Abstract
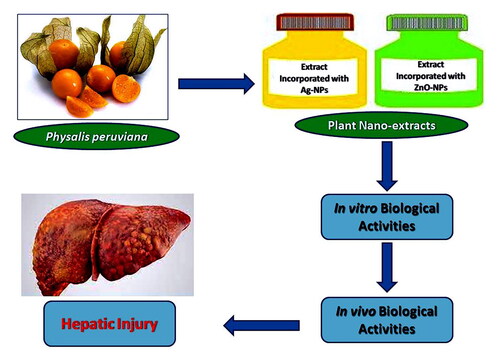
The study aimed to investigate the role of metal nanoparticles (M-NPs) in improving the efficiency of Physalis peruviana (Cape gooseberry) juice, which is rich in numerous important therapeutic phytochemicals. Therefore, it was subsequently studied against chemically-induced toxicity in rats. The present study demonstrated that C. gooseberry juice was used for the biosynthesis of silver (Ag-NPs) and zinc oxide nanoparticles (ZnO-NPs). The ZnO-C. gooseberry nano-extract exhibited higher in vitro biological activities compared to the other extracts. It was also found to be safer when administered orally. Moreover, it demonstrated a greater ameliorative effect against hepatotoxicity induced by carbon tetrachloride (CCl4) in rats. It restored the integrity of the liver tissue by increasing levels of antioxidant enzymes and reducing the inflammatory markers significantly (p ≤ 0.05). The study found that the ZnO-C. gooseberry nano-extract demonstrated greater efficacy in combating CCl4-induced hepatotoxicity compared to the other extracts.
1. Introduction
Physalis, a member of the Solanaceae family, is commonly cultivated in tropical and subtropical regions (Oliveira et al., Citation2016). It has a variety of traditional uses worldwide, including consumption in raw form, salads, sauces, compotes, and jams (Figueiredo et al., Citation2020). Certain Physalis species produce edible fruits that contain important therapeutic phytochemicals such as physalins, anolides, phenolics, phytosterols, carotenoids, vitamins, and minerals (Ramakrishna Pillai et al., Citation2022). These polyphenolic components are responsible for the medicinal value and physiological effects of Physalis fruits in the body. Alkaloids, tannins, flavonoids, and phenolic acids are the primary phytochemicals found in most Physalis species (Narváez-Cuenca et al., Citation2014).
Physalis peruviana, commonly known as Cape gooseberry, is the most widely cultivated and consumed species of Physalis due to its tangy fruits (Tammu and Ramana, Citation2015). It is highly intriguing as a promising fruit because of its exceptional nutritional value, delightful organoleptic quality, and abundant bioactive contents (Ballesteros-Vivas et al., Citation2019; Kasali et al., Citation2021). Polyunsaturated fatty acids, 𝛽-carotene, and vitamins are bioactive compounds that can contribute, along with essential minerals, to overall therapeutic properties such as antioxidant, anti-inflammatory, and anti-ulcer effects (Embaby and Mokhtar, Citation2019; Fuente et al., Citation2020). The ethanolic extract of P. peruviana fruit has shown potential in the treatment of various chronic disorders, including cancer, leukaemia, hepatitis, asthma, rheumatism, and dermatitis (Mirzaee et al., Citation2017; Jouybari et al., Citation2018).
The efficiency and bioavailability of phyto-constituents with high molecular weights are limited because they struggle to cross the cellular membrane. To address this issue, medicinal plants have been combined with nanotechnology. This integration aims to enhance the absorption of these biologically active components, increase their bioavailability, and reduce the need for repeated administration, ultimately minimising their toxicity (Mamillapalli et al., Citation2016).
Plant extracts are widely recognised as biosynthetic laboratories for a diverse range of phytochemicals, including phenolics, alkaloids, and flavonoids. These phytochemicals can be utilised in the biosynthesis of metal nanoparticles (M-NPs) (Thatyana et al., Citation2023). The M-NPs, which were synthesised using biologically derived extracts through the application of green nanotechnology, have been thoroughly reviewed and studied by various authors (Pandit et al., Citation2022). The major advantages offered by M-NPs include high yields, rapid synthesis, and their ability to be used for large-scale production of commercially attractive nanoparticles (Dikshit et al., Citation2021). Silver nanoparticles (Ag-NPs) have gained significant interest in antimicrobial research among various metal nanoparticles (M-NPs). They have been widely recognised for their ability to inhibit the growth of numerous bacterial strains and microorganisms (Alharbi et al., Citation2023). In many instances, plants have been utilised for the biosynthesis of Ag-NPs due to their advantageous characteristics, including availability, safety, and the presence of metabolites that facilitate the rapid reduction of silver ions during the biosynthesis process, surpassing the efficiency of microbes (Aboulthana et al., Citation2021).
It is widely recognised that zinc oxide (ZnO) falls into the category of metal oxides that possess a large specific area, high sensitivity, non-toxicity, and good compatibility. Due to the low toxicity and biodegradability of ZnO nanoparticles (ZnO-NPs), ZnO nanospheres have found numerous applications as nanosensors, biosensors, and catalysts in biomedical science and cosmetology (Zhou et al., Citation2023). Additionally, the Food and Drug Administration (FDA) has classified them as a safe material due to their stability and inherent ability to neutralise ionising radiation (Bashir et al., Citation2022). The aim of this study was to evaluate the role of M-NPs incorporation in improving the biological effectiveness of C. gooseberry juice, which was subsequently tested against chemically-induced hepatic toxicity in rats.
