1. Introduction
Cardiac pacemakers have become the cornerstone of bradyarrhythmia management with an improvement of both morbidity and mortality. Nevertheless, conventional pacemakers come with the complications associated with transvenous leads and the cutaneous pocket. The concept of a wholly self-contained intracardiac pacemaker has been under consideration since the 1970s [Citation1]. It was only in 2012 that the inaugural implantation of a fully self-contained leadless cardiac pacemaker, Nanostim (St Jude Medical Inc.; now Abbott Medical Inc., MN, U.S.A.), occurred [Citation2]. However, Nanostim devices were subject to a recall in 2016 due to critical battery failure [Citation3]. Nanostim was later redesigned into the AVEIR pacing system. Prior to the introduction of the AVEIR DR dual chamber leadless pacemaker system (Abbott Medical Inc., MN, U.S.A.), Micra (Medtronic Inc., MN, U.S.A.) stood as the sole commercially available leadless pacemaker. Micra operates as a single-chamber ventricular leadless pacemaker, incorporating an atrioventricular (AV) synchronization algorithm facilitated by analysis of the signal generated by the built-in accelerometer. Nevertheless, Micra exhibits limitations in AV synchrony, higher tracking capability, mode switches, and device retrieval [Citation4].
2. AVEIR DR dual chamber leadless pacemaker system
The AVEIR DR stands as a ground-breaking advancement, representing the world’s first dual-chamber leadless pacing system. This innovation achieves dual-chamber pacing and sensing capabilities by integrating an atrial leadless pacemaker (AVEIR AR) and a ventricular leadless pacemaker (AVEIR VR) [Citation5]. The dimensions of the AVEIR VR and AVEIR AR are 38 mm and 32.2 mm in length, respectively, with both leadless pacemakers having a diameter of 6.5 mm. The fixation mechanism involves a helix on the distal end of the leadless pacemaker (LP), which penetrates the myocardium. The helix projection measures 1.63 mm. Additionally, three small sutures on the outside of the LP nosecone, mitigate counter-rotation post-deployment. Situated on the proximal end of the device is a docking button, serving as the interface between the delivery catheter and the leadless pacemaker. This feature facilitates both delivery and, when necessary, retrieval of the AVEIR LP. Pacing electrodes are located at the tip of the LP and the proximal portion of the device body separated by parylene coating to ensure electrical isolation. The tip electrode (cathode) at the center of the helix incorporates a single dose of dexamethasone sodium phosphate (DSP), to mitigate inflammation [Citation6]. Patient therapy customization is achieved by implanting an atrial or ventricular device singularly or combining both for dual-chamber support. The provision for upgrades accommodates evolving patient needs over time.
Significant improvements from the preceding Nanostim [Citation7] device includes 1) alterations to the docking button, transitioning from flexible cables to fixed, 2) modifications to the pacemaker length (42.3 mm to 38 mm) and diameter (18F to 19.5F), 3) updated battery chemistry to improve stability, 4) Enhanced battery capacity, and 5) updates to the electronic components while preserving the device functionality. With a pacing output of 1.25 V @ 0.4 ms and 100% pacing, the AVEIR VR device is anticipated to endure 16–18 years [Citation8].
The proprietary implant-to-implant (i2i) communication technology [Citation5] serves as the key to AVEIR DR dual-chamber leadless pacemaker functionality. It constitutes wireless bidirectional communication between atrial and ventricular pacemakers on a beat-to-beat basis, ensuring continuous AV synchrony. This pioneering technology leverages low-energy, subthreshold pulses between implanted devices using the blood pool and myocardial tissue. These high-frequency pulses of data are delivered seamlessly with each locally paced or sensed event, without compromising pacing or intrinsic sensing.
2.1. Implantation and retrieval of AVEIR
Each AVEIR LP is delivered percutaneously by catheter via the femoral vein into the designated chamber [Citation6]. The AVEIR Introducer is utilized to facilitate the introduction of the AVEIR Delivery and Retrieval Catheters into the patient’s vasculature (). The introducer, with dimensions of 25F inner diameter (ID)/27F outer diameter (OD), is available in lengths of 30 and 50 centimeters. At the distal end of the catheter, a pair of tethers is present, which inserts into the LP docking button. These tethers enable the delivery catheter to manipulate and drive the LP within the body. The pliable protective sleeve, capable of advancing to cover the leadless pacemaker during intravenous transit, can be securely locked and unlocked in position through the hub lock on the catheter shaft. The targeted implantation site for the AVEIR VR is in the lower to mid-septal area of the right ventricle, while the AVEIR AR is positioned at the base of the right atrial appendage ().
