Abstract
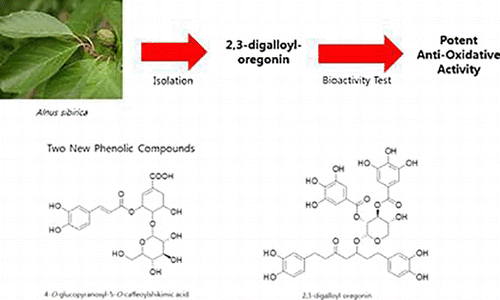
Two new phenolic compounds, 4-O-glucopyranosyl-5-O-caffeoylshikimic acid (1) and 2,3-digalloyl oregonin (2), were isolated along with eight known phenolic compounds (3–10) from an 80% acetone extract of Alnus sibirica leaves. The chemical structures of these compounds were elucidated using 1D/2D nuclear magnetic resonance and high resolution-MS. The anti-oxidative activities of these compounds were determined by assaying their 1,1-diphenyl-2-picrylhydrazyl radical and nitroblue tetrazolium superoxide anion scavenging activity. All of the isolated phenolic compounds (1–10) exhibited potent anti-oxidative activities. In particular, 2 and 4, which are diarylheptanoids, and 10 which is ellagitannin exhibited excellent anti-oxidative activities with almost the same potency as that of the positive controls L-ascorbic acid and allopurinol.
1. Introduction
Members of the Alnus species have been used in a number of traditional medicines such as cathartics, emetics, galactogogues, febrifuges, hemostatics, parasiticides, vermifuges, skin tonics and astringents (Guo et al. Citation2001). Alnus sibirica Fisch. ex Turcz. (AS) is geographically distributed in Korea, Japan, Northeast China and Russia, and the bark of this plant has been used as an antipyretic, expectorant, antiasthmatic and a health tea for alcoholism (Lee Citation1966). Previous studies on the chemical constituents of the Alnus species have led to the isolation of various tannins, flavonoids, diarylheptanoids and triterpenoids (Suga et al. Citation1972; Terazawa et al. Citation1984; Aoki et al. Citation1990; Lee et al. Citation1992, Citation1999; Jeong et al. Citation2000; Choi et al. Citation2012). These studies have shown that the Alnus species is a good source of diarylheptanoids, and that plants of this genus exhibit anti-oxidative, anti-inflammatory, anti-atopic, anti-bacterial and anti-adipogenic activities (Joo et al. Citation2009; Lee et al. Citation2010, Citation2013; Choi et al. Citation2012). This paper describes the isolation and structure elucidation of two new phenolic compounds along with eight known phenolic compounds. In addition, the anti-oxidative activities of these compounds were determining by assessing their 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical and nitroblue tetrazolium (NBT) superoxide anion scavenging activity.
2. Results and discussion
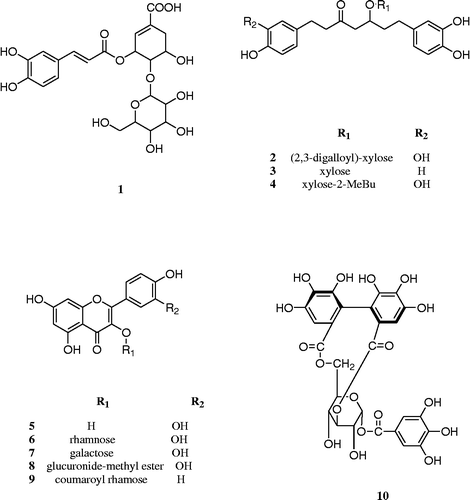
The 80% acetone extract of AS leaves was dissolved in water and filtered using Celite. The resulting filtrate was concentrated and applied to column chromatography using Ambelite XAD-2, Sephadex LH-20, MCI-gel, CHP 20P and ODS-B gel with a reversed phase medium pressure liquid chromatography (MPLC) system, which afforded 10 compounds including two new phenolic compounds (1 and 2) (Figure ). The known compounds (3–10) were identified as alnuside A (3, Kuroyanagi et al. Citation2005), alnuside C (4, Kuroyanagi et al. Citation2005), quercetin (5, Dutta et al. Citation2007), quercitrin (6, Lee et al. Citation2003), hyperoside (7, Lee et al. Citation2003), quercetin-3-O-β-d-glucuronide-methylester (8, Pacifico et al. Citation2013), kaempferol-3-O-α-l-(4″E-p-coumaroyl)-rhamnoside (9, Yang et al. Citation2010) and isocorilagin (10, Liu et al. Citation2008), respectively, by comparing their spectroscopic (MS and nuclear magnetic resonance (NMR)) data with literature values.
