ABSTRACT
Background
Triple antithrombotic therapy (TAT) with aspirin, a P2Y12 inhibitor, and oral anticoagulation in patients with atrial fibrillation (AF) undergoing percutaneous coronary intervention (PCI) raises concerns about increased bleeding. Regimens incorporating more potent P2Y12 inhibitors over clopidogrel have not been investigated adequately.
Research design and methods
A retrospective observational study was performed on 387 patients with AF receiving TAT for 1 month (n = 236) or ≤1 week (n = 151) after PCI. Major and clinically relevant non-major bleeding and major adverse cardiac and cerebrovascular events (MACCE) were assessed up to 30 days post-procedure.
Results
Bleeding was less frequent with ≤1 week versus 1 month of TAT (3.3 vs 9.3%; p = 0.025) while MACCE were similar (4.6 vs 4.7%; p = 0.998). No differences in bleeding or MACCE were observed between ticagrelor/prasugrel and clopidogrel regimens. For patients receiving ≤1 week of TAT, no excess of MACCE was seen in the subgroup given no further aspirin post-PCI compared with those given aspirin for up to 1 week (3.6 vs 5.2%).
Conclusions
TAT post-PCI for ≤1 week was associated with less bleeding despite greater use of ticagrelor/prasugrel but similar MACCE versus 1-month TAT. These findings support further studies on safety and efficacy of dual therapy with ticagrelor/prasugrel immediately after PCI.
1. Introduction
Patients with atrial fibrillation (AF) who undergo percutaneous coronary intervention (PCI) with stenting often require an antithrombotic regimen comprising a period of antiplatelet therapy in addition to long-term oral anticoagulation (OAC) [Citation1,Citation2]. Dual antiplatelet therapy (DAPT) is inferior to OAC for stroke prophylaxis in patients with AF while OAC is inferior to DAPT for prevention of coronary stent thrombosis, but combining them to simultaneously address both issues leads to an increased risk of major and clinically relevant non-major (CRNM) bleeding [Citation3–5]. Randomized controlled trials have compared the safety and efficacy of triple antithrombotic therapy (TAT), consisting of aspirin, a P2Y12 inhibitor, and OAC, with dual antithrombotic therapy (DAT) without aspirin [Citation6–9]. Meta-analyses of these trials demonstrate superior safety of DAT in regard to lower rates of bleeding but are less congruent on efficacy outcomes, with some suggestion of higher risk of myocardial infarction (MI) and stent thrombosis in patients receiving this regimen [Citation10–14]. Current guidelines therefore recommend a default regimen of DAT after up to 1 week of TAT following PCI, with variation depending on high bleeding or ischemic risk [Citation1]. However, this evidence is mostly based on trials comparing a vitamin K antagonist with a direct oral anticoagulant (DOAC) in their respective TAT and DAT groups. The AUGUSTUS trial specifically assessed the effect of discontinuing aspirin within 2 weeks (median of 1 week) after PCI and/or acute coronary syndrome (ACS), demonstrating an 89% relative increase in major or CRNM bleeding with continuation versus early cessation of aspirin [Citation8]. There are sparse data concerning the efficacy and safety of stopping aspirin and using DAT immediately after PCI.
Uncertainty remains about the impact of the type of P2Y12 inhibitor used in combination with OAC after PCI. Clopidogrel was the P2Y12 inhibitor of choice in the majority of trial patients, with the more potent P2Y12 inhibitors ticagrelor and prasugrel only used in ≤12%. Clopidogrel is, therefore, the recommended P2Y12 inhibitor in this setting [Citation1]. However, ticagrelor and prasugrel have both demonstrated more consistent P2Y12 inhibition over clopidogrel as well as superior cardiovascular outcomes in patients after ACS, albeit in the absence of OAC [Citation15–18]. Further investigation is required to clarify whether ticagrelor or prasugrel would be better suited to accompany OAC than clopidogrel, especially in DAT after PCI. This should also be reflective of more contemporary management of AF with DOACs over warfarin.
International guideline recommendations present a range of options for managing antithrombotic therapy in PCI patients with an indication for OAC and this has led to heterogeneity of practice, including within our own institution, which provides the opportunity for observational studies that compare different strategies. Reflecting gaps in the evidence base, we aimed to investigate the efficacy and safety of antithrombotic regimens commenced after PCI with stenting from two main angles: the duration of TAT and the type of P2Y12 inhibitor used.
