Abstract
The oral administration is probably the most used and largely applicable method, even if absorption across the intestinal epithelium is a limiting factor that can invalidate the achievement of a therapy. The aim of this study was to assess the steady state bioavailability of very low molecular weight hyaluronic acid (vLMW-HA) and its distribution in different districts of mice. Adult female C57BL6/J mice (n = 26) were divided in three groups and orally treated for 7 days with: saline solution (SHAM-HA), high dose of vLMW-HA (5 kDa; 500 mg/kg/day; HD-vLMW-HA), and low dose of vLMW-HA (5 kDa; 100 mg/kg/day; LD-vLMW-HA). HA content was quantified in plasma, skin, bladder, gut, rectum, vagina, and eyes with ELISA assay at the end of treatment. HA level significantly increased after treatment with HD-vLMW-HA in all analyzed tissues and plasma. Therefore, vLMW-HA easy absorption and distribution after the oral intake opens new possibilities for future biomedical applications.
1. Introduction
The nature of drugs or supplements along with the absorption efficacy are fundamental components for a successful therapy. Among the several delivery routes commonly used for the administration of drugs or supplements, the oral one is probably the most used and represents a widely applicable method for the ease of administration and the high patient compliance (Homayun et al. Citation2019). Nevertheless, when oral drugs are ingested, they encounter different challenges before reaching their target (Leal et al. Citation2017) and may have some limitations due to the low bioavailability or short half-life. Hyaluronic acid (HA) is one of the most interesting, versatile, and useful natural macromolecules. Despite its simple chemical structure, HA plays a key role in several biological processes and in different cell types and tissues, both in normal and pathological conditions (Fraser et al. Citation1997). In the last few years this molecule has gained great attention and found an extraordinarily broad range of biomedical applications (Vasvani et al. Citation2020; Valachová and Šoltés Citation2021). The most common administration route of HA is intra-articular injection for the treatment of osteoarthritis (OA) and rheumatoid arthritis (Battaglia et al. Citation2013), or filler injection in the field of aesthetic medicine (Lipko-Godlewska et al. Citation2021). Over the last years, also oral administration of exogenous HA has gained attention as supplementary therapy to treat local diseases, as atrophic vaginitis, or relieving chronic pain (La Galia et al. Citation2014; Jensen et al. Citation2015; Göllner et al. Citation2017). In this regard, the molecular weight of hyaluronic acid is crucial for the functions exerted and can determine its fate once ingested (Kimura et al. Citation2016) given that several factors may influence the gastrointestinal absorption. Not many reports directly measured HA absorption. Some studies examined HA in the gastrointestinal tract and its translocation to thyroid glands, stomach, kidney, and bladder using radioisotopes (Balogh et al. Citation2008), particularly focusing on the high molecular weight form (HMW-HA >500kDa). Nevertheless, despite these few studies, the distribution of HA is still unclear, especially for the low molecular weight form (LMW-HA <500 kDa). LMWHA when locally applied, can be easier absorbed by epithelia compared with HMWHA (Essendoubi et al. Citation2016). This property can be useful to alleviate, for example, all the symptoms associated with vaginal atrophy, as severe grade of dryness, itching, burning and dyspareunia (Costantino and Guaraldi Citation2008). Moreover, by interacting with several cells involved in proliferation processes (Jiang et al. Citation2011) and the immune system, LMW-HA can contribute to restore the physiological architecture of the damaged mucosa (Nakamura et al. Citation2004; Chang et al. Citation2007; Kavasi et al. Citation2017). Despite local application has been widely investigated, oral administration lacks evidence.
Therefore, the aim of the study was to assess the bioavailability and distribution of the very low molecular weight hyaluronic acid (vLMW-HA <10kDa) in various districts of mice, after oral supplementation.
2. Results and discussion
Quantikine™ Hyaluronic acid Immunoassay were performed to evaluate the HA levels in the plasma, skin, bladder, gut, rectum, vagina, and eye after oral treatment of mice with HD-vLMW-HA and LD-vLMW-HA. In the plasma, the mean value of HA was 420,3 ng/ml. After 7 days of treatment with the lower dose of vLMW-HA (100 mg/kg), the steady state HA in plasma did not significantly change compared to SHAM-HA group; instead, the higher dose of vLMW-HA (500 mg/kg) induced a significant increase of HA level compared to SHAM-HA group and LD-vLMW-HA-treated group (682,7 ng/ml) ().
Figure 1. HA levels evaluated by Quantikine™ Hyaluronic acid Immunoassay in plasma (a) and skin (b) samples from SHAM-HA group, LD-vLMW-HA treated group and HD-vLMW-HA treated group.