Compound 1 was isolated as a pale yellow amorphous powder. High-resolution (HR)-negative FAB-MS (m/z 497.1295 [M − H]− , calcd for C22H25O13, 497.1299) indicated its molecular formula was C22H26O13. In thin-layer chromatography (TLC), 1 was detected using a UV lamp at 254 nm as a dark brown spot by spraying with FeCl3 solution and a yellow spot by spraying with H2SO4 solution and heating.
The 1H NMR spectrum of 1 revealed three aromatic protons [δH 7.18 (d, J = 2.4 Hz, H-2′), 7.03 (dd, J = 2.4, 7.8 Hz, H-6′), 6.84 (d, J = 7.8 Hz, H-5′)] in an ABX spin system and two doublets at δH 7.70 (d, J = 16.2 Hz, H-7′), 6.33 (d, J = 16.2 Hz, H-8′) of a trans-double bond. The 13C NMR spectrum of 1 exhibited one carboxyl group (δC 167.3) and two hydroxyl bearing aromatic carbons (δC 148.2 and 146.2). These findings suggested the presence of a caffeoyl moiety.
The 1H NMR spectrum of 1 showed the presence of a proton singlet at δH 6.72 (1H, m, H-6), two coupled germinal protons at δH 2.79 (1H, dd, J = 5.4, 18.6 Hz, H-2a) and 2.30 (1H, dd, J = 5.4, 18.6 Hz, H-2b) and three oxymethine groups appearing at δH 5.83 (1H, brs, H-5), 4.24 (1H, dd, J = 5.1, 12.6 Hz, H-3) and 4.10 (1H, dd, J = 5.1, 12.6 Hz, H-4). 13C NMR spectrum of 1 revealed the presence of a shikimic acid moiety [δC 167.7 (C-7), 133.4 (C-1), 132.5 (C-2), 78.6 (C-4), 68.2 (C-5), 66.3 (C-3), 31.2 (C-6)].
The 1H and 13C NMR spectra of 1 also revealed a glucopyranosyl moiety, one methylene [δH 3.85 (m, H-6″a), 3.65 (m, H-6″b)], and five additional methine groups [δH 4.50 (d, J = 7.8 Hz, H-1″), 3.45 (m, H-3″), 3.34 (m, H-4″, H-5″), 3.23 (m, H-2″)] and at δC 103.6 (C-1″), 76.8 (C-5″), 76.4 (C-3″), 73.6 (C-2″), 70.2 (C-4″), 61.5 (C-6″). In addition, the large coupling constant (J = 7.8 Hz) of H-1″ at δH 4.50 in the 1H NMR spectrum of 1 suggested that the glucopyranoside was in a β-configuration.
The connectivities of caffeoyl and shikimic acid and glucose were elucidated by heteronuclear multiple bond coherence (HMBC) correlation signals. The HMBC spectrum of 1 showed a correlation between the H-1″ of glucose and C-4 of shikimic acid, and also showed the correlation between H-5 of shikimic acid and carbonyl C-9′ of caffeoyl moiety. Based on these results, compound 1 was elucidated as 4-O-β-d-glucopyranosyl-5-O-caffeoylshikimic acid. Compound 1 is the first reported shikimic acid conjugated with glucose and caffeic acid.
Compound 2 was isolated as a dark yellow amorphous powder. HR-negative FAB-MS (m/z 781.1978 [M − H]− , calcd for C38H37O18, 781.1980) indicated its molecular formula was C38H38O18. In TLC, 2 was detected as a dark blue spot by spraying with FeCl3 solution, and a brown spot by spraying with H2SO4 solution and heating.