2. Patients and methods
2.1. Study population
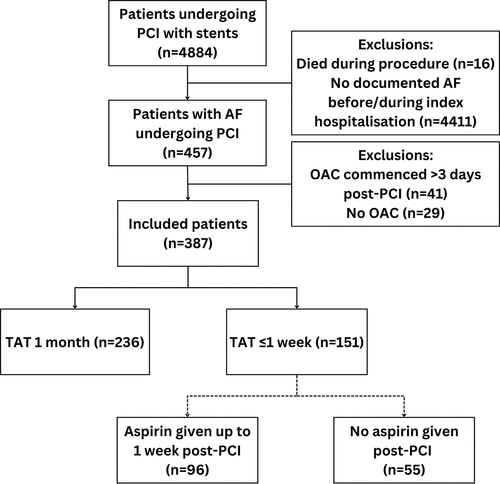
We performed a retrospective observational study on all consecutive patients with AF taking OAC who underwent PCI with stenting between January 2020 and December 2022 in the South Yorkshire Cardiothoracic Centre, Northern General Hospital, Sheffield, United Kingdom. Patients with a new diagnosis of AF during admission were included if OAC was commenced ≤72 h from PCI. Exclusion criteria for analysis related to scenarios in which the bleeding and ischemic risks were less clear: PCI with balloon angioplasty or drug-coated balloon only; deaths during the procedure; doses of OAC below those indicated for the management of AF; long-term OAC commenced or restarted >72 h post-PCI; and OAC for an indication other than AF. The analysis considered only the first PCI undertaken for each patient during this period; staged or repeat procedures were excluded.
2.2. Antithrombotic regimens
Patients were categorized according to the antithrombotic regimen determined by the operator and other attending physicians post-PCI or prior to hospital discharge based on procedure reports, discharge summaries and prescriptions issued. Consequently, patients were assigned to one of the two main groups: TAT for 1 month followed by DAT or TAT for ≤1 week followed by DAT (). Patients who received no further aspirin post-PCI (and therefore received DAT from the outset) were also included in the ≤1-week TAT group. The distribution of TAT duration in the ≤1-week group is described in Supplementary Figure S1 (median 3 days). No patients were commenced on TAT for an intended period of between 1 week and 1 month. Adherence to the prescribed regimen was confirmed at patients’ first follow-up clinic visit 6–12 weeks after PCI.
2.3. Clinical outcomes
Bleeding and ischemic outcomes were recorded up to 30 days after PCI based on subsequent admission and attendance records to hospital or primary care. Bleeding events were defined according to the International Society on Thrombosis and Hemostasis (ISTH) criteria. Major bleeding was defined as fatal, symptomatic in a critical area or organ (such as intracranial, intraspinal, intraocular, retroperitoneal, intraarticular, intramuscular with compartment syndrome or pericardial), or causing a fall in hemoglobin level of ≥20 g/L or requiring a transfusion of ≥2 units of whole blood or red cells [Citation19]. Bleeding events within the first 24 h after PCI were not included due to the possible confounding effects of intraprocedural heparin and/or glycoprotein IIb/IIIa receptor antagonists. CRNM bleeding was defined as requiring medical intervention by a healthcare professional, leading to hospitalization or increased level of care, or prompting a face-to-face evaluation [Citation20]. Major adverse cardiac and cerebrovascular events (MACCE) included cardiovascular death, MI, stroke, or transient ischemic attack, and definite stent thrombosis as defined by the Academic Research Consortium (ARC)-2 [Citation21]. Only the first bleeding event or MACCE was considered for each patient at primary analysis.
Bleeding risk was estimated using the PRECISE-DAPT (PREdicting bleeding Complications In patients undergoing Stent implantation and subsEquent Dual Anti Platelet Therapy) score, which incorporates age, creatinine clearance, hemoglobin level, white blood cell count, and a history of previous bleeding as variables [Citation22]. A score of ≥25 was considered high bleeding risk. Complex PCI was defined similar to the TWILIGHT (Ticagrelor With Aspirin or Alone in High-Risk Patients after Coronary Intervention) study as any of the following: three vessels treated; ≥3 lesions treated; ≥3 stents implanted; total stent length ≥60 mm; bifurcation with 2 stents implanted; left main PCI; use of atherectomy; coronary artery bypass graft PCI; or chronic total occlusion PCI [Citation23]. Risk scores for ischemic and bleeding risk to guide OAC in patients with AF (CHA2DS2-VASc and HAS-BLED, respectively) were also calculated.
