ABSTRACT
Introduction
Ischemic stroke is a leading cause of morbidity and mortality worldwide. Emerging evidence suggests that left atrial (LA) dysfunction could play a role in the pathophysiology of ischemic stroke, as a possible contributor and as a predictive biomarker.
Areas covered
This narrative review details the intricate relationship between LA function, atrial fibrillation (AF), and ischemic stroke. We discuss imaging techniques used to assess LA function, the mechanisms by which impaired LA function may contribute to stroke, and its potential as a prognostic marker of stroke.
Expert opinion
There is a lack of evidence-based treatments of LA dysfunction in both primary and secondary stroke prevention. This is partly due to the lack of a practical clinical definition and unanswered questions concerning the clinical implications of LA dysfunction in patients without AF. Until such questions are resolved, addressing well-known cardiovascular risk factors, like hypertension and obesity, should be prioritized for preventing AF and ischemic stroke. These risk factors are closely tied to atrial remodeling, emphasizing the importance of targeting primary modifiable factors for preventing future morbidity and mortality.
1. Introduction
Stroke is a leading cause of death, morbidity, and disability worldwide [Citation1,Citation2]. Identifying the root cause of a stroke is crucial for implementing primary and secondary prevention strategies tailored to the specific underlying condition [Citation3]. However, a substantial proportion of strokes, ranging from 20% to 30%, elude a precise mechanism and are deemed cryptogenic according to the Trial of ORG 10,172 in Acute Stroke Treatment (TOAST) classification or more recently as embolic stroke of unknown source (ESUS) [Citation4,Citation5]. In these instances, paroxysmal atrial fibrillation (AF) often emerges as a suspected culprit.
Two large-scale randomized clinical trials have investigated whether oral anticoagulation (OAC) is beneficial for stroke prevention in patients with ESUS but failed to demonstrate such a benefit, which was attributed to unexpected heterogeneity of the ESUS population and the fact that occult AF was less common than anticipated [Citation6,Citation7]. This underlines that identification of AF is crucial since not all patients with ESUS benefit from anticoagulation. Surprisingly, less than one-third of individuals with cryptogenic strokes exhibit any form of manifest AF within 3 years of continuous heart rhythm monitoring [Citation8]. In addition, among patients with paroxysmal AF and pacemakers, only 29% experience runs of AF in the month preceding a stroke event [Citation9]. Thus, the relationship between AF and ischemic stroke is far from straightforward and evidence suggests that other factors, including left atrial (LA) dysfunction, may play an important role beyond the arrhythmia in the development of ischemic stroke [Citation10–15].
Pathological changes in the LA in the context of AF involves fibrosis, enlargement, and reduced atrial emptying fractions and strain, resulting in structural and flow-related abnormalities [Citation16]. These changes, when coupled with vascular risk factors, is believed to contribute to the development of thrombus primarily in the LA appendage [Citation16]. In recent years, several studies have demonstrated that LA abnormalities associated with the risk of ischemic stroke are not limited to persons with AF [Citation17–31]. The concept of atrial cardiomyopathy, which has been defined in an expert consensus document from 2016 in a collaboration of transatlantic and transpacific cardiac scientific societies has been proposed as a framework to understand how atrial pathological changes may ‘impact cardiac performance, arrhythmia occurrence, and stroke risk,’ regardless of whether patients have AF or not. [Citation32] The definition of atrial cardiomyopathy encompasses electrical, structural, contractile, and architectural pathological changes in the atria. These changes can lead to clinical conditions where AF is considered a primary manifestation and a mediating factor. Gaining insight into the intricate interplay between atrial function, AF, and ischemic stroke holds potential clinical significance. Even though observational studies have linked LA function to AF and stroke, translating these findings to clinical benefit in clinical trials has proven challenging. A trial sub study has suggested a potential benefit from anticoagulation over antiplatelet therapy for stroke prevention in ESUS patients with signs of atrial cardiomyopathy, [Citation23] but recently dedicated trials have been published that failed to substantiate this hypothesis [Citation33,Citation34]. This review provides an in-depth exploration of the noninvasive assessments of LA function within the context of atrial cardiomyopathy and its relationship with AF and ischemic stroke ().
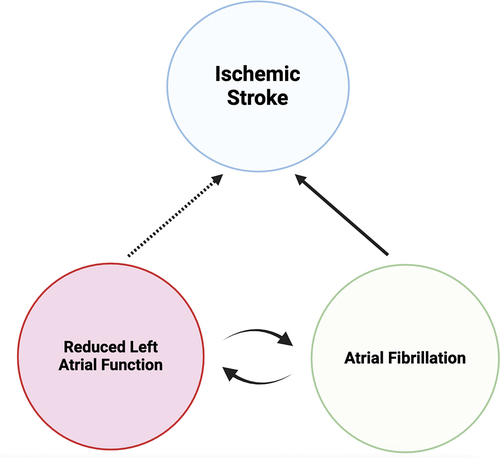
Figure 1. Proposed relationship between reduced left atrial function, atrial fibrillation (AF), and ischemic stroke.AF is a well-established causal factor for ischemic stroke, with anti-coagulant treatment reducing stroke risk. Reduced left atrial function, both a product and risk marker for AF, is associated with increased stroke risk, although the underlying mechanism remains unclear. It is uncertain whether treating reduced left atrial function, in the absence of AF, reduces ischemic stroke risk.
2. LA anatomy and function
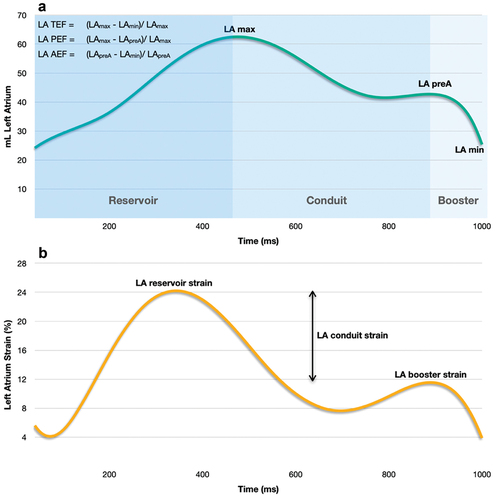
The LA is the most posteriorly situated of the cardiac chambers with a cuboidal shape [Citation35]. It has a venous component with the entrance of the four pulmonary veins, a trabeculated appendage, an interatrial septum, and a vestibule marking the outlet near the mitral valve [Citation36,Citation37]. The LA has thin muscular walls with varied transmural thickness depending on location (ranges 2.5 to 6.5 mm), [Citation35] and is primarily composed of atrial cardiomyocytes, fibroblasts, and extracellular matrix. [Citation32] Functionally, the LA plays an essential role in global cardiac performance through three linked mechanisms: 1) A reservoir function for pulmonary venous return during left ventricular (LV) systole and isovolumetric relaxation and is determined by LA compliance and by the descent of the LV base during ventricular contraction [Citation38]. 2) A conduit function, allowing passive blood flow into the left ventricle during early LV diastole and diastasis, and is inversely related to reservoir function and dependent on LV relaxation and compliance, resulting in a pressure gradient that drives blood into the LV and 3) a booster-pump function during late LV diastole that augments LV filling which is mainly determined by intrinsic atrial contractility, venous return (atrial preload), and LV end-diastolic pressure (atrial afterload), respecting the Frank-Starling mechanism [Citation39]. The three functional phases of the LA align with total emptying fraction or reservoir strain, passive emptying fraction or conduit strain, and active emptying fraction or booster strain ().
