?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The focus of this research is to determine the conditions under which passivity of Cu is (with the possibility of pitting) or is not possible, in anticipated bentonite pore water compositions expected to contact a Cu-coated container in a deep geological repository. Cyclic voltammetry has been used to deduce the active/passive maps that define the environmental conditions (in terms of [Cl−], [], [
], pH, and temperature) under which the Cu surface could be active or passive. A range of temperatures (up to 80°C), pH values (neutral to alkaline), and electrolyte compositions have been investigated. Such a database of maps shows that active/passive conditions change with the pH, temperature, and the type and concentration of anions. These maps will provide a basis for container corrosion models, as well as guidelines regarding the water compositions used when compacting the bentonite, the repository saturation time, and the optimum container spacing.
This paper is part of a supplement on the 6th International Workshop on Long-Term Prediction of Corrosion Damage in Nuclear Waste Systems.
Introduction
The present design of Canadian nuclear fuel waste containers for the permanent disposal of high level nuclear waste comprises a thick-walled steel vessel coated with a thin Cu layer (∼3 mm) to provide corrosion protection [Citation1]. After emplacement in a deep geologic repository (DGR), where it will be surrounded with compacted bentonite clay, the container will be subjected to a number of corrosion processes [Citation2–4]. Since exposure conditions will initially be oxidising due to the presence of O2 trapped on sealing the DGR and the presence of oxidants produced by the radiolysis of the groundwater, there is a possibility of localised corrosion, in the form of pitting. A requirement for pitting to occur is that the Cu surface must be passive, the condition required to support the separation of anodes and cathodes which is a prerequisite for this form of corrosion.
While the redox conditions established at the container surface are key in determining the susceptibility to localised corrosion, the composition of the bentonite pore water in contact with the container (near-field pore water), and the surface temperature established by the radiation decay processes in the wasteform inside the container will have a major influence on whether the required passive conditions can be established. The near-field pore water composition will depend on the water added to compact the clay, the solubility of minerals within the clay and how they change as the surface temperature evolves, and the longer term influence of the encroaching groundwater. Attempts to measure and calculate the near-field pore water concentrations have been undertaken [Citation5]. For the critical early emplacement period when conditions will be oxidising, the near-field pore water composition is expected to be Cl−-dominated, with small [] and [
] and a pH close to neutral, .
Table 1. Predicted Canadian near-field pore water chemistry.
A number of studies in potable waters show that these anions (Cl−, ,
) and the pH are dominant chemical influences on the pitting of Cu [Citation6]. Increases in pH and [
] support the required passivation whereas Cl− and
typically promote the breakdown of passivating oxides that can result in pit initiation. Pitting has been demonstrated in more saline environments, but generally at high pH, although at sufficiently high [Cl−] extensive film breakdown leads to active general corrosion conditions. Thus, whether or not pitting occurs is commonly a balance between factors that promote passivation and factors that lead to film breakdown and the establishment of general corrosion.
A common method of assessing the possibility of localised corrosion is to measure and compare the corrosion potential (Ecorr), the pitting breakdown potential (Eb), and the repassivation potential (Erp). This is commonly achieved by applying a potentiodynamic scan to the metal immersed in the solution of interest [Citation7]. Many such measurements have been made in Cu systems by Cong et al. [Citation6], who determined the effects of Cl−, ,
and OH− on Eb and Erp and derived simple expressions for the relationships between the potentials and anion concentrations. These expressions were developed from measurements at room temperature, pH 8.3 and pH 9.5, and with anion concentrations in the range 10−4–0.1 mol L−1, and the authors caution that the applicability of these expressions outside the range of measurements is uncertain.
The majority of these measurements suggest pitting should not occur under DGR conditions, an observation supported by a small number of long-term exposure measurements which indicate general corrosion leading to surface roughening is dominant [Citation8–10]. However, Eb and Erp are known to be distributed parameters, and considerable uncertainty remains with regard to the susceptibility to pitting. Owing to the small corrosion allowance of the Cu-coated container design, it is important to increase the level of confidence that pitting will not occur and that the Cu coating will corrode actively. The first step in establishing such confidence is to determine whether the container surface will be active or passive under DGR conditions. If active behaviour is assured then the possibility of pitting can be dismissed. If passive behaviour is possible then a more thorough evaluation of the relative values of Ecorr, Eb and Erp is required to determine the susceptibility to pitting.