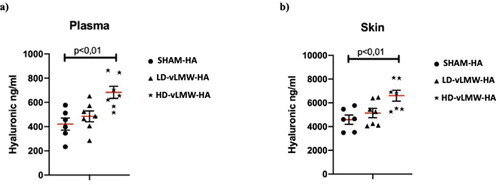
Regarding tissue distribution samples analyzed at the end of the study demonstrated a similar pattern of content: the mean level of HA in the skin was 4584 ng/ml. The dose of 100 mg/kg did not significantly change HA content (5138 ng/ml), while the 500 mg/kg dose caused a significant increase (6600 ng/ml) (p < 0.01, ).
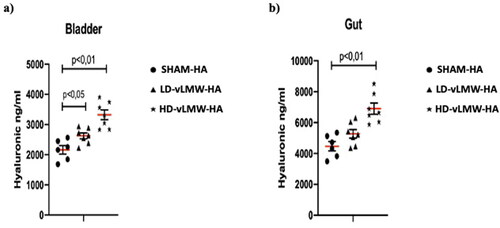
In bladder samples, the mean level of HA was 2161 ng/ml. Both LD-vLMW-HA (2626 ng/ml, p < 0,05) and HD-vLMW-HA (3323 ng/ml p < 0,01) treatments markedly increased HA levels compared to the SHAM-HA group (), probably due to the accumulation of hyaluronic fragments during excretion. On the other hand, HA mean level in gut samples were 4470 ng/ml: they significantly increased only in the HD-vLMW-HA-treated group (6901 ng/ml p < 0,01) compared to the LD-vLMW-HA-treated group (5272 ng/ml), and the SHAM-HA group ().
Figure 2. HA levels evaluated by Quantikine™ Hyaluronic acid Immunoassay in bladder (a) and gut (b) samples from SHAM-HA group, LD-vLMW-HA treated group and HD-vLMW-HA treated group.
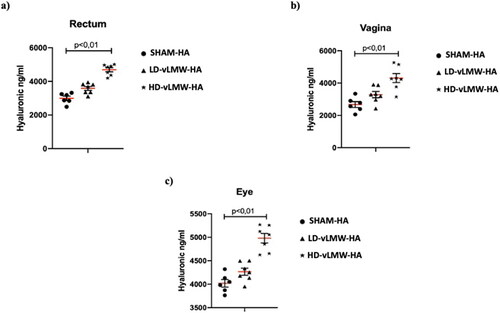
HA levels were also evaluated in rectal mucosa, vagina, and eye samples. The mean values of HA were 2993 ng/ml, 2674 ng/ml, and 4020 ng/ml respectively. LD-vLMW-HA administration did not modify HA levels compared to SHAM-HA group whereas, mice treated with HD-vLMW-HA showed a significantly increase in HA levels (4692 ng/ml, 4314 ng/ml, and 4979 ng/ml respectively; p < 0,01) compared to the SHAM-HA group and the LD-vLMW-HA-treated group () in all evaluated samples.
Figure 3. HA levels evaluated by Quantikine™ Hyaluronic acid Immunoassay in rectum (a), vagina (b) and eye (c) samples from SHAM-HA group, LD-vLMW-HA treated group and HD-vLMW-HA treated group.
This study investigated distribution of vLMW-HA in plasma and different tissues of adult female mice, after its oral uptake. Our results highlighted that hyaluronic acid with a very low molecular weight (5kDA) exhibits a good biodistribution. Indeed, HA level increased in all the analyzed tissues, especially when animals were treated with the highest dose of vLMW-HA (500 mg/kg). In the first part of this study, we quantified HA level in plasma at the steady state after oral administration. In the SHAM-HA group, an average of 420.3 ng/ml of HA was detected. A significant increase of HA level was observed in the HD-vLMW-HA-treated group (682.7 ng/mL, p < 0.01) with respect to LD-vLMW-HA-treated group (484.6 ng/ml). In all the analyzed districts (skin, bladder, gut, rectum, vagina, eyes), the higher dose of low molecular weight HA ensured a better absorption with respect to the lower dose.
Different drugs’ delivery routes are provided for different purposes and each of them encounters specific barriers before reaching their target. The oral route is probably the simplest way to delivery drugs or supplements given the ease of administration and the high patient compliance. Interestingly, the most challenging step in the oral uptake of a substance is its absorption across the intestinal epithelium. The absorptive properties of HA are still unclear, despite few experiments tested the high molecular weight HA absorption in the gastrointestinal tract and its translocation to tissues by using radiolabeled molecules (Balogh et al. Citation2008). However, information about the distribution in the various tissues of HA with different molecular weights is still very limited, and results from different studies are sometimes controversial, due to the nature of radioisotopes (Laurent et al. Citation1991; Komatsu et al. Citation1999; Kimura et al. Citation2016). Our study overcomes this limit because no radioisotope was used, instead HA level was directly quantified by using an enzyme-linked immunosorbent assay (ELISA).