Figure 2. Volumetric and functional phases of the LA. (a) Volume curve, (b) Strain curve. LA, left atrial; LAmax, left atrial maximal volume; LApreA, left atrial volume before atrial contraction; LAmin, left atrial minimal volume; LA TEF, left atrial total emptying fraction; LA PEF, left atrial passive emptying fraction; L A AEF, left atrial active emptying fraction.
3. LA remodeling, atrial cardiomyopathy, and atrial failure
LA remodeling has been defined as the time-dependent response of atrial cardiomyocytes to electrical, mechanical, and metabolic cardiac ‘stressors’ [Citation40]. Main causes are primary cardiac conditions such as atrial arrhythmias, ventricular dysfunction, or valvular heart disease resulting in pressure and volume overload. Secondary stressors include hypertension, diabetes, genetic factors, or lifestyle factors [Citation40]. A continuing stress will eventually affect the structure, electrical properties, and contractile function of the LA. Structural and architectural remodeling is the process of increased fibrosis formation and deposition in the extracellular matrix, compromising the architectural integrity and eventually resulting in LA dilatation [Citation41]. Fibrosis is broadly categorized into two categories. ‘Replacement’ or ‘reparative fibrosis’ is the loss of cardiomyocytes replaced by fibrous tissue [Citation42]. The scar from myocardial infarction is a typical example. Replacement fibrosis is mainly thought irreversible [Citation43]. In contrast, ‘reactive’ fibrosis is associated with fibroblast proliferation and collagen deposition in the interstitial and perivascular space surrounding the cardiac muscle strands. Reactive fibrosis may result from pressure or volume overload, or systemic inflammation [Citation41]. LA fibrosis is believed to be a key feature in the development and manifestations of LA dysfunction [Citation32]. Electrical remodeling promotes triggers of ectopic beats and a substrate that favors reentry and automaticity [Citation44,Citation45]. Intrinsic functional remodeling, with or without LA volume changes, is inversely correlated to architectural and electrical remodeling [Citation46–48]. Components of LA remodeling will overlap, and manifestations and degrees of remodeling may reflect the underlying etiology [Citation49]. LA remodeling is assumed to be reversible in the early stages but eventually becomes persistent [Citation50]. Atrial cardiomyopathy, as defined previously, can be considered the result of the LA remodeling process.
A distinction between LA remodeling and atrial cardiomyopathy is that atrial cardiomyopathy, by definition, implies a condition that manifests clinically [Citation32]. In contrast, LA remodeling seems a more neutral description and, is often used in conjunction with diastolic dysfunction and heart failure (HF) [Citation51–53]. Recently, to distinguish atrial dysfunction from being only a consequence of primary cardiac diseases, the concept of ‘atrial failure’ was introduced [Citation54]. Atrial failure is defined similarly to atrial cardiomyopathy, with the primary difference being that atrial failure is a manifestation that appears only in the absence of significant valvular or ventricular abnormalities. Nonetheless, deterioration of LA function is rarely an isolated phenomenon. While the atrial cardiomyopathy and atrial failure concepts were partly coined to decipher atrial disease from confounding factors, precisely defining such an independent primary disease entity may prove challenging.
4. LA imaging
Several imaging modalities are available for assessing the function of the LA, with different strengths and weaknesses in terms of acquisition and post-processing. These are summarized in .
Table 1. Comparison of imaging modalities and strength and weakness of specific echocardiographic LA measures.
4.1. Echocardiography
Echocardiography is one of the most used cardiac imaging modalities, owing to its availability, low cost, absence of absolute contraindications, and relative ease of use [Citation55,Citation56]. Echocardiography has limitations with its angle dependency and patient-derived factors, such as obesity or lung disease, that can result in poor acoustic windows. With two-dimensional echocardiography (2DE), the first LA parameter of choice was one-dimensional size that was assessed in a parasternal long axis view by measuring the anteroposterior diameter. Several studies have shown that this one-dimensional measure of LA size is imprecise, particularly in those with an enlarged LA, as it assumes that the LA is a symmetrically shaped structure, and that dimensions increase similarly in all directions if the LA enlarges, which is seldomly the case [Citation57,Citation58]. Thus, current clinical practice and guideline recommendations are to estimate LA volume, which is considered a superior measure of LA size and also facilitates functional measures of LA emptying fractions [Citation59]. Quantifying LA volume has also shown to be superior to LA diameter or LA area in predicting cardiovascular outcomes [Citation60]. Guidelines recommend volume estimations using either the biplane area-length method or Simpson’s biplane method of discs [Citation59]. However, volume estimation with 2DE has limitations due to the geometrical assumptions of the LA being either an ellipsoid shape using the area-length method or an oval shape using the disk summation method. Furthermore, dedicated apical LA views without foreshortening of the LA cavity should be used, which are often not a part of a standard examination affecting reproducibility [Citation61]. These limitations are partly overcome by 3D echocardiography (3DE) which is better equipped to capture the geometric complexity of the LA, provides details on both LA size and function in a time-efficient manner, and has demonstrated superior reproducibility [Citation62]. 3DE has also shown a good correlation with cardiac magnetic resonance (CMR) derived LA measures [Citation63,Citation64]. However, acquiring 3D images with echocardiography relies on high-quality imaging for accurate measurements and frame-stitching over multiple cardiac cycles, as well as dedicated software. The choice of using either 2DE or 3DE affects the reported volumes and normal values, with 2DE systematically underestimating LA volumes compared to 3DE, which is why they are not interchangeable in terms of reference values [Citation63,Citation65].
LA strain measurements with echocardiography are becoming more accessible, with several vendors providing dedicated software solutions. LA strain is, as its ventricular counterpart, used to assess the magnitude and rate of LA myocardial deformation [Citation66]. Strain measurements assess the relative changes and velocities in tissue length or deformation. They compare how much the tissue deforms during contraction and relaxation phases of the cardiac cycle without considering the absolute dimensions of the heart. This relative assessment allows to capture the intrinsic contractile properties of the myocardium, and irrespective of the variations in filling and volume status [Citation61].
While normal values for transthoracic echocardiography (TTE)-derived left atrial (LA) function parameters have been proposed, [Citation65,Citation67–69] they have yet to be widely adopted in clinical practice or guidelines. This may be due to the lack of prospective data with relevant interventions in appropriate populations. Notably, these proposed normal values demonstrate minimal sex-based variation in both LA functional parameters and in LA volumes when adjusted for body surface area [Citation65]. However, age has been linked to increased LA volumes and decreased LA function, underscoring the importance of considering age in interpretation [Citation70,Citation71].
4.2. Cardiac magnetic resonance
CMR is considered the ‘gold standard’ for assessing cardiac chamber volumes and allows for high contrast angle-independent assessment with excellent reproducibility, addressing some of the shortcomings of echocardiography [Citation72,Citation73]. Furthermore, feature tracking techniques allows for measurements of LA strain [Citation74]. However, CMR has limited availability and higher costs compared to echocardiography limiting the use in a general cardiology population. Implanted ferromagnetic metals represent an absolute contraindication to CMR, while implanted cardiac devices and claustrophobia are considered relative contraindications. Current guidelines for LA volume assessment with CMR do not provide specific recommendations for which volumetric method should be used [Citation75]. The most commonly used methods are biplane methods, similar to those in 2DE, which include either the biplane area-length or biplane disc summation method [Citation72,Citation75]. However, as with echocardiography, it has been demonstrated that these methods may underestimate LA volumes and overestimate LA emptying fractions compared to multi-slice cine images in the transversal or short-axis plane using the Simpson’s disc summation approach [Citation76,Citation77]. The studies that compared LA volumes as assessed by CMR and echocardiography and demonstrated a higher correlation between CMR and 3DE compared to 2DE have utilized the Simpson’s method of discs in either a transversal or short axis orientation for estimating the LA volume by CMR [Citation63,Citation64].