Here we present our determination of active/passive (A/P) maps, which define the conditions (in terms of [Cl−], [], [
], pH and T) under which passivity of Cu is (with the possibility of pitting) or is not possible. These A/P maps were determined from a series of cyclic voltammograms (CV), performed over a wide range of anion concentrations, pH and temperatures encompassing those anticipated in a DGR, and will provide a basis for container corrosion models, as well as guidelines regarding the water compositions used when compacting the bentonite, the repository saturation time, and the optimum container spacing.
Experimental
Electrodes and electrochemical cell
Phosphorous-doped, oxygen-free wrought copper supplied by the Swedish Nuclear Fuel and Waste Management Company (SKB-Cu) was used in the present experiments. The composition is presented in . The SKB-Cu was machined into bars (0.7 × 0.7 × 19.4 cm3) and a bare metal bar without casting and/or coating was used as the working electrode to avoid crevice corrosion occurring at the interface between the electrode and resin holder. Prior to an experiment, the Cu electrode was first ground with 1200 grit SiC papers lubricated with water, then rinsed thoroughly with Type I water (resistivity >18.2 MΩ cm) to remove any polishing residue, and dried in a stream of ultrapure Ar gas.
Table 2. Composition of SKB-Cu.
All electrochemical measurements were performed using a conventional three-electrode glass cell. Approximately 3.5 cm of the Cu bar electrode was immersed in solution, yielding an exposed area of ca. 10 cm2. A Pt sheet rolled into a cylinder was used as the counter electrode and a standard calomel electrode (SCE, 0.242 V vs. SHE) was used as the reference electrode; both were separated from the main compartment by porous frits. The cell was housed inside a Faraday cage to reduce electrical noise from external sources. Temperature (up to 80°C) was controlled by water circulating in a heating jacket surrounding the cell.
Electrolytes and environment
Electrolyte solutions were prepared with analytical grade reagents and Type I water obtained using a NANOPure system. All water was purged with Ar gas for one hour prior to the preparation of solutions. Once in the electrochemical cell, the solution was purged for a minimum of 20 min prior to an experiment to ensure an anoxic environment (a trace of O2 remained however). Subsequently, the solution was continuously purged with a stream of Ar gas during the whole course of an experiment.
The following electrolytes were investigated, . Since we expected that alkalinity would promote the passivity of copper, the solution pH was gradually increased from neutral until passivity was induced by adding NaOH and measuring with a pH metre (Thermo Scientific, Orion Star A211) at room temperature.
Table 3. Electrolyte systems and concentrations tested.
Electrochemical procedures
The Cu electrode was first cathodically treated to remove easily reduced oxides and desorbable contaminants, at −1.5 V/SCE and then at −1.15 V/SCE, each for 60 s. The electrode was then maintained at an open circuit condition for 30 min until Ecorr was well established. The potential was then swept from Ecorr in the positive (anodic) direction until the current reached 0.1 mA. The scan was then reversed (i.e. the potential swept in the negative direction) to avoid severe damage to the electrode, and the reverse scan terminated when the current dropped below −3 µA. To achieve reversibility/quasi-reversibility, the potential was swept at a low rate of 10 mV min−1. All electrochemical data were collected using a 1287 Solartron potentiostat connected to a computer equipped with CorrWare software.
Results and discussion
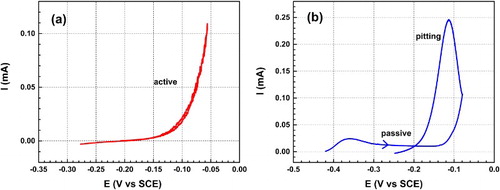
For an active system, the current increases exponentially with potential, indicating Cu to Cu+ oxidation and dissolution. In addition, the current almost retraces itself on the reverse scan, (a). On the other hand, for a passive system, an anodic peak is observed indicating formation of Cu2O, followed by a constant or even decreasing current through the passive region as the potential becomes more positive, until a critical potential (Eb) where the current abruptly increases, (b). This fairly sharp break (a rapid increase in current with only a small change in potential) in the polarisation curve usually indicates the breakdown of passivation and the commencement of pitting.
Once localised corrosion occurs, the pits remain active for some time after the potential scan direction is reversed, resulting in a higher current at potentials where passive behaviour was previously observed (producing hysteresis). The existence of a positive current hysteresis during the negative-going potential scan, as shown in (b), is considered to be indicative of pitting corrosion. The repassivation potential, Erp, is the potential where pits cease activity, and is generally taken to be the potential at which the current decreases back to the original passive current during the reverse scan.
