Abstract
Phytochemical examination of Clausena lenis Drake (Rutaceae), collected in Thailand, led to the isolation of seven coumarins, four furoquinolines, two amides, and one flavonoid glycoside. Four of these compounds, one coumarine derivative named as gravelliferone A (3), two furoquinoline derivatives (kokusagenin A (8) and B (9)) and one amide, clausenalansamide H (13), are reported for the first time. Compound 3 was isolated from the root bark, compound 8 from the stem bark and compounds 9 and 13 from the leaves. The molecular structures of all isolated compounds were established by means of NMR experiments combined with mass spectrometry. Preliminary tests of the lipophilic stem bark extract against various human pathogenic bacteria strains revealed promising effects against Staphylococcus aureus ATCC 43300.
1. Introduction
The small palaeotropically distributed genus Clausena (Aurantioideae, Rutaceae) consists of twenty-five woody species occurring in various forest types (Molino Citation1994). In Thailand, eight taxa occur (Molino Citation1994). Several species, such as C. excavata Burm. f., C. harmandiana (Pierre) Guillaumin and C. anisata (Willd.) Hook. f. ex Benth., are widely used by local people for ethnomedicinal and other purposes (Albaayit et al. Citation2016; Mukandiwa et al. Citation2016a, Citation2016b). Phytochemical studies on Clausena species revealed the preponderance of prenylated/geranylated coumarins and carbazole alkaloids with interesting bioactivities (Ito et al. Citation2000; Sripisut et al. Citation2012; Cao et al. Citation2018; Yan et al. Citation2019). Generally, prenylation of specialised metabolites seems to be common within rutaceous species and might be of chemotaxonomic importance for the subfamily Aurantioideae (Lukaseder et al. Citation2009; Sakunpak et al. Citation2013; Ma et al. Citation2018a). The studied species Clausena lenis occurs in the border region between China, Thailand and Burma. Phytochemical data are only available from individuals occurring in China (He et al. Citation2003a, Citation2006; Liu et al. Citation2019; Yan et al. Citation2019) so far. Herein we report four hitherto undescribed compounds from C. lenis and screening results of antibacterial activities.
2. Results and discussion
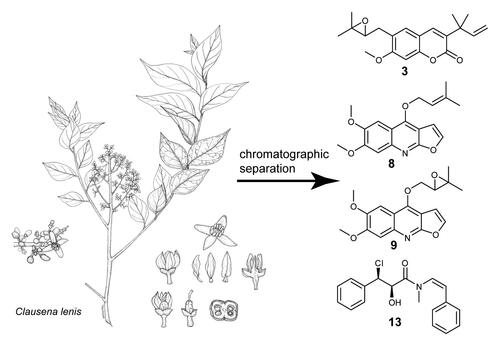
Chromatographic separation of leaf, stem bark and root bark extracts afforded 14 compounds, four of them (3, 8, 9 and 13) being described for the first time (). The root bark yielded the coumarins 3-(1,1-dimethylallyl) xanthyletin (1), xanthyletin (2), gravelliferone A (3), gravelliferone (4), 5-isopentenyloxy-8-(2',3'-epoxyisopentenyloxy)-psoralen (5), imperatorin (6), and heliettin (7). The stem bark afforded the furoquinoline kokusagenin A (8) and the leaf extract the furoquinolines kokusagenin B (9), kokusagenin (10), 1-[(6,7-dimethoxyfuro[2,3-b]quinolin-4-yl)oxy]-3-methylbutane-2,3-diol (11) together with the amides (2S*,3R*)-N-methyl-N-[(Z)-styryl]-3-phenyloxirane-2-carboxamide (12), clausenalansamide F (13) and the flavonoid glucoside myricitrin (14).
Figure 1. Structural formulae of the isolated compounds from Clausena lenis (1–14). 3-(1,1-dimethylallyl) xanthyletin (1), xanthyletin (2), gravelliferone A (3), gravelliferone (4), 5-isopentenyloxy-8-(2',3'-epoxyisopentenyloxy)-psoralen (5), imperatorin (6), heliettin (7), kokusagenin A (8), kokusagenin B (9), kokusagenin (10), 1-[(6,7-dimethoxyfuro[2,3b]quinolin-4-yl)oxy]-3-methylbutane-2,3-diol (11), (2S*,3R*)-N-methyl-N-[(Z)-styryl]-3-phenyloxirane-2carboxamide (12), clausenalansamide H (13) and myricitrin (14).