5. LA function and AF
Several prospective studies have linked LA volumes and function to incident clinical and subclinical AF. Below is a brief outline of these studies with further study details presented in [Citation78–86]. In a cohort from the general population, Olsen et al. demonstrated that LA minimal volume and total LAEF, but not LA maximal volume, was associated with AF [Citation78]. Similar results have been reported from the Multi-Ethnic Study of Atherosclerosis (MESA) study with the addition that reduced LA reservoir strain was likewise correlated with incident AF, [Citation84] findings which have also been replicated in the Copenhagen City Heart Study [Citation82]. Bertelsen et al. showed in a CMR cohort from the Danish LOOP study that in elderly patients with risk factors but no history of AF, higher LA volumes and lower function (both LA strain and emptying fractions) predicted subclinical AF as detected by continuous monitoring [Citation81]. These findings were corroborated in a larger cohort using TTE examining the association between LA strain and subclinical AF, within the LOOP study [Citation86]. Another prospective study in a cryptogenic stroke cohort showed that total LAEF < 50% identified 92% of patients developing subclinical AF using continuous monitoring [Citation87]. In the same cohort, LA reservoir strain has also been shown to be associated with subclinical AF, [Citation88] and has also been shown to be associated with AHRE in patients with cardiac implantable electronic devices [Citation89].
Table 2. Measures of left atrial function and subsequent risk of atrial fibrillation or ischemic stroke.
6. LA function and stroke
In 1995, Benjamin et al. [Citation28] demonstrated an association between LA anteroposterior diameter measured from M-mode paper strip charts and ischemic stroke in the widely influential Framingham Heart Study. Since then, several studies have examined the association between LA volume measures and the risk of ischemic stroke in diverse populations and demonstrated associations between LA size, LA volumetric measures, and ischemic stroke [Citation26–31]. Nevertheless, recent studies appear to be unable to replicate these results in modern cohorts, suggesting a potential benefit to incorporate LA functional measures than using volumetric assessments alone. Below is a summary of studies using LA functional measures, with more study information provided in .
The MESA study found that in 4,261 participants, as assessed with CMR, only reduced LAEF, and not LA volumes, was associated with incident ischemic cerebrovascular events [Citation17]. A similar study in a community-based cohort derived from the general population, free of known AF or prior stroke demonstrated a longitudinal association between echocardiography assessed reduced LAEF and ischemic stroke. This association remained significant, irrespective of the development of AF and the competing risk of death. A recent study from the Atherosclerosis Risk in Communities (ARIC) study examined the association between ischemic stroke and echocardiographic LA strain parameters and LA volumes [Citation22]. They found that in an elderly cohort (mean age 75 years), LA reservoir strain was significantly associated with ischemic stroke which was not the case for LA passive strain, active strain or LA maximal volume. When added to the CHA₂DS₂-VASc score (Congestive Heart Failure, Hypertension, Age ≥ 75 Years, Diabetes Mellitus, Prior Stroke or Transient Ischemic Attack or Thromboembolism, Vascular Disease, Age 65 to 74 Years, Sex), LA reservoir strain improved stroke prediction and yielded a small net benefit in decision curve analysis. Interestingly, in a sensitivity analysis, the association was primarily driven by non-lacunar and non-carotid embolic strokes. Similar results were seen in the Cardiovascular Abnormalities and Brain Lesions (CABL) study cohort, a community-based study with participants aged 55 years or older without a history of stroke or AF [Citation20]. They demonstrated that reduced LA reservoir strain was independently linked to an increased risk of ischemic stroke after accounting for relevant risk factors such as LA volumes, LV global longitudinal strain, and incident AF.
Recent studies suggest that assessing LA function might serve as an earlier and more sensitive indicator of LA pathology compared to LA enlargement. In a study involving AF patients, it was observed that LA function declined before any enlargement of the LA became apparent [Citation90]. Moreover, LA enlargement does not always coincide with a decrease in LA function, as evident in a study on the athlete’s heart suggesting that functional decline is in some cases a separate entity [Citation91].
However, the shift in predictive capabilities of LA measures could also be interpreted to suggest that individuals enrolled in earlier decades had a higher risk profile when LA enlargement was detected compared to contemporary study cohorts where imaging is more easily accessible in earlier stages of disease.
6.1. Clinical trials of stroke prevention
Limited research has explored the effectiveness of OAC for reducing stroke risk among individuals exhibiting markers of atrial cardiomyopathy including LA dysfunction, with a notable absence of studies in a primary prevention context. Relevant trials in terms of secondary stroke prevention are detailed in and summarized below. A secondary analysis of the NAVIGATE ESUS trial suggested that ESUS patients with an LA diameter > 4.6 cm had a moderate benefit of OAC compared to a standard practice using an antiplatelet agent for preventing recurrent stroke [Citation23]. However, subclinical AF in those with enlarged LA cannot be ruled out as the mediating factor of this exploratory result. In contrast to these exploratory findings, the large multicenter randomized control trial ARCADIA (AtRial Cardiopathy and Antithrombotic Drugs In prevention After cryptogenic stroke) trial investigated whether patients with ESUS and indices of atrial cardio(myo)pathy (P-wave terminal force >5000 mV × ms in ECG lead V1, serum NT-proBNP > 250 pg/mL or LA diameter index ≥ 3 cm/m2) would benefit from OAC compared to standard antiplatelet treatment failed to demonstrate efficacy in secondary stroke prevention [Citation34,Citation92]. These findings may reflect the heterogeneous atrial cardiomyopathy inclusion criteria, with only a minor subset fulfilling the LA diameter criteria (1.6%), whereas 54.4% were included from ECG criteria, which may be a suboptimal marker of LA cardiomyopathy [Citation93]. The ATTICUS trial, investigated whether an ‘enriched’ ESUS population, also with attributes of atrial cardiomyopathy (LA size > 45 mm, spontaneous echo contrast in LA appendage, LA appendage flow velocity ≤ 0.2 m/s, atrial high-rate episodes, CHA2DS2-VASc ≥ 4, patent foramen ovale) failed to demonstrate a benefit from anticoagulation in terms of new ischemic lesions on 12-month follow-up magnetic resonance imaging [Citation33].
Table 3. Studies examining atrial cardiomyopathy, subclinical atrial fibrillation, and anticoagulation for stroke prevention.
If trials on secondary prevention of stroke in individuals exhibiting markers of atrial cardiomyopathy are truly neutral, the prospect of orchestrating trials for primary prevention with OAC in such a population seems futile. Indirect evidence of primary prevention trials of atrial cardiomyopathy are studies that have selectively screened for subclinical AF or AHRE in individuals possessing risk factors correlated with markers of atrial cardiomyopathy and instigated OAC If found () [Citation94–96]. These trials have not consistently demonstrated concordant outcomes. The singular exception is the ARTESIA trial, which has hinted at a potential benefit arising from AF screening [Citation96].
