Abstract
A new phenanthrene compound, 7-(4-hydroxybenzyl)-8-methoxy-9,10- dihydrophenanthrene-2,5-diol (HMD), along with five known compounds (Coelonin, DD, Shancidin, HDP and MDD) were isolated from the roots of Cymbidium faberi Rolfe. (CFR). Their structures were identified using various spectroscopic methods. These compounds were reported for the first time in the genus. All isolated compounds were tested by radical-scavenging ability against 1,1-diphenyl-2-picryl-hydrazyl (DPPH), cytotoxic activity against three human cancer cell lines and inflammatory activity. Among them, Shancidin exhibited the stronger DPPH-scavenging activity (IC50=6.67 ± 0.84 μΜ) and cytotoxic activity against three tumour cell lines. Except for HDP, all compounds dose-dependently suppressed production of NO, TNF-α, IL-6 in LPS induced mouse primary peritoneal macrophage and showed anti-inflammatory activity. Moreover, 18 compounds were identified by UHPLC-LTQ-Orbitrap-MS combined with MS database, which provides a basis for further research.
1. Introduction
Cymbidium faberi Rolfe. (CFR) is a kind of herbaceous plant in the genus of Cymbidium (Orchidaceae), mainly distributed in the vast areas of China. It is also largely cultivated in China for ornamental purpose. Though it was not recorded in Chinese Pharmacopoeia, the roots of CFR have been used as a folk herbal medicine for decades (Li Citation1985). The roots of CFR were recorded for resolving phlegm, relieving cough, etc. (Wang Citation2014).
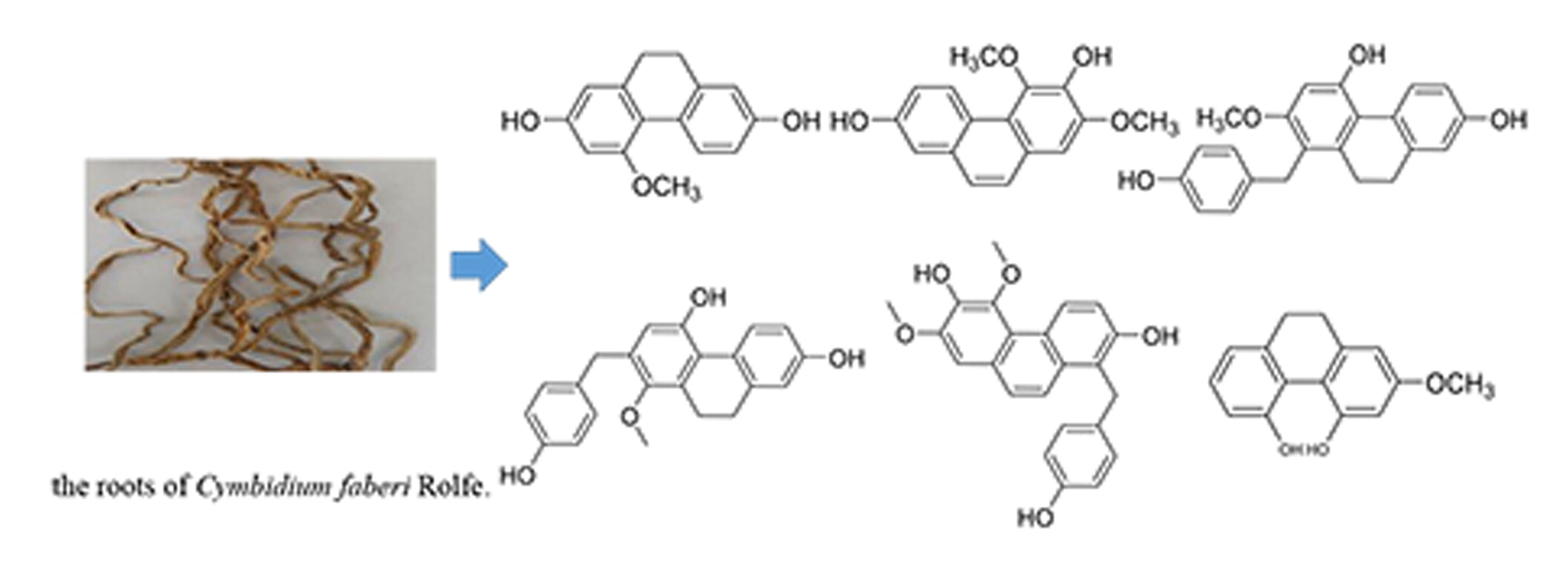
Until now, various compounds, such as phenols, phenanthrenes, stilbenoid derivatives and steroids, have been isolated in the Orchidaceae. For example, phenolic compounds were isolated from Himantoglossum robertianum and Rhynchostele rossii (Yang et al. Citation2006; Bazzicalupo et al. Citation2019). Phenanthrene compounds were also found in orchidaceae, but most of them were isolated from Dendrobium Sw. and Bletilla Rchb. f. (Zhao et al. Citation2018; Zhou et al. Citation2019). Bibenzyl compounds were commonly isolated in Dendrobium Sw. plants, such as Dendrobium sinense (Chen et al. Citation2014). These compounds have been reported to possess a variety of pharmacological activities, including anti-bacterial (Yang et al. Citation2012), anti-neoplastic (Lee et al. Citation2009), anti-oxidative (Paudel et al. Citation2018) and anti-inflammatory effects (Lin et al. Citation2013). Among these compounds, phenanthrenes with a C-6-C-2-C-6 skeleton were regarded as characteristic chemical constituents in the Orchidaceae, and