Figure 1. AVEIR ventricular leadless pacemaker, AVEIR leadless pacemaker Delivery and Retrieval catheter. (a) AVEIR ventricular leadless pacemaker, (b) Delivery catheter (c) Tri-loop snare of the Retrieval catheter grabbing the docking button of the leadless pacemaker. (Reproduced by the permission of Abbott Medical Inc., MN, U.S.A.).
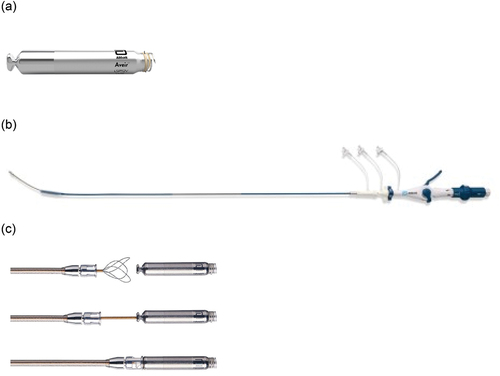
Figure 2. Chest radiographs of a patient who underwent implantation of this leadless cardiac pacemaker are shown in the posteroanterior (Panel a) and lateral (Panel b) projections.
Preceding fixation, the AVEIR Leadless Pacemakers can assess R-waves, impedance, and an initial capture threshold by direct contact of the electrode with the endocardial tissue. This electrical mapping before fixation aims to minimize the repositioning attempts in both the atrium and ventricle [Citation5]. For a dual-chamber implant procedure, the two LPs are paired using the programmer while the second LP is in tether mode. Once they are paired, the system is programmed to the appropriate dual-chamber settings and i2i performance is accessed. i2i settings are adjusted as needed to achieve acceptable i2i performance. If acceptable i2i performance cannot be obtained, repositioning of an LP may be considered at that point [Citation6].
The AVEIR devices feature an active fixation design employing a screw-in mechanism, to facilitate both implantation and long-term retrieval of the atrial and ventricular devices. The device is actively engaged with the endocardium with a mechanism on the delivery catheter that allows very careful and controlled rotation of 1.5 turns to fixate. The retrieval catheter shares similarities with the delivery catheter, with a notable difference being the inclusion of a tri-loop snare at the distal end. This snare is utilized to grasp the docking button of the LP during the retrieval process. Consequently, the retrieval catheter allows for the counter-rotation and subsequent removal of the LP [Citation6].
The AVEIR device is intended to be fully retrievable. When either the ventricular or atrial device reaches the end of its battery life the device would be retrieved, and a new device implanted. This contrasts with the Micra device where it is anticipated that most devices would be fully encapsulated at 12 months. It is widely accepted that the Micra device would be left in-situ and an additional device implanted [Citation9].
2.2. Clinical efficacy and safety
The LEADLESS Phase I trial [Citation10] investigated the safety and performance of the Nanostim LP. Despite demonstrating highly stable performance and providing reassuring safety results during intermediate-term follow-up, the Nanostim device faced a market recall due to critical battery failure.
LEADLESS Phase II Investigational Device Exemption study [Citation7] assessed the safety and efficacy of the redesigned AVEIR VR pacing system in patients with standard VVI(R) pacing indications. The study enrolled 200 patients across 43 centers in the U.S., Canada, and Europe, with a six-week follow-up period. The Implant success reached 98%, compared to 96.3% in Phase I, with 83.2% of the successful implants not requiring repositioning, in contrast to 70.2% in Phase I. Therapy efficacy, defined as a pacing capture threshold ≤2.0 V @ 0.4 ms and R-waves ≥5.0 mV was achieved in 96% of patients at six weeks post-implant. The study demonstrated a 95.9% safety endpoint achievement, supporting the use of the novel AVEIR pacing system for right ventricular pacing as an alternative to transvenous pacemakers.
The AVEIR DR i2i Investigational Device Exemption (IDE) study [Citation11] marked the first prospective, multicentre, single-group evaluation of the safety and performance of a dual-chamber leadless pacemaker system. The study enrolled 300 patients, with a successful implantation rate of 98.3%. Two pericardial effusion events were reported, and both were related to the atrial leadless pacemaker. Six intraprocedural and five post-procedural dislodgements of a leadless pacemaker occurred, and 10 of these dislodgements were attributed to the atrial device. A total of 271 patients (90.3%) were free from complications and the primary safety endpoint, freedom from complications (i.e. device- or procedure-related serious adverse events) at 90 days, was met, surpassing the performance goal of 78%. Both primary performance endpoints, adequate atrial capture threshold, and sensing amplitude were successfully achieved. Atrioventricular synchrony in 70% or more of the cardiac cycles was achieved in 97.3% of the patients. The study’s positive outcomes led to the U.S FDA approval of the AVEIR DR dual chamber pacing system.