An important limitation of the mentioned studies are the lack of diversity in terms of race or ethnic group with all studies primarily including White Europeans which limits the generalizability to other ethnic groups. Stroke risk factors, treatment responses to anticoagulation, and outcomes can vary across different racial and ethnic groups due to genetic factors, cultural differences, and disparities in healthcare access and quality [Citation97,Citation98]. In summary, the optimal secondary stroke prevention measures for patients with markers of atrial cardiomyopathy are still unclear and ambiguity still exists as to whether OAC could be beneficial for subclinical AF in patients with risk factors.
6.2. LA dysfunction and biological plausibility of thromboembolic stroke
In patients with AF, the risk of ischemic stroke is modulated by the presence of risk factors that determine treatment with OAC. Currently, a CHA2DS2-VASc score of 0 and a diagnosis of AF translates to a very low risk of stroke and OAC is not recommended in current guidelines [Citation1]. This indirectly implies that AF alone is insufficient for thrombogenesis. Individual components of the CHA2DS2-VASc scheme are associated with atrial cardiomyopathy, reflecting that a higher burden of atrial pathology could mediate the increased risk associated with a higher score [Citation99]. The risk of stroke increases with the burden of AF, suggesting a dose-response relationship, [Citation100] and recent studies indicate that early AF rhythm control may effectively lower the risk of stroke [Citation101]. These findings could be consistent with the theory of an atrial cardiomyopathy substrate that worsens under the influence of AF. Intriguingly, explaining some discrepancies in the search for the temporality of AF and stroke, it could be argued that in some cases, thromboembolism may not be temporally associated with episodes of AF but still be a consequence of AF-induced atrial cardiomyopathy. Thus, as proposed by Shen et al., atrial cardiomyopathy may lead to intermittent AF that lacks a timely association with stroke, but the underlying atrial cardiomyopathy is always present and thrombogenic [Citation50].
However, the available evidence substantiating a biological plausibility of thrombus formation in the LA or LA appendage, leading to stroke in individuals with LA dysfunction, without concurrent AF, is presently limited. As previously mentioned, several population-based observational studies have shown a relationship between LA dysfunction and the risk of ischemic stroke [Citation17–31]. However, none of the studies were able to exclude episodes of subclinical AF, as no continuous rhythm monitoring was used. Furthermore, no studies have incorporated the adjudicated mechanism of stroke to ascertain whether measures of atrial cardiomyopathy are genuinely linked to cryptogenic stroke or if they function as confounding factors associated with shared cardiovascular risk factors linked to other recognized stroke pathologies. LA structural and functional remodeling can result from a variety of reasons, and the underlying pathology undoubtedly modifies clinical outcomes. Residual confounding from shared upstream risk factors such as hypertension, higher age, diabetes, and vascular disease are also associated with non-cardioembolic pathways of ischemic stroke. Since AF is highly prevalent, not all patients with AF necessarily have a thromboembolic stroke from the LA. It is likely that some strokes in patients with AF are mediated by shared vascular risk factors such as large-artery atherosclerosis with artery-to-artery embolism or hypertension-induced cerebral small-vessel occlusion [Citation13].
Some of the strongest evidence indicating a causal link between atrial cardiomyopathy and ischemic stroke emerges from observations of elevated stroke risk in individuals with cardiac amyloidosis [Citation102,Citation103]. Cardiac amyloidosis, primarily comprising amyloid transthyretin amyloidosis (ATTR) and light chain amyloidosis (AL), frequently involves LA infiltration, resulting in increased stiffness and reduced LA function [Citation104,Citation105]. In a study encompassing 324 patients with both AL and ATTR amyloidosis, Martinez-Naharro et al. detected intracardiac thrombi in 6.2% of cases using CMR with LGE [Citation106]. Notably, in this study thrombi were also identified in some patients presenting in sinus rhythm without known AF.
7. Conclusion
Evidence suggests that LA functional measures are relevant markers of atrial cardiomyopathy, associated with clinically relevant outcomes and provide prognostic information both in patients with and without AF. LA dysfunction in the context of ischemic stroke could serve as a prognostic biomarker irrespective of AF status, a risk modifier in AF patients, and, perhaps most speculatively, a potential causative factor for ischemic stroke. It is essential to note that these features are not mutually exclusive. However, few trials have yet to incorporate LA dysfunction as a means to guide management strategies, and the available trials have not supported a benefit from considering atrial cardiomyopathy in a clinical context. A refined and more operative definition is needed to guide future clinical trials, which are necessary to establish the clinical practical importance of LA dysfunction and atrial cardiomyopathy.
8. Expert opinion
Translational perspectives of actively treating atrial cardiomyopathy-associated changes, including LA dysfunction, and the impact on clinical outcomes remain limited both in patients with and without AF. The criteria for meaningful reverse remodeling of the LA, with a significant clinical impact, and the optimal approach for achieving this remain unknown. Thus, no evidence-based specific treatment of LA dysfunction exists in primary or secondary prevention of stroke. This is partly the consequence of LA dysfunction lacking a practical clinical definition creating a void in its clinical utility outside research purposes. In patients with ESUS and markers of atrial cardiomyopathy, not including LA dysfunction, initial findings suggest that OAC does not outperform a standard antiplatelet strategy. This implies that the presence of AF may be necessary to derive benefit from OAC for secondary stroke prevention. These findings also attenuate optimism concerning the primary prevention of ischemic stroke with OAC in individuals with atrial dysfunction in the absence of AF.
One crucial area of interest, which will become increasingly important as the population ages, is how shared cardiovascular risk factors can lead to multiple competing causes of stroke. Patients might exhibit multiple mechanisms for a stroke, but the chosen mechanism for classification often overlooks this complexity. The presence of numerous stroke classifications further exacerbates this limitation [Citation107]. Thus, stroke classifications may not adequately identify patients with potential LA-related stroke and their subsequent risk of AF. Therefore, a key focus area should be to identify individuals who should be prioritized for long-term monitoring of AF, utilizing a multimodality approach identifying those who are most likely to benefit from OAC in prevention of first-time and recurrent strokes [Citation108]. Improvements are also necessary in our understanding of the pathophysiology behind thrombus formation in the LA and to determine whether thrombus formation is an event that can happen in the absence of AF [Citation109]. There is also a need to identify the primary contributors to thrombogenicity in patients with confirmed AF. In silico models incorporating LA fluid dynamics, electrical abnormalities, structure, and function show promise to answer some of these questions and will become more feasible as computational power increases [Citation110]. Yet, among all the competing reasons for stroke, there is a chance that only a small proportion of strokes labeled as ‘of undetermined cause’ are truly linked to atrial cardiomyopathy. Atrial cardiomyopathy and LA dysfunction might merely indicate a higher stroke risk from other factors, rather than directly causing it. This underscores the importance of prioritizing the search of alternative mechanisms for stroke [Citation111].
Machine learning techniques hold promise in tackling the complexities of stroke and AF prediction, as well as stroke classification. Advancement in machine learning may pave the path toward a more personalized approach to treatment and prevention, moving away from the limitations of the current ‘one size fits all’ prediction models [Citation112]. Until current challenges are addressed, it is well documented that treating cardiovascular risk factors, such as hypertension or obesity, can prevent AF or ischemic stroke [Citation113,Citation114]. Both conditions are related to atrial remodeling, atrial dysfunction, and vascular pathology, indicating that targeted risk factor management may prevent stroke through multiple pathways.
In the absence of conclusive evidence establishing effectiveness of interventions aimed at addressing LA dysfunction, emphasis should persist on addressing primary modifiable risk factors, such as hypertension and obesity, to mitigate future morbidity and mortality.