2.4. Statistical analysis
The continuous variables are presented as mean ± standard deviation or median (interquartile range), as appropriate. The Kolmogorov–Smirnov test was used to assess normality. The unpaired Student’s t-test for normally distributed data and Mann–Whitney U-test for non-parametric data were used to compare continuous variables. Categorical variables are presented as total numbers (proportions) and compared using the chi-square test. Bleeding and ischemic events were compared between groups using Kaplan–Meier curve and log-rank analysis. Cox proportional-hazards regression was used to evaluate the risk of bleeding and ischemic events, including antithrombotic regimen groups and P2Y12 inhibitors as the two covariates. All calculations were performed using IBM SPSS Statistics 29 (SPSS Statistics for Macintosh, Version 29.0. Armonk, NY, U.S.A.: IBM Corp) and GraphPad Prism 10 (GraphPad Prism version 10.0.0 for Macintosh, GraphPad Software, Boston, Massachusetts, U.S.A.). A P-value <0.05 was considered statistically significant. No adjustment was made for multiple testing and so the results are considered exploratory.
2.5. Ethical approval
This study was approved by the ethics committee of the University of Sheffield (reference 059596) and by the Clinical Research and Innovation Office of Sheffield Teaching Hospitals National Health Service (NHS) Foundation Trust. Informed patient consent was not required due to the nature of the study’s design.
3. Results
Three hundred and eighty-seven patients were included in the primary analysis; 236 (61%) were planned for 1 month of TAT and 151 (39%) were planned for ≤1 week of TAT after PCI. Of the latter group, 96 patients were scheduled to receive TAT for up to 1 week and 55 patients received DAT without aspirin straight after PCI. Baseline clinical characteristics were well balanced between the groups, with the exception that clopidogrel was the most favored P2Y12 inhibitor in the 1-month TAT group (78% of these patients), while ticagrelor was the preferred option in the ≤1-week TAT group (59% of these patients) (). Prasugrel was used in only 2% of patients. Apixaban was the OAC used in the majority of patients (82%). Rivaroxaban was the second commonest OAC (12%) and a small number of patients were taking dabigatran (3%), edoxaban (1%) or warfarin (1%). Over half of patients were at high bleeding risk (52% with PRECISE-DAPT score ≥25) and the mean HAS-BLED score was 2.0 ± 0.6. PCI was performed for ACS in 79%, including ST-segment elevation MI (28%), non-ST-segment elevation MI (46%) and unstable angina (5%), while PCI was performed for chronic coronary syndromes in 21%, including stable angina (18%) and significant coronary artery disease prior to transcatheter aortic valve implantation (3%).
Table 1. Baseline characteristics of the patients.
3.1. Impact of antithrombotic therapy duration
Patients receiving ≤1 week of TAT had significantly lower rates of ISTH-defined major or CRNM bleeding at 30 days post-PCI compared with patients receiving 1 month of TAT (3.3 versus 9.3%, p = 0.025) ( and ). Multivariate Cox regression analysis demonstrated independent association of ≤1 week of TAT with reduced rates of clinically relevant bleeding (adjusted hazard ratio [HR] 0.31, 95% confidence intervals [CI] 0.11–0.88; p = 0.028).
Figure 2. Cumulative incidence of major and clinically relevant non-major bleeding at 30 days after percutaneous coronary intervention; TAT = triple antithrombotic therapy.
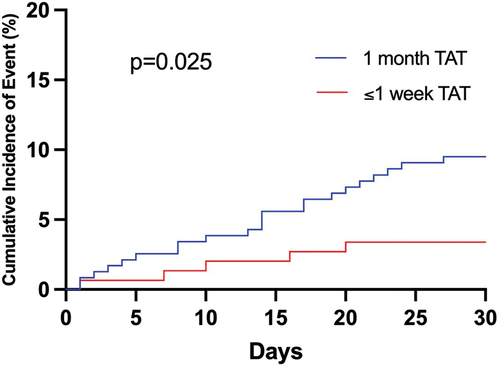
Table 2. Bleeding and major adverse cardiac and cerebrovascular events by group at 30 days after percutaneous coronary intervention.