have been isolated from Dendrobium nobile and Cremastra appendiculata (Zhang et al. Citation2008; Wang et al. Citation2013). Literatures reported that phenanthrenes displayed diverse and promising biological activities, including anti-inflammatory, anti-proliferative, anti-microbial, spasmolytic, anti-platelet aggregative, anti-oxidant and anti-allergic activities (Kovacs et al. Citation2008). These reports prompted us the presence of phenanthrenes in CFR. However, to our knowledge, research on the chemical constituents and activity of CFR was limited. Headspace solid phase microextraction (HS-SPME) and gas chromatography-mass spectrometry (GC-MS) were applied to analyse the aromatic composition and relative content of CFR (Feng et al. Citation2009). In addition, paper disk diffusion method was applied to measure their anti-bacterial activity of the chemical components of CFR (Chen et al. Citation2012). The system isolation and activity test of phenanthrenes in the roots of CFR were rarer. In the present study, systematic isolation and structure elucidation of the phenanthrenes from the roots of CFR were carried out. Six phenanthrene compounds, including a new compound, were isolated from the roots of CFR, and the anti-oxidant, anti-tumor and anti-inflammatory activities of these compounds were measured. Meanwhile, the chemical profile of the roots of CFR was carried out by UHPLC-LTQ-Orbitrap-MS combined with MS database.
2. Results and discussion
2.1. Structural elucidation of the isolated phenanthrene compounds
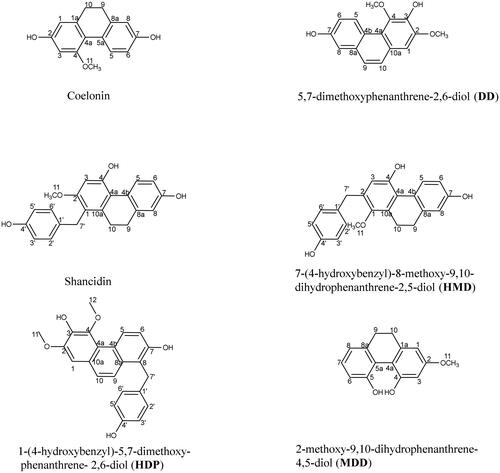
Six phenanthrene compounds were isolated from the roots of CFR by column chromatography. Their structures () were determined by UHPLC-LTQ-Orbitrap-MS and NMR spectroscopic analysis and consistent with that reported in the literature. Among them, the structures of known compounds, Coelonin (Juneja et al. Citation1987; Yuan Wah et al. Citation1997), 5,7- dimethoxyphenanthrene-2,6-diol (DD) (Yuan Wah et al. Citation1997), Shancidin (Takagi et al. Citation1983), 1-(4-hydroxybenzyl)-5,7-dimethoxy- phenanthrene-2,6-diol (HDP) (Li, Yang, et al. Citation2018) and 2-methoxy-9,10-dihydro- phenanthrene-4,5-diol (MDD) (Lee et al. Citation1978; Matsuda et al. Citation2004), were identified and consistent with those reported in the literatures (1H NMR, 13C NMR, HSQC and HMBC spectrum of above compounds in Supplementary information Figure S5–20). 7-(4-hydroxybenzyl)-8-methoxy-9,10-dihydrophenanthrene-2,5-diol (HMD) is a new compound whose structure has been identified as follows.