Article highlights
Left atrial (LA) dysfunction is prospectively associated with atrial fibrillation (AF) and ischemic stroke and provide relevant prognostic information.
The biological plausibility of thrombus formation in LA dysfunction without AF is uncertain, with potential confounding from shared cardiovascular risk factors resulting in non-cardiac related stroke.
Limited translational perspectives exist for actively treating LA dysfunction, with unknown criteria for meaningful reverse remodeling and a lack of evidence-based treatments in stroke prevention.
Treating cardiovascular risk factors, such as hypertension and obesity, is well-documented in preventing atrial fibrillation and ischemic stroke, emphasizing the importance of addressing primary modifiable risk factors in the absence of conclusive evidence for interventions targeting LA dysfunction.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or material discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or mending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Disclosures
BSL: none
TBS: research grants from Sanofi Pasteur, GSK, Novo Nordisk, AstraZeneca, Boston Scientific and GE Healthcare, consulting fees from Novo Nordisk, IQVIA, Parexel, Amgen, CSL Seqirus, GSK and Sanofi Pasteur, and lecture fees from Bayer, Novartis, Sanofi Pasteur, GE Healthcare and GSK.
FJO: none.
Additional information
Funding
References
- Hindricks G, Potpara T, Dagres N, et al. ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for cardio-thoracic surgery (EACTS). Eur Heart J. 2021;42(5):373–498. doi: 10.1093/eurheartj/ehaa612
- Feigin VL, Stark BA, Johnson CO. Global, regional, and national burden of stroke and its risk factors, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet Neurol. 2021;20(10):795–820. doi: 10.1016/S1474-4422(21)00252-0
- Guercini F, Acciarresi M, Agnelli G, et al. Cryptogenic stroke: time to determine aetiology. J Thromb Haemost. 2008;6(4):549–554. doi: 10.1111/j.1538-7836.2008.02903.x
- Hart RG, Diener H-C, Coutts SB, et al. Embolic strokes of undetermined source: the case for a new clinical construct. Lancet Neurol. 2014;13(4):429–438. doi: 10.1016/S1474-4422(13)70310-7
- Adams HP, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of org 10172 in acute stroke treatment. Stroke. 1993;24(1):35–41. doi: 10.1161/01.STR.24.1.35
- Hart RG, Sharma M, Mundl H, et al. Rivaroxaban for stroke prevention after embolic stroke of undetermined source. N Engl J Med. 2018;378(23):2191–2201. doi: 10.1056/NEJMoa1802686
- Diener H-C, Sacco RL, Easton JD, et al. Dabigatran for prevention of stroke after embolic stroke of undetermined source. N Engl J Med. 2019;380(20):1906–1917. doi: 10.1056/NEJMoa1813959
- Sanna T, Diener H-C, Passman RS, et al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med. 2014;370(26):2478–2486. doi: 10.1056/NEJMoa1313600
- Mahajan R, Perera T, Elliott AD, et al. Subclinical device-detected atrial fibrillation and stroke risk: a systematic review and meta-analysis. Eur Heart J. 2018;39(16):1407–1415. doi: 10.1093/eurheartj/ehx731
- Kamel H, Okin PM, Elkind MV, et al. Atrial fibrillation and mechanisms of stroke: time for a new model. Stroke. 2016;47(3):895–900. doi: 10.1161/STROKEAHA.115.012004
- Yaghi S, Kamel H, Elkind MV. Atrial cardiopathy: a mechanism of cryptogenic stroke. Expert Rev Cardiovasc Ther. 2017;15(8):591–599. doi: 10.1080/14779072.2017.1355238
- Kamel H, Okin PM, Longstreth W, et al. Atrial cardiopathy: a broadened concept of left atrial thromboembolism beyond atrial fibrillation. Future Cardiol. 2015;11(3):323–331. doi: 10.2217/fca.15.22
- Freedman B, Kamel H, Van Gelder IC, et al. Atrial fibrillation: villain or bystander in vascular brain injury. Eur Hear J Suppl. 2020;22(Supplement_M):M51–M59. doi: 10.1093/eurheartj/suaa166
- Kamel H, Healey JS. Cardioembolic stroke. Circ Res. 2017;120(3):514–526. doi: 10.1161/CIRCRESAHA.116.308407
- Hirsh BJ, Copeland-Halperin RS, Halperin JL. Fibrotic atrial cardiomyopathy, atrial fibrillation, and thromboembolism. J Am Coll Cardiol. 2015;65(20):2239–2251. doi: 10.1016/j.jacc.2015.03.557
- Choi SE, Sagris D, Hill A, et al. Atrial fibrillation and stroke. Expert Rev Cardiovasc Ther. 2023;21(1):35–56. doi: 10.1080/14779072.2023.2160319
- Habibi M, Zareian M, Ambale Venkatesh B, et al. Left atrial mechanical function and incident ischemic cerebrovascular events Independent of AF. JACC Cardiovasc Imaging. 2019;12(12):2417–2427. doi: 10.1016/j.jcmg.2019.02.021
- Janga C, Madhavan M. Premature atrial contractions. Mayo Clin Proc. 2021;96(5):1111–1113. doi: 10.1016/j.mayocp.2021.03.029
- Russo C, Jin Z, Liu R, et al. LA volumes and reservoir function are associated with subclinical cerebrovascular disease: the CABL (cardiovascular abnormalities and brain lesions) study. JACC Cardiovasc Imaging. 2013;6(3):313–323. doi: 10.1016/j.jcmg.2012.10.019
- Mannina C, Ito K, Jin Z, et al. Association of left atrial strain with ischemic stroke risk in older adults. JAMA Cardiol. 2023;8(4):317–325. doi: 10.1001/jamacardio.2022.5449
- Larsen BS, Olsen FJ, Andersen DM, et al. Left atrial volumes and function, and long‐term incidence of ischemic stroke in the general population. J Am Heart Assoc. 2022;11(18):27031. doi: 10.1161/JAHA.122.027031
- Maheshwari A, Norby FL, Inciardi RM, et al. Left atrial mechanical dysfunction and the risk for ischemic stroke in people without prevalent atrial fibrillation or stroke: a prospective cohort study. Ann Intern Med. 2023;176(1):39–48. doi: 10.7326/M22-1638
- Healey JS, Gladstone DJ, Swaminathan B, et al. Recurrent stroke with rivaroxaban compared with aspirin according to predictors of atrial fibrillation: secondary analysis of the NAVIGATE ESUS randomized clinical trial. JAMA Neurol. 2019;76(7):764–773. doi: 10.1001/jamaneurol.2019.0617
- Karas MG, Devereux RB, Wiebers DO, et al. Incremental value of biochemical and echocardiographic measures in prediction of ischemic stroke: the strong heart study. Stroke. 2012;43(3):720–726. doi: 10.1161/STROKEAHA.111.631168
- Wong JM, Welles CC, Azarbal F, et al. Relation of left atrial dysfunction to ischemic stroke in patients with coronary heart disease (from the heart and soul study). Am J Cardiol. 2014;113(10):1679–1684. doi: 10.1016/j.amjcard.2014.02.021
- Edwards JD, Healey JS, Fang J, et al. Atrial cardiopathy in the absence of atrial fibrillation increases risk of ischemic stroke, incident atrial fibrillation, and mortality and improves stroke risk prediction. J Am Heart Assoc. 2020;9(11):e013227. doi: 10.1161/JAHA.119.013227
- Di Tullio MR, Sacco RL, Sciacca RR, et al. Left atrial size and the risk of ischemic stroke in an ethnically mixed population. Stroke. 1999;30(10):2019–2024. doi: 10.1161/01.STR.30.10.2019
- Benjamin EJ, D’Agostino RB, Belanger AJ, et al. Left atrial size and the risk of stroke and death. The Framingham heart study. Circulation. 1995;92(4):835–841. doi: 10.1161/01.CIR.92.4.835
- Kamel H, Bartz TM, Longstreth WT, et al. Cardiac mechanics and incident ischemic stroke: the cardiovascular health study. Sci Rep. 2021;11(1):1–9. doi: 10.1038/s41598-021-96702-z
- Barnes ME, Miyasaka Y, Seward JB, et al. Left atrial volume in the prediction of first ischemic stroke in an elderly cohort without atrial fibrillation. Mayo Clin Proc. 2004;79(8):1008–1014. doi: 10.4065/79.8.1008
