 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Gaseous nitriding of ferritic Fe–Cr and austenitic Ni–Ti solid solutions reveals that the extent of the uptake of dissolved nitrogen depends on the crystallographic orientation of the surface grains of the substrate. In both ferritic and austenitic substrates, the surface nitrogen concentration and the nitriding depth decrease upon increasing the smallest angle between the surface normal and the normal of a {1 0 0} plane of the surface grain considered. This phenomenon could be ascribed to the residual compressive macrostress developed during nitriding which varies as a function of crystallographic orientation of the (surface) grains due to the elastically anisotropic nature of ferrite and austenite solid solutions investigated in this study.
1. Introduction
Nitriding is a thermochemical surface treatment employed to pronouncedly enhance the wear, fatigue and corrosion resistances of metallic, especially steel components [Citation1,2]. To arrive at fundamental understanding of the nitriding behaviour of multicomponent ferritic (bcc) steels, research has been focused on ferritic model alloys such as iron-based binary Fe–Cr alloys [Citation3–9]. Upon nitriding of Fe–Me (Me: Cr, V, Al, Ti, Mo) alloys, the nitrogen dissolves interstitially in the matrix and, after surpassing the solubility product of Me-nitride, precipitation of nanosized, (semi-) coherent MeN nitride platelets develop in the matrix, leading to distinct enhancement of particularly mechanical properties [Citation10–15].
In the case of austenitic (fcc), stainless steels, the nitriding treatment is usually performed at low temperatures (<450 °C) and for relatively short times, in order to avoid distinct diffusion of Me and thus formation of MeN precipitates; in this way, a high level of N supersaturation can be realized, while preserving Me (in this case, Me usually is Cr) in dissolved state. Austenite with such high level of N supersaturation is known in the literature as expanded austenite (previously indicated by the unfortunate name S-phase) [Citation16–27]. The extremely high content of N (i.e. far above the amount compatible with equilibrium) absorbed by the alloyed austenitic matrix has been attributed to the trapping of N, at octahedral interstitial sites, by alloying element atoms dissolved in the matrix with high affinity for N (such as Cr and Ti) [Citation18,25,28]. A similar, expanded austenite (fcc) phase was also observed during low-temperature nitriding/carburizing of nonferrous Ni-based [Citation29–31] and Co-based [Citation32] alloys.
The surface nitrogen content and the thickness of the case of expanded austenite were shown to depend on the crystallographic orientation of the grain considered with respect to the specimen surface: the nitrided depth and the surface nitrogen content are the larger; the smaller the angle is between the surface normal and the normal of the {1 0 0} lattice plane [Citation16,30,33]; the smallest nitrided zone depth and the smallest surface nitrogen content occur for the grains with a {1 1 1} plane parallel to the surface. The following explanations for this phenomenon have been offered:
| (1) | Some researchers have attributed this phenomenon to a supposed intrinsic anisotropy of the nitrogen diffusivity [Citation16,18]. However, the diffusivity in cubic lattices is essentially isotropic [Citation34]. | ||||
| (2) | The rate of the nitriding reaction between a nitriding medium and a solid substrate can depend on the crystallographic structure of the solid surface exposed to the nitriding medium [Citation35,36]. As a result, the nitrogen fluxes passing through the surface adjacent grains of different crystallographic orientations can be different. | ||||
| (3) | Finally, the magnitude of the residual (compressive) macrostress parallel to the surface of the heterogeneously nitrided specimens [Citation9,30,37] can depend on the crystallographic orientation of the grain adjacent to the surface with respect to the surface (note the anisotropy of the intrinsic, single-crystal elastic constants). As a consequence, the nitrogen solubility level of the surface [Citation26] and the nitrogen diffusivity [Citation38] can depend on the crystallographic orientation of the surface adjacent grains with regard to the surface. | ||||
Against the above background and recognizing that there appears to be no reason that the effect discussed should be restricted to austenitic substrates only, in the present work, low-temperature gaseous nitriding experiments have been carried out on substrates of both ferritic Fe–Cr and austenitic Ni–Ti alloys. This study shows, for the first time, the occurrence of the surface grain, crystallographic orientation-dependent nitriding rate of ferritic alloys. A general explanation for the phenomenon discussed is offered, which is based on the effect of the residual, nitriding-induced macrostress on the nitrogen solubility for differently oriented surface grains of an intrinsically elastically anisotropic polycrystalline substrate.
2. Theoretical background
2.1. Stressed solid-/gas-phase thermodynamic equilibrium
The chemical potential of the nitrogen dissolved in a stress-free (iron) matrix phase ( is related to the chemical potential of nitrogen in the standard state (
) and the activity of the dissolved nitrogen (
) [Citation39]:
(1)
(1)
where R is the gas constant and T is the absolute temperature. The nitrogen activity can be written as the product of the corresponding nitrogen concentration () and the nitrogen activity coefficient (
).
The chemical potential of nitrogen dissolved in the stressed solid matrix phase () is described as:
(2)
(2)
where is the activity of nitrogen in the stressed matrix phase, and
and
are the corresponding nitrogen concentration and nitrogen activity coefficient. Equation (2) can be rewritten according to [Citation40,41] as:
(3)
(3)
where is the partial molar volume of nitrogen in the matrix phase, and
is the hydrostatic component of the state of stress acting on the matrix-phase grain considered.
Once equilibrium has been attained at the surface of a (hydrostatically) stressed specimen and of a stress-free specimen with the same nitriding atmosphere of defined nitriding potential (at a certain temperature; [Citation1]), the chemical potentials of N in the stress-free specimen () and of N in the stressed specimen (
) are necessarily the same and equal to the chemical potential of N in the gaseous nitriding medium (
):
(4)
(4)
Accordingly, it follows from Equations (1) and (3) [Citation41,42]:(5)
(5)
where and
are the equilibrium N solubilities in the matrix phase in the presence and in the absence of stress, respectively.
In the following discussion, it will be argued how different values for the (hydrostatic component of the state of) stress can occur for differently oriented surface adjacent grains.
Upon nitriding a residual, biaxial, rotationally symmetric compressive state of stress develops due to the volume misfit of the nitrided case and the unnitrided core of the specimen [Citation43]: and
0 with x, y and z as the principal axes for the state of stress (equal to the principal axes for the state of strain for cubic crystals) with the x and y axes in the plane of the surface and the z axis perpendicular to the surface. Usually, the residual stress σ
// is considered to be the same in every surface adjacent grain of the loaded specimen. However, for intrinsically elastically anisotropic materials, the (state of) stress will actually vary from grain to grain by the elastic interaction of each grain with its surroundings: so-called elastic grain interaction [Citation44,45].