2.1.1. 7-(4-Hydroxybenzyl)-8-methoxy-9,10-dihydrophenanthrene-2,5-diol (HMD)
Light brown powder solid; HR-ESI-MS (-) at m/z 347.1280 [M-H]−. 1H-NMR (500 MHz, CD3OD) δ (ppm): 8.05 (1H, d, J = 8.6 Hz, 5-H), 7.08 (2H, d, J = 8.0 Hz, 2′,6′-H), 6.64 (4H, br. d, J = 5.9 Hz, 6, 8-H and 3′, 5′-H), 6.51 (1H, s, 3-H), 3.91 (2H, s, 7′-H), 3.28 (3H, s, 11-H), 2.63 (4H, br. m, J = 2.9 Hz, 9-H and 10-H). 13C NMR (125 MHz, CD3OD) δ (ppm): 158.1 (1-C), 134.5 (1′-C), 121.8 (2-C), 130.5 (2′,6′-C), 111.6 (3-C), 115.6 (3′, 5′-C), 155.6 (4-C), 156.0 (4′-C), 120.3 (4a-C), 126.2 (4b-C), 128.9 (5-C), 114.3 (6-C), 156.6 (7-C), 29.5 (7′-C), 115.2 (8-C), 140.6 (8a-C), 31.2 (9-C), 31.4 (10-C), 139.6 (10a-C), 60.3 (11-C) (Figure S21, S22). The DEPT-135 spectra exhibited 12 carbon resonances including one methyls, three methylenes, eight methines. Combined with carbon spectrum analysis, it can be inferred that the chemical shift values of the three methylene groups at positions 7′, 9 and 10 are δ 29.5, 31.2 and 31.4, respectively (Supplementary information Figure S23). In the HMBC spectrum, the correlations of 9-H, 10-H to 9-C, 10-C, 10a-C, 8a-C further verified the existence of –CH2 at 9 or 10-C. The long-range correlations of 7′-H to 1′-C, 2-C, 1-C, 2′-C and 6-C; 3′-H, 5′-H to 4′-C and 1′-C; 6-H, 8-H to 4b-C, 6-C, 8-C and 9-C; 2′-H, 6′-H to 4′-C and 7′-C; 5-H to 4a-C, 7-C and 8a-C further supported the deduction (Supplementary information Figure S24, S25 and Table S1). Based on the above information, HMD was a new phenanthrene and elucidated as 7-(4-hydroxybenzyl)- 8-methoxy-9,10-dihydro- phenanthrene-2,5-diol.
All compounds were first isolated from the roots of CFR. Among these compounds, HDP was previously isolated only from Bletilla ochracea Schltr (Li, Yang, et al. Citation2018). Coelonin was separated from a variety of orchids, such as Bletilla formosana and Dendrobium plicatile (Lin et al. Citation2016; Chen et al. Citation2020). DD, Shancidin and MDD had been also reported presence in some orchidaceous plants (Cretton et al. Citation2018; Zhou et al. Citation2019; Lertnitikul et al. Citation2020). Though HMD possessed the similar structure with Shancidin, the structure of HMD was hitherto unreported in the literature.
2.2. DPPH radical scavenging capacity assay
The radical scavenging capacity of the six phenanthrenes was measured by the DPPH assay in vitro. DPPH is a stable radical with an odd electron. When reacted with anti-oxidants, DPPH could acquire an electron or a hydrogen from anti-oxidants. The absorbance at 517 nm could decrease. So the change in absorbance at 517 nm is used as a measurement for the scavenging effect of a particular sample for DPPH radicals (Yamaguchi et al. Citation1998; Zhang et al. Citation2007).
The anti-oxidant activity results of phenanthrene compounds were shown in the Supplementary information Table S2. Six compounds possessed different scavenging ability on DPPH. Compared with the positive control Vitamin C, Coelonin, DD, Shancidin, HDP and MDD showed stronger anti-oxidant activity. Especially, the IC50 of Shancidin was 6.67 ± 0.84 μM. However, HMD, which was isomeric with Shancidin, had no free radical scavenging ability. Among them, the anti-oxidant activity of MDD was reported in the literature (Wang et al. Citation2007), and our result was consistent with that of literature. The structure-activity relationship showed that Coelonin, Shancidin, HDP and MDD possessed a hydroxyl or methoxy as the substituents at the C-2 position, while a benzyl group instead of hydroxyl or methoxy in C-2 position for HMD. The steric hindrance might have a certain influence on the anti-oxidant activity. Literatures showed that the anti-oxidant capacity of phenanthrene was related to the number of phenolic hydroxyl groups or methoxy groups, as well as other specific structural features (Zhang et al. Citation2007; Citation2008; Ma et al. Citation2016). Our research results are consistent with literature reports.
2.3. Cytotoxic activity
In order to understand the effects of six phenanthrenes on human cancer cell lines, cytotoxic experiments were carried using cultured SMMC-7721, A549, MGC80-3 cell lines by MTT assay. As shown in Supplementary information Table S2, Coelonin, DD, Shancidin and HDP showed inhibitory activities against at least one cell line. Among these compounds, Shancidin displayed a remarkable effect against all three cancer cell lines. In addition, literatures also reported the inhibitory activity of these compounds on other cancer cell lines, such as HL-60, MCF-7, HCT-8 (Lee et al. Citation2009; Li, Kuang, et al. Citation2018). Interestingly, HMD possessed the same substituents as Shancidin, but the difference in the position made HMD exhibited little anti-tumor activity. The result analysis might be that steric hindrance has a certain influence. Similarly, Coelonin and MDD have similar structures, but MDD exhibited no cytotoxic activity because of the position change of the hydroxyl group on C-2 and C-7. Based on the above results, steric hindrance and position of group in the phenanthrenes have a greater impact on the anti-tumor activity. Besides, some literatures also reported the cytotoxic activity of other orchid plants, which rich in phenanthrene and dihydrophenanthrene compounds (Yang et al. Citation2010; Paudel et al. Citation2018).