- Bouzas-Mosquera A, Broullón FJ, Álvarez-García N, et al. Left atrial size and risk for all-cause mortality and ischemic stroke. CMAJ. 2011;183(10):E657–64. doi: 10.1503/cmaj.091688
- Goette A, Kalman JM, Aguinaga L, et al. EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: definition, characterization, and clinical implication. Europace. 2016;18(10):1455–1490. doi: 10.1093/europace/euw161
- Geisler T, Keller T, Martus P, et al. Apixaban versus aspirin for embolic stroke of undetermined source. NEJM Evid. 2023;3(1):1–12. doi: 10.1056/EVIDoa2300235
- Kamel H, Longstreth WT, Tirschwell DL, et al. Apixaban to prevent recurrence after cryptogenic stroke in patients with atrial cardiopathy: the ARCADIA randomized clinical trial. JAMA. 2024;331(7):573–581. doi: 10.1001/jama.2023.27188
- Ho SY, Cabrera JA, Sanchez-Quintana D. Left atrial anatomy revisited. Circ Arrhythmia Electrophysiol. 2012;5(1):220–228. doi: 10.1161/CIRCEP.111.962720
- Cabrera JA, Saremi F, Sánchez-Quintana D. Left atrial appendage: anatomy and imaging landmarks pertinent to percutaneous transcatheter occlusion. Heart. 2014;100(20):1636–1650. doi: 10.1136/heartjnl-2013-304464
- Whiteman S, Saker E, Courant V, et al. An anatomical review of the left atrium. Trans Res Anat. 2019;17. doi: 10.1016/j.tria.2019.100052
- Barbier P, Solomon SB, Schiller NB, et al. Left atrial relaxation and left ventricular systolic function determine left atrial reservoir function. Circulation. 1999;100(4):427–436. doi: 10.1161/01.CIR.100.4.427
- Hoit BD. Left atrial size and function: role in prognosis. J Am Coll Cardiol. 2014;63(6):493–505. doi: 10.1016/j.jacc.2013.10.055
- Thomas L, Abhayaratna WP. Left atrial reverse remodeling: mechanisms, evaluation, and clinical significance. JACC Cardiovasc Imaging. 2017;10(1):65–77. doi: 10.1016/j.jcmg.2016.11.003
- Xintarakou A, Tzeis S, Psarras S, et al. Atrial fibrosis as a dominant factor for the development of atrial fibrillation: facts and gaps. EP Europace. 2020;22(3):342–351. doi: 10.1093/europace/euaa009
- de Boer RA, De Keulenaer G, Bauersachs J, et al. Towards better definition, quantification and treatment of fibrosis in heart failure. A scientific roadmap by the committee of translational research of the heart failure association (HFA) of the European society of cardiology. Eur J Heart Fail. 2019;21(3):272–285. doi: 10.1002/ejhf.1406
- Nattel S. Molecular and cellular mechanisms of atrial fibrosis in atrial fibrillation. JACC Clin Electrophysiol. 2017;3(5):425–435. doi: 10.1016/j.jacep.2017.03.002
- Haïssaguerre M, Jaïs P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339(10):659–666. doi: 10.1056/NEJM199809033391003
- Everett TH, Olgin JE, Everett Iv TH, et al. Atrial fibrosis and the mechanisms of atrial fibrillation. Heart Rhythm. 2007;4(3):S24–7. doi: 10.1016/j.hrthm.2006.12.040
- Hopman LHGA, Mulder MJ, van der Laan AM, et al. Impaired left atrial reservoir and conduit strain in patients with atrial fibrillation and extensive left atrial fibrosis. J Cardiovasc Magn Reson. 2021;23(1):131. doi: 10.1186/s12968-021-00820-6
- Kuppahally SS, Akoum N, Burgon NS, et al. Left atrial strain and strain rate in patients with paroxysmal and persistent atrial fibrillation: relationship to left atrial structural remodeling detected by delayed-enhancement MRI. Circ Cardiovasc Imaging. 2010;3(3):231–239. doi: 10.1161/CIRCIMAGING.109.865683
- Heckbert SR, Jensen PN, Austin TR, et al. Associations of left atrial function and structure with supraventricular ectopy: the multi-ethnic study of atherosclerosis. J Am Heart Assoc. 2021;10(4):1–19. doi: 10.1161/JAHA.120.018093
- Guichard JB, Nattel S. Atrial cardiomyopathy: a useful notion in cardiac disease management or a passing fad? J Am Coll Cardiol. 2017;70(6):756–765. doi: 10.1016/j.jacc.2017.06.033
- Shen MJ, Arora R, Jalife J. Atrial myopathy. JACC Basic To Transl Sci. 2019;4(5):640–654. doi: 10.1016/j.jacbts.2019.05.005
- Thomas L, Marwick TH, Popescu BA, et al. Left atrial structure and function, and left ventricular diastolic dysfunction: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73(15):1961–1977. doi: 10.1016/j.jacc.2019.01.059
- Triposkiadis F, Pieske B, Butler J, et al. Global left atrial failure in heart failure. Eur J Heart Fail. 2016;18(11):1307–1320. doi: 10.1002/ejhf.645
- Inciardi RM, Bonelli A, Biering-Sorensen T, et al. Left atrial disease and left atrial reverse remodelling across different stages of heart failure development and progression: a new target for prevention and treatment. Eur J Heart Fail. 2022;24(6):959–975. doi: 10.1002/ejhf.2562
- Bisbal F, Baranchuk A, Braunwald E, et al. Atrial failure as a clinical entity: JACC review topic of the week. J Am Coll Cardiol. 2020;75(2):222–232. doi: 10.1016/j.jacc.2019.11.013
- Mitchell C, Rahko PS, Blauwet LA, et al. Guidelines for performing a comprehensive transthoracic echocardiographic examination in adults: recommendations from the American society of echocardiography. J Am Soc Echocardiogr. 2019;32(1):1–64. doi: 10.1016/j.echo.2018.06.004
- Reeves RA, Halpern EJ, Rao VM. Cardiac imaging trends from 2010 to 2019 in the medicare population. Radiol Cardiothorac Imag. 2021;3(5):3. doi: 10.1148/ryct.2021210156
- Lester SJ, Ryan EW, Schiller NB, et al. Best method in clinical practice and in research studies to determine left atrial size. Am J Cardiol. 1999;84(7):829–832. doi: 10.1016/S0002-9149(99)00446-4
- Maddukuri PV, Vieira MLC, DeCastro S, et al. What Is the best approach for the assessment of left atrial size? Comparison of various unidimensional and two-dimensional parameters with three-dimensional echocardiographically determined left atrial volume. J Am Soc Echocardiogr. 2006;19(8):1026–1032. doi: 10.1016/j.echo.2006.03.011
- Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European Association of cardiovascular imaging. Eur Heart J Cardiovasc Imaging. 2015;16(3):233–271. doi: 10.1093/ehjci/jev014
- Tsang TSMM, Abhayaratna WP, Barnes ME, et al. Prediction of cardiovascular outcomes with left atrial size: is volume superior to area or diameter? J Am Coll Cardiol. 2006;47(5):1018–1023. doi: 10.1016/j.jacc.2005.08.077
- Thomas L, Muraru D, Popescu BA, et al. Evaluation of left atrial size and function: relevance for clinical practice. J Am Soc Echocardiogr. 2020;33(8):934–952. doi: 10.1016/j.echo.2020.03.021
- Badano LP, Miglioranza MH, MihǍilǍ S, et al. Left atrial volumes and function by three-dimensional echocardiography: reference values, accuracy, reproducibility, and comparison with two-dimensional echocardiographic measurements. Circ Cardiovasc Imaging. 2016;9(7). doi: 10.1161/CIRCIMAGING.115.004229
- Buechel RR, Stephan FP, Sommer G, et al. Head-to-head comparison of two-dimensional and three-dimensional echocardiographic methods for left atrial chamber quantification with magnetic resonance imaging. J Am Soc Echocardiogr. 2013;26(4):428–435. doi: 10.1016/j.echo.2013.01.001
- Mor-Avi V, Yodwut C, Jenkins C, et al. Real-time 3D echocardiographic quantification of left atrial volume: multicenter study for validation with CMR. JACC Cardiovasc Imaging. 2012;5(8):769–777. doi: 10.1016/j.jcmg.2012.05.011
- Singh A, Carvalho Singulane C, Miyoshi T, et al. Normal values of left atrial size and function and the impact of age: results of the world alliance societies of echocardiography study. J Am Soc Echocardiogr. 2021;35:154–164.