2. Results and discussion
2.1. Vitamins, minerals and pigment content
As presented in Supplementary Table 2, composition of vitamins showed that vitamin C (9.13 mg/100g) and vitamin B3 (3.15 mg/100g) are the most abundant in C. gooseberry juice followed by vitamin A (1.86 mg/100g). However, vitamins B1, B2, B6, B9 and B12 exist with lower concentrations. The mineral composition showed that potassium (985 mg/100g) was considered as the major identified macro element followed by phosphorus (473 mg/100g). While zinc (1.9 mg/100g) proved to be the main micro element in C. gooseberry juice. These values agreed with Aamer (Citation2018), who postulated that C. gooseberry juice is used safely as a potential nutraceutical agent due to the presence of a wide range of vitamins and minerals. Regarding the pigment content, high amounts of total chlorophylls, as well as chlorophyll a and b (17.93, 6.28 and 9.35 mg/g, respectively), were noticed in the juice. These values were in accordance with the study reported previously by Abou-Gharbia and Abou-Tour (Citation2001). Additionally, a significant concentration of β-carotene (10.15 mg/g) was found, which agreed with that mentioned by Puente et al. (Citation2011) and Briones-Labarca et al. (Citation2013), who stated that this fruit contains 10.75 mg/g β-carotene.
2.2. Phenolics and flavonoids
As depicted in Supplementary Table 3, composition of the polyphenolic compounds was determined in C. gooseberry juice quantitatively and qualitatively by HPLC/DAD. The HPLC/DAD analysis showed that 14 phenolic compounds, including derivatives of both hydroxybenzoic acid and hydroxycinammic acid were identified. Furthermore, 25 flavonoids were detected. Ferulic acid (946.21 ppm), ρ-coumaric acid (863.73 ppm), and ellagic acid (667.28 ppm) were considered as the main identified phenolic acids, while catechein (699.25 ppm), quercetin 3-O-rhamnoside (2371.74 mg/100 g), apigenin 7-O-glucoside (1846.58 mg/100 g), rutin (1734.82 mg/100 g) and apigenin 7-neohespiroside (1274.53 mg/100 g) were determined as major identified flavonoids in C. gooseberry juice.
2.3. Structure elucidation of the major isolated flavonoids
Data of the present study showed that quercetin 3-O-rhamnoside (Quercitrin), kaempferol 3-O-rhamnoside (Afzelin), luteolin 7-O-glucoside and chrysoeriol 7-O-glucoside are the most common flavonoids elucidated in C. gooseberry juice. These results are consistent with prior research suggesting that C. gooseberry juice provides medicinal advantages because of its chlorophylls, β-carotene (Abou-Gharbia and Abou-Tour, Citation2001), vitamins (da Silva et al., Citation2016), minerals (Mazova et al., Citation2020), as well as phenolics and flavonoids (Medina-Medrano et al., Citation2015).
2.4. The structural properties of the biosynthesized M-NPs
The biosynthesized M-NPs exhibit good stability due to the presence of secondary (active) metabolites that act as reducing (capping) agents during the biosynthesis process (Raja et al., Citation2017). The size, morphological shape, and crystalline characteristics of the biosynthesized M-NPs were assessed using TEM analysis. The results indicated that the Ag-NPs were mainly spherical in shape and well dispersed, as shown in Supplementary Fig. 2a. Some Ag-NPs displayed irregular shapes without any aggregations. In the case of ZnO-NPs, Supplementary Fig. 2b confirmed that they were well dispersed with a variety of morphological shapes and sizes ranging from 25 to 75 nm. Additionally, they exhibited a polycrystalline (hexagonal) structure. They were mostly spherical in shape with a slight variation in thickness, consistent with our previous findings (Aboulthana et al., Citation2022). Some ZnO-NPs also exhibited irregular shapes.
Dynamic Light Scattering (DLS) is a precise and non-destructive technique primarily used to determine particle size (ranging from 5 nm to 5 mm) and size distributions in diluted aqueous monodisperse solutions. It is widely recognised that the particle size measured by DLS is often larger than that determined by TEM due to the influence of Brownian motion. This finding is consistent with the research conducted by Hassan et al. (Citation2019), which demonstrated the presence of repulsion forces between identically charged particles in dispersions, as indicated by the zeta potential. The data presented in Supplementary Fig. 2c reveals that the primary diameter of the particle size distribution for the fabricated Ag-NPs is approximately 184.5 nm. Additionally, Supplementary Fig. 2d illustrates that the hydrodynamic size and distribution of the particle size have a main diameter of around 812 nm for the fabricated ZnO-NPs. These findings align with the findings of Kim et al. (Citation2012), who proposed that the increase in average diameter of biosynthesized ZnO-NPs in a liquid dispersion may be attributed to their tendency to form agglomerates or aggregates.
2.5. In vitro study on C. gooseberry juice incorporated with M-NPs
The levels of the most common active phyto-constituents (total polyphenolic compounds and condensed tannins) were measured in C. gooseberry juice mixed with M-NPs. The biological activities of the juice, including antioxidant, radical scavenging, anti-diabetic, anti-alzheimer, and anti-inflammatory activities, were also assessed and compared to the juice without M-NPs. The results showed that the levels of these phyto-constituents were significantly (p ≤ 0.05) higher in the ZnO-C. gooseberry nano-extract compared to both the native juice and the Ag-C. gooseberry nano-extract (Supplementary Table 4). As a result, the antioxidant activities (TAC and iron reducing power) and scavenging activities (against DPPH and ABTS radicals) were significantly higher in the ZnO-C. gooseberry nano-extract compared to the other extracts (p ≤ 0.05). In terms of anti-diabetic and anti-alzheimer activities, the ZnO-C. gooseberry nano-extract exhibited the highest inhibitory effect on α-amylase (73.05 ± 0.07%) and AChE (51.91 ± 0.09%) activities, followed by the Ag-C. gooseberry nano-extract (53.02 ± 0.08% and 39.91 ± 0.11%, respectively), and then the native juice (40.99 ± 0.11% and 20.89 ± 0.04%, respectively). Both the Ag- and ZnO-C. gooseberry nano-extracts (at equal concentrations) demonstrated the same anti-inflammatory activity (the same inhibitory effect on proteinase denaturation and proteinase activity) as diclofenac sodium, which was used as the standard.