The 1H and 13C NMR spectra of 2 showed two galloyl groups in the aromatic region [δH 7.04 (2H, s, galloyl-2, 6) and 7.06 (2H, s, galloyl-2′, 6′)] and [(δC 165.7 (C-7″″), 165.0 (C-7″″′), 145.0 (C-3″″, 3″″′, 5″″, 5″″′), 138.1 (C-4″″), 138.0 (C-4″″′), 120.4 (C-1″″), 120.3 (C-1″″′), 109.2 (C-6″″, 6″″′), 109.1 (C-2″″, 2″″′)] and one diarylheptanoid glycoside moiety compose of five methylenes [δH 1.70–1.75 (2H in total, m, H-6), 2.33–2.68 (8H, m, H-1, 2, 4, 6, 7] and a methine [δH 4.15 (1H, m, H-5) and 1.70–1.75 (2H in total, m, H-6)] and carbonyl carbon C-3 (δC 207.7), a secondary carbonyl carbon C-5 (δC 75.2), and five carbons [δC 28.6 (C-1), 44.8 (C-2), 47.6 (C-4), 37.3 (C-6) and 30.5 (C-7)] as heptanes moiety and two sets of caffeoyl groups [δH 6.67 (1H, d, J = 7.8 Hz, H-5′), 6.69 (1H, d, J = 7.8 Hz, H-5″, 6.59 (1H, d, J = 2.4 Hz, H-2′), 6.67 (1H, d, J = 2.4 Hz, H-2″), 6.39 (1H, dd, J = 2.4, 7.8 Hz, H-6′) and 6.48 (1H, dd, J = 2.4, 7.8 Hz, H-6″; δC 115.0 (C-2′), 115.1 (C-2″), 115.3 (C-5′, C-5″) and 119.4 (C-6′, C-6″), 144.6 (C-3′), 144.7 (C-3″), 143.0 (C-4″) and 142.9 (C-4′)] and a xylopyranosyl moiety [δH 5.32 (1H, m, H-3″′), 5.07 (1H dd, J = 7.8, 9.6 Hz, H-2″′), 4.81 (1H, d, J = 7.8 Hz, H-1″′), 4.04 (1H, m, H-5″′a), 3.95 (1H, m, H-4″′), and 3.48 (1H, m, H-5′b), and δC 101.2 (C-1″′), 75.7 (C-3″′), 71.7 (C-2″′), 68.4 (C-4″′) and 65.7 (C-5″′)] were observed.
These results indicated that 2 consisted of a diarylheptanoid glycoside (oregonin) and two galloyl groups. The locations of the galloyl units were determined as the C-3″′ and C-2″′ of the xylopyranosyl moiety by a downfield shift of C-3″′ and C-2″′ at δC 75.7 (C-3″′) and 71.7 (C-2″′) together with an upfield shift of C-4″′ to δC 68.4 (C-4″′) compared with oregonin (Lee et al. Citation1992).
The connectivity of the diarylheptanoid with xylose and galloyl groups was further confirmed by HMBC correlations. Especially, the HMBC spectrum of 2 showed a correlation between the H-1″′ of xylose and C-5 of the diarylheptanoid moiety. The HMBC spectrum of 2 also revealed correlations between H-2″′ and H-3″′ of xylose and each C-7″″, 7″″′ of the galloly moiety. Based on these results, the structure of compound 2 was elucidated as 2,3-digalloyl oregonin. Interestingly, a diarylheptanoid-conjugated ellagitannin from the leaves of Alnus hirsuta var. microphylla was previously reported (Lee et al. Citation1992); however, compound 2 is the first report of a diarylheptanoid-conjugated gallotannin.
Most of the isolated phenolic compounds from the leaves of AS exhibited potent scavenging activities of DPPH radical and NBT superoxide anion (Table ). The new compound (2) which is diarylheptanoid with galloyl moiety and isocorilagin (10) which is ellagitannin showed more strong anti-oxidative activity than L-ascorbic acid and almost same potency compared with allopurinol (Table ).
Table 1 DPPH radical and superoxide anion scavenging activities of compounds 1–10.
In particular, the anti-oxidative activity of 2, which contains a diarylheptanoid with two galloyl moieties, is more potent than that of 3 and 4 whose structures are similar to 2 (Table ). This result indicates the importance of polyhydroxy groups in the structure for the anti-oxidative effect.