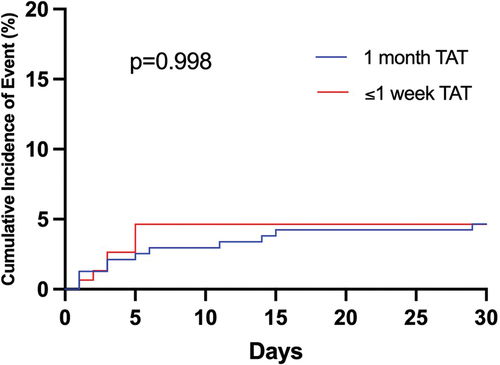
MACCE within 30 days post-PCI were similar in both groups: 4.6% with ≤1 week of TAT versus 4.7% with 1 month of TAT (p = 0.998) ( and ). Adjustment for P2Y12 inhibitor with multivariate Cox regression analysis did not alter this result (adjusted HR 0.97, 95% CI 0.34–2.72; p = 0.966). There was only one case of definite stent thrombosis in the entire cohort, occurring 2 weeks post-PCI in the 1-month TAT group.
Figure 3. Cumulative incidence of major adverse cardiac and cerebrovascular events at 30 days after percutaneous coronary intervention; TAT = triple antithrombotic therapy.
Within the group receiving ≤1 week of TAT, the incidence of MACCE in those who discontinued aspirin immediately after PCI (n = 55) was 3.6% versus 5.2% in those who received up to 1 week of aspirin after PCI (n = 96), with no definite stent thrombosis in either subgroup. Rates of clinically relevant bleeding were 1.8% versus 4.2%, respectively.
3.2. Impact of P2Y12 inhibitor
Owing to different P2Y12 inhibitor use in both groups, baseline clinical characteristics were also compared between patients taking clopidogrel and ticagrelor/prasugrel (Supplementary Table S1). Ticagrelor/prasugrel was used more commonly than clopidogrel after PCI in patients who were younger (p = 0.002), had a lower CHA2DS2-VASc score (p = 0.042) and presented with ACS (p < 0.001). There were nonsignificant trends toward increased major or CRNM bleeding events (adjusted HR 1.28, 95% CI 0.55–3.00; p = 0.568) as well as MACCE (adjusted HR 1.09, 95% CI 0.39–3.06; p = 0.875) with ticagrelor/prasugrel compared with clopidogrel. In patients undergoing PCI for ACS (Supplementary Table S2), MACCE occurred in 5.2% patients taking ticagrelor/prasugrel (n = 135) and in 5.8% patients taking clopidogrel (n = 171). In the ≤1-week TAT group, incidence of major or CRNM bleeding and incidence of MACCE at 30 days were 5.2% and 5.2% on TAT with ticagrelor/prasugrel (n = 58), 2.6% and 5.3% on TAT with clopidogrel (n = 38), 0% and 5.7% on DAT with ticagrelor/prasugrel (n = 35), and 5.0% and 0% on DAT with clopidogrel (n = 20), respectively.
4. Discussion
In this retrospective observational study, patients with AF planned for ≤1 week of TAT after PCI had less major or CRNM bleeding at 30 days than patients planned for 1 month of TAT. This lower incidence of bleeding with shorter duration or avoidance of TAT was seen despite significantly greater use of ticagrelor or prasugrel instead of clopidogrel. There was no difference in MACCE between the different antithrombotic regimens and no clear difference in clinical outcomes according to the P2Y12 inhibitor used but the confidence intervals were wide, and the findings can only be considered to provide pilot data in support of larger studies that explore the use of DAT immediately after PCI.
AF has a rising prevalence and is also associated with coronary artery disease, therefore the number of patients with both conditions is likely to increase [Citation24]. The optimal antithrombotic strategy for patients with AF undergoing PCI remains a contemporary issue that is highly debated due to fears of excessive bleeding with prolonged TAT and inadequate protection against thrombotic events with shorter TAT or DAT, especially stent-related [Citation25]. Increasing use of the more effective P2Y12 inhibitors ticagrelor and prasugrel over clopidogrel, especially in ACS, as well as the almost ubiquitous use of DOACs over warfarin for nonvalvular AF have added extra layers to the debate that the original trials did not fully address. Only AUGUSTUS, with its 2 × 2 factorial design, compared TAT and DAT using the same DOAC (apixaban) [Citation8]. Latest ACS guidelines remain relatively unchanged from previous versions, citing limited evidence for recommending DAT with OAC and ticagrelor or prasugrel as an alternative to TAT [1]. Our study includes a majority of patients taking apixaban and a much higher proportion of ticagrelor use than seen in the large clinical trials. The median age was also higher than in the original trials and probably reflects a more real-world representation of this patient population. Even in more recently-published observational studies comparing DAT with TAT in clinical practice, the proportion of patients receiving ticagrelor/prasugrel remains relatively small: 6% in the WOEST 2 registry, 3% in a multicenter German registry, and 12% in a multicenter Japanese registry [Citation26–28].