![Figure 1. Structural formulae of the isolated compounds from Clausena lenis (1–14). 3-(1,1-dimethylallyl) xanthyletin (1), xanthyletin (2), gravelliferone A (3), gravelliferone (4), 5-isopentenyloxy-8-(2',3'-epoxyisopentenyloxy)-psoralen (5), imperatorin (6), heliettin (7), kokusagenin A (8), kokusagenin B (9), kokusagenin (10), 1-[(6,7-dimethoxyfuro[2,3b]quinolin-4-yl)oxy]-3-methylbutane-2,3-diol (11), (2S*,3R*)-N-methyl-N-[(Z)-styryl]-3-phenyloxirane-2carboxamide (12), clausenalansamide H (13) and myricitrin (14).](/cms/asset/e79f8794-007f-4de2-88ed-0e103601f3a2/gnpl_a_1747455_f0001_b.jpg)
The presence of bioactive C- and O-prenylated pyrano- and furocoumarins is quite common within Clausena species (Ouyang et al. Citation2016; Ma et al. Citation2018b; Wongthet et al. 2018) and was also reported for C. lenis (Liu et al. Citation2018). In contrast, furoquinoline derivatives (8–11) seem to be an extraordinary feature of this species, since there has been only one report of such compounds within the genus Clausena (He et al. Citation2003b). Within Aurantioideae, furoquinoline derivatives are reported only for Aegle marmelos (Linn.) Correa (Mohammed et al. Citation2016). They are also known to occur in Vepris (Kouam et al. Citation2018) and Melicope species (Chen et al. Citation2003; Rasamison et al. Citation2016), which both belong to the subfamily Toddalioideae.
2.1. Structure elucidation of the new compounds
The molecular formula of 3 was determined as C20H24O4 based both on the [M + H]+ peak at m/z = 329.1754 (calcd 329.1747 for C20H25O4) and the [M + Na]+ peak at m/z = 351.1575 (calcd 351.1567 for C20H24O4Na). The 1H NMR spectra revealed three aromatic singlets at δH 7.52, 7.29 and 6.78 ppm lacking the typical doublet of ca J = 10 Hz for H-3 and H-4 between 6.0–6.5 ppm (H-3) and 7.7–8.2 ppm (H-4) of coumarins (Szabó et al. Citation1985). This indicated (1) a substitution at one of these two positions and (2) the presence of a trisubstituted coumarin. Based on the aromatic protons being singlets only a 3,6,7- or 4,6,7-substitution pattern was possible. A NOESY crosspeak between the signals at δH 7.52 and 7.29 ppm eliminated the 4,6,7-type, and allowed the assignment of these resonances to H-4 and H-5, respectively. As substituents, one methoxyl (δH 3.89 ppm), one epoxyprenyl and one dimethylallyl group were identified. The epoxyprenyl sidechain is characterised by two methyl groups at δH 1.34 and 1.40 ppm which show HMBC crosspeaks to one quarternary oxygenated carbon at δC 58.9 and an oxymethine carbon at δC 63.4 ppm. These two carbons show additional long-range crosspeaks to the remaining CH2-protons at δH 2.77 & 2.98 ppm. The dimethylallyl group is characterised by its vinyl signals at δH/δC 6.17/145.6 ppm and δH/δC 5.08 & 5.09/112.1 ppm, a 6H-singlet at δH/δC 1.47/26.1 ppm and a quarternary carbon at δC 40.1 ppm. Analyses of NOESY and HMBC spectra resolved the subsitution pattern: in the NOESY spectra the methoxyl group showed a crosspeak to the singlet at δH 6.78 ppm (H-8), whereas the CH2-group of the epoxyprenyl moiety had a correlation to δH 7.29 ppm (H-5) and finally both methyl groups of the dimethylallyl sidechain gave crosspeaks to H-4. This pattern was also proved by relevant HMBC correlations, e.g., crosspeaks δH/δC 7.52/160.1 (H-4/C-2), δH/δC 7.52/128.4 (H-4/C-5) or δH/δC 2.77 & 2.98/128.5 (H-1′′/C-5). Because no X-ray data were available the absolute stereochemistry of the epoxy substructure remains unclear. Therefore, the structure of compound 3 was elucidated as given in and denominated gravelliferone A. Significant HMBC and NOESY correlations of 3 are presented in Figure S1.