Hisada investigated the intestinal permeability of hyaluronic acid with low molecular weight in a CaCo2 cell line model and demonstrated that HA permeability was dose dependent and increased with the reduction of molecular size (Hisada et al. Citation2008). This is consistent with our results, which highlighted a similar dose dependent absorption rate observable both with low and high dose of vLMW-HA in all the analyzed tissues. Hence, a significant increase in HA content was observed only in the HD-vLMW-HA-treated mice.
Hyaluronic acid is one of the most interesting, peculiar, and useful macromolecules with an important role in almost all areas of biology. Currently, HA is used in several branches of medicine (pulmonology, orthopedics, aesthetic medicine, gynecology, ophthalmology, etc.) and no contraindications or adverse interactions with other drugs have been documented (Bray Citation2001; Nolan et al. Citation2006; Ciofalo et al. Citation2017; Huynh and Priefer Citation2020). Hyaluronic acid can modulate several pathways and its modulatory function not only depends on the balance between its synthesis and degradation, but it is mainly due to its molecular weight which determines hyaluronic acid activities. In some case high molecular (HMW) and very low/low molecular weight (LMW) hyaluronic acid can exert opposite effects (Cyphert et al. Citation2015). HMW-HA is a pivotal component of skin integrity and thanks to its hydrophilic nature can increase moisture retention and smoothness of the skin by incorporating water molecules (Sato et al. Citation2002). Thanks to its water retention properties and viscoelasticity, HA favors the healing of corneal and conjunctival epithelium (Aragona et al. Citation2002; Gomes et al. Citation2004), determining a reduction of ocular surface inflammation for the treatment of signs and symptoms of moderate to severe dry eye syndrome. Furthermore, HMW-HA exhibits anti-inflammatory and antiangiogenic properties, promotes epithelial cell integrity and, thanks to the high viscosity, it is a suitable lubricant agent in the synovial joint fluid favoring the protection of the articular cartilage (Tamer Citation2013). Moreover, HMW-HA has beneficial roles in wound healing repair, and immunosuppression, thus controlling the levels of inflammatory cytokines and the migration of stem cells (Jiang et al. Citation2011). On the other hand, LMW-HA, during some environmental and pathological conditions including asthma, pulmonary fibrosis, and hypertension, exhibits pro-inflammatory and pro-angiogenic properties, by promoting extracellular matrix (ECM) remodeling (Heldin et al. Citation2019). Experimental studies demonstrated that treatment with vLMW-HA could be appropriate for different applications. For example, vLMW-HA exerts a reparatory action in dermal excisional wounds (Gao et al. Citation2008). In sites of inflammation or tissue injury, it induces activation of keratinocytes and improves the release of β-defensins, without an inflammatory response, thus ameliorating the self-defense of the skin for the protection of cutaneous tissue from infection by microorganisms (Gariboldi et al. Citation2008). Oral administration of vLMW-HA ameliorates vaginal atrophy, improves epithelium thickness, and increases the number of epithelial layers in postmenopausal women (La Galia et al. Citation2014). Moreover, vLMW-HA could also be used as preventive treatment in pathological conditions such as patients who undergo radiotherapy (RT) for prostate cancers (Piro and Marafioti Citation2018). Often, urinary toxicity is an unpleasant side effect of postoperative radiotherapy, in a pilot study, treatment with oral vLMW-HA completely reduced toxicity in 89% of patients affected by prostate cancer and undergoing RT (Piro and Marafioti Citation2018).
3. Conclusion
Hyaluronic acid is a key molecule that can cover a wide range of clinical applications, thanks to its different molecular weights. The success of a therapy often depends on how a supplement is distributed after its absorption. Oral delivery route is often chosen, because of the ease of administration and the high patient compliance; nevertheless, many challenges are encountered before supplement reaches its target. So, it is necessary to increase knowledge of the exact mechanism by which oral hyaluronic acid is distributed when orally administrated. Several pieces of evidence exist about the ‘fate’ of HMW-HA, while lacks exist regarding the very low molecular weight. Given its properties and its usefulness in many branches of medicine, we analyzed its level and distribution in plasma and other tissues, to open new possibilities for future biomedical and clinical perspectives.