We restricted primary outcomes to the first 30 days after PCI as most patients were expected to have de-escalated from TAT to DAT by this time and use of TAT beyond 30 days is not recommended. There was a marked difference in P2Y12 inhibitor between the groups, with clopidogrel used more commonly in longer TAT regimens and ticagrelor (and, to a much smaller extent, prasugrel) used more commonly in shorter TAT or DAT regimens, consistent with guideline recommendations to avoid TAT with ticagrelor or prasugrel due to concerns over excessive bleeding risk. For this reason, multivariate analysis of bleeding and MACCE outcomes included adjustment for P2Y12 inhibitor use. TAT for ≤1 week was independently associated with a 69% risk reduction in major or CRNM bleeding compared with a longer TAT duration of 1 month. Patients receiving DAT straight after PCI had the lowest incidence of bleeding, but direct comparison with TAT of any duration was limited by small numbers. Nevertheless, dropping aspirin immediately or shortly after PCI expectedly resulted in less bleeding and is in keeping with the intentions of current guidelines to appropriately minimize this risk. Ticagrelor and prasugrel were associated with a nonsignificant increase in bleeding compared with clopidogrel, mostly driven by their use in TAT regimens. This further reinforces the recommendation not to use a combination of aspirin, ticagrelor/prasugrel, and OAC [Citation29,Citation30].
Using ≤1 week of TAT was not associated with an increase in MACCE; however, this study was underpowered to detect a difference, as were all the trials to date in this field. Meta-analyses including all or most of the relevant trials report contrasting results: Lopes et al. and Capodanno et al. reported no significant differences in major adverse cardiovascular events between TAT and DAT while Gargiulo et al. and Galli et al. report borderline increased rates of MI and stent thrombosis with DAT [Citation10–14]. Thus, the optimal antithrombotic strategy and duration to reduce thrombotic risk remain unclear. The slight increase in MACCE with ticagrelor/prasugrel observed in this study appears to contradict their superior efficacy over clopidogrel in the PLATO and TRITON-TIMI 38 trials [Citation15,Citation17]. However, there was significantly higher use of ticagrelor/prasugrel among patients undergoing PCI after ACS, which may have confounded outcomes due to higher ischemic risk, similar to observations made in a subgroup analysis of RE-DUAL PCI [Citation30].
Stent thrombosis is rare but carries a high mortality risk; hence, why it is very challenging to design studies with enough power to investigate such an important complication [Citation31]. We observed one case of subacute stent thrombosis out of the 387 patients. There was no clear association with antithrombotic regimen but stent underexpansion and calcification are known predictors [Citation31,Citation32]. Operators may think they are erring on the side of caution by choosing TAT after PCI to provide maximum protection against stent thrombosis and other ischemic events but bleeding, including minor bleeding, can cause a reduction in quality of life, issues with antithrombotic compliance and itself precipitate an ischemic event [Citation33–35]. Furthermore, clopidogrel provides less consistent platelet inhibition than ticagrelor due to pharmacokinetic and pharmacogenomic differences, and thus less predictable efficacy [Citation18,Citation36,Citation37]. DAT with clopidogrel very early after PCI in patients with an impaired antiplatelet response could explain the increased risk of early stent thrombosis observed in aforementioned pooled trial analyses. Our study supports the safety of shorter TAT or DAT after PCI, with ticagrelor or prasugrel being suitable P2Y12 inhibitors in a DAT regimen but not a TAT regimen. The indirect antiplatelet effect of OAC through the inhibition of thrombin-mediated platelet activation (via the protease-activated receptor-1 and 4 pathways) may sufficiently substitute for the inhibition of thromboxane A2 release by aspirin in patients also receiving a P2Y12 inhibitor but further evidence is needed to establish optimum antithrombotic combinations and durations [Citation25,Citation38]. Results from ongoing trials (NCT03234114, NCT04981041 and NCT04695106) investigating DAT with ticagrelor/prasugrel after ACS are not expected for a few years.