2.4. Anti-inflammatory activity
Mouse primary peritoneal macrophage was used in the anti-inflammatory activity test. Compared with cell lines, the primary peritoneal macrophage possesses stronger sensitivity and more similar with the micro-environment in vivo. It releases NO and pro-inflammatory cytokines such as TNF-α and IL-6, when induced by LPS. Thus it could provide a suitable model for the anti-inflammatory activity study of phenanthrenes.
According to the results shown in Supplementary information Figure S1, Figure S2 and Figure S3, these compounds exhibited different effects on LPS-induced mouse primary peritoneal macrophage, and the viability of cells exposed to their corresponding concentrations of the compounds was over 80%. Except HDP, all compounds could effectively inhibit NO, TNF-α and IL-6 production induced by LPS in a dose-dependent manner. The anti-inflammatory activity of MDD has been reported (Lin et al. Citation2013). According to the literature, Coelonin might partially inhibit the activation of NF-κB through the PTEN/AKT pathway, thereby achieving anti-inflammatory effects (Jiang et al. Citation2019). Other compounds have the same skeleton as Coelonin. It was preliminarily speculated that other compounds might also act through this mechanism.
The structure–activity relationship shows that the six compounds have a same skeleton, but the difference in the substituent group produces a difference in anti- inflammatory activity. Among them, HDP possesses a larger benzyl substituent at position C-8, and the steric hindrance might cause its loss of anti-inflammatory activity. Based on the above analysis, the presence of the phenanthrene framework would contribute to the increase of anti-inflammatory activity, but the steric hindrance also has a certain influence on the anti-inflammatory activity.
2.5. Constituents identification of the roots of CFR by UHPLC-LTQ-Orbitrap-MS
UHPLC-LTQ-Orbitrap-MS has been widely used in identification of chemical composition in herbal medicine (Sun et al. Citation2016; Stojkovic et al. Citation2020). In order to further understand the chemical composition of the roots of CFR, UHPLC-LTQ-Orbitrap-MS method was employed to investigate extract of the roots of CFR. In the first, the sample was analysed in both positive and negative modes to provide sufficient information for structural identification. The result showed that negative mode produced moderate response mass and extensive fragmentation information. Therefore, the [M-H]− ion in negative mode was selected for MS/MS analysis.
The analysis of all compounds was combined with the natural product OTCML database and mzCloud network database (all from Thermo Fisher Scientific). The OTCML database contains MS and MS/MS spectrometry information of more than 1200 standard reference substance. The mzCloud network database is a state of the mass spectral database that assists analysts in identifying compounds in many areas. Because of continuously update, the database contains more than 15,000 compounds information, including high-resolution and accurate-mass MS and MSn spectra.
The total ion chromatogram (TIC) of the roots of CFR extract was shown in Figure S4. Combined with the database, 18 compounds were identified, including 9 organic acids and their derivatives, 6 phenanthrene compounds, 1 glycoside, 1 alcohol derivative, and 1 pyrimidine compound (Supplementary information Table S3). Six phenanthrenes are marked in Supplementary information Figure S4. Among the identified ingredients, organic acids and phenanthrenes accounted for the majority. It was reported that there were numerous organic acids in orchid plants. 4-hydroxy-3,5-dimethoxybenzoic acid (Tuchinda et al. Citation1988), 4-hydroxy-cinnamic acid, 3,4,5-trimethoxybenzyl acid (Tezuka et al. Citation1993), linolenic acid and L-norleucine (Kikuchi et al. Citation1981) were also isolated from other orchid plants. In addition, phenanthrenes, which is considered as characteristic constituents in orchids, has been also reported in many literatures (Yang et al. Citation2007; Kovacs et al. Citation2008; Lertnitikul et al. Citation2020).
3. Experimental
3.1. General experimental procedures
The high-resolution electrospray ionisation mass spectra (HR-ESI-MS) was obtained from a Thermo Fisher LTQ-Orbitrap XL Hybrid mass spectrometer (Thermo Fisher Scientific, Bremen, Germany). 1H and 13C NMR spectra were determined using AV-500 Superconducting Nuclear Magnetic Resonance System (Bruker, Switzerland). Column chromatography was established using silica gel (100–200 mesh and 200–300 mesh, Qingdao Haiyang Chemistry Co., Ltd., China), MCI GEL CHP-20 (Mitsubishi Chemical Corporation, Japan) and Sephadex LH-20 (GE, USA). Thin-layer chromatography (TLC) separations (Qingdao Haiyang Chemistry Co., Ltd., China) were detected under UV light at 210, 254 and 365 nm. Reversed-phase semipreparative HPLC (LC-52, Japan Analytical Industry Co. Ltd., Tokyo, Japan) with a YMC-Pack ODS-A column (AA12S05-2510WT, Tokyo, Japan) was used for the final purification. The chemical reagents were supplied by Tianjin zhiyuan Chemical Reagent Co., Ltd. (Tianjin, China) or Sigma (USA). Biological reagents were supplied by Hangzhou Multi Science Biotech Co., Ltd. (Wuhan, China) or Beijing Solarbio Technology Co., Ltd. (Beijing, China). The OD value of each well in the biological activity assays was read in multifunctional microplate reader (Varioskan™ LUX, Thermo Fisher Scientific, USA).