Supplementary Table 5 and Supplementary Figure 3 show the maximum cytotoxic activity (at Conc. 1000 µg/ml) and IC50 of plant juice (before and after incorporating M-NPs) compared to control CACO-2 cells. The ZnO-C. gooseberry nano-extract exhibited the highest cytotoxic activity with the lowest IC50 value (IC50 232.60 μg/mL), followed by Ag-C. gooseberry nano-extract (IC50 479.20 μg/mL), and then the native juice (IC50 2643.00 μg/mL). Similarly, regarding the cytotoxic activity against HEPG-2 cells, the maximum cytotoxic activity (at Conc. 1000 µg/ml) and IC50 of the plant juice (before and after incorporating M-NPs) compared to control HEPG-2 cells were observed (Supplementary Table 6 and Supplementary Figure 4). The ZnO-C. gooseberry nano-extract showed the highest cytotoxic activity (IC50 204.90 μg/mL), followed by the Ag-C. gooseberry nano-extract (IC50 458.00 μg/mL), and then the native plant juice (IC50 735.60 μg/mL). These findings suggest that the ZnO-C. gooseberry nano-extract has higher cytotoxic activity against HEPG-2 cells compared to CACO-2 cells, possibly due to the presence of ZnO-NPs that can target tumour cells specifically. Further studies were conducted to investigate the cytotoxic activity of ZnO-C. gooseberry nano-extract against HEPG-2 cells and compare it to native plant juice. The results showed that the ZnO-C. gooseberry nano-extract caused a decrease in the fold changes of both EGFR and Bcl2 genes, while there was an increase in the fold change of the Casp3 gene in HEPG-2 cells (Supplementary Figure 5). The data compiled in Supplementary Table 7 demonstrated that ZnO-C. gooseberry nano-extract significantly altered the expression of EGFR, Bcl2, and Casp3 genes in HEPG-2 cells compared to control HEPG-2 cells or those treated with native plant juice. These findings are consistent with previous studies (Ibrahim et al., Citation2021; Aboulthana et al., Citation2022) that highlighted the potential anticancer activity of plant-derived ZnO-NPs by disrupting signal transduction pathways through the generation of reactive oxygen species (ROS) and promoting cellular death.
As depicted in Supplementary Figure 6, the ZnO-C. gooseberry nano-extract resulted in an increased accumulation of HEPG-2 cells at the G2/M and Pre-G1 phases compared to control HEPG-2 cells or cells treated with native plant juice alone. The percentages of cells at these phases were approximately 30.60% and 22.31%, respectively. Conversely, this nano-extract led to a decreased accumulation of HEPG-2 cells at the G0-G1 and S phases compared to control HEPG-2 cells or cells treated with native plant juice alone. The percentages of cells at these phases were approximately 39.52% and 29.88%, respectively. This agreed with the recent studies demonstrated by Hussien et al. (Citation2024).
The ZnO-C. gooseberry nano-extract significantly increased the percentage of total (22.31%), early (3.29%), and late apoptotic cells (11.47%) in HEPG-2 cells compared to control HEPG-2 cells or those treated with native plant juice itself (Supplementary Figure 7). Flow cytometric analysis (Supplementary Figure 8) and apoptosis assay using Annexin V-FITC (Supplementary Figure 9) data showed that the ZnO-C. gooseberry nano-extract increased apoptosis in HEPG-2 cells compared to control HEPG-2 cells or those treated with native plant juice itself. This increase in apoptosis may be attributed to the presence of a wide range of phyto-constituents (phenolic and flavonoid compounds) as detected by HPLC technique. According to Srivastava et al. (Citation2007), flavonoids in plant juice have been shown to play a role in regulating apoptosis and preventing the incidence and progression of cancer cells. Additionally, Vasanth et al. (Citation2014) suggested that the incorporation of M-NPs into plant juice induced apoptosis in human cervical carcinoma cells by increasing the generation of ROS and inhibiting cellular replication.
2.6. In vivo study on C. gooseberry juice incorporated with M-NPs
The study found that the addition of M-NPs (Ag- and ZnO-NPs) to C. gooseberry juice did not result in toxicity when orally administered to mice. This finding is consistent with the research of Aboulthana et al. (Citation2019) and is further supported by Aboulthana et al. (Citation2022), who suggested that M-NPs are eliminated from the bloodstream before undergoing catabolic pathways to prevent adverse side effects. The efficiency of elimination depends on the dose and size of the M-NPs (Shaban et al., Citation2021). The ZnO-C. gooseberry nano-extract had the highest LD50 value (11333.33 mg/Kg), followed by Ag-C. gooseberry nano-extract (8083.33 mg/Kg), and then the native plant juice (5333.33 mg/Kg) (Supplementary Figure 10). In this study, the effectiveness of C. gooseberry juice incorporated with Ag- and ZnO-NPs was evaluated against CCl4-induced hepatotoxicity in rats and compared to the native juice itself.
