Abstract
Carbon redistribution is known to occur during martensite ageing. The two associated processes most discussed in the literature are spinodal decomposition and carbon segregation to defects. In order to elucidate the topic, the ageing and tempering of two Fe–Ni–C alloys have been characterised by means of atom probe tomography and synchrotron radiation diffraction. Upon ageing at room temperature, carbon redistribution is clearly observed, where the process of carbon segregation to defects appears to be most likely to occur. Nevertheless, the possibility of spinodal decomposition is not entirely discarded, and the current work presents a series of discussion points that challenge our current understanding of the thermodynamic of ferrite in steels.
1. Introduction
Understanding carbon redistribution in steels poses a challenge, especially in complex microstructures, where there are numerous microstructural features that can affect carbon behaviour. Even carbon redistribution within ferrite still remains a topic of debate. Vast experimental evidence shows carbon redistribution taking place during room temperature ageing of freshly quenched Fe–C martensite, where carbon atoms diffuse into more stable configurations that lead to a reduction in the overall system’s free energy. At the microstructural level, such redistributions are known to give rise to fine modulations in carbon content across martensite. Earlier electron microscopy studies on aged martensite led some researchers to conclude that the origin of these local variations was spinodal decomposition [Citation1,Citation2], while others have interpreted the observations as carbon segregating to defects within the microstructure [Citation3,Citation4]. Whether these two mechanisms of carbon redistribution take place simultaneously and interact, or one occurs predominantly, is still not understood [Citation5].
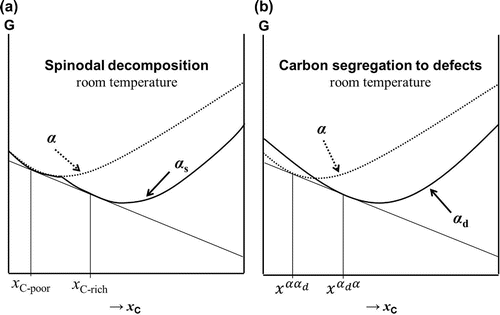
In terms of the system’s free energy, both processes lead to a reduction in the free energy. However, a clear distinction must be made between the two, regarding the cause for which carbon atoms redistribute. In simple terms, spinodal decomposition is thought to be the process by which the free energy is lowered by separating the ferritic phase into a high-carbon and a low-carbon component. For this process to take place, the total free energy of these two components should be lower than that of the phase with average composition. On the other hand, segregation to defect sites is based on the clustering of carbon atoms along dislocations, grain boundaries and vacant sites. Before going into more specifics, it is first necessary to understand where the carbon atoms are found within the lattice.
Figure 1. (colour online) Schematics of the location of the (a) tetrahedral (TIS) and (b) octahedral interstitial sites (OIS) for possible carbon occupancy in -Fe; (c) the classification of the OIS into ‘a’, ‘b’ and ‘c’ sites, and (d) representation of the so-called
-Fe
C
supercell structure, which accounts for the ordered product of spinodal decomposition, adapted from [Citation5].
![Figure 1. (colour online) Schematics of the location of the (a) tetrahedral (TIS) and (b) octahedral interstitial sites (OIS) for possible carbon occupancy in -Fe; (c) the classification of the OIS into ‘a’, ‘b’ and ‘c’ sites, and (d) representation of the so-called -FeC supercell structure, which accounts for the ordered product of spinodal decomposition, adapted from [Citation5].](/cms/asset/3715be6b-15bc-4620-9a9a-916f36582d55/tphm_a_1211790_f0001_oc.gif)
Carbon atoms occupy interstitial sites within the Fe-lattice, where the two types of interstitial sites are tetrahedral and octahedral interstitial sites (TIS and OIS, respectively), Figure (a) and (b), respectively. Amongst these sites, it is generally accepted that carbon occupies OIS [Citation6,Citation7]. Within the OIS, there are three sub-lattice sites ‘a’,‘b’ and ‘c’, based on the orientational variants in the [1 0 0], [0 1 0] and [0 0 1] directions, respectively, Figure (c). Depending on which of these sites is occupied, owing to the local distortion of the lattice created around the interstitial, an expansion of the lattice in one of the originally cubic directions is observed. The widely accepted view is that the crystallographic tetragonality is due to carbon’s preferential occupancy of one of these variants (for which we take ‘c’ sites) by a ‘self-induced preferential distribution’ of carbon atoms during martensitic transformation from austenite [Citation8], referred to as Zener-ordering.