3. Experimental
3.1. General experimental procedures
Column chromatographic isolations were performed using Ambelite XAD-2 (20–50 μm, Fluka AG, Buchs, Switzerland), Sephadex LH-20 (10–25 μm, GE Healthcare Bio-Science AB, Uppsala, Sweden), MCI-gel CHP 20P (75–150 μm, Mitsubishi Chemical, Tokyo, Japan), Toyopearl HW-40F (30–60 μm, Tosoh Corp., Tokyo, Japan) and ODS-B gel (40–60 μm, Daiso, Osaka, Japan). ODS-B gel was also used as a stationary phase for the middle pressure liquid chromatography (MPLC) system. TLC was carried out using a pre-coated silica gel 60 F254 plate (Merck, Darmastadt, Germany) with chloroform, methanol, and water (70:30:4, 80:20:2, volume ratio). Spots were detected under UV radiation (254 nm) and spraying with FeCl3 and 10% H2SO4 or anisaldehyde–H2SO4 followed by heating. The chemical structures were elucidated by several instrumental analyses. 1D NMR such as 1H (300 or 600 MHz) and 13C (75 or 150 MHz) NMR, 2D NMR such as proton–proton correlation spectroscopy (1H–1H COSY), heteronuclear single quantum coherence, and HMBC experiments were recorded with Gemini 2000 and VNS instruments (Varian, Palo Alto, CA, USA) at the centre for research facilities of Chung-Ang University. HR fast atom bombardment mass spectra were recorded with JMS-600W and JMS-700 instruments (JEOL, Tokyo, Japan) at the National Center for Inter-University Research facilities at Seoul National University.
3.2. Plant material
AS leaves were collected from Mt. Guksabong in Dongjak-gu, Seoul, Korea in August 2010 and its identity was confirmed by Prof. M.W. Lee (Pharmacognosy Lab, Laboratory of Pharmacognosy and Natural Product Derived Medicine, College of Pharmacy, Chung-Ang University) and C.I. Lee (Kwang-Leung Korean National Arboretum in Pocheon, Korea). A voucher specimen (MR2010-08) has been deposited at the herbarium of the College of Pharmacy, Chung-Ang University.
3.3. Extraction and isolation
AS leaves (15 kg) were extracted with 80% acetone at room temperature. The resulting extract was concentrated by removing the acetone under vacuum, which afforded 1062 g of material. After acetone evaporation, water liquid was filtered through Celite 545 (Duksan Pure Chemicals Co. Ltd, Korea). The resulting filtrate (764 g) was applied to Ambelite XAD-2 (20–50 μm, 10 kg, 70 × 50 cm) and eluted using a graded H2O, 50–100% MeOH solvent system yielding six fractions (AS-1 to AS-6). Repeated column chromatography of fraction AS-4 (225 g) using a Sephadex LH-20 column (25–100 μm, 2000 g, 10 × 120 cm, 0–100% MeOH in H2O) yielded seven subfractions (AS-4-1 to AS-4-7). Fraction AS-4-3 (94 g) was applied to a Sephadex LH-20 column (25–100 μm, 2000 g, 10 × 120 cm, 0–100% MeOH in H2O), which yielded eight subfractions (AS-4-3-1 to AS-4-3-8). Repeated column chromatography of fraction AS-4-3-2 using an ODS gel column (50 μm, 150 g, 3 × 50 cm, 0–100% MeOH in H2O) and Toyopearl HW-40F (40 μm, 120 g, 3 × 40 cm) yielded [4-O-glucopyranosyl-5-O-caffeoylshikimic acid] (1, 370 mg). Fraction AS-4-3-4 (9.79 g) was applied to an MCI gel column (50 μm, 400 g, 3 × 50 cm, 0–100% MeOH in H2O) and an ODS gel column (50 μm, 250 g, 3 × 50 cm, 10–100% MeOH in H2O) to yield alnuside A (3, 80 mg). Subfraction AS-4-3-7 (3.78 g) was applied to an ODS gel column (50 μm, 250 g, 3 × 50 cm, 20–100% MeOH in H2O), which yielded quercitrin (6, 300 mg) and hyperoside (7, 22 mg). Subfraction AS-4-4 was applied to an MCI gel column (50 μm, 400 g, 3 × 50 cm, 0–100% MeOH in H2O), from which 13 subfractions (AS-4-4-1 to AS-4-4-13) were obtained. Subfraction AS-4-4-3 was sequentially applied to an ODS gel column (50 μm, 250 g, 3 × 50 cm, 0–100% MeOH in H2O), of which subfraction AS-4-4-3-11 was applied to a Toyopearl HW-40F column (40 μm, 120 g, 3 × 40 cm, 0–100% MeOH in H2O) to yield isocorilagin (10, 55 mg). Likewise, the subfraction AS-4-4-5 was applied to an ODS gel column (50 μm, 250 g, 3 × 50 cm, 0–100% MeOH in H2O), which yielded quercetin-3-O-β-d-glucuronide-methylester (8, 62 mg). In addition, 2,3-digalloyl oregonin (2, 66 mg) was obtained from subfraction AS-4-4-8 by column chromatography with an ODS gel column (50 μm, 250 g, 3 × 50 cm, 0–100% MeOH in H2O). Repeated column chromatography of fraction AS-5 (40.85 g) using a Sephadex LH-20 column (25–100 μm, 2000 g, 10 × 120 cm, 50–100% MeOH in H2O), yielded seven subfractions (AS-5-1 to AS-5-7). The subfraction AS-5-2 (1.37 g) was applied to an MCI gel column (50 μm, 400 g, 3 × 50 cm, 40–100% MeOH in H2O) and an ODS gel column (50 μm, 150 g, 3 × 50 cm, 20–100% MeOH in H2O), which yielded alnuside C (4, 45 mg). Fraction AS-5-5 (0.97 g) was continuously applied to an ODS gel column (50 μm, 150 g, 3 × 50 cm, 50–100% MeOH in H2O), which resulted in kaempferol-3-O-α-l-(4″E-p-coumaroyl)-rhamnoside (9, 800 mg). Lastly, quercetin (5, 125 mg) was obtained by recrystallisation of AS-5-6 (0.49 g).
3.3.1. 4-O-Glucopyranosyl-5-O-caffeoylshikimic acid (1)
Pale yellow amorphous powder. [α]D: − 149.7° (c = 0.01, MeOH). IR (KBr) cm–1: 3391, 1697, 1601, 1517. HR-negative FAB-MS m/z: 497.1295 [M − H]– (calcd C22H25O13, 497.1299). 1H NMR (600 MHz, acetone-d6 + D2O): δ 7.70 (1H, d, J = 16.2 Hz, H-7′), 7.18 (1H, d, J = 2.4 Hz, H-2′), 7.03 (1H, dd, J = 2.4, 7.8 Hz, H-6′), 6.84 (1H d, J = 7.8 Hz, H-5′), 6.72 (1H, m, H-6), 6.33 (1H, d, J = 16.2 Hz, H-8′), 5.83 (1H, brs, H-5), 4.50 (1H, d, J = 7.8 Hz, H-1″), 4.24 (1H, m, H-3), 4.10 (1H, m, H-4), 3.85 (1H, m, H-6″a), 3.65 (1H, m, H-6″b), 3.45 (1H, t, J = 8.7 Hz, H-3″), 3.34 (2H, m, H-4″, H-5″), 3.23 (1H, m, H-2″), 2.79 (1H, m, H-2a), 2.30 (1H, m, H-2b). 13C NMR (150 MHz, acetone-d6 + D2O): δ 167.3 (C-7), 166.9 (C-9′), 148.2 (C-4′), 146.2 (C-3′), 145.4 (C-7′), 133.0 (C-1), 132.1 (C-2), 126.4 (C-1′), 121.9 (C-6′), 115.5 (C-5′), 114.3 (C-2′), 114.0 (C-8′), 103.6 (C-1″), 78.1 (C-4), 76.8 (C-5″), 76.4 (C-3″), 73.6 (C-2″), 70.2 (C-4″), 67.8 (C-5), 65.9 (C-3), 61.5 (C-6″), 30.7 (C-6).