This study had a number of limitations. First, it was inherently limited by its retrospective and observational nature. Second, the small number of events in a relatively short period of time limited the ability to compare antithrombotic regimens, and the study was expectedly underpowered to detect differences in MACCE. Few covariates could be tested in multivariate analysis due to the possibility of overfitting. In particular, we were unable to account for the effect of different bleeding and ischemic risk profiles. A longer follow-up period may have increased the number of events; however, this would risk diluting the true difference between the regimens which hardly ever consist of TAT beyond 1 month in modern practice. Despite this, we were still able to observe a significant difference in bleeding. Third, patient adherence with the assigned antithrombotic therapy could not be accurately assessed except by confirming it remained unchanged at hospital discharge and at first clinic follow-up 6–12 weeks post-PCI or upon readmission for a bleeding/ischemic event. Fourth, no adjustment was made for multiple testing, and the results should be considered hypothesis-generating.
5. Conclusions
In patients with AF undergoing PCI with stents, ≤1 week of TAT was associated with a lower 30-day incidence of clinically relevant bleeding compared with 1 month of TAT. No significant difference in MACCE between these two groups was observed. Our findings support the rationale for prospective studies investigating the safety and efficacy of DAT regimens with ticagrelor or prasugrel immediately after PCI.
Declaration of interest
AMK Rothman reports research support from Abbott, Medtronic, Endotonix, SoniVie, Gradient, NXT Biomedical, Novartis, JnJ, and Apollo. RF Storey reports research grants and personal fees from AstraZeneca and Cytosorbents, and personal fees from Alfasigma, Chiesi, Daiichi Sankyo, Idorsia, Novartis, Novo Nordisk, Pfizer, PhaseBio, and Tabuk. PD Morris was funded by the Wellcome Trust [214567/Z/18/Z]. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
MA Sammut was involved in the conception and design, analysis and interpretation of data, drafting of the paper, and the final approval of the version to be published. RF Storey was involved in the conception and design, interpretation of data, critical revision for intellectual content, and the final approval of the version to be published. D Conway, J Iqbal, A Krishnamurthy, KP Morgan, PD Morris, JD Richardson, AMK Rothman, and JP Gunn were involved in the critical revision for intellectual content and final approval of the version to be published. All authors agree to be accountable for all aspects of the work.
Ethical approval
This study was approved by the ethics committee of The University of Sheffield (reference 059596) and by the Clinical Research and Innovation Office of Sheffield Teaching Hospitals National Health Service (NHS) Foundation Trust. Informed patient consent was not required due to the nature of the study’s design.
Supplemental Material
Download Zip (70 KB)Acknowledgements
The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14779072.2024.2374366.
Additional information
Funding
References
- Byrne RA, Rossello X, Coughlan JJ, et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur Heart J. 2023 Aug 25; 44(38):3720–3826. doi: 10.1093/eurheartj/ehad191
- Knuuti J, Wijns W, Saraste A, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020 Jan 14;41(3):407–477. doi: 10.1093/eurheartj/ehz425
- Connolly S, Pogue J, Hart R, et al. Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the Atrial fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events (ACTIVE W): a randomised controlled trial. Lancet. 2006 Jun 10;367(9526):1903–12.
- Schömig A, Neumann FJ, Kastrati A, et al. A randomized comparison of antiplatelet and anticoagulant therapy after the placement of coronary-artery stents. N Engl J Med. 1996 Apr 25;334(17):1084–9.
- van Rein N, Heide-Jørgensen U, Lijfering WM, et al. Major bleeding rates in atrial fibrillation patients on single, dual, or triple antithrombotic therapy. Circulation. 2019 Feb 5;139(6):775–786.