Nonetheless, there is supporting evidence that Fe–C undergoes further ordering beyond that proposed by Zener, in conjunction with spinodal decomposition [Citation5]. Advanced simulation techniques have provided quite some insight into this matter. Naraghi et al. [Citation9] corroborated in ThermoCalc that indeed the lowest free energy is obtained if spinodal decomposition is considered. Similar conclusions were presented by Sinclair et al. [Citation10] by means of molecular dynamics simulations. The ordered product of spinodal decomposition that corresponds to the carbon-rich part of the modulation is generally described as a superlattice structure that in literature is referred to as -Fe
C
, illustrated in Figure (d).
Despite several studies postulating the occurrence of spinodal decomposition, there are still a few aspects that remain unclear. For instance, the carbon-rich product of spinodal decomposition is generally expressed in the form of FeC
, sometimes referred to as a phase of its own, even as a carbide [Citation11]. This is inconsistent with spinodal decomposition, which by definition takes place within a single phase, where parent and product phases need to have the same crystallographic property. Such requirement of spinodal decomposition fundamentally challenges the support of spinodal decomposition in Fe–Ni–C systems, as will be discussed later on. Furthermore, the possibility of spinodal decomposition in Fe–C systems is directly related to one fundamental question: Is there a miscibility gap in the Fe–C system necessary for spinodal decomposition to occur?
On the other hand, there is also literature on how interstitial carbon interacts with various defects within the microstructure. Carbon–defect interactions have gained acceptance, where it is well known that the creation of dislocations in the materials (e.g. as-quenched martensite) leads to a rapid stress-induced ordering of the solute carbon atoms, where these migrate towards dislocation stress fields [Citation12]. Atomistic and first-principle calculations typically report the diffusion path of carbon atoms to be from OIS to vacant sites, via TIS [Citation13–Citation17].
The simulations such as those in [Citation9,Citation10,Citation16,Citation17] give an account for a specific phenomenon, either spinodal decomposition or carbon segregation to defects. However, the actual interaction between the two processes is unknown. As discussed in a previous study by the authors, both defect segregation and spinodal decomposition have important implications to the thermodynamic description of the ferrite phase [Citation18]. It is thus the aim of this study to critically assess both interpretations of carbon redistribution. The literature on spinodal decomposition and carbon segregation to defects will be briefly reviewed in order to understand the origin of the discrepancy regarding carbon redistribution during ageing. Experimental work conducted on Fe–Ni–C systems will then be presented in order to raise further questions that challenge our current understanding of the Fe–C and Fe–Ni–C systems.
2. Spinodal decomposition and carbon segregation to defects
2.1. The thermodynamics of the two processes
In this section, the differences in thermodynamics between spinodal decomposition and segregation to defects will be discussed. Figure (a) represents an Fe–C system undergoing spinodal decomposition, where the curve has the characteristic double minima that gives rise to compositional modulations of carbon within the phase. This results in carbon-rich and carbon-poor regions. As a reference, the free energy curve for the hypothetical case of a perfect
phase without miscibility gap is given. Figure (b) shows the free energy involved in the process of carbon segregation to defects. Relative to a defect-free system, when ferrite has a high density of defects (e.g. following a diffusionless transformation such as in the case of martensite), at low-carbon concentrations the free energy curve for
will be higher than for defect-free ferrite (in the following,
) due to the presence of strain fields in the vicinity of the defects. However, at higher carbon concentrations, once carbon segregation to defect sites occurs and the strain fields are relieved,
will acquire a lower free energy when compared to defect-free
with the same carbon concentration, where the carbon atoms cause significant lattice strain. The system in Figure (b) will produce two concentrations of carbon,
and
(where
is the carbon concentration in phase i when in equilibrium with phase j), which would also result in carbon-rich and carbon-poor regions, analogous to a system undergoing spinodal decomposition.