3.3.2. 2,3-Digalloyl oregonin (2)
Dark yellow amorphous powder. IR (KBr) cm− 1: 3346, 1708, 1611, 1526. HR-negative FAB-MS m/z: 781.1978 [M − H]− (calcd C38H37O18, 781.1980) 1H NMR (600 MHz, acetone-d6 + D2O): δ 7.06 (2H, s, H-2″″′, 6″″′), 7.04 (2H, s, H-2″″, 6″″), 6.69 (1H, d, J = 7.8 Hz, H-5″, 6.67 (1H, d, J = 2.4 Hz, H-2′), 6.67 (1H, d, J = 7.8 Hz, H-5′), 6.59 (1H, d, J = 2.4 Hz, H-2″), 6.48 (1H, dd, J = 2.4, 7.8 Hz, H-6″), 6.39 (1H, dd, J = 2.4, 7.8 Hz, H-6′), 5.32 (1H, m, H-3″′), 5.07 (1H, dd, J = 7.8, 9.6 Hz, H-2″′), 4.81 (1H, d, J = 7.8 Hz, H-1″′), 4.15 (1H, m, H-5), 4.04 (1H, m, H-5″′a), 3.95 (1H, m, H-4″′), 3.48 (1H, m, H-5″′b), 2.33–2.68 (8H in total, m, H-1, 2, 4, 7), 1.70–1.75 (2H in total, m, H-6). 13C NMR (150 MHz, acetone-d6 + D2O): δ 207.7 (C-3), 165.7 (C-7″″), 165.0 (C-7″″′), 145.0 (C-3″″, 3″″′, 5″″, 5″″′), 144.7 (C-3″), 144.6 (C-3′), 143.0 (C-4″), 142.9 (C-4′), 138.1 (C-4″″), 138.0 (C-4″″′), 133.7 (C-1″), 132.8 (C-1′), 120.4 (C-1″″), 120.3 (C-1″″′), 119.4 (C-6′, 6″), 115.3 (C-5′, 5″), 115.1 (C-2″), 115.0 (C-2′), 109.2(C-6″″, 6″″′), 109.1 (C-2″″, 2″″′), 101.2 (C-1″′), 75.7 (C-3″′), 75.2 (C-5), 71.7 (C-2″′), 68.4 (C-4″′), 65.7 (C-5″′), 47.6(C-4), 44.8 (C-2), 37.3 (C-6), 30.5 (C-7), 28.6 (C-1).
3.4. Measurement of DPPH radical scavenging activity
Each sample was dissolved in absolute EtOH and added to a DPPH solution (0.1 mM, in absolute EtOH). After mixing gently for 30 min, optical densities were measured at 518 nm using a microplate reader (TECAN, Salzburg, Austria). L-ascorbic acid was used as a positive control.
3.5. Measurement of NBT/superoxide anion scavenging activity
Each sample was dissolved in 50 mM phosphate buffer (pH 7.5) containing 0.05 mM EDTA, 0.2 mM hypoxanthine, and 0.1 mM NBT. Next, xanthine oxidase (1.2 U/μL) was added to the mixture. After mixing gently for 30 min, optical densities were measured at 612 nm using a microplate reader (TECAN). Allopurinol was used as a positive control.
3.6. Statistical analysis
All data were expressed as the mean ± SD. Values were analysed by Student–Newman–Keuls test, and values of p < 0.05 were considered to be significantly different.
4. Conclusion
The activity-guided isolation of A. sibirica yielded 10 phenolic compounds (1–10) including two new compounds, 4-O-β-d-glucopyranosyl-5-O-caffeoylshikimic acid (1) and 2,3-digalloyl oregonin (2) which are the first reported shikimic acid conjugated with glucose and caffeic acid (1) and diarylheptanoid conjugated with gallotannin (2).
The phenolic compounds (1–10) showed potent antioxidative activities against DPPH and NBT radicals. Especially, 2 and 4 which are diarylheptaoid and 10 which is ellagitannin showed excellent anti-oxidative activities. The results suggest that the leaves of A. sibirica and the phenolic compounds isolated from these leaves are promising source of natural products that can be developed as anti-oxidant agents.
Supplementary material
The underlying research materials for this article can be accessed at http://dx.doi.org/10.1080/14786419.2015.1053087
Disclosure statement
No potential conflict of interest was reported by the authors.
1053087_Supplementary_material.pdf
Download PDF (676.2 KB)Additional information
Funding
Notes
1. These authors contributed equally to this work and should be considered as co-first authors.
References
- Aoki T, Ohta S, Suga T. 1990. Triterpenoids, diarylheptanoids and their glycosides in the flowers of Alnus speies. Phytochemistry. 29:3611–3614.