Hematological measurements, including RBCs, HB, HCT, platelets, and WBCs count, were significantly decreased in the CCl4 intoxicated group (p ≤ 0.05). This finding aligns with the study by Madthi et al. (Citation2018), which demonstrated that CCl4 caused a general reduction in blood cellular elements, known as pancytopenia, as evidenced by lower measurements. The decrease in these blood measurements may be attributed to the excessive generation of ROS in the erythrocytes, which act as oxygen carrier protein haemoglobin (AL-Mashhadani, Citation2017). Additionally, the abnormalities in total and differential leukocytes count may occur as a result of role of CCl4 in the inflammatory response, leading to the stimulation of the release of numerous bone marrow cells, including neutrophils, and the subsequent release of H2O2, which may induce damage to surrounding cells and tissues (Abuharfeil et al., Citation2001). The administration of plant juice before and after incorporating M-NPs with CCl4 improved the efficiency of the active components, especially ZnO-NPs, and resulted in an anti-inflammatory effect due to free radical scavenging activity (Sule et al., Citation2012). Additionally, the presence of flavonoids, which are powerful antioxidants and vasculo-protectors, reduced the accumulation of toxic CCl4 derived metabolites (Mada et al., Citation2014). Treatment with native C. gooseberry juice had no effect on these measurements. Levels of these measurements significantly (p ≤ 0.05) increased after treatment with Ag-C. gooseberry nano-extract, but they could not be restored to normal values. However, their levels were restored to normal after administration of ZnO-C. gooseberry nano-extract (Supplementary Table 8).
The liver is susceptible to harmful physiological and pathological changes due to its essential role in the detoxification process, which involves the transformation of various xenobiotics and therapeutic agents (Wanjari et al., Citation2016; Aboulthana et al., Citation2023). CCl4, an industrial solvent commonly used in the production of semiconductors, the synthesis of chlorinated organic compounds, the processing of fats and oils, and other laboratory applications, is well-known for its toxic effects on the liver (Guven et al., Citation2003; Szymonik-Lesiuk et al., Citation2003). The experimental model demonstrated that liver injury was induced by chemical exposure to CCl4. The metabolism of CCl4 led to the production of highly toxic metabolites, specifically trichloromethyl radicals (CCl3. and CCl3O2.), in the liver by cytochrome P-450 (Muriel and Moreno, Citation2004). These radicals are responsible for initiating lipid peroxidation and fatty changes in the liver. Furthermore, their ability to oxidize essential macromolecules (DNA, proteins, and lipids) can lead to cytotoxicity (Lee and Lim, Citation2008). In the Ayurvedic system of medicine, various medicinal herbs have been studied for their potential to treat liver dysfunctions, leading to a focus on hepatoprotective substances of plant origin (Ahmed, Citation2015). In this study, the effectiveness of C. gooseberry juice incorporated with Ag- and ZnO-NPs was evaluated against CCl4-induced hepatotoxicity in rats and compared to the native juice itself. Intoxication with CCl4 led to a significant (p ≤ 0.05) increase in the activities of serum hepatic enzymes (ALT, AST, ALP, GGT, and LDH) as indicated in Supplementary Table 9. Additionally, the levels of total protein and albumin decreased significantly (p ≤ 0.05) following CCl4 injection compared to control rats. This result aligns with the findings of Al-Sayed et al. (Citation2014), who suggested that levels of these enzymes were elevated in the sera of the CCl4-injected group due to extensive liver damage and loss of hepatic architecture. Dahiru et al. (Citation2010) also observed that liver toxicity induced by CCl4 is partly mediated by the generation of excessive ROS. Additionally, the levels of total protein and albumin decreased following CCl4 injection compared to control rats. The administration of native plant juice did not show any ameliorative effect on these measurements. However, the Ag-C. gooseberry nano-extract improved levels of these biochemical measurements, although they could not be restored to normal levels. Conversely, the ZnO-C. gooseberry nano-extract countered the adverse effect induced by CCl4 injection by restoring levels of these measurements to normal.
In the liver tissues of the CCl4 injected group, there was a significant decrease (p ≤ 0.05) in markers of the antioxidant system (TAC, SOD, CAT, GPx, and GSH). This aligns with Srivastava and Shivanandappa’s (Citation2010) findings, which indicated that the active metabolites (CCl3. and CCl3O2. radicals) alter the antioxidant state of the liver by inhibiting the antioxidant enzymes in hepatic tissue. Furthermore, the deactivation of GSH was linked to the reaction of trichloromethyl radicals with the sulfhydryl groups of GSH. As for the products of the peroxidation reactions (LPO and TPC), the levels of these significantly increased (p ≤ 0.05) in liver tissues due to CCl4 intoxication. This finding is consistent with Tipoe et al. (Citation2010), who suggested that the levels of LPO and TPC markedly increased in liver tissues in response to liver damage induced by oxidative stress, leading to a decrease in the markers of the antioxidant system.
The study focused on the use of antioxidants, both enzymatic and non-enzymatic, to prevent various toxicity conditions. Previous research has shown that decreased levels of antioxidants are implicated in the pathogenesis process (Abulyazid et al., Citation2017; Seif et al., Citation2019). The present study found that the ZnO-C. gooseberry nano-extract was able to restore markers of oxidative stress to normal levels. The present study found that incorporating C. gooseberry juice with M-NPs helped minimise CCl4-induced hepatotoxicity through antioxidant activities. The administration of C. gooseberry juice with M-NPs improved the antioxidant status of liver tissues by increasing antioxidants and decreasing products of peroxidation reactions significantly (p ≤ 0.05) compared to the CCl4 injected group. Additionally, ZnO-C. gooseberry nano-extract was able to restore markers of oxidative stress to normal levels (Supplementary Table 10).
The TNF-α and IL-6 are considered central regulators of inflammation and are categorised as markers of the inflammatory response (Esposito and Cuzzocrea, Citation2009). The present study found that levels of these biomarkers were significantly elevated in the liver tissues of the CCl4 injected group (p ≤ 0.05) (Supplementary Table 11). This finding is consistent with Ouyang et al. (Citation2009), who suggested that levels of these measurements were elevated in the CCl4 intoxicated group due to the inflammatory conditions related to the production of excessive CCl4-generated free radicals. In comparison to the CCl4 injected group, the native plant juice improved levels of these measurements as evidenced by the reduction in their levels. Both Ag- and ZnO-C. gooseberry nano-extracts were capable of restoring levels of these markers to normalcy. Injection of CCl4 caused no deleterious effect on the marker of liver fibrosis (Hydroxyproline), which was approximately equal in liver tissue homogenates of all treated groups.