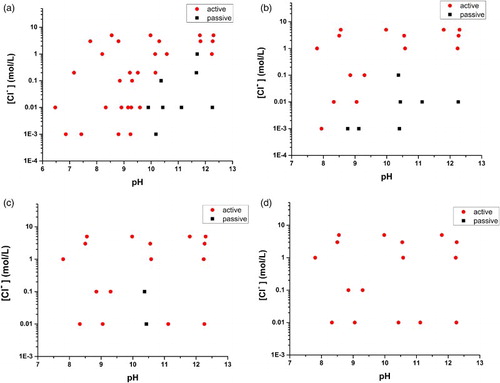
We have been using these criteria to construct A/P maps, maps which show whether the Cu behaves actively or passively in a given environment (in terms of [Cl−], [], [
], pH and T). Examples of these maps in a unary chloride system are shown in , which shows that, at room temperature, passivity is to be expected in alkaline solutions except at extreme [Cl−] values, and that the active-to-passive transition shifts to higher pH with [Cl−]. The observations that active conditions prevail for pH < 9.5 and that passivity does not occur for [Cl−] > 1 mol L−1 are consistent with thermodynamics, since no Cu2O nor CuO/CuOH2 forms below pH 9.5 at [Cl−] = 0.2 mol kg−1 and not at all at [Cl−] = 1.5 mol kg−1 as shown in the E-pH (Pourbaix) diagram [Citation11]. As the temperature increases, the regions within which passivity is possible contract, and completely disappear at 80°C, the highest temperature tested. This could be explained as the enhancement of the difference between the rates of Cu+ dissolution (Reaction (1)) and passive film formation in favour of dissolution when the temperature is increased,
(1)
(1) Whether the Cu will be active or passive depends on the anion concentration, the pH (OH− concentration), and the temperature. Generally speaking, alkalinity and carbonate are beneficial to passivity whereas salinity and higher temperature promote active corrosion. The location of the boundary between regions where only active behaviour is possible and those where passivation is possible (the A/P boundary) can be conveniently estimated by assuming the demarcation lies approximately half-way between the measured points where only active behaviour is observed and those where passive behaviour is indicated as possible in the A/P maps. The derived A/P boundaries are summarised in . Although these A/P boundaries are presented in as curves, they should be interpreted as associated with grey areas on both sides of the boundary, since the exact transition points have not been located.
Figure 3. A/P boundaries derived from A/P maps for (a) chloride-only, (b) sulphate-only, (c) chloride with 0.01 M sulphate, (d) carbonate/bicarbonate with 0.01 M sulphate, (e) sulphate with 0.01 M chloride, (f) carbonate/bicarbonate with 0.01 M chloride, (g) carbonate/bicarbonate with 0.1 M chloride, and (h) carbonate/bicarbonate with 0.01 M sulphate and 0.1 M chloride systems.
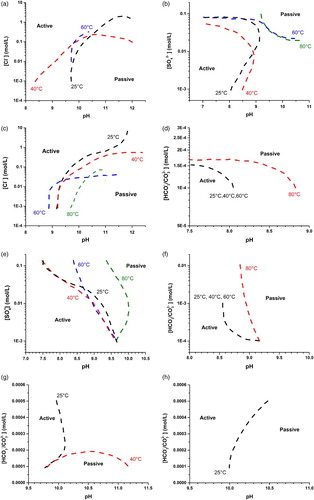
On examining , it is found that (Note that the list labels correspond to the sub-figures of ):
A/P boundaries shift to higher pH values as [Cl−] is increased. The region within which passivity is possible contracts and disappears as the temperature is increased;
A/P boundaries shift to lower pH when [
] is high (>0.01 mol L−1) suggesting that high [
] promotes passivity. Passivity is most likely at high [
] and high pH, but less likely as the temperature increases;
As for chloride-only solutions, regions within which passivity is possible decrease as [Cl−] and temperature increase, and sulphate expands the passive area slightly compared to the situation in chloride-only solutions;
Passive conditions dominate in the absence of chloride, except at very low carbonate concentrations and/or very high temperatures. Carbonate anion is generally considered to promote passivity, due to either its buffering capability, which prevents local acidity from developing, or the possible formation of a protective copper carbonate film, or both [Citation12–13];
Increased sulphate shifts A/P boundaries to lower pH, and temperature exerts a minor effect on the A/P boundary except at the highest temperature;
Active conditions prevail at low pH and low carbonate concentrations, and an increase in the carbonate concentration increases the likelihood of passivity. Temperature exerts little influence on the A/P boundary except at the highest temperature;
As [Cl−] increases to 0.1 mol L−1, passivity is only observed at low temperatures, irrespective of [
];
At a [Cl−] of 0.1 mol L−1, passivity is possible only at room temperature and when pH > 10, even if the solution contains bicarbonate/carbonate and sulphate.