3.2. Plant material
The roots of CFR was collected from Tongbai County, Nanyang City, Henan Province. A voucher sample (No. 20170422) was identified by one of authors, Professor Suiqing Chen and deposited in the Department of Pharmacognosy, Henan University of Chinese Medicine, Zhengzhou, China.
3.3. Extraction and isolation
The roots of CFR (8 kg) was extracted by repeated heating and reflux with 95% ethanol as solvent. The extract was filtered and concentrated, then extracted by EtOAc. After filtered and concentrated, the EtOAc extract (156.3 g) was chromatographed on a silica gel column (Φ 10 × 100 cm, 100–200 mesh, 1.5 kg) with a gradient of petroleum ether/Acetone (10:1 to 1:1). The fraction 3–7 was further separated on silica gel column chromatography, eluted with petroleum ether/EtOAc (10:1 to 2:1, v/v) to yield DD (21 mg). The fraction 4 (7.2 g) was eluted by silica gel column. And the fraction 4-10-1 was continuously purified on a Sephadex LH-20 column and eluted with CH2Cl2–MeOH (1:1, v/v) to afford Coelonin (28.1 mg). And a semi-preparative liquid chromatography column elution and purification were performed to obtain HDP (5.1 mg) and MDD (67.7 mg) from fraction 4-10-2. The fraction 4-10-3 was purified by semi-preparative liquid chromatography column elution to give Shancidin (27.4 mg) and HMD (5.6 mg).
3.4. In vitro anti-oxidative activity
The radical-scavenging activities of six phenanthrene compounds were determined using DPPH method (Yamaguchi et al. Citation1998; Zhang et al. Citation2007). In brief, a series concentration of sample in methanol was prepared, and then 100 µL of each sample solution was mixed with 100 µL of 0.1 mM DPPH solution freshly prepared in methanol. After 30 min in the dark, absorbance was read at 517 nm using a spectrophotometer. The radical scavenging activity of each sample was expressed in terms of the IC50 (the effective concentration at which DPPH radicals were scavenged by 50%), which was calculated from the log–dose inhibition curve.
3.5. Cytotoxic assay
Cytotoxicity of six phenanthrene compounds was evaluated against three human tumorous cell lines—namely, SMMC-7721, A549, MGC80-3. The three cell lines were obtained from the Type Culture Collection of the Chinese Academy of Sciences and were cultured in DMEM supplemented with 10% FBS, 1% l-glutamine, and 1% penicillin–streptomycin, and maintained at 37 °C and 5% CO2. Cisplatin was used as the positive control. Cells were seeded at a density of 2 × 104 cells/well in a 96-well plate and incubated for 12 h, followed by treatment with the different concentrations of test compounds. The control culture was subjected to the carrier solvent (0.1% DMSO). After 24 h, medium of each well was aspirated and the cells were washed. Then the 100 μL fresh medium with 20 μL (5 mg/mL) MTT solution was added to each well. After 4 h of incubation at 37 °C, the medium was discarded, and the formazan blue formed in the cells was dissolved in DMSO. Each sample was tested in triplicate at five different concentrations. The optical density at 490 nm was determined with a microplate reader, and IC50 values were calculated through nonlinear regression analysis using Graphpad 7.0 software.
3.6. Anti-inflammatory activity
In this article, three indicators of inflammation were measured as follows: nitric oxide (NO); tumour necrosis factor (TNF-α); interleukin- 6 (IL-6).
3.6.1. Preparation of mouse peritoneal macrophages
Thioglycolate induced peritoneal macrophages were used for anti-inflammatory activity test (Wang et al. Citation2000; Aparicio-Soto et al. Citation2015). Briefly, KunMing mouse (male, 18-22 g) were injected with prepared 6% thioglycolate broth (Solarbio Science&Technology Co., Ltd., China) into the peritoneal cavity. Peritoneal cells were obtained from the peritoneal cavity lavage with a cold RPMI 1640 medium (Solarbio Science&Technology Co., Ltd., China). Peritoneal lavage was pooled and centrifuged at 1000 rpm for 5 min at 4 °C, and the pellet was resuspended in RPMI 1640 medium supplemented with 10% heat-inactivated FBS and 1% penicillin/streptomycin. Peritoneal exudate cells containing 5.5 × 106 macrophages/ml were incubated in petri dish overnight at 37 °C in 5% CO2 for later use.
3.6.2. No determination
Mouse peritoneal macrophages were seeded in 96-well plate at a density of 1 × 105 cells/well for 12 h prior to the sample treatment. The cells were then simultaneously stimulated with 1 µg/mL LPS (Solarbio Science&Technology Co., Ltd., China) and different concentrations of the test compounds for 24 h. The nitrite, accumulated in the culture medium, was measured as an indicator of NO production based on a diazotization reaction using the Griess reagent system (Grisham et al. Citation1996).