Now, consider hypothetically a set of non-interacting surface adjacent single crystals with a (hkl) plane parallel to the surface, of variable rotation around the surface normal, called ‘(hkl) set’ in the following. Then, the state of stress as described in the specimen frame of reference (x, y, z) is the same for each crystal of the (hkl) set, independent of its angle of rotation around the surface normal, as . For the relationship between σ
// and ɛ
⊥ (=strain in the surface-normal direction), it straightforwardly follows:
(6)
(6)
with as a single-crystal elastic constant in the specimen frame of reference which is related to the intrinsic single-crystal elastic constants in the crystal frame of reference (for cubic crystalline materials) by [Citation46]:
(7)
(7)
with as the so-called orientation factor given by [Citation44]:
(8)
(8)
Considering the same set of (hkl) crystals in the real, massive polycrystalline specimen, the elastic interaction occurring in reality of each grain with its specific surroundings will lead to different values for σ // and ɛ ⊥ for each grain of the (hkl) set. (e.g. see Refs. [Citation47,48]). It will now be assumed that the averaging of the grain interaction in the specimen [Citation45,49] brings about that the principal features of the intrinsic elastic anisotropy do prevail in the polycrystalline aggregate [Citation50], so that Equation (7) remains valid by interpreting σ // and ɛ ⊥ as average values for the (hkl) set. Hence, in this article, different (average) states of stress are adopted for different (hkl) sets.
The dependence of is shown in Figure as a function of
for pure bcc (ferritic) iron and pure fcc Ni. Evidently (note that both σ
// (see above) and
are negative), for the same value of ɛ
⊥, the absolute value of the σ
// is smallest for (hkl) = (1 0 0) (
= 0) and largest for (hkl) = (1 1 1) (
= 0.33).Footnote1
Figure 1. (colour online) The elastic compliance dependent term, , as a function of orientation factor (Гhkl varies from 0 for (1 0 0) plane parallel to the surface to 0.33 for (1 1 1) plane parallel to the surface) for pure ferrite and pure Ni. The elastic compliances are considered as follows: for pure ferrite; S
11 = 7.57 TPa −1, S
12 = –2.82 TPa −1 and S
44 = 8.62 TPa −1 and for pure Ni; S
11 = 7.69 TPa −1, S
12 = –2.92 TPa −1, S
44 = 8.36 TPa −1 [Citation60].
![Figure 1. (colour online) The elastic compliance dependent term, 12S12xyz(hkl), as a function of orientation factor (Гhkl varies from 0 for (1 0 0) plane parallel to the surface to 0.33 for (1 1 1) plane parallel to the surface) for pure ferrite and pure Ni. The elastic compliances are considered as follows: for pure ferrite; S 11 = 7.57 TPa −1, S 12 = –2.82 TPa −1 and S 44 = 8.62 TPa −1 and for pure Ni; S 11 = 7.69 TPa −1, S 12 = –2.92 TPa −1, S 44 = 8.36 TPa −1 [Citation60].](/cms/asset/702896cf-1844-4df3-b4f3-8bf908677cae/tphm_a_1115906_f0001_oc.gif)
Figure 2. (colour online) Light optical macrograph taken from the cross section of nitrided Ni-5 at.% Ti alloy nitrided at 400 °C for 65 h using r N = 500 atm−1/2. Thickness of the expanded austenite layer is different for differently oriented grains. The thickness of the expanded austenite layers developed in (3 3 4), (1 1 7) and (1 1 1) oriented grains are 9.8, 13.2 and 9.2 µm, respectively.

Figure 3. (colour online) Nitrogen-depth profiles measured by EPMA for differently oriented grains (with the different orientation factors (Г)) on a cross section of a Ni-5 at.% Ti specimen nitrided for 65 h at 400 °C using r N = 500 atm−1/2. Both the surface N content and the nitrided zone depth are different for differently oriented grains.
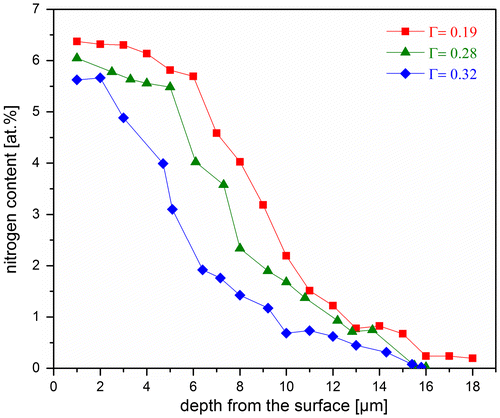
Figure 4. (colour online) EPMA and EBSD (with incorporated corresponding [0 0 1] inverse pole figure (IPF)) measurements performed on the surface of a nitrided Fe-4.5 at.% Cr specimen (450 °C, r N = 0.1 atm−1/2 for 3 h). The dotted white line shows the line scan of EPMA measurement. Nitrogen content is low in grains with blue colour (i.e. with the (1 1 1) plane parallel to the surface).
![Figure 4. (colour online) EPMA and EBSD (with incorporated corresponding [0 0 1] inverse pole figure (IPF)) measurements performed on the surface of a nitrided Fe-4.5 at.% Cr specimen (450 °C, r N = 0.1 atm−1/2 for 3 h). The dotted white line shows the line scan of EPMA measurement. Nitrogen content is low in grains with blue colour (i.e. with the (1 1 1) plane parallel to the surface).](/cms/asset/ccfc76cc-37de-44ac-a97b-76218adf811b/tphm_a_1115906_f0004_oc.gif)
Figure 5. (colour online) Nitrogen content measured on the surface of various grains of Fe-4.5 at.% Cr as a function of the orientation factor of the corresponding grains, as measured from specimen nitrided for different times. Nitriding experiments were performed at 450 °C and r N = 0.1 atm−1/2 for 1, 3, 6, 15 and 30 h. Single N content for each grain was obtained by averaging EPMA data points measured within each grain (measured points on the grain boundaries were neglected; nucleation of precipitates is faster at grain boundaries as compared to areas far from grain boundaries). To exemplify the error of the measured averaged N content for each grain, error bars for two nitriding times (1 and 3 h) have been shown. The polynomial fits have been given only to guide the eye. Evidently, the surface N content decreases with increasing orientation factor.