The hepatic mRNA and protein levels of antioxidant enzymes (SOD1, CAT, and GPX) were significantly elevated (p ≤ 0.05) in the CCl4 intoxicated group compared to the control group. Rats injected with CCl4 and given plant juice (before and after incorporating M-NPs) showed a significant decrease in these levels compared to the CCl4 intoxicated group, highlighting the effective role of these extracts in mitigating the cellular complications caused by CCl4 toxicity. The ZnO-C gooseberry nano-extract demonstrated the most ameliorative effect compared to the other studied extracts (Supplementary Figure 11). The injection of CCl4 triggered severe inflammation in the liver tissue, as evidenced by the significant (p ≤ 0.05) increase in levels of the pro-inflammatory biomarkers (TNF-α and IL-6) compared to the control group. Administration of plant juice (before and after incorporating M-NPs) reduced levels of these biomarkers compared to CCl4 treated rats. The ZnO-C gooseberry nano-extract showed the most ameliorative effect on levels of these biomarkers compared to the other treated groups. The current study suggests that inducing systems of antioxidant defense and suppressing peroxidation reactions by C. gooseberry juice incorporated with M-NPs could be effective in inhibiting apoptosis by caspase cascades triggered by injection of CCl4, as supported by previous findings obtained by El-Mahdy et al. (Citation2008).
The scanning electron microscopy (SEM) is a powerful tool for examining ultrastructural changes in tissues under study (Vodenicharov, Citation2007). In this study, the ultrastructural examination was carried out by SEM to investigate the effects of CCl4 injection on liver architecture and the potential ameliorative effects of plant juice incorporated with M-NPs. The liver ultrastructure of control rats showed normal tissue architecture and hepatocytes (Supplementary Fig. 12a). Rats treated with the juice before and after incorporating M-NPs also displayed normal liver architecture and no evidence of hepatic lesions (Supplementary Figs. 12b, c, and d). However, CCl4 injection caused severe lesions, including fragmentation of hepatic cells, irregular cracks on hepatocyte surfaces, and fat accumulation on the liver surface (Supplementary Figs. 12e, f, and g). The CCl4 injection caused severe lesions, including fragmentation of hepatic cells, irregular cracks on hepatocyte surfaces, and fat accumulation on the liver surface. This is consistent with Ikeda et al. (Citation2007), who suggested that CCl4 injection disrupts the structural integrity of hepatic cell membranes, leading to oxidative stress and potential hepatic dysfunctions. Rats treated with native plant juice showed superficial regular cracks with cellular bridging and loss (Supplementary Fig. 12h). Rats treated with Ag-C. gooseberry nano-extract showed superficial regular cracks without tissue debris (Supplementary Fig. 12i). In rats treated with ZnO-C. gooseberry nano-extract, the liver architecture appeared to have restored to a normal state and appeared smoother compared to the other treated groups (Supplementary Fig. 12j). The liver architecture appeared to have restored to a normal state and appeared smoother after the treatment with ZnO-C. gooseberry nano-extract compared to the other treated groups. Our findings showed that combining C. gooseberry juice with M-NPs and administering it to rats with CCl4 intoxication effectively reduced the release of enzymes from hepatocytes. This may be partly due to the antioxidant enzyme activities mentioned earlier. Interestingly, evaluation of liver ultrastructure by SEM supported these findings. Liver tissues from the CCl4-injected group treated with ZnO-C. gooseberry nano-extract showed significantly fewer signs of liver injury compared to the CCl4-injected group. Treatment with the plant juice incorporated with M-NPs prevented fat accumulation and reduced the severity of tissue lesions. Markers of the antioxidant system significantly increased, while markers of inflammatory reactions significantly decreased, approaching normal values in liver tissues from CCl4-injected rats treated with metal C. gooseberry nano-extracts. This indicates that these extracts were able to inhibit apoptosis in hepatocytes, supporting the hepatic ultrastructural findings and liver function results obtained during the experiment.
3. Conclusion
A recent study has found that the ZnO-C. gooseberry nano-extract demonstrated superior in vitro biological activities, including antioxidant, scavenging, anti-diabetic, anti-alzheimer, anti-inflammatory, and cytotoxic activities. Following closely behind is the Ag-C. gooseberry nano-extract, with the native juice showing the lowest activity. Additionally, when taken orally, the ZnO-C. gooseberry nano-extract was found to be safer than the other extracts. Moreover, it showed the highest effectiveness in protecting against hepatotoxicity induced by CCl4 in rats and improved the antioxidant status of the liver by reducing elevated biochemical measurements, increasing the activity of antioxidant enzymes, and decreasing markers of the inflammatory response.
Ethical approval
The experimental design for the experimental animals was conducted in accordance with the guidelines outlined in the ‘Guide for the Care and Use of Laboratory Animals’. This was done following the approved protocol by the Institutional Animal Ethical Committee of the National Research Centre, located in Dokki, Giza, Egypt (No: 444102022).
Author contributions
WMA, NIO, and EAH proposed and compiled relevant previously published papers on the idea, and developed a practical plan. AME conducted phytochemical screening and prepared plant extracts, while AMY focused on biosynthesis of M-NPs and preparation of plant nano-extracts. WMA, AKH, and MS conducted biochemical and molecular experiments. All authors approved the final draft of the manuscript, with WMA corresponding with journal editors to request publication.
Supplemental Material
Download PDF (3.9 MB)Acknowledgments
The authors from diverse scientific fields wish to extend their thanks to the National Research Centre in Dokki, Cairo, Egypt for their invaluable technical support during the completion of this research.