- Gibson CM, Mehran R, Bode C, et al. Prevention of bleeding in patients with atrial fibrillation undergoing PCI. N Engl J Med. 2016 Dec 22;375(25):2423–2434. doi: 10.1056/NEJMoa1611594
- Cannon CP, Bhatt DL, Oldgren J, et al. Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. N Engl J Med. 2017 Oct 19;377(16):1513–1524. doi: 10.1056/NEJMoa1708454
- Lopes RD, Heizer G, Aronson R, et al. Antithrombotic therapy after acute coronary syndrome or PCI in atrial fibrillation. N Engl J Med. 2019 Apr 18;380(16):1509–1524. doi: 10.1056/NEJMoa1817083
- Vranckx P, Valgimigli M, Eckardt L, et al. Edoxaban-based versus vitamin K antagonist-based antithrombotic regimen after successful coronary stenting in patients with atrial fibrillation (ENTRUST-AF PCI): a randomised, open-label, phase 3b trial. Lancet. 2019 Oct 12;394(10206):1335–1343. doi: 10.1016/S0140-6736(19)31872-0
- Gargiulo G, Goette A, Tijssen J, et al. Safety and efficacy outcomes of double vs. triple antithrombotic therapy in patients with atrial fibrillation following percutaneous coronary intervention: a systematic review and meta-analysis of non-vitamin K antagonist oral anticoagulant-based randomized clinical trials. Eur Heart J. 2019 Dec 7;40(46):3757–3767. doi: 10.1093/eurheartj/ehz732
- Lopes RD, Hong H, Harskamp RE, et al. Optimal antithrombotic regimens for patients with atrial fibrillation undergoing percutaneous coronary intervention: an updated network meta-analysis. JAMA Cardiol. 2020 May 1;5(5):582–589. doi: 10.1001/jamacardio.2019.6175
- Galli M, Andreotti F, D’Amario D, et al. Dual therapy with direct oral anticoagulants significantly increases the risk of stent thrombosis compared to triple therapy. Eur Heart J Cardiovasc Pharmacother. 2020 Apr 1;6(2):128–129. doi: 10.1093/ehjcvp/pvz030
- Galli M, Andreotti F, Porto I, et al. Intracranial haemorrhages vs. stent thromboses with direct oral anticoagulant plus single antiplatelet agent or triple antithrombotic therapy: a meta-analysis of randomized trials in atrial fibrillation and percutaneous coronary intervention/acute coronary syndrome patients. Europace. 2020 Apr 1;22(4):538–546. doi: 10.1093/europace/euz345
- Capodanno D, Di Maio M, Greco A, et al. Safety and efficacy of double antithrombotic therapy with non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation undergoing percutaneous coronary intervention: a systematic review and meta-analysis. J Am Heart Assoc. 2020 Aug 18;9(16):e017212.
- Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007 Nov 15;357(20):2001–2015. doi: 10.1056/NEJMoa0706482
- Wallentin L, Varenhorst C, James S, et al. Prasugrel achieves greater and faster P2Y12receptor-mediated platelet inhibition than clopidogrel due to more efficient generation of its active metabolite in aspirin-treated patients with coronary artery disease. Eur Heart J. 2008 Jan;29(1):21–30. doi: 10.1093/eurheartj/ehm545
- Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009 Sep 10;361(11):1045–1057. doi: 10.1056/NEJMoa0904327
- Storey RF, Angiolillo DJ, Patil SB, et al. Inhibitory effects of ticagrelor compared with clopidogrel on platelet function in patients with acute coronary syndromes: the PLATO (PLATelet inhibition and patient Outcomes) PLATELET substudy. J Am Coll Cardiol. 2010 Oct 26;56(18):1456–1462. doi: 10.1016/j.jacc.2010.03.100
- Schulman S, Kearon C Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005 Apr;3(4):692–694. doi: 10.1111/j.1538-7836.2005.01204.x
- Kaatz S, Ahmad D, Spyropoulos AC, et al. Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: communication from the SSC of the ISTH. J Thromb Haemost. 2015 Nov;13(11):2119–2126. doi: 10.1111/jth.13140
- Garcia-Garcia HM, McFadden EP, Farb A, et al. Standardized end point definitions for coronary intervention trials: the academic research consortium-2 consensus document. Circulation. 2018 Jun 12;137(24):2635–2650. doi: 10.1161/CIRCULATIONAHA.117.029289
- Costa F, van Klaveren D, James S, et al. Derivation and validation of the predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy (PRECISE-DAPT) score: a pooled analysis of individual-patient datasets from clinical trials. Lancet. 2017 Mar 11;389(10073):1025–1034. doi: 10.1016/S0140-6736(17)30397-5
- Dangas G, Baber U, Sharma S, et al. Ticagrelor with or without aspirin after complex PCI. J Am Coll Cardiol. 2020 May 19;75(19):2414–2424. doi: 10.1016/j.jacc.2020.03.011
- Benjamin EJ, Muntner P, Alonso A, et al. Heart disease and stroke statistics-2019 update: a report from the american heart association. Circulation. 2019 Mar 5;139(10):e56–e528.