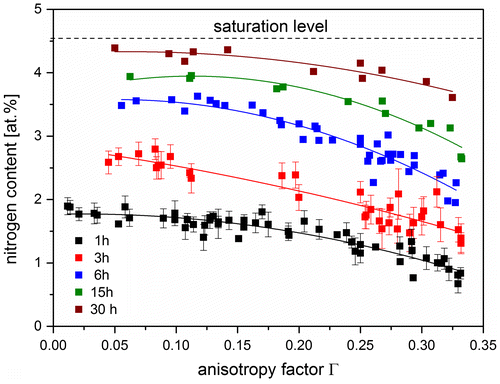
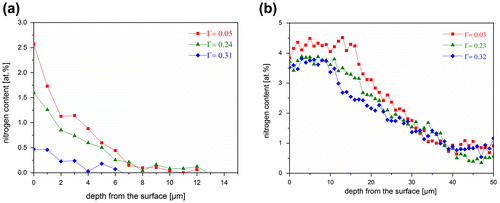
Figure 6. (colour online) Nitrogen-depth profiles measured for differently oriented grains (with the different orientation factors (Г)) on the cross section of a Fe-4.5 at.% Cr specimen nitrided for a) 1 h and b) 30 h. Both the surface N content and the nitrided zone depth are different for differently oriented grains.
Finally, it should be recognized that the treatment leading to Equation (5) is valid for a hydrostatic state of stress ( then can be considered as a scalar). At the moment, no practically applicable, comprehensive treatment of the thermodynamics of stressed solids exists (for a rigorous consideration, based on so-called ‘network solids’, incompatible with common practice, see Ref. [Citation51]). It is proposed here, for the case of a non-hydrostatic state of stress, to apply Equation (5) by equating with the equivalent hydrostatic stress given by the average of the principal components of the stress tensor, i.e.
, which here becomes (see above):
.
Because will in general be different for different (hkl) sets, it follows that different equilibrium nitrogen solubilities occur at the surface for the different (hkl) sets (see Equation (5)). Then, also the depths of the nitrided zones of different (hkl) sets will be different as the diffusional flux increases with increasing nitrogen-concentration gradient. The remainder of this article presents experimental validation for these conclusions.
To determine σ
h for each (hkl) set, the strain ɛ
⊥, as caused by the stress parallel to the surface as consequence of the macroscopic misfit of nitrided case and unnitrided core, is measured for each (hkl) set and then follows by application of Equations (6–8). The strain ɛ
⊥(hkl) can be determined from (i) the average lattice spacing of the (hkl) planes parallel to the surface as determined for the (hkl) set of the macroscopically stressed specimen (a heterogeneously nitrided specimen),
and (ii) the corresponding lattice spacing of a macroscopically nitrided specimen devoid of the planar state of stress considered (reference specimen; further see Section 4.2),
, as follows:
(9)
(9)
3. Experimental
3.1. Specimen preparation
Melts of two alloys of Fe–Cr (Fe-2 at.% Cr and Fe-4.5 at.% Cr) and a melt of Ni–Ti alloy (Ni-5 at.% Ti) were prepared in Al2O3 crucibles, under a protective argon gas (99.999 vol.%) atmosphere, from pure Fe (99.98 wt.%) and pure Cr (99.999 wt.%), and pure Ni (99.98 wt. %) and pure Ti (99.999 wt.%), respectively. Subsequently, casts of Fe–Cr (of cylindrical shape with a diameter of 10 mm and a length of 100 mm) and of Ni–Ti (of rectangular shape with width dimensions of 15 and 30 mm and a length of 150 mm) were obtained by pouring the melts in appropriate Cu moulds. The results of chemical analyses of the produced casts have been presented in Refs. [Citation31,52]. The cast Fe–Cr ingots were encapsulated in a quartz tube filled with argon gas and were homogenized at 1000 °C for 1 h. The cast Ni–Ti ingots were homogenized in a horizontal quartz-tube furnace at 1050 °C for 24 h under a protective flowing (50 ml/min) hydrogen atmosphere by placing the ingot in the middle of the furnace together with a Zr plate as oxygen getter. Afterwards, the Fe–Cr and Ni–Ti casts were cold-rolled to sheets of 1 mm thickness.
Specimens with lateral dimensions of 10 × 15 and 25 × 20 mm2 were cut from these 1-mm-thick sheets of Fe-4.5 at.% Cr and Ni–Ti alloys, respectively. In order to obtain relatively large grains (approx. 50 μm), the Fe-4.5 at.% Cr specimens were recrystallized at 800 °C for 3 h after encapsulating the specimens in a quartz tube filled with Ar gas and the Ni–Ti specimens were recrystallized at 850 °C for 3 h in a hydrogen atmosphere, followed by slow cooling to room temperature for both types of specimens. A thin foil of the Fe-2 at.% Cr alloy, to be homogenously nitrided (of Section 5), was produced as described in Ref. [Citation52].
Immediately prior to nitriding, the surfaces of all specimens were ground and polished (final polishing step: 1 μm diamond suspension) and subsequently cleaned with ethanol in an ultrasonic bath.
3.2. Nitriding experiments
Controlled gaseous nitriding was performed in a vertical quartz-tube furnace with an inner-tube diameter of 28 mm in an ammonia/hydrogen gas flux. The flow rate of each gas component was adjusted separately using mass-flow controllers. The Fe–Cr alloy specimens were nitrided at 450 °C using a nitriding potential (r N; see Ref. [Citation1]) of 0.104 atm−1/2 for 216 h (Fe-2 at.% Cr thin foil) and for 1, 3, 6, 15 and 30 h (1-mm-thick specimens of Fe-4.5 at.% Cr). The Ni–Ti alloy specimens were nitrided at 400 °C for 65 h using a nitriding potential of 500 atm−1/2. The nitriding potentials were chosen such that no iron or nickel nitrides could develop (i.e. the development of only a diffusion zone occurs in all nitrided alloys [Citation1]). Nitriding experiments for the thick specimens were ended by quenching the specimens to room temperature which was achieved by dropping the specimens into nitrogen-flushed water. To avoid breakage of the thin foil of Fe-2 at.% Cr during water quenching, the nitrided thin foil was lifted up into the low-temperature zone of the furnace where the foil was allowed to cool slowly in the nitriding atmosphere.