Disclosure statement
The authors declare that there are no conflicts of interest, whether financial or non-financial, among them.
Data availability statement
No Data associated in the manuscript.
Additional information
Funding
References
- Aamer RA. 2018. Evaluation of new non-traditional products processed from cape gooseberry (Physalis peruviana L.). Egypt J Agric Res. 96(4):1493–1511.
- Abou-Gharbia HA, Abou-Tour EM. 2001. Properties and processing of husk tomato (Physalis pruinosa L.). Minufiya J Agric Res. 26:761–781.
- Aboulthana WM, Youssef A, El-Feky AM, Ibrahim NE, Seif MM, Hassan AK. 2019. Evaluation of Antioxidant Efficiency of Croton tiglium L. Seeds Extracts after Incorporating Silver Nanoparticles. Egyptian Journal of Chemistry. 62(2):181–200. doi:10.21608/ejchem.2018.4960.1442.
- Aboulthana WM, Ibrahim NE, Hassan AK, Bassaly WK, Abdel-Gawad H, Taha HA, Ahmed KA. 2023. The hepato- and neuroprotective effect of gold Casuarina equisetifolia bark nano-extract against Chlorpyrifos-induced toxicity in rats. J Genet Eng Biotechnol. 21(1):158. doi:10.1186/s43141-023-00595-6.
- Aboulthana WM, Omar NI, El-Feky AM, Hasan EA, Ibrahim NE, Youssef AM. 2022. In Vitro study on effect of zinc oxide nanoparticles on the biological activities of Croton tiglium L. seeds extracts. Asian Pac J Cancer Prev. 23(8):2671–2686. doi:10.31557/APJCP.2022.23.8.2671.
- Aboulthana WM, Youssef AM, Seif MM, Osman NM, Sahu RK, Ismael M, El-Baz HA, Omar NI. 2021. Comparative study between Croton tiglium Seeds and Moringa oleifera leaves extracts, after incorporating silver nanoparticles, on murine brains. Egypt J Chem. 64(4):1709–1731. doi:10.21608/ejchem.2021.53777.3113.
- Abuharfeil N, Sarsour E, Hassuneh M. 2001. The effect of sodium nitrite on some parameters of the immune system. Food Chem Toxicol. 39(2):119–124. doi:10.1016/s0278-6915(00)00122-8.
- Abulyazid I, Abd Elhalim SA, Sharada HM, Aboulthana WM, Abd Elhalim STA. 2017. Hepatoprotective Effect of Carob Pods Extract (Ceratonia siliqua L.) against Cyclophosphamide Induced Alterations in Rats. ijcprr. 8(02):149–162. doi:10.25258/ijcprr.v8i02.9199.
- Ahmed NG. 2015. Hepatoprotective and anti hepatotoxic drugs from plant sources. 5th International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems. Pharm Anal Acta. 6:1.
- Alharbi NS, Khaled JM, Alanazi K, Kadaikunnan S, Alobaidi AS. 2023. Biosynthesis of silver nanoparticles (Ag-NPs) using Senna alexandrina grown in Saudi Arabia and their bioactivity against multidrug-resistant pathogens and cancer cells. Saudi Pharm J. 31(6):911–920. doi:10.1016/j.jsps.2023.04.015.
- Al-Mashhadani FA. 2017. Effect of fenugreek seed and leaves on some hematological and biochemical parameters in CCl4-induced liver injury. Int J Curr Microbiol App Sci. 6(4):2328–2337.
- Al-Sayed E, Martiskainen O, Seif el-Din SH, Sabra AA, Hammam OA, El-Lakkany NM, Abdel-Daim MM. 2014. Hepatoprotective and Antioxidant Effect of Bauhinia hookeri Extract against Carbon Tetrachloride-Induced Hepatotoxicity in Mice and Characterization of Its Bioactive Compounds by HPLC-PDA-ESI-MS/MS. Biomed Res Int. 2014:245171–245179. doi:10.1155/2014/245171.
- Ballesteros-Vivas D, Alvarez-Rivera G, León C, Morantes SJ, Ibánez E, Parada-Alfonso F, Cifuentes A, Valdés A. 2019. Anti-proliferative bioactivity against HT-29 colon cancer cells of a with anolides-rich extract from golden berry (Physalis peruviana L.) calyx investigated by Foodomics. J Functional Foods. 63:1756–4646.
- Bashir S, Awan MS, Farrukh MA, Naidu R, Khan SA, Rafique N, Ali S, Hayat I, Hussain I, Khan MZ. 2022. In-vivo (Albino Mice) and in-vitro assimilation and toxicity of zinc oxide nanoparticles in food materials. Int J Nanomedicine. 17:4073–4085. doi:10.2147/IJN.S372343.
- Briones-Labarca V, Giovagnoli-Vicuña C, Figueroa-Alvarez P, Quispe-Fuentes I, Pérez-Won M. 2013. Extraction of Carotene, vitamin c and antioxidant compounds from Physalis peruviana (Cape Gooseberry) assisted by high hydrostatic pressure. FNS. 04(08):109–118. doi:10.4236/fns.2013.48A014.
- da Silva DF, Pio R, Soares JDR, Elias HHS, Villa F, Vilas Boas EVB. 2016. Light spectrum on the quality of fruits of Physalis species in subtropical area. Bragantia. 75(3):371–376. doi:10.1590/1678-4499.463.
- Dahiru D, Mamman DN, Wakawa HY. 2010. Ziziphus mauritiana fruit extract inhibits CCl4-induced hepatotoxicity in male rats. Pak J Nutr. 9:990–993.
- Dikshit PK, Kumar J, Das AK, Sadhu S, Sharma S, Singh S, Gupta PK, Kim BS. 2021. Green Synthesis of Metallic Nanoparticles: applications and Limitations. Catalysts. 11(8):902. doi:10.3390/catal11080902.