2.2. Discrepancies observed in the atom probe literature
Recently, some researchers have sought clues on this issue in the atom probe tomography (APT) technique. In steel research, APT is a powerful tool for studying carbon composition profiles and is used to quantitatively measure the local carbon concentration. Nevertheless, one should bear in mind the following: taking the product of spinodal decomposition to be -Fe
C
,
in Figure (a) would yield a composition in carbon equivalent to
11 at.%. Similarly, a local concentration of
at.% by APT is also feasible for carbon segregation to defects [Citation19]. The values for
and
are very similar and one could argue that the differences are within experimental error. Based solely on the local carbon enrichment observed in APT, it would thus be difficult to determine the thermodynamic origin of the carbon redistribution process.
Taylor et al. [Citation1] tested a series of Fe–Ni–C alloys and reported the occurrence of spinodal decomposition in alloys containing 0.2 to 2 wt.% carbon. The alloy systems studied by Taylor et al. in which spinodal decomposition was reported to occur were Fe-25.5Ni-0.19C, Fe-24.9Ni-0.39C and Fe-14.8Ni-0.88C wt.%. Typically, nickel is added in relatively large quantities in order to decrease the martensitic start temperature below room temperature, thus avoiding unintentional carbon segregation to defects or carbide precipitation during autotempering. Taylor et al. [Citation1] claimed that ‘the development of structural modulations by spinodal decomposition thus appears to be a general feature of the aging behaviour of Fe-X-C martensite’. In their work, the ageing of Fe–15Ni–0.88C wt.% was followed by APT. The evolution in the carbon content between the carbon-poor and carbon-rich regions was measured as a function of time. Surprisingly, already after eight days of room temperature ageing, the carbon concentration in the carbon-rich zone reached a plateau concentration of 11 at.%.
More recently, Danoix et al. [Citation20] carried out atom probe studies on Fe–25Ni–0.4C wt.% (a system that was previously investigated by Taylor et al. [Citation1]) and showed similar conclusions. After ageing at room temperature for 4.5 years, the carbon content of the carbon-rich product reached 11 at.% C. Furthermore, they have shown that tempering at 75 C for 16 hours also had the equivalent effect as room temperature ageing for 4.5 years. The authors in [Citation20] proposed Fe
C
to be a strong candidate for the carbon-rich product of spinodal decomposition.
On the other hand, Herbig et al. [Citation21] conducted similar experiments on an Fe–15Ni–1C wt.%C alloy, similar to one of the alloys investigated by Taylor et al. [Citation1]. Herbig et al. [Citation21] emphasised that there are still doubts regarding the exact microstructural features under observation in APT. This still leaves doubts whether the carbon redistribution owes to spinodal decomposition or segregation to defects. Based on the literature, a series of experiments have been designed in the present work in order to provide further insight into the matter.
3. Experimental procedure
In order to further investigate the occurrence of spinodal decomposition in Fe–Ni–C systems, two alloys similar to those studied earlier by Taylor et al. [Citation1] were selected for this study, a low-carbon (LC) and high-carbon (HC) alloy. The measured chemical compositions are summarised in Table , where the carbon content was measured by LECO. Both alloys were homogenised at 1250 C for 48 hours in vacuum in order to ensure a uniform distribution of nickel throughout the material. The two main techniques employed for characterising the ageing of these materials were high-resolution X-ray diffraction (HR-XRD) using synchrotron radiation and APT.
Table 1. Chemical composition of alloys studied.
Synchrotron experiments were carried out at the I11 beamline at the Diamond Light Source (Didcot, UK) under reflection geometry using flat plate samples, recorded with MAC detectors [Citation22]. A 15 keV beam energy (measured wavelength 0.0825866 nm) was used for this study. Rietveld refinement was performed on the XRD spectra using MAUD software [Citation23].