- De Caterina R, Agewall S, Andreotti F, et al. Great debate: triple antithrombotic therapy in patients with atrial fibrillation undergoing coronary stenting should be limited to 1 week. Eur Heart J. 2022 Oct 7;43(37):3512–3527. doi: 10.1093/eurheartj/ehac294
- de Veer A, Bennaghmouch N, Bor WL, et al. The WOEST 2 registry: a prospective registry on antithrombotic therapy in atrial fibrillation patients undergoing percutaneous coronary intervention. Neth Heart J. 2022 Jun;30(6):302–311.
- Zeymer U, Toelg R, Wienbergen H, et al. Current status of antithrombotic therapy and in-hospital outcomes in patients with atrial fibrillation undergoing percutaneous coronary intervention in Germany. Herz. 2023 Mar;48(2):134–140.
- Kitahara H, Yamashita D, Sato T, et al. Dual antithrombotic therapy with oral anticoagulant and P2Y12 inhibitors in patients with atrial fibrillation after percutaneous coronary intervention. J Cardiol. 2023 Sep;82(3):207–214. doi: 10.1016/j.jjcc.2023.06.002
- Verlinden NJ, Coons JC, Iasella CJ, et al. Triple antithrombotic therapy with aspirin, P2Y12 inhibitor, and warfarin after percutaneous coronary intervention: an evaluation of prasugrel or ticagrelor versus clopidogrel. J Cardiovasc Pharmacol Ther. 2017 Nov;22(6):546–551. doi: 10.1177/1074248417698042
- Oldgren J, Steg PG, Hohnloser SH, et al. Dabigatran dual therapy with ticagrelor or clopidogrel after percutaneous coronary intervention in atrial fibrillation patients with or without acute coronary syndrome: a subgroup analysis from the RE-DUAL PCI trial. Eur Heart J. 2019 May 14;40(19):1553–1562. doi: 10.1093/eurheartj/ehz059
- Claessen BE, Henriques JP, Jaffer FA, et al. Stent thrombosis: a clinical perspective. JACC: Cardiovasc Interv. 2014 Oct;7(10):1081–92.
- Rheude T, Koch T, Joner M, et al. Ten-year clinical outcomes of drug-eluting stents with different polymer coating strategies by degree of coronary calcification: a pooled analysis of the ISAR-TEST 4 and 5 randomised trials. EuroIntervention. 2023 Feb 20;18(14):1188–1196. doi: 10.4244/EIJ-D-22-00781
- Amin AP, Wang TY, McCoy L, et al. Impact of bleeding on quality of life in patients on DAPT: insights from TRANSLATE-ACS. J Am Coll Cardiol. 2016 Jan 5;67(1):59–65. doi: 10.1016/j.jacc.2015.10.034
- Sorrentino S, Sartori S, Baber U, et al. Bleeding risk, dual antiplatelet therapy cessation, and adverse events after percutaneous coronary intervention: the PARIS registry. Circ Cardiovasc Interv. 2020 Apr;13(4):e008226. doi: 10.1161/CIRCINTERVENTIONS.119.008226
- Mehran R, Pocock S, Nikolsky E, et al. Impact of bleeding on mortality after percutaneous coronary intervention results from a patient-level pooled analysis of the REPLACE-2 (randomized evaluation of PCI linking angiomax to reduced clinical events), ACUITY (acute catheterization and urgent intervention triage strategy), and HORIZONS-AMI (harmonizing outcomes with revascularization and stents in acute myocardial infarction) trials. JACC: Cardiovasc Interv. 2011 Jun;4(6):654–664. doi: 10.1016/j.jcin.2011.02.011
- Orme RC, Parker WAE, Thomas MR, et al. Study of two dose regimens of ticagrelor compared with clopidogrel in patients undergoing percutaneous coronary intervention for stable coronary artery disease (STEEL-PCI). Circulation. 2018 Jun 21;138(13):1290–1300. doi: 10.1161/CIRCULATIONAHA.118.034790
- Ortega-Paz L, Bor W, Franchi F, et al. P2Y(12) inhibition in patients requiring oral anticoagulation after percutaneous coronary intervention: the SWAP-AC-2 study. JACC: Cardiovasc Interv. 2024 Apr 1.
- Capodanno D, Bhatt DL, Eikelboom JW, et al. Dual-pathway inhibition for secondary and tertiary antithrombotic prevention in cardiovascular disease. Nat Rev Cardiol. 2020 Apr;17(4):242–257. doi: 10.1038/s41569-019-0314-y