- El-Mahdy MA, Zhu Q, Wang QE, Wani G, Patnaik S, Zhao Q, Arafa E, Barakat B, Mir SN, Wani AA. 2008. Naringenin protects HaCaT human keratinocytes against UVB induced apoptosis and enhances the removal of cyclobutane pyrimidine dimers from the genome. Photochem Photobiol. 84(2):307–316. doi:10.1111/j.1751-1097.2007.00255.x.
- Embaby HES, Mokhtar SM. 2019. Impact of adding goldenberry (Physalis peruviana L.) on some quality characteristics and bio-functional properties of pasteurized carrot (Daucus carota L.) nectar. J Food Sci Technol. 56(2):966–975. doi:10.1007/s13197-018-03563-y.
- Esposito E, Cuzzocrea S. 2009. Superoxide, NO, peroxynitrite and PARP in circulatory shock and inflammation. Front Biosci (Landmark Ed). 14(1):263–296. doi:10.2741/3244.
- Figueiredo MCC, Passos AR, Hughes FM, dos Santos KS, da Silva AL, Soares TL. 2020. Reproductive biology of Physalis angulata L. (Solanaceae). Sci Hortic. 267:109307. doi:10.1016/j.scienta.2020.109307.
- Fuente FP, Nocetti D, Sacristán C, Ruiz P, Guerrero J, Jorquera G, Uribe E, Bucarey JL, Espinosa A, Puente L. 2020. Physalis peruviana L. Pulp Prevents Liver Inflammation and Insulin Resistance in Skeletal Muscles of Diet-Induced Obese Mice. Nutrients. 12(3):700. doi:10.3390/nu12030700.
- Guven A, Guven A, Gulmez M. 2003. The effect of kefir on the activities of GSHPx, GST, CAT, GSH and LPO levels in carbon tetrachloride-induced mice tissues. J Vet Med B Infect Dis Vet Public Health. 50:412–416.
- Hassan SSM, Abdel-Shafy HI, Mansour MSM. 2019. Removal of pharmaceutical compounds from urine via chemical coagulation by green synthesized ZnO-nanoparticles followed by microfiltration for safe reuse. Arabian J Chem. 12(8):4074–4083.
- Hussien AG, Aboulthana WM, Youssef AM. 2024. In vitro evaluation of Saussurea costus gold nano-extract as antioxidant, anti-diabetic, anti-alzheimer, and anti-inflammatory agent. Egypt J Chem. 67(5):257–283.
- Ibrahim NE, Morsy H, Abdelgwad M. 2021. The comparative effect of nisin and thioridazine as potential anticancer agents on hepatocellular carcinoma. Rep Biochem Mol Biol. 9(4):452–462.
- Ikeda H, Kume Y, Tejima K, Tomiya T, Nishikawa T, Watanabe N. 2007. Rho-kinase inhibitor prevents hepatocyte damage in acute liver injury induced by carbon tetrachloride in rats. Am J Physiol Gastrointest Liver Physiol. 293(4):G911–G917.
- Jouybari HB, Hosseini AS, Davoodi A, Mirzaee F, Azadbakht M. 2018. Materia medica used in jaundice based on Persian medicine. Res J Pharmacogn. 5(4):83–93.
- Kasali FM, Tusiimire J, Kadima JN, Tolo CU, Weisheit A, Agaba AG. 2021. Ethnotherapeutic Uses and Phytochemical Composition of Physalis peruviana L.: an Overview. Sci World J. 2021:1–22.
- Kim KM, Kim TH, Kim HM, Kim HJ, Gwak GH, Paek SM, Oh JM. 2012. Colloidal behaviors of ZnO nanoparticles in various aqueous media. J Toxicol Environ Health Sci. 4(2):121–131.
- Lee S-J, Lim K-T. 2008. Glycoprotein of Zanthoxylum piperitum DC has a hepatoprotective effect via anti-oxidative character in vivo and in vitro. Toxicol in Vitro. 22(2):376–385. doi:10.1016/j.tiv.2007.10.002.
- Mada SB, Inuwa HM, Abarshi MM, Mohammed HA, Aliya A. 2014. Hepatoprotective effect of Momordica charantia extract against CCL4 induced liver damage in rats. Br J Pharmaceutic Res. 4(3):368–380.
- Madthi AS, Al-Diwan MA, Al-Jadaan SAN. 2018. Hematological profile of rats treated with quercetin derivative against carbon tetrachloride (CCl4) toxicity. Bas J Vet Res. 17(2):70–84.
- Mamillapalli V, Atmakuri AM, Khantamneni P. 2016. Nanoparticles for Herbal Extracts. Asian J Pharm. 10(2):S54–S60.
- Mazova N, Popova V, Stoyanova A. 2020. Phytochemical composition and biological activity of Physalis spp.: a mini-review. FSAB. 3(1):56–70. doi:10.30721/fsab2020.v3.i1.80.
- Medina-Medrano JR, Almaraz-Abarca N, González-Elizondo MS, Uribe-Soto JN, González-Valdez LS, Herrera-Arrieta Y. 2015. Phenolic constituents andantioxidant properties of five wild species of Physalis (Solanaceae). Bot Stud. 56(1):24. doi:10.1186/s40529-015-0101-y.
- Mirzaee F, Hosseini A, Jouybari HB, Davoodi A, Azadbakht M. 2017. Medicinal, biological and phytochemical properties of Gentiana species. J Tradit Complement Med. 7(4):400–408. doi:10.1016/j.jtcme.2016.12.013.
- Muriel P, Moreno MG. 2004. Effects of silymarin and vitamins E and C on liver damage induced by prolonged biliary obstruction in the rat. Basic Clin Pharmacol Toxicol. 94:99–104.