The molecular formula of 9 was determined as C18H19NO5 based on its [M + Na]+ peak at m/z = 352.1155 (calcd 352.1155 for C18H19NO5Na) and the [M + H]+ peak at m/z = 330.1334 (calcd 330.1336 for C18H20NO5). The 1H as well as the 13C NMR spectra revealed the typical signals of a furan moiety with resonances at δH 7.59/δC 142.93 ppm and δH 6.93/δC 104.16 ppm, where the protons showed the characteristic small doublet coupling of J = 2.7 Hz (Nunes et al. Citation2005). Additionally two aromatic singlet signals at δH 7.51 and 7.39 ppm were observed, suggesting the presence of a trisubstituted furoquinoline. This assumption was supported by the detection of two methoxyl groups (both δH 4.03 ppm) and one oxy-epoxyprenyl group, the latter characterised by two methyl groups, epoxycarbons at δC 58.3 and 61.4 ppm, and the oxymethylene signal at δH/δC 4.69 & 4.82/70.6 ppm. The substitution pattern was determined with the information extracted from 2 D NMR spectra, allowing complete signal assignment. The methoxyl groups showed NOESY crosspeaks to δH 7.51 ppm (H-5) and 7.39 ppm (H-8), locating them at positions C-6 and C-7, whereas H-3 (δH 6.96 ppm) gave a NOESY correlation to the OCH2-protons of the epoxyprenyl sidechain. Therefore, the structure of compound 9 was established as given in and denominated kokusagenin B.
Compound 8 had a molecular formula of C18H19NO4 based on its HR-ESI-MS data at m/z = 336.1223 [M + Na]+ (calcd. 336.1206 for C18H19NO4Na) and showed 1H NMR spectra similar to 9, with the only difference in the isoprenyl sidechain. The epoxide functionality was replaced by a double bond, characterised by the olefinic signal at δH/δC 5.64/119.3 ppm. NOESY correlations from the methoxyl groups to H-5 and H-8 and from the prenyl sidechain to H-3 revealed the same substitution pattern as for compound 9. Similar to 3, the absolute configuration of the oxirane remains unclear. Compound 8 was shown to be a new furoquinoline alkaloid as well, and was denominated kokusagenin A. The 1H and 13C NMR data of 8 and 9 are listed in Table S1.
Compound 13 was denominated as clausenalansamide H. This compound showed NMR spectra similar to those of clausenalansamide E (Shen et al. Citation2017) and gave also the same molecular formula of C18H18NO2Cl, calcd. from its [M + Na]+ peak at m/z = 338.0917 (calcd 338.0918 for C18H18NO2ClNa). In the HMBC spectrum, a hydroxyl proton at δH 3.82 ppm showed a long-range correlation to the carbonyl carbon at δC 170.93 ppm, the oxymethin proton at δH 4.70 ppm gave a correlation to only a quarternary aromatic carbon at δC 137.92 ppm, whereas the chloromethin proton at δH 5.27 ppm had also long range crosspeaks to protonated aromatic carbons. These data confirmed 13 to be also a 3-hydroxy-4-chloro derivative, but compared to clausenalansamide E there are significant shift differences in the 1H as well as in the 13C spectra. So, the oxymethin resonances reported for clausenalansamide E are at δH/δC 4.86/72.6 ppm (for 13: 4.70/73.2 ppm) whereas those of the chloromethin are at δH/δC 5.04/62.5 ppm (for 13: 5.27/63.4 ppm). Therefore we assume that 13 is a diastereomer of clausenalansamide E with either R,R- or S,S-configuration. As we were also able to isolate the epoxide 12, it is hence likely that 13 was formed by addition of HCl, which is often present in traces in CHCl3 used in the isolation process.
2.2. Antibacterial assays
Lipophilic extracts from leaves and stem bark of C. lenis were tested against Staphylococcus aureus ATCC 25923, S. aureus ATCC 43300, Enterococcus faecium UCLA192, and E. faecalis ATCC 29212 (all gram-positive) as well as the gram-negative strains Escherichia coli ATCC 25922, Pseudomonas aeruginosa DMST 37166, Klebsiella pneumonia ATCC-BAA 1705, K. pneumoniae ATCC-BAA 1706, K. pneumoniae ATCC 700603, Acinetobacter baumannii ATCC 19606, Stenotrophomonas maltophilia DMST 19079, and Salmonella choleraesuis ATCC 10708. The antibiotics ciprofloxacin and ampicillin were used for positive control. The leaf extract showed only moderate activities, but the stem bark extract showed good results against S. aureus ATCC 43300 (IC50 24 µg/mL), E. faecium UCLA192 (IC50 24 µg/mL) and E. faecalis ATCC 29212 (IC50 24 µg/mL) (Table S2) but lack of plant material prevented identification of the active compounds.
3. Experimental
3.1. General experimental procedures
Technical details are provided in the supplementary material.