3.6.3. Cytokines determination
TNF-α level in the culture medium was measured by a Mouse TNF-α High Sensitivity ELISA Kit (Multi sceince, China, Cat No. EK282HS). IL-6 in the culture medium was quantified by ELISA Kits (Multi sceince, China, Cat No. EK206HS) according to the manufacturer’s protocols (Singh et al. Citation2005).
3.7. Constituents identification of the roots of CFR by UHPLC-LTQ-Orbitrap-MS
2 mg ethyl acetate extract of the roots of CFR was dissolved in a 20 mL methanol and sonicated for 30 min. After cooling, the lost weight was compensated by adding 70% methanol. The resultant solution was filtered through a 0.22 μm filter for UHPLC-LTQ-Orbitrap-MS analysis.
The chromatographic analysis was performed on a Dionex UltiMate 3000 UHPLC system (Thermo Scientific, Germering, Bavaria, Germany) equipped with a binary pump, an online degasser, a autosampler, a thermostatically controlled column compartment, and a diode array detector (DAD). Chromatographic separation was performed on a reverse-phase column Syncronis C18 column (4.6 × 250 mm, 5 μm, Thermo Scientific) maintained at 30 °C. The mobile phases consisted of water containing 0.1% formic acid (A) and acetonitrile (B), and the elution gradient was set as follows: 0.0–3.0 min, 15.0% B; 3.0–48.0 min, 15.0–100.0% B; 48.0–50.0 min, 100.0–15.0% B. The flow rate was set at 0.5 mL/min, and the injection volume was 5 μL. For the LC-ESI-MS experiments, a Thermo Fisher LTQ-Orbitrap XL Hybrid mass spectrometer (Thermo Fisher Scientific, Bremen, Germany) equipped with an electrospray ionization (ESI) source was connected to the UHPLC instrument. The ESI source parameters were set as follows: ion spray voltage, 4.5 kV; capillary temperature, 300; capillary voltage, 35 V; tube lens voltage, 160 V; and sheath (N2) and auxiliary gas (He) flow rates, 38 and 8 arbitrary units, respectively. The Orbitrap mass analyser was operated in both negative and positive ion mode, with a mass range of 80–1000.
The data was recorded and processed using the Xcalibur 3.0 software (Thermo Fisher Scientific) and Compound Discovery 2.1 software (Thermo Fisher Scientific, Waltham, MA, USA).
3.8. Statistical analysis
All tests were carried out in triplicates and the results were expressed in mean ± standard deviation (SD). Graphpad Prism 7.0 software (San Diego, CA, USA) was used for the statistical and graphical analysis.
4. Conclusions
In this paper, six phenanthrenes were isolated, then the anti-tumor, anti-oxidant, anti-inflammatory activities of the isolates were investigated employing various systems in vitro. All of the compounds were first isolated in the roots of CFR. Among the six compounds, Shancidin exhibited the stronger DPPH-scavenging activity (IC50=6.67 ± 0.84 μΜ) and cytotoxic activity against SMMC-7721, A549 and MGC80-3 (IC50=12.57 ± 0.90 μΜ, 18.21 ± 0.93 μΜ, 11.60 ± 0.75 μΜ). Coelonin, DD, Shancidin, HDP and MDD displayed inhibitory effect on NO, TNF-α and IL-6 production in LPS-stimulated mouse peritoneal macrophages and showed stronger anti-inflammatory activity, which indicates that they may be a valuable therapeutic agent for the treatment of inflammatory diseases. In addition, UHPLC-LTQ-Orbitrap-MS method was used to identify the components in the roots of CFR. As a result, 18 compounds, including organic acids and their derivatives, phenanthrene compounds, glycoside, alcohol derivative, and 1 pyrimidine compound, were identified, which provides abundant information for further research on the roots of CFR.
SUPPLEMENTARY_MATERIAL.docx
Download MS Word (1.9 MB)Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Aparicio-Soto M, Sanchez-Fidalgo S, Gonzalez-Benjumea A, Maya I, Fernandez- Bolanos JG, Alarcon-de-la-Lastra C. 2015. Naturally occurring hydroxytyrosol derivatives: hydroxytyrosyl acetate and 3,4-dihydroxy- phenylglycol modulate inflammatory response in murine peritoneal macrophages. Potential utility as new dietary supplements. J Agric Food Chem. 63(3):836–846.
- Bazzicalupo M, Burlando B, Denaro M, Barreca D, Trombetta D, Smeriglio A, Cornara L. 2019. Polyphenol characterization and skin-preserving properties of hydroalcoholic flower extract from Himantoglossum robertianum (Orchidaceae). Plants. 8(11):502–515.