The retrieval data of the Nanostim device [Citation3,Citation12] indicated an overall retrieval success rate of 87.6% over 9 years irrespective of implant duration. The AVEIR VR device employs an active fixation helix mechanism similar to its Nanostim device, designed for chronic retrievability.
Furthermore, AVEIR leadless pacemakers are designated as MR Conditional for full-body scans employing a 1.5T or 3T field strength MRI scanner.
2.3. Limitation of the AVEIR device
Presently, the available literature on the AVEIR device comprises short-term follow-up data, thereby leaving a gap in our understanding of its long-term safety profile and battery longevity. The ideal location for implantation of AVEIR ventricular LP is the lower- to mid-septal area [Citation6], as opposed to the high/mid-septal area commonly targeted for Micra implantation, potentially leading to variation in pacing behavior based on the chosen implantation site. Furthermore, comprehensive data regarding the retrieval of AVEIR LP devices after a decade of implantation remains absent from the existing research literature.
3. Conclusion
The AVEIR DR dual chamber pacing system stands as a programmable, modular innovation comprising two devices, collectively delivering dual-chamber rate-responsive bradycardia pacing. With encouraging outcomes and enhanced battery longevity, the AVEIR system holds significant promise in reshaping prevailing clinical practices and has the potential to emerge as the predominant choice for bradycardia management.
Declaration of interest
LT Toon has received an unrestricted research grant from Boston Scientific. PR Roberts receives consultancy fees from Boston Scientific and Medtronic. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
One peer reviewer has received consulting or presenter fees from Abbott, Bayer Healthcare, Biotronik, Biosense Webster, BMS/Pfizer, Boston Scientific, Cook Medical, Daiichi Sankyo, and Medtronic. Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Additional information
Funding
References
- Spickler JW, Rasor NS, Kezdi P. Totally self-contained intracardiac pacemaker. J Electrocardiol. 1970;3(3–4):325–331. doi: 10.1016/S0022-0736(70)80059-0
- Reddy, Knops, Sperzel, et al. Permanent leadless cardiac pacing: results of the LEADLESS trial. Circulation. 2014;129(14):1466–1471. doi: 10.1161/CIRCULATIONAHA.113.006987
- Neužil P, Petrů J, Chovanec M. Retrieval and replacement of a helix-fixation leadless pacemaker at 9 years post-implant. HeartRhythm Case Rep. 2023;9(4):258–262. doi: 10.1016/j.hrcr.2023.01.012
- Mitacchione, Schiavone, Gasperetti, et al. Atrioventricular synchronous leadless pacemaker: state of art and broadened indications. Rev Cardiovasc Med. 2021;22(2):395–401. doi: 10.31083/j.rcm2202045
- Abbott. Abbott DR brochure. 2023 [cited 2023 Dec 1]. Available from: https://www.cardiovascular.abbott/content/dam/cv/cardiovascular/hcp/products/cardiac-rhythm-management/pacemakers/aveir-dr/AVEIR-DR-Brochure.pdf
- Abbott. AVEIR dual chamber leadless pacemaker and delivery catheter instructions for use. 2023 [cited 2023 Dec 2]. Available from: https://manuals.sjm.com/Search-Form?re=North-America&cc=US&ln=all&ct=professional&qry=Aveir&ipp=10
- Reddy, Exner, Doshi, et al. Primary results on safety and efficacy from the LEADLESS II–phase 2 worldwide clinical trial. JACC: Clin Electrophysiol. 2022;8(1):115–117. doi: 10.1016/j.jacep.2021.11.002
- Reddy, Exner, Cantillon, et al. Percutaneous implantation of an entirely intracardiac leadless pacemaker. N Engl J Med. 2015;373(12):1125–1135. doi: 10.1056/NEJMoa1507192
- Medtronic. Micra™ AV MC1AVR1 device manual. 2023 [cited 2024 Jan 1]. Available from: https://manuals.medtronic.com/manuals/main/en_GB/manual/otherlang#
- Knops, Tjong, Neuzil, et al. Chronic performance of a leadless cardiac pacemaker: 1-year follow-up of the LEADLESS trial. J Am Coll Cardiol. 2015;65(15):1497–1504. doi: 10.1016/j.jacc.2015.02.022
- Knops, Reddy, Ip, et al. A dual-chamber leadless pacemaker. N Engl J Med. 2023;388(25):2360–2370. doi: 10.1056/NEJMoa2300080
- Reddy VY. Worldwide experience with leadless pacemaker retrievals: a worldwide nanostim experience out of 9y. In: Conference proceeding at APHRS Singapore 2022.; 2022 [cited 2023 Now 30].