- Choi SE, Park KH, Kim MH, Song JH, Jin HY, Lee MW. 2012. Diarylheptanoids from the bark of Alnus pendula Matsumura. Nat Prod Sci. 18:106–110.
- Dutta NK, Mazumdar K, Mishra US, Dastidar SG, Park JH. 2007. Isolation and identification of a flavone (quercetin) from Butea frondosa bark. Khimiko-Farmatsevtischeskii Zhurnal. 41:37–39.
- Guo JX, Kimura T, But PPH, Sung CK. 2001. International collation of traditional and folk medicine. Vol. 4. Singapore: World Scientific Publishing.
- Jeong DW, Kim JS, Cho SM, Lee YA, Kim KH, Kim SW, Lee MW. 2000. Diarylheptanoids from the stem barks of Alnus hirsuta var sibirica. Korean J Pharmacogn. 31:28–33.
- Joo SS, Kim SG, Choi SE, Kim YB, Park HY, Seo SJ, Choi YW, Lee MW, Lee DI. 2009. Suppression of T cell activation by hirsutenone, isolated from the bark of Alnus japonica, and its therapeutic advantages for atopic dermatitis. Eur J Pharmacol. 614:98–105.
- Kuroyanagi M, Shimomae M, Nagashima Y, Muto N, Okuda T, Kawahara N, Nakane T, Sano T. 2005. New diarylheptanoids Alnus japonica and their antioxidative activity. Chem Pharm Bull. 53:1519–1523.
- Lee SJ. 1966. Korea folk medicine. Seoul: Seoul National University Publishing Center Press.
- Lee O, Choi MH, Ha SH, Lee GW, Kim JY, Park GM, Lee MW, Choi YW, Kim MG, Oh CH. 2010. Effect of pedunculagin investigated by non-invasive evaluation on atopic-like dermatitis in NC/Nga mice. Skin Res Technol. 16:371–377.
- Lee MW, Jeong DW, Lee YA, Park MS, Toh SH. 1999. Flavonoids from the leaves of Alnus hirsuta. YakhakHoegi. 43:547–552.
- Lee JH, Lee KN, Lee CW, Chun HJ, You IS, Lim JN, Baek SH. 2003. The inhibitory effects of quercitrin from Houttuynia cordata against cadmium induced cytotoxicity. J Korean Chem Soc. 47:175–178.
- Lee MN, Song JY, Chin YW, Sung SH. 2013. Anti-adipogenic diarylheptanoids from Alnus hirsuta var sibirica on 3T3-L1 cells. Bioorg Med Chem Lett. 23:2069–2073.
- Lee MW, Tanaka T, Nonaka GI, Nishioka I. 1992. Hirsunin, an ellagitannin with a diarylheptanoid moiety, from Alnus hirsuta var microphylla. Phytochemistry. 31:967–970.
- Liu X, Cui C, Zhao M, Wang J, Luo W, Yang B, Jiang Y. 2008. Identification of phenolics in the fruit of emblica (Phyllanthus emblica L.) and their antioxidant activities. Food Chem. 109:909–915.
- Pacifico S, D'Abrosca B, Scognamiglio M, Gallicchio M, Galasso S, Monaco P, Fiorentino A. 2013. Antioxidant polyphenolic constituents of Vitis × Labruscana cv. ‘Isabella’ leaves. Open Nat Prod J. 6:5–11.
- Suga T, Iwata T, Asakawa Y. 1972. Chemical constituents of the male flower of Alnus pendula (Betulaceae). Bull Chem Soc Jpn. 45:2058–2060.
- Terazawa M, Miyake M, Okuyama H. 1984. Phenolic compounds in living tissue of woods. V. Teddish orange staining in keyamahannoki (Alnus hirsuta) and hannoki (A. japonica) [Betulaceae] caused by the interaction of hirsutoside and catechol oxidase after cutting the woods. Mokuzai Gakkaishi. 30:601–607.
- Yang NY, Tao WW, Duan JA. 2010. Antithrombotic flavonoids from the faeces of Trogopterus xanthipes. Nat Prod Res. 24:1843–1849.