3.3. Specimen characterization
3.3.1. Light microscopy
To characterize the nitrided zone of the Ni–Ti and the Fe–Cr specimens, pieces from the nitrided specimens were cut normal to the specimen surface and were electroplated with a nickel layer using a Watts bath at 70 °C. Purpose of the Ni-plating is to protect the nitrided layer during the following specimen preparation process. Subsequently, the specimen was embedded in Struers PolyFast. The cross section was then ground and polished (final polishing step using 1 μm diamond suspension) and etched using an aqueous solution composed of 1 part of 10 wt.% NaCN in water and 1 part of 10 wt.% (NH4)2S2O8 in water for 35 s at room temperature in the case of Ni–Ti specimen, and using 1% Nital (1 vol.% HNO3 in ethanol) at room temperature during about 10 s for the Fe-2.0 at.% Cr thin foil and for about 30 s for the Fe-4.5 at.% Cr 1-mm-thick specimens. Optical micrographs from the etched cross sections were recorded to measure the nitrided zone depth on each grain in the cross section using a Zeiss Axiophot microscope equipped with an Olympus ColorView IIIu digital camera. An accurate determination of nitrided zone depth on various grains in the cross section of nitrided Fe–Cr specimens was impossible by light optical micrography due to the unclear interface between the nitrided and unnitrided regions. Therefore, optical micrographs recorded from these specimens are not presented in this article.
3.3.2. X-ray diffraction
XRD patterns were recorded from the surface of the 1 h nitrided Fe-4.5 at.% Cr specimen using a PANalytical X’Pert diffractometer in Bragg–Brentano configuration equipped with a Co tube and a graphite monochromator in the diffracted beam. XRD pattern was recorded from the surface of the 65 h nitrided Ni-5 at.% Ti specimen using a PANalytical X’Pert diffractometer in Bragg–Brentano configuration equipped with a Cu tube and a Johansson monochromator in the primary beam. The lattice spacings corresponding to the (hkl) reflections were calculated from their peak positions determined by fitting pseudo-Voigt functions using the software TOPAS (Version 4.2, Bruker AXS).
3.3.3. Electron probe microanalysis
To determine the elemental (Cr, N and Fe) concentrations of the nitrided zone of the Fe–Cr specimens and the elemental (Ti, N and Ni) concentrations of the nitrided zone of the Ni–Ti specimens, electron probe microanalysis (EPMA) was performed. To obtain the element contents at each measurement point, the intensities of the characteristic X-ray emission peaks were measured and divided by the corresponding intensities obtained from standard samples of pure Fe, Cr, Ti, Ni and γʹ-Fe4 N (for N-Kα). Elemental concentrations were calculated from the intensity ratios applying the Ф(ρz) approach [Citation53] (note that in the case of nitrided Ni–Ti alloy, due to the overlapping of the N-Kα and Ti-L1 emission lines, for the accurate determination of N content, the procedure described in Ref. [Citation31] was applied). For these measurements, a Cameca SX100 microprobe (acceleration voltage U = 15 kV, current I = 100 nA, spot size about 1 μm) was used.
3.3.4. Electron backscatter diffraction
Electron backscatter diffraction (EBSD) was performed on the polished surface of an unnitrided Fe-4.5 at.% Cr (1 mm thick) specimen (final polishing step: 0.05 μm OPS-suspension) and the cross sections of a nitrided Fe-2 at.% Cr thin foil and the nitrided Fe-4.5 at.% Cr and Ni–Ti specimens (final polishing step: 0.05 μm OPS-suspension) with a Zeiss Leo 438 VP scanning electron microscope equipped with an EDAX TSL EBSD measurement system. The data were analyzed using the software OIM version 5. For determining the crystallographic orientation adjacent to the surface of grains, (i) for EBSD measurements performed on the surface of unnitrided specimens, the specimen surface-normal direction (ND) was considered as reference direction and (ii) for EBSD measurements presented on the cross section of nitrided specimens, the specimen rolling direction (RD) was considered as reference direction. In this way, it was possible to identify the same grains (adjacent to the surface of specimen) in both the surface and the cross section of the same specimen (see also Ref. [Citation30]).
4. Results and evaluation
4.1. Dependence of surface nitrogen content and nitrided zone depth on crystallographic orientation of surface grains
4.1.1. Ni–Ti (fcc) specimens
Formation of an expanded austenite layer has been observed during nitriding of Ni–Ti alloy specimen [Citation31]. The thickness of this layer formed in different grains adjacent to the surface varies. In order to trace the surface-grain orientation dependence of the extent of nitriding, the crystallographic orientation of each surface adjacent grain with respect to the surface was determined by EBSD (cf. Section 3.3.4) and expressed by the orientation factor, Г hkl with (hkl) being the lattice plane parallel to the surface (cf. Equation (8)). The orientation factor provides a measure of how large the deviation of the (0 0 1) planes of a particular grain from the surface is. Thus, for example, orientation factor values of 0.1, 0.2 and 0.3 represent (1 0 0) planes inclined at angles of 19°, 30° and 44.5°, respectively, with respect to the specimen surface. As illustrated in Figure , the nitrided zone depth is distinctly larger for the grain with a (1 0 0) plane nearly parallel to the specimen surface (i.e. the grain with a (1 1 7) plane parallel to the surface; Г = 0.04) than for the grain with a (1 1 1) plane parallel to the specimen surface (Г = 0.33). Moreover, the nitrogen-concentration depth profiles measured by EPMA across three surface adjacent grains of this specimen, with orientation factors of 0.19, 0.28 and 0.32, reveal that not only the nitrided depth but also the nitrogen content in the solid at the surface depends on the orientation of the surface adjacent grains Figure : it is concluded that both the surface nitrogen content and the nitrided depth decrease with increasing orientation factor. It is noted that similar observations have been made for austenitic steel (e.g. [Citation26,27]).
Figure 7. (colour online) N-depth profiles measured along two grains with largely different orientations (with respect to the surface of the specimen; Г = 0.02 and Г = 0.30) on the cross section of nitrided Fe- 2at.% Cr thin foil (450 °C, r N = 0.1 atm−1/2 and 216 h). EBSD orientation map and [1 0 0] inverse pole figure (IPF) were also recorded.