- Narváez-Cuenca CE, Mateus-Gómez Á, Restrepo-Sánchez LP. 2014. Antioxidant capacity and total phenolic content of air-dried cape gooseberry (Physalis peruviana L.). Agron Colomb. 32(2):232–237. doi:10.15446/agron.colomb.v32n2.43731.
- Oliveira SF, Gonçalves FJ, Correia PM, Guiné RP. 2016. Physical properties of Physalis peruviana L. Open Agric. 1(1):56–62.
- Ouyang W, Beckett O, Flavell RA, Li MO. 2009. An essential role of the forkhead-box transcription factor foxo1 in control of T cell homeostasis and tolerance. Immunity. 30(3):358–371. doi:10.1016/j.immuni.2009.02.003.
- Pandit C, Roy A, Ghotekar S, Khusro A, Islam MN, Bin Emran T, Lam SE, Khandaker MU, Bradley DA. 2022. Biological agents for synthesis of nanoparticles and their applications. J King Saud Univ Sci. 34(3):101869. doi:10.1016/j.jksus.2022.101869.
- Puente LA, Pinto-Muñoz CA, Castro ES, Cortés M. 2011. Physalis peruviana Linnaeus, the multiple properties of a highly functional fruit: a review. Food Res Inter J. 44(7):1733–1740. doi:10.1016/j.foodres.2010.09.034.
- Raja S, Ramesh V, Thivaharan V. 2017. Green bio synthesis of silver nanoparticles using Calliandra haematocephala leaf extract, their antibacterial activity and hydrogen peroxide sensing capability. Arab J Chem. 10:253–261.
- Ramakrishna Pillai J, Wali AF, Menezes GA, Rehman MU, Wani TA, Arafah A, Zargar S, Mir TM. 2022. Chemical composition analysis, cytotoxic, antimicrobial and antioxidant activities of Physalis angulata L.: a comparative study of leaves and fruit. Molecules. 27(5):1480. doi:10.3390/molecules27051480.
- Seif MM, Madboli A, Marrez DA, Aboulthana WMK. 2019. Hepato-renal protective effects of egyptian purslane extract against experimental cadmium toxicity in rats with special emphasis on the functional and histopathological changes. Toxicol Rep. 6:625–631. doi:10.1016/j.toxrep.2019.06.013.
- Shaban E, Salaheldin K, El Sayed E, Abd El-Aziz ME, Nasr S, Desouky HM, Elbakry HF. 2021. Evaluation of acute oral toxicity of zinc oxide nanoparticles in rats. Egypt J Chem. 0(0):0–0. doi:10.21608/ejchem.2021.80810.4003.
- Srivastava A, Shivanandappa T. 2010. Hepatoprotective effect of the root extract of Decalepis hamiltonii against carbon tetrachloride-induced oxidative stress in rats. Food Chem. 118(2):411–417. doi:10.1016/j.foodchem.2009.05.014.
- Srivastava A, Akoh CC, Fischer J, Krewer G. 2007. Effect of anthocyanin fractions from selected cultivars of Georgia-grown blueberries on apoptosis and phase II enzymes. J Agric Food Chem. 55(8):3180–3185. doi:10.1021/jf062915o.
- Sule OJ, Elekwa I, Ayalogu EO. 2012. Effect of Acalypha wilkesiana muell arg. on haematological parameters in wistar albino rats. Int J Biol Med Res. 3(1):1234–1237.
- Szymonik-Lesiuk S, Czechowska G, Stryjecka-Zimmer M, Słomka M, Madro A, Celiński K, Wielosz M. 2003. Catalase, superoxide dismutase, and glutathione peroxidase activities in various rat tissues after carbon tetrachloride intoxication. J Hepatobiliary Pancreat Surg. 10(4):309–315. doi:10.1007/s00534-002-0824-5.
- Tammu J, Ramana KV. 2015. Pharmacological review on Physalis species: A potential herbal cure-all. World J Pharm Res. 4(2):247–256.
- Thatyana M, Dube NP, Kemboi D, Manicum AE, Mokgalaka-Fleischmann NS, Tembu JV. 2023. Advances in phytonanotechnology: a plant-mediated green synthesis of metal nanoparticles using phyllanthus plant extracts and their antimicrobial and anticancer applications. Nanomaterials (Basel). 13(19):2616. doi:10.3390/nano13192616.
- Tipoe GL, Leung TM, Liong EC, Lau TYH, Fung ML, Nanji AA. 2010. Epigallocatechin-3-gallate (EGCG) reduces liver inflammation, oxidative stress and fibrosis in carbon tetrachloride (CCl4)-induced liver injury in mice. Toxicology. 273(1-3):45–52. doi:10.1016/j.tox.2010.04.014.
- Vasanth K, Ilango K, MohanKumar R, Agrawal A, Dubey GP. 2014. Anticancer activity of Moringa oleifera mediated silver nanoparticles on human cervical carcinoma cells by apoptosis induction. Colloids Surf B Biointerfaces. 117:354–359. doi:10.1016/j.colsurfb.2014.02.052.
- Vodenicharov A. 2007. Scanning electron microscopic study on renal glomerular arterioles in pigs. Bulg J Veter Med. 10(3):147–154.
- Wanjari MM, Gangoria R, Dey YN, Gaidhani SN, Pandey NK, Jadhav AD. 2016. Hepatoprotective and antioxidant activity of Bombax ceiba flowers against carbon tetrachloride-induced hepatotoxicity in rats. Hepatoma Res. 2:144–150.
- Zhou X-Q, Hayat Z, Zhang D-D, Li M-Y, Hu S, Wu Q, Cao Y-F, Yuan Y. 2023. Zinc oxide nanoparticles: synthesis, characterization, modification, and applications in food and agriculture. Processes. 11(4):1193. doi:10.3390/pr11041193.