Based on the A/P maps developed in the present work, active conditions should prevail for the anticipated ranges of near-field pore water compositions and pH in a Canadian DGR site (). Passive conditions are only expected to dominate in carbonate and moderate sulphate content environments with very little or no chloride at low temperatures, conditions which are universally unattainable in a Canadian DGR.
Conclusions
The conditions under which Cu is only active and those under which Cu can sometimes display passivity are summarised in A/P maps, which depend on the type of anions, the anion concentration, the pH, and the temperature.
A database of A/P maps has been built for Cu corrosion under a wide range of environmental conditions.
While chloride promotes active dissolution, carbonate favours the formation of a passive film
An increase in alkalinity increases the tendency towards passivation.
Passivity becomes less likely at higher temperatures.
Passivity is only expected in
/
solutions at extremely low Cl− concentrations.
Disclosure statements
No potential conflict of interest was reported by the authors.
ORCID
Additional information
Funding
References
- Implementing Adaptive Phased Management 2016 to 2020, Nuclear Waste Management Organization Report, March, 2016. Available from: https://www.nwmo.ca/~/media/Site/Reports/2016/03/30/14/24/Implementing_APM_2016_to_2020_March29.ashx?la=en
- Martino T, Partovi-Nia R, Chen J, et al. Mechanisms of film growth on copper in aqueous solutions containing sulphide and chloride under voltammetric conditions. Electrochim Acta. 2014;127:439–447. doi: 10.1016/j.electacta.2014.02.050
- Kwong G. Status of corrosion studies for copper used fuel containers under low salinity conditions. Toronto: Nuclear Waste Management Organization; 2011. (NWMO Technical Report TR 2011–14).
- Raiko H, Salo J-P. Design report of the disposal canister for twelve fuel assemblies. Olkiluoto: Posiva Oy; 1999. (Technical Report POSIVA-99-18).
- King F, Lilja C. Localised corrosion of copper canisters in bentonite pore water. Stockholm: Swedish Nuclear Fuel and Waste Management Company; 2014 . (SKB Technical Report, TR 13-27).
- Cong H, Michels HT, Scully JR. Passivity and pit stability behavior of copper as a function of selected water chemistry variables. J Electrochem Soc. 2009;156:C16–C27. doi: 10.1149/1.2999351
- Standard test method for conducting cyclic potentiodynamic polarization measurements for localized corrosion susceptibility of iron, nickel, or cobalt based alloys. ASTM G-61-86, ASTM International. 2014.
- King F. Overview of the corrosion behaviour of copper and steel used fuel containers in a deep geologic repository in the sedimentary rock of the Michigan Basin, Ontario. Toronto: Ontario Power Generation Nuclear Waste Management Division; 2005 . (OPG Technical Report No: 06819-REP-01300-10101).
- King F, Lilja C, Pedersen K, et al. An update of the state-of-the-art report on the corrosion of copper under expected conditions in a deep geologic repository. Stockholm: Swedish Nuclear Fuel and Waste Management Company; 2010 . (SKB Technical Report TR 10 67).
- Werme L, Sellin P, Kjellbert N. Copper canisters for nuclear high level waste disposal. Corrosion aspects. Stockholm: Swedish Nuclear Fuel and Waste Management Company; 1992 . (SKB Technical Report TR 92 26).
- Beverskog B, Puigdomenech I. Pourbaix diagrams for the system copper-chlorine at 5–100°C. Stockholm: Swedish Nuclear Power Inspectorate; 1998 . (SKI Technical Report 98:19).
- Adeloju SB, Duan YY. Influence of bicarbonate ions on stability of copper oxides and copper pitting corrosion. Br Corros J London. 1994;29:315–320. doi: 10.1179/000705994798267520
- Harrison DB, Nicholas DM, Evans GM. Remedial treatment for soft water copper pitting corrosion. Corros Mater. 2005;30(5):S1–S4.