![Figure 7. (colour online) N-depth profiles measured along two grains with largely different orientations (with respect to the surface of the specimen; Г = 0.02 and Г = 0.30) on the cross section of nitrided Fe- 2at.% Cr thin foil (450 °C, r N = 0.1 atm−1/2 and 216 h). EBSD orientation map and [1 0 0] inverse pole figure (IPF) were also recorded.](/cms/asset/5ac60f8a-eb22-422e-b95c-872329cbc5ab/tphm_a_1115906_f0007_oc.gif)
4.1.2. Fe–Cr (bcc) specimens
EPMA measurements carried out on the surface of a Fe-4.5 at.% Cr specimen (Figure ) nitrided for 3 h indicate that the nitrogen surface content is different for different surface adjacent grains of this ferritic specimen: The lowest surface nitrogen content is observed for surface adjacent grains with a (1 1 1) plane parallel to the surface (Г = 0.33).
The crystallographic orientation of each surface adjacent grain with respect to the surface was determined by EBSD from the surface of nitrided specimens and expressed by the orientation factor, Г hkl . Thus, the quantitative relationship between the nitrogen content in the solid at the surface as a function of the orientation factor was determined for specimens nitrided for different times. The results are shown in Figure . Evidently, the surface nitrogen content decreases with increasing orientation factor. With increasing nitriding time, a saturation level of N is approached and, at the same time, the differences between the surface nitrogen contents for the differently oriented grains reduce (compare also Figure (a) and (b); see Section 5 for discussion).
Several nitrogen-concentration depth profiles were determined by EPMA for differently oriented grains, on cross sections of Fe-4.5 at.% Cr specimens nitrided for 1 and 30 h (Figure ). According to Figure (a), the depth of the nitrided zone is larger for the grain with a (1 0 0) lattice plane (closely) parallel to the surface (Г = 0.05) as compared to the grain with a (1 1 1) lattice plane (closely) parallel to the surface (Г = 0.31). As for the (fcc) Ni–Ti specimens, it can be concluded that also for the (bcc) Fe–Cr specimens, both the surface N content and the nitrided depth decreases with increasing orientation factor. This is the first time that this effect has been observed for ferritic specimens.
4.2. Dependence of residual stress on crystallographic orientation of surface grains
Values of the strains for grains with (0 0 1) lattice planes and grains with (1 1 1) lattice planes parallel to the surface (i.e. a (1 0 0) set and a (1 1 1) set) of Ni-5 at.% Ti specimen nitrided for 65 h and for grains with (0 0 1) lattice planes and grains with (1 1 2) lattice planes parallel to the surface (i.e. a (1 0 0) set and a (2 1 1) set) of a Fe-4.5 at.% Cr specimen nitrided for 1 h have been determined from
and
according to Equation (9). Values of
as measured from the positions of the intensity maxima of the (2 0 0) and (1 1 1) surface adjacent expanded austenite component reflections in the X-ray diffractogram of Ni–Ti specimen nitrided for 65 h (see Figure (b) in [Citation31]) are also shown in Table . Values of
and lattice spacings as measured from the positions of the intensity maxima of the (2 0 0) and (2 1 1) ferrite reflections in the X-ray diffractogram of the Fe–Cr specimen nitrided for 1 h are also shown in Table . Values of
must represent a (hypothetical) homogenous specimen which is devoid of the planar state of stress. Values of
have been calculated for the Ni–Ti and Fe–Cr specimens as follows:
Table 1. Values used for the calculation of ɛ ⊥ (strain in the surface-normal direction, Equation (9)) for grains with (1 0 0) lattice planes and for grains with (1 1 1) lattice planes parallel to the surface of the 65 h nitrided Ni-5 at.% Ti specimen. The error in the lattice-spacing values (column 1), obtained by fitting the measured diffractograms, is ± 0.0001 Å.
Table 2. Values used for the calculation of ɛ ⊥ (strain in the surface-normal direction, Equation (9)) for grains with (1 0 0) lattice planes and for grains with (2 1 1) lattice planes parallel to the surface of the 1 h nitrided Fe-4.5 at.% Cr specimen. The error in the lattice-spacing values (column 1), obtained by fitting the measured diffractograms, is ±0.0001 Å.
4.2.1. Ni–Ti (fcc) specimens
Values of for a homogenous, stress-free specimen, containing the same amount of dissolved N and dissolved Ti as in the matrix of the nitrided region of the surface grains of the heterogeneously nitrided specimen, have been calculated as follows:
(10)
(10)
where is the lattice spacing of pure Ni [Citation54], and
and
are the lattice-spacing changes of pure Ni due to dissolved N and dissolved Ti, respectively.
For calculation of for the (1 0 0) and (1 1 1) sets, the amounts of dissolved nitrogen atoms in the Ni-rich matrix of these two sets are required. The average nitrogen contents measured in the nitrided region (thickness about 6 μm) of a (1 0 0) grain and in the nitrided region (thickness about 4 μm) of a (1 1 1) grain of the specimen nitrided for 65 h, as determined by EPMA, are 6.2 and 4.6 at.%, respectively (see Figure : the red curve corresponds to a (1 0 0) grain and the blue curve corresponds to a (1 1 1) grain). The corresponding changes of the Ni-lattice spacing,
, for the (1 0 0) and (1 1 1) sets follow from a lattice parameter increase of + 0.012Å per at.% of dissolved N [Citation31].
The values of for the (1 0 0) and (1 1 1) sets, for 5 at.% Ti dissolved in Ni matrix, follow from a lattice parameter increase of + 0.0020 Å per at.% of dissolved Ti [Citation55].
4.2.2. Fe–Cr (bcc) specimens
Values of for a homogenous specimen, devoid of the planar state of stress, containing the same amounts of CrN precipitates, dissolved N and (remaining) dissolved Cr as in the matrix of the nitrided region of the surface grains of the heterogeneously nitrided specimen, have been calculated as follows:
(11)
(11)
where is the lattice spacing of pure Fe [Citation54], and
and
are the lattice-spacing changes of pure Fe due to dissolved N and (remaining) dissolved Cr in the matrix, respectively.
represents the lattice-spacing change due to a hydrostatic component of stress (see below).
