,Citation13].
Large prospective studies and trials are needed here to validate the above criteria. However, important questions remain. For instance, if multiples of the URL are used as thresholds for the hs-cTn assays, it is important that the correct URL is used as population, statistical and analytical issues can affect the derivation of the healthy population URL [Citation14]. Moreover, should the overall population URL, or the sex-specific URL or even an age specific URL (i.e. pediatric versus adult) be used in this setting [Citation14,Citation15]? And how would kidney function after surgery affect the interpretation? Several URLs for each of the above scenarios have been published, so collaboration between the cardiac surgery team, the intensive care team, the clinical chemistry core laboratory team and cardiology will be essential.
Declaration of interest
PA Kavsak has received grants/reagents/consultant/advisor/honoraria from Abbott Laboratories, Abbott Point of Care, Beckman Coulter, Ortho Clinical Diagnostics, Quidel, Randox Laboratories, Roche Diagnostics, Siemens Healthcare Diagnostics, and Thermo Fisher Scientific.
McMaster University has the following patent with PA Kavsak listed as an inventor: ‘METHOD OF DETERMINING RISK OF AN ADVERSE CARDIAC EVENT.’ McMaster University has also filed the following patent: ‘QUALITY CONTROL MATERIALS FOR CARDIAC TROPONIN TESTING’ with PA Kavsak listed as an inventor and both McMaster University and Yale University have licensed patent on a Method and panel for determining acute kidney injury where PA Kavsak is listed as an Inventor.
R Whitlock reports honorarium and grants from Boeringer-Ingelheim, consultancy and grant from Atricure, consultancy from Phasebio, grants from Bayer, Roche, Boston Scientific and Cytosorbent Inc., and research grant from BMS-Pfizer.
E Belley-Cote reports grants from Bayer and Roche, research grant from BMS-Pfizer and consulting honoraria from Trimedic Therapeutics Inc.
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial relationships or otherwise to disclose.
Additional information
Funding
References
- Thygesen K, Alpert JS, Jaffe AS, et al. Fourth Universal definition of myocardial infarction (2018). J Am Coll Cardiol. 2018;72(18):2231–2264. doi: 10.1016/j.jacc.2018.08.1038
- Bucholz EM, Whitlock RP, Zappitelli M, et al. TRIBE-AKI Consortium. Cardiac biomarkers and acute kidney injury after cardiac surgery. Pediatrics. 2015 Apr;135(4):e945–56. doi: 10.1542/peds.2014-2949
- Belley-Côté EP, Parikh CR, Shortt CR, et al. TRIBE-AKI consortium. Association of cardiac biomarkers with acute kidney injury after cardiac surgery: a multicenter cohort study. J Thorac Cardiovasc Surg. 2016 Jul;152(1):245–251.e4.
- Belley-Cote EP, Lamy A, Devereaux PJ, et al. Definitions of post-coronary artery bypass grafting myocardial infarction: variations in incidence and prognostic significance. Eur J Cardiothorac Surg. 2020;57(1):168–175. doi: 10.1093/ejcts/ezz161
- Devereaux PJ, Lamy A, Chan MTV, et al. VISION cardiac surgery investigators. High-sensitivity troponin I after cardiac surgery and 30-day mortality. N Engl J Med. 2022;386(9):827–836. doi: 10.1056/NEJMoa2000803
- Omran H, Deutsch MA, Groezinger E, et al. High-sensitivity cardiac troponin I after coronary artery bypass grafting for post-operative decision-making. Eur Heart J. 2022;43(25):2388–2403. doi: 10.1093/eurheartj/ehab918
- Pölzl L, Thielmann M, Cymorek S, et al. Impact of myocardial injury after coronary artery bypass grafting on long-term prognosis. Eur Heart J. 2022;43(25):2407–2417. doi: 10.1093/eurheartj/ehac054
- Neumann JT, Twerenbold R, Ojeda F, et al. COMPASS-MI Study Group, Application of high-sensitivity troponin in suspected myocardial infarction. N Engl J Med. 2019;380(26):2529–2540.
- Kavsak PA, Clark L, Arnoldo S, et al. Analytic Result Variation for High-Sensitivity Cardiac Troponin: Interpretation and Consequences. Can J Cardiol. 2023 Jul;39(7):947–951. doi: 10.1016/j.cjca.2023.04.013
- Kavsak PA. Lot-to-lot bias for high-sensitivity cardiac troponin I concentrations ≥1000 ng/L. Clin Chem Lab Med. 2023;61(7):e105–e107. doi: 10.1515/cclm-2023-0017
- Kavsak PA, Sharif S, Globe I, et al. The clinical validation of a common analytical change criteria for cardiac troponin for ruling in an acute cardiovascular outcome in patients presenting with ischemic chest pain symptoms. J Cardiovasc Dev Dis. 2023;10(8):335. doi: 10.3390/jcdd10080335
- Kavsak PA, Clark L, Martin J, et al. Acute phase response and non-reproducible elevated concentrations with a high-sensitivity cardiac troponin I assay. J Clin Med. 2021;10(5):1014. doi: 10.3390/jcm10051014
- Lurati Buse G, Bollen Pinto B, Abelha F, et al. ESAIC focused guideline for the use of cardiac biomarkers in perioperative risk evaluation. Eur J Anaesthesiol. 2023 Jun 2;40(12): 888–927. Epub ahead of print. PMID: 37265332. doi: 10.1097/EJA.0000000000001865
- Aakre KM, Saenger AK, Body R, et al. Analytical considerations in deriving 99th percentile upper reference limits for high-sensitivity cardiac troponin assays: educational recommendations from the IFCC committee on clinical application of cardiac bio-markers. Clin Chem. 2022 Jul 27;68(8):1022–1030. doi: 10.1093/clinchem/hvac092
- McEvoy JW, Wang D, Brady T, et al. Myocardial injury thresholds for 4 high-sensitivity troponin assays in a population-based sample of US children and adolescents. Circulation. 2023 Jul 4;148(1):7–16. doi: 10.1161/CIRCULATIONAHA.122.063281