3.2. Plant material
The plant material was collected and identified by W. Aiyakool and N. Wongthet near Mueang Khong, Chiang Dao, Chiang Mai province, Thailand, in 2017. A voucher specimen (Aiyakool, W. No. 2017-186, BKF No. 194891) was deposited at the Bangkok Forest Herbarium (BKF) in 10900 Bangkok, Thailand.
3.3. Extraction and isolation
A detailed description is provided in the supplementary material.
3.4. Isolated compounds
Gravelliferone A (3): White amorphous powder; HR-ESI-MS m/z = 329.1754 [M + H]+ (calcd. 329.1747) and m/z = 351.1575 [M + Na]+ (calcd. 351.1567); UV max(MeOH/H2O) 222, 296 sh, 238 nm; NMR data are given in the supplementary material.
Kokusaginine A (8): White amorphous powder; HR-ESI-MS m/z = 336.1223 [M + Na]+ (calcd. 336. 1206). UV max(MeOH/H2O) 246, 250, 308 and 322 nm; NMR data see Table S1.
Kokusaginine B (9): White amorphous powder; HR-ESI-MS m/z = 330.1334 [M + H]+ (calcd. 330.1336) and m/z = 352.1155 [M + Na]+ (calcd. 352.1155); UV max(MeOH/H2O) 246, 250, 308 and 322 nm; NMR data see Table S1.
Clausenalansamide H (13): White amorphous powder; HR-ESI-MS m/z = 338.0917 [M + Na]+ (calcd. 338.0918); UV max(MeOH/H2O) 214 sh, 262 nm; NMR data are given in the supplementary material.
4. Conclusion
Thirteen lipophilic compounds, belonging to coumarins, furoquinolines, amides and one flavonoid glucoside, were isolated from root bark, stem bark and leaves of Clausena lenis. All these compounds were herein described for the first time for this plant species. Except the furoquinoline derivatives, the described compounds fit well to the array of compounds known from Clausena and related species from this subfamily. The antibacterial activity of the tested lipophilic extract from the stem bark suggests the presence of potential antibacterial agents but identification of these compounds was prevented by lack of plant material.
Supplemental Material
Download PDF (3.5 MB)Acknowledgements
We gratefully acknowledge Susanne Felsinger (NMR Center) and Peter Unteregger (MS Center), both Faculty of Chemistry, University of Vienna for recording NMR and mass spectra. Grateful acknowledgment is made to Erasmus plus scholarship to N. Wongthet, the Science Achievement Scholarship of Thailand (SAST) and Kasetsart University Research and Development Institute (KURDI) for funding.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Albaayit SF, Abba Y, Abdullah R, Abdullah N. 2016. Prophylactic effects of Clausena excavata Burum. f. leaf extract in ethanol-induced gastric ulcers. Drug Des Devel Ther. 10:1973–1986.
- Cao N, Chen Y, Ma X, Zeng K, Zhao M, Tu P, Li J, Jiang Y. 2018. Bioactive carbazole and quinoline alkaloids from Clausena dunniana. Phytochemistry. 151:1–8.
- Chen JJ, Duh CY, Huang HY, Chen IS. 2003. Furoquinoline alkaloids and cytotoxic constituents from the leaves of Melicope semecarpifolia. Planta Med. 69:542–546.
- He HP, Chen ST, Shen YM, Chen CX, Zhao YB, Hao XJ. 2003a. A novel dimeric coumarin from Clausena lenis. Chin Chem Lett. 14:1150–1153.
- He HP, Shen YM, Chen ST, He YN, Hao XJ. 2006. Dimeric coumarin and phenylpropanoids from Clausena lenis. Helv Chim Acta. 89(11):2836–2840.
- He HP, Shen YM, Zuo GY, Yang XS, Hao XJ. 2003b. Dinorditerpene, diterpenes, alkaloids, and coumarins from Clausena dunniana. Helv Chim Acta. 86(9):3187–3193.
- Ito C, Itoigawa M, Katsuno S, Omura M, Tokuda H, Nishino H, Furukawa H. 2000. Chemical constituents of Clausena excavata: isolation and structure elucidation of novel furanone-coumarins with inhibitory effects for tumor-promotion. J Nat Prod. 63(9):1218–1224.
- Kouam ADK, Bissoue AN, Tcho AT, Happi EN, Waffo AFK, Sewald N, Wansi JD. 2018. Antimicrobial furoquinoline alkaloids from Vepris lecomteana (Pierre) Cheek & T. Heller (Rutaceae). Molecules. 23(1):13.