For calculation of for the (1 0 0) and (2 1 1) sets, the amounts of dissolved nitrogen atoms in the ferrite matrix of these two sets are required. The maximum amount of dissolved nitrogen after complete nitriding of an Fe-4.5 at.% Cr specimen (implying all Cr has precipitated) at 450 °C and r
N = 0.1 atm−0.5 is 0.42 at.% (as deduced from weight-uptake measurements as described in Ref. [Citation52]). The average nitrogen contents measured in the nitrided region of a (1 0 0) grain and in the nitrided region of a (2 1 1) grain of the specimen nitrided for 1 h, as determined by EPMA, are 1.4 at.% and 0.7 at.%, respectively (see Figure (a): the red curve corresponds to a (1 0 0) grain and the green curve corresponds to a (2 1 1) grain). Correspondingly, the amounts of dissolved N in the surface region of the (1 0 0) grain and in the surface region of the (2 1 1) grain are now estimated as [(1.4/(4.5 + 0.42))×0.42) at.%]=0.12 at.% and [(0.7/(4.5 + 0.42))×0.42) at.%] = 0.06 at.%, respectively. The corresponding changes of the ferrite-lattice spacing,
for the (1 0 0) and (2 1 1) sets, follow from a lattice parameter increase of + 0.0079Å per at.% of dissolved N [Citation56].
For calculation of for the (1 0 0) and (2 1 1) sets, the amounts of remaining dissolved Cr in the ferrite matrix of these two sets are required. These data can be obtained by subtracting the Cr content contributing to the CrN precipitation from the total Cr content of the alloy (4.5 at.%). Thus, the amounts of remaining dissolved Cr in the surface regions of the (1 0 0) and the (2 1 1) grains are 3.2 at.% and 3.9 at.%, respectively. The corresponding changes of the ferrite-lattice spacing
for the (1 0 0) and (2 1 1) sets follow from a lattice parameter increase of + 0.0005Å per at.% of dissolved Cr [Citation57].
The nitrided regions contain tiny, misfitting coherent CrN precipitates which evoke a (tensile) hydrostatic stress component in the ferrite matrix [Citation52,58], also in the reference specimen, which leads to a corresponding change of the lattice spacing, . Therefore, in order to determine the sole effect of the stress parallel to the surface as caused by the macroscopic misfit of nitrided case and unnitrided core in the actual heterogeneously nitrided specimen,
has to be incorporated in the calculation of
(see Equation (11)). For the current case (coherent precipitates of CrN in the ferrite matrix),
can be straightforwardly calculated using the model described in Ref. [Citation52]. To this end, the average amount of CrN precipitates in the nitrided regions of the surface adjacent grains with (1 0 0) and (2 1 1) planes parallel to specimen surface must be known. These data follow from the nitrogen content contributing to the CrN precipitation (i.e. the total measured nitrogen content minus the amount of dissolved nitrogen; see above) in the nitrided regions of the (1 0 0) grains and the (2 1 1) grains: 1.28 at.% N for the (1 0 0) grains and 0.64 at.% N for the (2 1 1) grains.
The thus determined values of ,
,
and finally
for the (1 0 0) and (1 1 1) sets of Ni–Ti specimen are shown in Table . The thus determined values of
,
,
,
and finally
for the (1 0 0) and (2 1 1) sets of Fe–Cr specimen are shown in Table .
The elastic strains can now be calculated applying Equation (9). The results have been gathered in Table , together with the corresponding values of
(calculated for pure nickel and iron according to Equation (7)) and the values of the acting biaxial stress obtained by use of Equation (6). The finally resulting values of the equilibrium N solubility ratio,
(calculated by application of Equation (5)), for the (1 0 0) and (1 1 1) sets of the Ni–Ti specimen and for the (1 0 0) and (2 1 1) sets of Fe–Cr specimen have been given in the last column of Table . It is remarked that these results should be considered to be of at most semi quantitative in view of the assumptions made (see Section 2).
Table 3. Values used for calculation of equilibrium nitrogen ratio (Equation (5)) for grains with (1 0 0) lattice planes and grains with (1 1 1) lattice planes parallel to the surface of the 65 h nitrided Ni-5 at.% Ti specimen and for grains with (1 0 0) lattice planes and grains with (2 1 1) lattice planes parallel to the surface of the 1 h nitrided Fe-4.5 at.% Cr specimen. For calculation of equilibrium N ratio (Equation (5)), the partial molar volume of nitrogen in iron (
 ) was deduced from the expansion of the ferrite lattice due to nitrogen dissolution [Citation61] as 5.12 cm3/mol and the partial molar volume of nitrogen in nickel was deduced from the expansion of the Ni lattice due to nitrogen dissolution [Citation31] as 6.73 cm3/mol.
) was deduced from the expansion of the ferrite lattice due to nitrogen dissolution [Citation61] as 5.12 cm3/mol and the partial molar volume of nitrogen in nickel was deduced from the expansion of the Ni lattice due to nitrogen dissolution [Citation31] as 6.73 cm3/mol.
It follows that the larger value of equilibrium N solubility is predicted (from the determined stress value) to occur for grains with {1 0 0} planes parallel to the surface of Ni–Ti and Fe–Cr specimens, as compared to grains with {2 1 1} or {1 1 1} planes parallel to the specimens surface. This well agrees with the experimental observations.
5. General discussion
The results presented in Sections 4.1 and 4.2 indicate that the stress and the surface nitrogen solubility in both nitrided fcc Ni–Ti and nitrided bcc Fe–Cr alloys depend on the crystallographic orientation of the surface adjacent grains, such that with increasing value of Г hkl , the stress and the surface nitrogen concentration decrease. Thereby, the correspondence of stress and equilibrium nitrogen solubility for a (hkl) set, as indicated by Equation (5), is experimentally validated. In fact, this last statement requires that ‘local equilibrium’ prevails at the surface. This may not be true for the stages of nitriding considered [Citation1]. However, the correspondence of stress and surface nitrogen concentration of the surface adjacent grains, as described by Equation (5), will, at least qualitatively, not be affected.
A consequence of the proposed interpretation of the current experimental results is that in a homogeneously nitrided specimen, i.e. without a biaxial state of stress imposed by the misfit of nitrided case and unnitrided core (as in the heterogeneously nitrided specimens considered until now), the results for nitrogen surface concentration should be the same for the different (hkl) sets.