- Buggey J, Hoit BD. Left atrial strain: measurement and clinical application. Curr Opin Cardiol. 2018;33(5):479–485. doi: 10.1097/HCO.0000000000000537
- Yafasov M, Olsen FJ, Skaarup KG, et al. Normal values for left atrial strain, volume, and function derived from 3D echocardiography: the Copenhagen city heart study. Eur Hear J - Cardiovasc Imaging. 2024;25(5):602–612. doi: 10.1093/ehjci/jeae018
- Nyberg J, Jakobsen EO, Østvik A, et al. Echocardiographic reference ranges of global longitudinal strain for all cardiac chambers using guideline-directed dedicated views. JACC Cardiovasc Imaging. 2023;16(12):1516–1531. doi: 10.1016/j.jcmg.2023.08.011
- Nielsen AB, Skaarup KG, Hauser R, et al. Normal values and reference ranges for left atrial strain by speckle-tracking echocardiography: the Copenhagen city heart study. Eur Heart J Cardiovasc Imaging. 2022;23(1):42–51. doi: 10.1093/ehjci/jeab201
- Olsen FJ, Johansen ND, Skaarup KG, et al. Changes in left atrial structure and function over a decade in the general population. Eur Hear J - Cardiovasc Imaging. 2021;23(1):124–136. doi: 10.1093/ehjci/jeab173
- Lim DJ, Ambale-Ventakesh B, Ostovaneh MR, et al. Change in left atrial function predicts incident atrial fibrillation: the multi-ethnic study of atherosclerosis. Eur Heart J Cardiovasc Imaging. 2019;20(9):979–987. doi: 10.1093/ehjci/jez176
- Kawel-Boehm N, Hetzel SJ, Ambale-Venkatesh B, et al. Reference ranges (“normal values”) for cardiovascular magnetic resonance (CMR) in adults and children: 2020 update. vol. 22. BioMed Central; 2020. J Cardiovasc Magn Reson. 2020;(1):87. doi: 10.1186/s12968-020-00683-3
- Grothues F, Smith GC, Moon JCC, et al. Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two-dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. Am J Cardiol. 2002;90(1):29–34. doi: 10.1016/S0002-9149(02)02381-0
- Claus P, Omar AMS, Pedrizzetti G, et al. Tissue tracking technology for assessing cardiac mechanics: principles, normal values, and clinical applications. JACC Cardiovasc Imaging. 2015;8(12):1444–1460. doi: 10.1016/j.jcmg.2015.11.001
- Petersen SE, Khanji MY, Plein S, et al. European Association of cardiovascular imaging expert consensus paper: a comprehensive review of cardiovascular magnetic resonance normal values of cardiac chamber size and aortic root in adults and recommendations for grading severity. Eur Hear J - Cardiovasc Imaging. 4419;20(12):1321–1331. doi: 10.1093/ehjci/jez232
- Wandelt LK, Kowallick JT, Schuster A, et al. Quantification of left atrial volume and phasic function using cardiovascular magnetic resonance imaging—comparison of biplane area-length method and Simpson’s method. Int J Cardiovasc Imaging. 2017;33(11):1761–1769. doi: 10.1007/s10554-017-1160-9
- Zareian M, Ciuffo L, Habibi M, et al. Left atrial structure and functional quantitation using cardiovascular magnetic resonance and multimodality tissue tracking: validation and reproducibility assessment. J Cardiovasc Magn Reson. 2015;17(1):1–13. doi: 10.1186/s12968-015-0152-y
- Olsen FJ, Møgelvang R, Jensen GB, et al. Relationship between left atrial functional measures and incident atrial fibrillation in the general population: the Copenhagen city heart study. JACC Cardiovasc Imaging. 2019;12(6):981–989. doi: 10.1016/j.jcmg.2017.12.016
- Schaaf M, Andre P, Altman M, et al. Left atrial remodelling assessed by 2D and 3D echocardiography identifies paroxysmal atrial fibrillation. Eur Heart J Cardiovasc Imaging. 2017;18(1):46–53. doi: 10.1093/ehjci/jew028
- Habibi M, Samiei S, Venkatesh BA, et al. Cardiac magnetic resonance–measured left atrial volume and function and incident atrial fibrillation. Circ Cardiovasc Imaging. 2016;9(8):1–8. doi: 10.1161/CIRCIMAGING.115.004299
- Bertelsen L, Diederichsen SZ, Haugan KJ, et al. Left atrial volume and function assessed by cardiac magnetic resonance imaging are markers of subclinical atrial fibrillation as detected by continuous monitoring. EP Europace. 2020;22(5):724–731. doi: 10.1093/europace/euaa035
- Hauser R, Nielsen AB, Skaarup KG, et al. Left atrial strain predicts incident atrial fibrillation in the general population: the Copenhagen city heart study. Eur Heart J Cardiovasc Imaging. 2022;23(1):52–60. doi: 10.1093/ehjci/jeab202
- Patel RB, Delaney JA, Hu M, et al. Characterization of cardiac mechanics and incident atrial fibrillation in participants of the cardiovascular health study. JCI Insight. 2020;5(19). doi: 10.1172/jci.insight.141656
- Huber MP, Pandit JA, Jensen PN, et al. Left atrial strain and the risk of atrial arrhythmias from extended ambulatory cardiac monitoring: MESA. J Am Heart Assoc. 2022;11(21):11. doi: 10.1161/JAHA.122.026875
- Mannina C, Ito K, Jin Z, et al. Left atrial strain and incident atrial fibrillation in older adults. Am J Cardiol. 2023;206:161–167. doi: 10.1016/j.amjcard.2023.08.060
- Olsen FJ, Diederichsen SZ, Jørgensen PG, et al. Response by Olsen et al to letter regarding article, “left atrial strain predicts subclinical atrial fibrillation detected by long-term continuous monitoring in elderly high-risk individuals”. Circ Cardiovasc Imaging. 2024;17(6):E016197. doi: 10.1161/CIRCIMAGING.124.016897
- Biering-Sørensen T, Christensen LM, Krieger DW, et al. LA emptying fraction improves diagnosis of paroxysmal AF after cryptogenic ischemic stroke: results from the SURPRISE study. JACC Cardiovasc Imaging. 2014;7(9):962–963. doi: 10.1016/j.jcmg.2014.02.003
- Olsen FJ, Christensen LM, Krieger DW, et al. Relationship between left atrial strain, diastolic dysfunction and subclinical atrial fibrillation in patients with cryptogenic stroke: the SURPRISE echo substudy. Int J Cardiovasc Imaging. 2020;36(1):79–89. doi: 10.1007/s10554-019-01700-y