Author contributions
Conceptualization, F.M.; supervision A.B.; writing—original draft, F.M.; writing—review and editing, A.B.; methodology, G.P.; validation, N.I. All authors have read and agreed to the published version of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Aragona P, Papa V, Micali A, Santocono M, Milazzo G. 2002. Long term treatment with sodium hyaluronate-containing artificial tears reduces ocular surface damage in patients with dry eye. Br J Ophthalmol. 86(2):181–184. PMID: 11815344; PMCID: PMC1771021.
- Balogh L, Polyak A, Mathe D, Kiraly R, Thuroczy J, Terez M, Janoki G, Ting Y, Bucci LR, Schauss AG. 2008. Absorption, uptake, and tissue affinity of high-molecular-weight hyaluronan after oral administration in rats and dogs. J Agric Food Chem. 56(22):10582–10593. PMID: 18959406.
- Battaglia M, Guaraldi F, Vannini F, Rossi G, Timoncini A, Buda R, Giannini S. 2013. Efficacy of ultrasound-guided intra-articular injections of platelet-rich plasma versus hyaluronic acid for hip osteoarthritis. Orthopedics. 36(12):e1501–e1508. PMID: 24579221.
- Bray BA. 2001. The role of hyaluronan in the pulmonary alveolus. J Theor Biol. 210(1):121–130. PMID: 11343436.
- Chang E-J, Kim HJ, Ha J, Kim HJ, Ryu J, Park K-H, Kim U-H, Lee ZH, Kim H-M, Fisher DE, et al. 2007. Hyaluronan inhibits osteoclast differentiation via Toll-like receptor 4. J Cell Sci. 120(Pt 1):166–176.
- Ciofalo A, Zambetti G, Altissimi G, Fusconi M, Soldo P, Gelardi M, Iannella G, Pasquariello B, Magliulo G. 2017. Pathological and cytological changes of the nasal mucosa in acute rhinosinusitis: the role of hyaluronic acid as supportive therapy. Eur Rev Med Pharmacol Sci. 21(19):4411–4418. PMID: 29077152.
- Costantino D, Guaraldi C. 2008. Effectiveness, and safety of vaginal suppositories for the treatment of the vaginal atrophy in postmenopausal women: an open, non-controlled clinical trial. Eur Rev Med Pharmacol Sci. 22(6):411–6.
- Cyphert JM, Trempus CS, Garantziotis S. 2015. Size matters: molecular weight specificity of hyaluronan effects in cell biology. Int J Cell Biol. 2015:563818. Epub 2015 Sep 10. PMID: 26448754; PMCID: PMC4581549.
- Essendoubi M, Gobinet C, Reynaud R, Angiboust JF, Manfait M, Piot O. 2016. Human skin penetration of hyaluronic acid of different molecular weights as probed by Raman spectroscopy. Skin Res Technol. 22(1):55–62.
- Fraser JR, Laurent TC, Laurent UB. 1997. Hyaluronan: its nature, distribution, functions and turnover. J Intern Med. 242(1):27–33. PMID: 9260563.
- Gao F, Yang CX, Mo W, Liu YW, He YQ. 2008. Hyaluronan oligosaccharides are potential stimulators to angiogenesis via RHAMM mediated signal pathway in wound healing. Clin Invest Med. 31(3):E106–16.
- Gariboldi S, Palazzo M, Zanobbio L, Selleri S, Sommariva M, Sfondrini L, Cavicchini S, Balsari A, Rumio C. 2008. Low molecular weight hyaluronic acid increases the self-defense of skin epithelium by induction of beta-defensin 2 via TLR2 and TLR4. J Immunol. 181(3):2103–2110.
- Göllner I, Voss W, von Hehn U, Kammerer S. 2017. Ingestion of an oral hyaluronan solution improves skin hydration, wrinkle reduction, elasticity, and skin roughness: results of a clinical study. J Evid Based Complementary Altern Med. 22(4):816–823. Epub 2017 Dec 4. PMID: 29228816; PMCID: PMC5871318.
- Gomes JA, Amankwah R, Powell-Richards A, Dua HS. 2004. Sodium hyaluronate (hyaluronic acid) promotes migration of human corneal epithelial cells in vitro. Br J Ophthalmol. 88(6):821–825. PMID: 15148219; PMCID: PMC1772195.
- Heldin P, Lin CY, Kolliopoulos C, Chen YH, Skandalis SS. 2019. Regulation of hyaluronan biosynthesis and clinical impact of excessive hyaluronan production. Matrix Biol. 78–79:100–117. Epub 2018 Jan 31. PMID: 29374576.