This prediction was verified by the following experiment: a thin foil of Fe-2 at.% Cr (of Section 3.1) alloy was homogeneously nitrided (i.e. the same amount of N occurs at all depths). A thin foil specimen of modest Cr content shows so-called, ‘weak Me-N interaction’ and as a consequence, the specimen is gradually enriched in N simultaneously at all depths [Citation1,59]. The thus obtained homogeneously nitrided thin foil with no residual macrostress (as verified by sin2 ψ stress measurement [Citation45] performed in this project) indeed showed the absence of surface-grain orientation dependence of the surface nitrogen content for two grains crossing the specimen thickness and of largely different orientations (Г = 0.02 and Г = 0.30); see EPMA and EBSD results shown in Figure , thereby providing full support for the interpretation offered above.
It is noted that the decrease upon continued nitriding of the differences between the surface nitrogen contents for the differently oriented surface adjacent grains, as shown in Figures and (a) and (b), is fully compatible with the present explanation: towards specimen homogenization, i.e. for increasing nitriding time, the residual stress in the surface region naturally decreases and thereby the difference in stress level of the (hkl) sets also decreases.
6. Conclusions
| • | The extent of nitriding (surface nitrogen concentration and nitrided depth) depends on the crystallographic orientation of the surface grains of the substrate. This effect not only was observed upon nitriding of austenitic (Ni–Ti) solid solutions but, for the first time, also upon nitriding of ferritic (Fe–Cr) solid solutions. | ||||
| • | The effect could be ascribed to the occurrence of a state of residual planar stress invoked in the nitrided surface region: | ||||
The value of this stress parallel to the surface is different for grains of crystallographic orientations different with respect to the surface. | |||||
A simple thermodynamic theory relates the values of stress parallel to the surface to the change of the equilibrium solubility of nitrogen. | |||||
| • | In the absence of stress, as holds for homogenously nitrided specimens, no differences between the variously oriented surface grains with respect to their equilibrium solubility of nitrogen are expected and do not occur indeed. | ||||
Acknowledgements
The authors thank Dr E. Bischoff for assistance with the EBSD measurements, Mrs S. Haug for assistance with the EPMA experiments, Mr P. Kress for assistance with the nitriding experiments and T. Steiner for assistance with analyzing the X-ray diffractograms.
Disclosure statement
No potential conflict of interest was reported by the authors.
Notes
1. Equation (7) can be rewritten as , where A =
represents Zener ´s anisotropy ratio. In the case of Fe and Ni, A > 1,
, and thus, according to Equation (6), for the same value of ɛ
⊥, the absolute value of stress is smallest for (hkl) = (1 0 0) (
= 0) and largest for (hkl) = (1 1 1) (
= 0.33). For the few cubic materials with A < 1 (e.g. Mo) and again with
, using the same treatment, the absolute value of stress is smallest for (hkl) = (1 1 1) (
= 0.33) and largest for (hkl) = (1 0 0) (
= 0). Cubic materials with A = 1 (e.g. W) are elastically isotropic and, in that case, the value of stress is hkl-independent.
References
- E.J. Mittemeijer , Fundamentals of nitriding and nitrocarburizing, in ASM Handbook . Steel Heat Treating Fundamentals and Processes, J. Dossett and G.E. Totten , eds., 2013, p. 619.
- D.H. Jack and K.H. Jack , Mater. Sci. Eng. 11 (1973) p.1.10.1016/0025-5416(73)90055-4
- B. Mortimer , P. Grieveson and K.H. Jack , Scand. J. Metall. 1 (1972) p.203.
- E.J. Mittemeijer , A.B.P. Vogels and P.J. Vanderschaaf , J. Mater. Sci. 15 (1980) p.3129.10.1007/BF00550386
- R.E. Schacherl , P.C.J. Graat and E.J. Mittemeijer , Z. MetaIlkd. 93 (2002) p.468.10.3139/146.020468
- M. Sennour , P.H. Jouneau and C. Esnouf , J. Mater. Sci. 39 (2004) p.4521.10.1023/B:JMSC.0000034146.64444.80
- G. Miyamoto , A. Yonemoto , Y. Tanaka , T. Furuhara and T. Maki , Acta Mater. 54 (2006) p.4771.10.1016/j.actamat.2006.06.006
- S.S. Hosmani , R.E. Schacherl and E.J. Mittemeijer , J. Mater. Sci. 43 (2008) p.2618.10.1007/s10853-008-2473-9
- E.J. Mittemeijer and M.A.J. Somers , Thermochemical Surface Engineering of Steels , Woodhead Publishing, Cambridge, 2015.
- H.H. Podgurski and H.E. Knechtel , Trans. Metall. Soc. of AIME 245 (1969) p.1595.
- J.H. Driver and J.M. Papazian , Acta Metall. 21 (1973) p.1139.10.1016/0001-6160(73)90030-8
- H.H. Podgurski and F.N. Davis , Acta Metall. 29 (1981) p.1.10.1016/0001-6160(81)90081-X
- P.M. Hekker , H.C.F. Rozendaal and E.J. Mittemeijer , J. Mater. Sci. 20 (1985) p.718.10.1007/BF01026547
- N.E.V. Diaz , S.S. Hosmani , R.E. Schacherl and E.J. Mittemeijer , Acta Mater. 56 (2008) p.4137.10.1016/j.actamat.2008.04.041
- S.R. Meka , E. Bischoff , S.S. Hosmani and E.J. Mittemeijer , Int. J. Mater. Res. 105 (2014) p.1057.10.3139/146.111127
- O. Ozturk and D.L. Williamson , J. Appl. Phys. 77 (1995) p.3839.10.1063/1.358561
- G.A. Collins , R. Hutchings , K.T. Short , J. Tendys , X. Li and M. Samandi , Surf. Coat. Technol. 74–75 (1995) p.417.10.1016/0257-8972(95)08370-7
- D.L. Williamson , J.A. Davis and P.J. Wilbur , Surf. Coat. Technol. 104 (1998) p.178.