- Liu YP, Wen Q, Hu S, Ma YL, Jiang ZH, Tang JY, Fu YH, Qiu SX. 2019. Furanocoumarins with potential antiproliferative activities from Clausena lenis. Nat Prod Res. 33(18):2631–2637.
- Lukaseder B, Vajrodaya S, Hehenberger T, Seger C, Nagl M, Lutz-Kutschera G, Robien W, Greger H, Hofer O. 2009. Prenylated flavanones and flavanonols as chemical markers in Glycosmis species (Rutaceae). Phytochemistry. 70(8):1030–1037.
- Ma YL, Liu YP, Zhang C, Zhao W-H, Shi S, He DN, Zhang P, Liu XH, Han TT, Fu YH. 2018a. Carbazole alkaloids from Clausena hainanensis with their potential antiproliferative activities. Bioorg Chem. 76:359–364.
- Ma YL, Zhang C, Zhao WH, Shi S, He DN, Zhang P, Liu XH, Han TT, Fu YH, Liu YP. 2018b. Bioactive furanocoumarins from the stems and leaves of Clausena hainanensis. Nat Prod Res. 32(18):2159–2164.
- Mohammed MMD, Ibrahim NA, El-Sakhawy FS, Mohamed KM, Deabes D. 2016. Two new cytotoxic furoquinoline alkaloids isolated from Aegle marmelos (Linn.) Correa. Nat Prod Rese. 30(22):2559–2566.
- Molino JF. 1994. Revision du genre Clausena Burm. f. (Rutaceae). Adansonia. 16:105–153.
- Mukandiwa L, Eloff JN, Naidoo V. 2016a. Repellent and mosquitocidal effects of leaf extracts of Clausena anisata against the Aedes aegypti mosquito (Diptera: Culicidae). Environ Sci Pollut Res. 23(11):11257–11266.
- Mukandiwa L, Naidoo V, Katerere DR. 2016b. The use of Clausena anisata in insect pest control in Africa: A review. J Ethnopharmacol. 194:1103–1111.
- Nunes FM, Barros-Filho BA, de Oliveira MCF, Andrade-Neto M, De Mattos MC, Mafezoli J, Pirani JR. 2005. 1H and 13C NMR spectra of 3,8-dimethoxyfuro[3,2-g]coumarin and maculine from Esenbeckia grandiflora Martius (Rutaceae). Magn Reson Chem. 43(10):864–866.
- Ouyang GQ, Li CJ, Yang JZ, Li L, Song XY, Jiang YN, Chen NH, Ma J, Zhang DM. 2016. Bioactive coumarins from the stems of Clausena emarginata. Chem Biodivers. 13(9):1178–1185.
- Rasamison VE, Brodie PJ, Merino EF, Cassera MB, Ratsimbason MA, Rakotonandrasana S, Rakotondrafara A, Rafidinarivo E, Kingston DGI, Rakotondraibe HL. 2016. Furoquinoline alkaloids and methoxyflavones from the stem bark of Melicope madagascariensis (Baker) T. G. Hartley. Nat Prod Bioprospect. 6(5):261–265.
- Sakunpak A, Matsunami K, Otsuka H, Panichayupakaranant P. 2013. Isolation of new monoterpene coumarins from Micromelum minutum leaves and their cytotoxic activity against Leishmania major and cancer cells. Food Chem. 139(1-4):458–463.
- Shen DY, Kuo PC, Huang SC, Hwang TL, Chan YY, Shieh PC, Ngan NT, Thang TD, Wu TS. 2017. Constituents from the leaves of Clausena lansium and their anti-inflammatory activity. J Nat Med. 71(1):96–104.
- Sripisut T, Cheenpracha S, Ritthiwigrom T, Prawat U, Laphookhieo S. 2012. Chemical constituents from the roots of Clausena excavata and their cytotoxicity. Rec Nat Prod. 6:386–389.
- Szabó G, Greger H, Hofer O. 1985. Coumarin-hemiterpene ethers from Artemisia species. Phytochemistry. 24(3):537–541.
- Wongthet N, Sanevas N, Schinnerl J, Valant-Vetschera K, Bacher M, Vajrodaya S. 2018. Chemodiversity of Clausena excavata (Rutaceae) and related species: coumarins and carbazoles. Biochem Syst Ecol. 80:84–90.
- Yan G, Li YJ, Zhao YY, Guo JM, Zhang WH, Zhang MM, Fu YH, Liu YP. 2019. Neuroprotective carbazole alkaloids from the stems and leaves of Clausena lenis. Nat Prod Res.:1–8. DOI:https://doi.org/10.1080/14786419.2019.1652285.