- Chen DN, Wang YY, Liu WJ, Chen YJ, Wu YP, Wang JX, He F, Jiang L. 2020. Stilbenoids from aerial parts of Dendrobium plicatile. Nat Prod Res. 34(3):323–328.
- Chen XJ, Mei WL, Cai CH, Guo ZK, Song XQ, Dai HF. 2014. Four new bibenzyl derivatives from Dendrobium sinense. Phytochem Lett. 9:107–112.
- Chen XJ, Zhong YF, Dai HF. 2012. Study on GC-MS analysis and anti-bacterial activity of volatile oil from Cymbidium hybridum 'dafeng. Guangdong Agric Sci. 39(16):113–115.
- Cretton S, Oyarzun A, Righi D, Sahib L, Kaiser M, Christen P, Fajardo V. 2018. A new antifungal and antiprotozoal bibenzyl derivative from Gavilea lutea. Nat Prod Res. 32(6):695–701.
- Feng LG, Zhou L, Tao J, Shen MX. 2009. Study on aromatic components in Cymbidium faberi. J Anhui Agric Sci. 37(35):17465–17466.
- Grisham MB, Johnson GG, Lancaster JR. Jr. 1996. Quantitation of nitrate and nitrite in extracellular fluids. Methods Enzymol. 268:237–246.
- Jiang F, Li M, Wang H, Ding B, Zhang C, Ding Z, Yu X, Lv G. 2019. Coelonin, an anti-inflammation active component of Bletilla striata and its potential mechanism. Int J Mol Sci. 20(18):4422.
- Juneja RK, Sharma SC, Tandon JS. 1987. Two substituted bibenzyls and a dihydrophenanthrene from Cymbidium Aloifolium. Phytochemistry. 26(4):1123–1125.
- Kikuchi T, Kadota S, Hanagaki S, Suehara H, Namba T, Lin C, Kan W. 1981. Studies on the constituents of orchidaceous plants. I. Constituents of Nervilia purpurea Schlechter and Nervilia aragoana Gaud. (L.). Chem Pharm Bull. 29(7):2073–2078.
- Kovacs A, Vasas A, Hohmann J. 2008. Natural phenanthrenes and their biological activity. Phytochemistry. 69(5):1084–1110.
- Lee CL, Chang FR, Yen MH, Yu D, Liu YN, Bastow KF, Morris-Natschke SL, Wu YC, Lee KH. 2009. Cytotoxic phenanthrenequinones and 9,10-dihydrophenanthrenes from Calanthe arisanensis. J Nat Prod. 72(2):210–213.
- Lee TT, Rock GL, Stoessl A. 1978. Effects of orchinol and related phenanthrenes on the enzymic degradation of indole-3-acetic acid. Phytochemistry. 17(10):1721–1726.
- Lertnitikul N, Pattamadilok C, Chansriniyom C, Suttisri R. 2020. A new dihydrophenanthrene from Cymbidium finlaysonianum and structure revision of cymbinodin-A. J Asian Nat Prod Res. 22(1):83–90.
- Li JY, Kuang MT, Yang L, Kong QH, Hou B, Liu ZH, Chi XQ, Yuan MY, Hu JM, Zhou J. 2018. Stilbenes with anti-inflammatory and cytotoxic activity from the rhizomes of Bletilla ochracea Schltr. Fitoterapia. 127:74–80.
- Li JY, Yang L, Hou B, Ren FC, Yang XB, Lv YF, Kuang MT, Hu JM, Zhou J. 2018. Poly p-hydroxybenzyl substituted bibenzyls and phenanthrenes from Bletilla ochracea Schltr with anti-inflammatory and cytotoxic activity. Fitoterapia. 129:241–248.
- Li SZ. 1985. Compendium of Materia Medica. Beijing: People's Medical Publishing House.
- Lin CW, Hwang TL, Chen FA, Huang CH, Hung HY, Wu TS. 2016. Chemical constituents of the rhizomes of Bletilla formosana and their potential anti-inflammatory activity. J Nat Prod. 79(8):1911–1921.
- Lin Y, Wang F, Yang LJ, Chun Z, Bao JK, Zhang GL. 2013. Anti-inflammatory phenanthrene derivatives from stems of Dendrobium denneanum. Phytochemistry. 95:242–251.
- Ma W, Zhang Y, Ding YY, Liu F, Li N. 2016. Cytotoxic and anti-inflammatory activities of phenanthrenes from the medullae of Juncus effusus L. Arch Pharm Res. 39(2):154–160.
- Matsuda H, Morikawa T, Xie H, Yoshikawa M. 2004. Antiallergic phenanthrenes and stilbenes from the tubers of Gymnadenia conopsea. Planta Med. 70(9):847–855.
- Paudel MR, Chand MB, Pant B, Pant B. 2018. Antioxidant and cytotoxic activities of Dendrobium moniliforme extracts and the detection of related compounds by GC-MS. BMC Complement Altern Med. 18(1):134–142.