- Y. Sun , X.Y. Li and T. Bell , J. Mater. Sci. 34 (1999) p.4793.10.1023/A:1004647423860
- S. Mandl and B. Rauschenbach , Defects Diffus. Metals III 188-1 (2001) p.125.10.4028/www.scientific.net/DDF.188-190
- T. Christiansen and M.A.J. Somers , Surf. Eng. 21 (2005) p.445.10.1179/174329405X68597
- T. Czerwiec , H. He , G. Marcos , T. Thiriet , S. Weber and H. Michel , Plasma Processes Polym. 6 (2009) p.401.10.1002/ppap.v6:6/7
- A. Martinavicius , G. Abrasonis , W. Moller , C. Templier , J.P. Riviere , A. Declemy and Y. Chumlyakov , J. Appl. Phys. 105 (2009) p.093502.10.1063/1.3120912
- J.C. Stinville , P. Villechaise , C. Templier , J.P. Riviere and M. Drouet , Acta Mater. 58 (2010) p.2814.10.1016/j.actamat.2010.01.002
- H. Dong , Int. Mater. Rev. 55 (2010) p.65.10.1179/095066009X12572530170589
- D. Wu , H. Kahn , J.C. Dalton , G.M. Michal , F. Ernst and A.H. Heuer , Acta Mater. 79 (2014) p.339.10.1016/j.actamat.2014.07.007
- H. Kahn , G.M. Michal , F. Ernst and A.H. Heuer , Metall. Mater. Trans. A 40A (2009) p.1799.
- T. Christiansen and M.A.J. Somers , Scr. Mater. 50 (2004) p.35.10.1016/j.scriptamat.2003.09.042
- C. Leroy , T. Czerwiec , C. Gabet , T. Belmonte and H. Michel , Surf. Coat. Technol. 142 (2001) p.241.
- H. He , T. Czerwiec , C. Dong and H. Michel , Surf. Coat. Technol. 163 (2003) p.331.
- M. Fonović , A. Leineweber , O. Robach , E.A. Jägle and E.J. Mittemeijer , Metall. Mater. Trans. A 46 (2015) p.4115.10.1007/s11661-015-2999-9
- X.Y. Li , N. Habibi , T. Bell and H. Dong , Surf. Eng. 23 (2007) p.45.10.1179/174329407X161564
- A. Martinavicius , G. Abrasonis and W. Moller , J. Appl. Phys. 110 (2011) p.074907.10.1063/1.3646493
- J.F. Nye , Physical Properties of Crystals: Their Representation by Tensors and Matrices , Oxford University Press, Oxford, 1985.
- M. Grunze , F. Bozso , G. Ertl and M. Weiss , Appl. Surf. Sci. 1 (1978) p.241.10.1016/0378-5963(78)90017-X
- J. McAllister and R.S. Hansen , J. Chem. Phys. 59 (1973) p.414.10.1063/1.1679821
- T. Christiansen , K.V. Dahl and M.A.J. Somers , Mater. Sci. Technol. 24 (2008) p.159.10.1179/026708307X232901
- H. He , J.X. Zou , C. Dong , T. Czerwiec and H. Michel , Pricm 5: The Fifth Pacific Rim International Conference on Advanced Materials and Processing, Pts 1-5 475–479, 2005, p. 3669–3672.
- E.J. Mittemeijer and J.T. Slycke , Surf. Eng. 12 (1996) p.152.10.1179/sur.1996.12.2.152
- J.C.M. Li , R.A. Oriani and L.S. Darken , Z. Phys. Chem. 49 (1966) p.271.10.1524/zpch.1966.49.3_5.271
- H.A. Wriedt and R.A. Oriani , Acta Metall. 18 (1970) p.753.10.1016/0001-6160(70)90039-8
- R.A. Swalin , Thermodynamics Solids , 2nd ed., Wiley, New York , 1972.
- N.E. Vives Diaz , R.E. Schacherl , L.F. Zagonel and E.J. Mittemeijer , Acta Mater. 56 (2008) p. 1196.10.1016/j.actamat.2007.11.012
- I.C. Noyan and J.B. Cohen , Residual Stress Measurement by Diffraction and Interpretation , Springer-Verlag, New York , 1987.
- U. Welzel , J. Ligot , P. Lamparter , A.C. Vermeulen and E.J. Mittemeijer , J. Appl. Crystallogr. 38 (2005) p.1.10.1107/S0021889804029516
- J. Turley and G. Sines , J. Phys. D: Appl. Phys. 4 (1971) p.1731.10.1088/0022-3727/4/11/317
- M.K.A. Koker , U. Welzel and E.J. Mittemeijer , Philos. Mag. 93 (2013) p.2967.10.1080/14786435.2013.793852
- M.K.A. Koker , U. Welzel and E.J. Mittemeijer , J. Appl. Crystallogr. 47 (2014) p.391.10.1107/S1600576713032202
- U. Welzel and E.J. Mittemeijer , J. Appl. Phys. 93 (2003) p.9001.10.1063/1.1569662
- T. Gnaupel-Herold , P.C. Brand and H.J. Prask , Adv. X-Ray Anal. 42 (1999) p.464.
- F.C. Larché and J.W. Cahn , J. Res. Nat. Bureau Stand. 89 (1984) p.467.10.6028/jres.089.026
- M. Akhlaghi , T. Steiner , S.R. Meka , A. Leineweber and E.J. Mittemeijer , Acta Mater. 98 (2015) p.254.10.1016/j.actamat.2015.07.017
- J.L. Pouchou and F. Pichoir , Recherche Aerospatiale [Aerospace research], 5 (1984), p.349.
- ICDD Data Base , in PCPDFWIN, 2002.
- W.B. Pearson , An Alphabetical Index of Work on Metals and Alloys , in A Handbook of Lattice Spacings and Structures of Metals and Alloys , W. B. Pearson , ed., Pergamon, London, 1958, p.634.
- H.A. Wriedt and L. Zwell , Trans. Metall. Soc. of AIME 224 (1962) p.1242.
- A. Sutton and W. Hume-Rothery , Philos. Mag. 46 (1955) p.1295.10.1080/14786441208521140
- M.A.J. Somers , R.M. Lankreijer and E.J. Mittemeijer , Philos. Mag. A 59 (1989) p.353.10.1080/01418618908205064
- M.H. Biglari , C.M. Brakman , M.A.J. Somers , W.G. Sloof and E.J. Mittemeijer , Z Metallkd 84 (1993) p.124.
- C.J. Smithells , Metals Reference Book , 5th ed., Butterworth, London, 1976.
- P. Ferguson and K.H. Jack , Quenched-aging and Strain-aging of Nitrogen–Ferrite, in Proceedings of Heat Treatment `81 , The Metals Society, London, 1983, p. 158.