- Choi HM, Yoon YE, Oh IY, et al. Global left atrial strain as a predictor of silent atrial fibrillation following dual chamber cardiac implantable electronic device implantation. JACC Cardiovasc Imaging. 2018;11(10):1537–1539. doi: 10.1016/j.jcmg.2017.12.013
- Kojima T, Kawasaki M, Tanaka R, et al. Left atrial global and regional function in patients with paroxysmal atrial fibrillation has already been impaired before enlargement of left atrium: velocity vector imaging echocardiography study. Eur Heart J Cardiovasc Imaging. 2012;13(3):227–234. doi: 10.1093/ejechocard/jer281
- D’Ascenzi F, Anselmi F, Focardi M, et al. Atrial enlargement in the Athlete’s heart: assessment of atrial function may help distinguish adaptive from pathologic remodeling. J Am Soc Echocardiogr. 2018;31(2):148–157. doi: 10.1016/j.echo.2017.11.009
- Kamel H, Longstreth WT, Tirschwell DL, et al. The AtRial cardiopathy and antithrombotic drugs in prevention after cryptogenic stroke randomized trial: rationale and methods. Int J Stroke. 2019;14(2):207–214. doi: 10.1177/1747493018799981
- Xing LY, Diederichsen SZ, Højberg S, et al. Electrocardiographic morphology-voltage-p-wave-duration (MVP) score to select patients for continuous atrial fibrillation screening to prevent stroke. Am J Cardiol. 2023;205:457–464. doi: 10.1016/j.amjcard.2023.08.042
- Svendsen JH, Diederichsen SZ, Højberg S, et al. Implantable loop recorder detection of atrial fibrillation to prevent stroke (the LOOP study): a randomised controlled trial. Lancet. 2021;398(10310):1507–1516. doi: 10.1016/S0140-6736(21)01698-6
- Kirchhof P, Toennis T, Goette A, et al. Anticoagulation with edoxaban in patients with atrial high-rate episodes. N Engl J Med. 2023;2023(13):1167–1179. doi: 10.1056/NEJMoa2303062
- Healey JS, Lopes RD, Granger CB, et al. Apixaban for stroke prevention in subclinical atrial fibrillation. N Engl J Med. 2023:107–117.
- Gibson CM, Yuet WC. Racial and ethnic differences in response to anticoagulation: a review of the literature. J Pharm Pract. 2021;34(5):685–693. doi: 10.1177/0897190019894142
- Gardener H, Sacco RL, Rundek T, et al. Race and ethnic disparities in stroke incidence in the Northern Manhattan study. Stroke. 2020;51(4):1064–1069. doi: 10.1161/STROKEAHA.119.028806
- Providência R, Trigo J, Paiva L, et al. The role of echocardiography in thromboembolic risk assessment of patients with nonvalvular atrial fibrillation. J Am Soc Echocardiogr. 2013;26(8):801–812. doi: 10.1016/j.echo.2013.05.010
- Ganesan AN, Chew DP, Hartshorne T, et al. The impact of atrial fibrillation type on the risk of thromboembolism, mortality, and bleeding: a systematic review and meta-analysis. Eur Heart J. 2016;37(20):1591–1602. doi: 10.1093/eurheartj/ehw007
- Kirchhof P, Camm AJ, Goette A, et al. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med. 2020;383(14):1305–1316. doi: 10.1056/NEJMoa2019422
- Cappelli F, Tini G, Russo D, et al. Arterial thrombo-embolic events in cardiac amyloidosis: a look beyond atrial fibrillation. Amyloid. 2021;28(1):12–18. doi: 10.1080/13506129.2020.1798922
- Vergaro G, Aimo A, Rapezzi C, et al. Atrial amyloidosis: mechanisms and clinical manifestations. Eur J Heart Fail. 2022;24(11):2019–2028. doi: 10.1002/ejhf.2650
- Bandera F, Martone R, Chacko L, et al. Clinical importance of left atrial infiltration in cardiac transthyretin amyloidosis. JACC Cardiovasc Imaging. 2022;15(1):17–29. doi: 10.1016/j.jcmg.2021.06.022
- Aimo A, Merlo M, Porcari A, et al. Redefining the epidemiology of cardiac amyloidosis. A systematic review and meta-analysis of screening studies. Eur J Heart Fail. 2022;24(12):2342–2351. doi: 10.1002/ejhf.2532
- Martinez-Naharro A, Gonzalez-Lopez E, Corovic A, et al. High prevalence of intracardiac thrombi in cardiac amyloidosis. J Am Coll Cardiol. 2019;73(13):1733–1734. doi: 10.1016/j.jacc.2019.01.035
- Alexandru R, Terecoasă EO, Băjenaru OA, et al. Etiologic classification of ischemic stroke: where do we stand? Clin Neurol Neurosur. 2017;159:93–106. doi: 10.1016/j.clineuro.2017.05.019
- Kotadia ID, O’Dowling R, Aboagye A, et al. Atrial CARdiac magnetic resonance imaging in patients with embolic stroke of unknown source without documented atrial fibrillation (CARM-AF): study design and clinical protocol. Hear Rhythm O2. 2022;3(2):196–203. doi: 10.1016/j.hroo.2022.01.005
- Spartera M, Stracquadanio A, Pessoa-Amorim G, et al. Reduced left atrial rotational flow is independently associated with embolic brain infarcts. JACC Cardiovasc Imaging. 2023;16(9):1149–1159. doi: 10.1016/j.jcmg.2023.03.006
- Boyle PM, Del Álamo JC, Akoum N. Fibrosis, atrial fibrillation and stroke: clinical updates and emerging mechanistic models. Heart. 2021;107:99–105. doi: 10.1136/heartjnl-2020-317455
- Fuentes B, Gutiérrez-Zúñiga R, Díez-Tejedor E. It’s time to say goodbye to the ESUS construct. Front Neurol. 2020;11:1–5. doi: 10.3389/fneur.2020.00653
- Chahine Y, Magoon MJ, Maidu B, et al. Machine learning and the conundrum of stroke risk prediction. Arrhythmia Electrophysiol Rev. 2023;12:12. doi: 10.15420/aer.2022.34
- Unger T, Borghi C, Charchar F, et al. International society of hypertension global hypertension practice guidelines. Hypertension. 2020;75(6):1334–1357. doi: 10.1161/HYPERTENSIONAHA.120.15026
- Hindricks G, Potpara T, Dagres N, et al. ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of cardio-thoracic surgery (EACTS). Eur Heart J. 2020;2020:1–126.