- Hisada N, Satsu H, Mori A, Totsuka M, Kamei J, Nozawa T, Shimizu M. 2008. Low-molecular-weight hyaluronan permeates through human intestinal Caco-2 cell monolayers via the paracellular pathway. Biosci Biotechnol Biochem. 72(4):1111–1114. Epub 2008 Apr 7. PMID: 18391466.
- Homayun B, Lin X, Choi HJ. 2019. Challenges and recent progress in oral drug delivery systems for biopharmaceuticals. Pharmaceutics. 11(3):129. PMID: 30893852; PMCID: PMC6471246.
- Huynh A, Priefer R. 2020. Hyaluronic acid applications in ophthalmology, rheumatology, and dermatology. Carbohydr Res. 489:107950. Epub 2020 Feb 18. PMID: 32070808.
- Jensen GS, Attridge VL, Lenninger MR, Benson KF. 2015. Oral intake of a liquid high-molecular-weight hyaluronan associated with relief of chronic pain and reduced use of pain medication: results of a randomized, placebo-controlled double-blind pilot study. J Med Food. 18(1):95–101. PMID: 25415767; PMCID: PMC4281855.
- Jiang D, Liang J, Noble PW. 2011. Hyaluronan as an immune regulator in human diseases. Physiol Rev. 91(1):221–264. PMID: 21248167; PMCID: PMC3051404.
- Kavasi R-M, Berdiaki A, Spyridaki I, Corsini E, Tsatsakis A, Tzanakakis G, Nikitovic D. 2017. HA metabolism in skin homeostasis and inflammatory disease. Food Chem Toxicol. 101:128–138. Mar
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. 2010. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 160(7):1577–1579.
- Kimura M, Maeshima T, Kubota T, Kurihara H, Masuda Y, Nomura Y. 2016. Absorption of orally administered hyaluronan. J Med Food. 19(12):1172–1179. PMID: 27982756.
- Komatsu S, Iwata H, Nabeshima T. 1999. Studies on the kinetics, metabolism and re-utilisation after intra-articular administration of hyaluronan to rabbits. Arzneimittelforschung. 49(5):427–433.
- La Galia T, Micali A, Puzzolo D, Cancellieri F. 2014. Oral low-molecular weight hyaluronic acid in the treatment of atrophic vaginitis. Indian J Community Med. 5(11):617–624.
- Laurent UBG, Dahl LB, Reed RK. 1991. Catabolism of hyaluronan in rabbit skin takes place locally in lymph nodes and liver. Exp Physiol. 76(5):695–703.
- Leal J, Smyth HDC, Ghosh D. 2017. Physicochemical properties of mucus and their impact on transmucosal drug delivery. Int J Pharm. 532(1):555–572.
- Lipko-Godlewska S, Bolanča Ž, Kalinová L, Kermen I, Onisak B, Papp I, Rebrov M, Valančienė G. 2021. Whole-face approach with hyaluronic acid fillers. Clin Cosmet Investig Dermatol. 14:169–178. PMID: 33633459; PMCID: PMC7901566.
- Nakamura K, Yokohama S, Yoneda M, Okamoto S, Tamaki Y, Ito T, Okada M, Aso K, Makino I. 2004. High, but not low, molecular weight hyaluronan prevents T-cell-mediated liver injury by reducing proinflammatory cytokines in mice. J Gastroenterol. 39(4):346–354.
- Nolan A, Baillie C, Badminton J, Rudralingham M, Seymour RA. 2006. The efficacy of topical hyaluronic acid in the management of recurrent aphthous ulceration. J Oral Pathol Med. 35(8):461–465. PMID: 16918596.
- Piro F, Marafioti L. 2018. Oral low molecular weight hyaluronic acid in the prevention and treatment of radiation-induced cystitis. IJMDAT. 1(1):e112.
- Sato T, Sakamoto W, Odanaka W. 2002. Clinical effect of hyaluronic acid diet for dry and rough skin. Aesthetic Dermatol. 12:109–120.
- Tamer TM. 2013. Hyaluronan and synovial joint: function, distribution, and healing. Interdiscip Toxicol. 6(3):111–125. PMID: 24678248; PMCID: PMC3967437.
- Valachová K, Šoltés L. 2021. Hyaluronan as a prominent biomolecule with numerous applications in medicine. Int J Mol Sci. 22(13):7077. PMID: 34209222; PMCID: PMC8269271.
- Vasvani S, Kulkarni P, Rawtani D. 2020. Hyaluronic acid: a review on its biology, aspects of drug delivery, route of administrations and a special emphasis on its approved marketed products and recent clinical studies. Int J Biol Macromol. 151:1012–1029. Epub 2019 Nov 9. PMID: 31715233.