- Singh U, Tabibian J, Venugopal SK, Devaraj S, Jialal I. 2005. Development of an in vitro screening assay to test the antiinflammatory properties of dietary supplements and pharmacologic agents. Clin Chem. 51(12):2252–2256.
- Stojkovic D, Drakulic D, Gasic U, Zengin G, Stevanovic M, Rajcevic N, Sokovic M. 2020. Ononis spinosa L., an edible and medicinal plant: UHPLC-LTQ-Orbitrap/MS chemical profiling and biological activities of the herbal extract. Food Funct. 11(8):7138–7151.
- Sun X, Zhang Y, Chen S, Fu Y. 2016. Characterization and identification of the chemical constituents in the root of Lindera reflexa Hemsl. using ultra-high performance liquid chromatography coupled with linear trap quadrupole orbitrap mass spectrometry. J Pharm Biomed Anal. 126:34–47.
- Takagi S, Yamaki M, Inoue K. 1983. Antimicrobial agents from Bletilla striata. Phytochemistry. 22(4):1011–1015.
- Tezuka Y, Yoshida Y, Kikuchi T, Xu G-J. 1993. Constituents of Ephemerantha fimbriata. Isolation and structure elucidation of two new phenanthrenes, Fimbriol-A and Fimbriol-B, and a new Dihydrophenanthrene, Ephemeranthol-C. Chem Pharm Bull. 41(8):1346–1349.
- Tuchinda P, Udchachon J, Khumtaveeporn K, Taylor WC, Engelhardt LM, White AH. 1988. Phenanthrenes of Eulophia nuda. Phytochemistry. 27(10):3267–3271.
- Wang GQ. 2014. National Chinese herbal medicine collection. Beijing: People's Medical Publishing House.
- Wang J, Wang L, Kitanaka S. 2007. Stilbene and dihydrophenanthrene derivatives from Pholidota chinensis and their nitric oxide inhibitory and radical-scavenging activities. J Nat Med. 61(4):381–386.
- Wang MJ, Jeng KC, Shih PC. 2000. Differential expression and regulation of macrophage inflammatory protein (MIP)-1alpha and MIP-2 genes by alveolar and peritoneal macrophages in LPS-hyporesponsive C3H/HeJ mice. Cell Immunol. 204(2):88–95.
- Wang Y, Guan SH, Meng YH, Zhang YB, Cheng CR, Shi YY, Feng RH, Zeng F, Wu ZY, Zhang JX, et al. 2013. Phenanthrenes, 9,10-dihydrophenanthrenes, bibenzyls with their derivatives, and malate or tartrate benzyl ester glucosides from tubers of Cremastra appendiculata. Phytochemistry. 94:268–276.
- Yamaguchi T, Takamura H, Matoba T, Terao J. 1998. HPLC method for evaluation of the free radical-scavenging activity of foods by using 1,1-diphenyl-2-picrylhydrazyl. Biosci Biotechnol Biochem. 62(6):1201–1204.
- Yang H, Sung SH, Kim YC. 2007. Antifibrotic phenanthrenes of Dendrobium nobile stems. J Nat Prod. 70(12):1925–1929.
- Yang L, Wang Z, Xu L. 2006. Phenols and a triterpene from Dendrobium aurantiacum var. denneanum (Orchidaceae). Biochem Syst Ecol. 34(8):658–660.
- Yang M, Cai L, Tai Z, Zeng X, Ding Z. 2010. Four new phenanthrenes from Monomeria barbata Lindl. Fitoterapia. 81(8):992–997.
- Yang X, Tang C, Zhao P, Shu G, Mei Z. 2012. Antimicrobial constituents from the tubers of Bletilla ochracea. Planta Med. 78(6):606–610.
- Yuan Wah L, Chiang Cheong K, Harrison LJ, Powell AD. 1997. Phenanthrenes, dihydrophenanthrenes and bibenzyls from the orchid bulbophyllum vaginatum. Phytochemistry. 44(1):157–165.
- Zhang X, Xu JK, Wang J, Wang NL, Kurihara H, Kitanaka S, Yao XS. 2007. Bioactive bibenzyl derivatives and fluorenones from Dendrobium nobile. J Nat Prod. 70(1):24–28.
- Zhang X, Xu JK, Wang NL, Kurihara H, Yao XS. 2008. Antioxidant phenanthrenes and lignans from Dendrobium nobile. J Chin Pharm Sci. 17:314–318.
- Zhao GY, Deng BW, Zhang CY, Cui YD, Bi JY, Zhang GG. 2018. New phenanthrene and 9, 10-dihydrophenanthrene derivatives from the stems of Dendrobium officinale with their cytotoxic activities. J Nat Med. 72(1):246–251.
- Zhou D, Chen G, Ma YP, Wang CG, Lin B, Yang YQ, Li W, Koike K, Hou Y, Li N. 2019. Isolation, structural elucidation, optical resolution, and antineuroinflammatory activity of phenanthrene and 9,10-dihydrophenanthrene derivatives from Bletilla striata. J Nat Prod. 82(8):2238–2245.