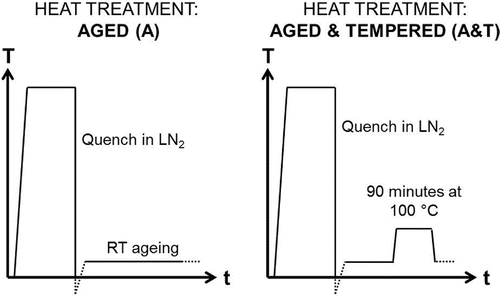
Two heat treatments were designed in order to observe carbon segregation in the samples: (i) aged at room temperature for two weeks prior beamtime, and (ii) aged for one week at room temperature, followed by tempering at 100 C for 90 min. Figure illustrates the thermal cycles that were applied. Austenitisation was carried out at 1000
C for 10 min. The samples were submerged in liquid nitrogen (LN
) until the quenching medium stopped boiling. The samples were brought back to room temperature, at which point the ‘room temperature’ ageing time started. For the aged & tempered sample, after the tempering stage, the sample was kept at room temperature for one week before measurement in the beamline.
Figure 3. Schematic representation of the thermal cycles for aged (A) and aged & tempered (A&T) heat treatments.
In order to study local variations in carbon contents during room temperature ageing, aged samples of the LC alloy (5 and 10 weeks) were examined by APT using a local electrode atom probe (LEAP 4000X HR) at Eindhoven University of Technology. The microtips were prepared by FIB, and LEAP analyses were performed using both voltage and laser pulse modes. The voltage mode was carried out at 50 K, 200 kHz pulse rate and 20% pulse fraction; and the laser mode was at 20 K, 30 pJ laser energy and 200 kHz pulse rate. The reconstructions were performed using the IVAS 3.6.8 software, where the peaks from the mass spectra were assigned to C, ,
,
, Ni and Fe. Where peak overlapping was detected, the peak decomposition algorithm within the IVAS software was applied to separate the peaks.
4. Results
4.1. HR-XRD on aged and aged & tempered LC and HC
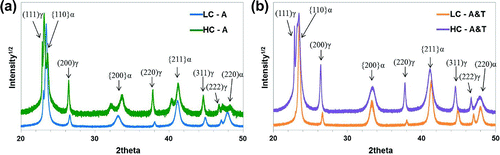
Diffractograms for four samples are presented in Figure : LC and HC samples that underwent ageing (A) and ageing & tempering (A&T). Consistent with the findings from Taylor et al. [Citation1], retained austenite () is still found in spite of quenching in liquid nitrogen. In LC,
was measured to be 0.05
0.01 and in HC
.
Figure 4. (colour online) Scans showing 20–50 2
, showing all labelled ferrite and austenite peaks where LC and HC refer to Fe–25Ni–0.19C and Fe–10Ni–0.87C wt.% alloys, and (a) shows the XRD patterns for samples that have undergone two weeks of room temperature ageing (A), and (b) shows XRD patterns after one week of room temperature ageing followed by tempering at 100
C for 90 min (A&T).
Rietveld refinement was carried out with the two following phases: -austenite (face-centred cubic, space group Fm
m) and
-ferrite (body-centred tetragonal, space group I4/mmm). The lattice parameters obtained for the ferrite phase were used in order to determine the tetragonality (c/a) of the phase. This gives an estimate of the carbon content in solid solution in weight % (
) by applying the following: c/
[Citation24], where
(c/a – 1). The values are summarised in Table .
Table 2. Summary of XRD Rietveld refinement analysis, showing the global carbon content in solid solution based on matrix tetragonality.
For the aged samples (LC - A and HC - A), the estimated carbon content in solid solution obtained from tetragonality measurements closely matches with the carbon composition obtained by LECO analysis given in Table . Tempering at 100 C for 90 min (A&T) significantly reduces the carbon content within the matrix to
0.13 wt.% C, which can be attributed to the precipitation of carbides.
Figure 5. (colour online) XRD scans shown for 13–37 2
where
,
and
carbides peak positions have been marked, for (a) Fe–25Ni–0.19C (LC) and (b) Fe–10Ni–0.87C wt.% (HC). The closed symbols represent the strongest reflections for each carbide.
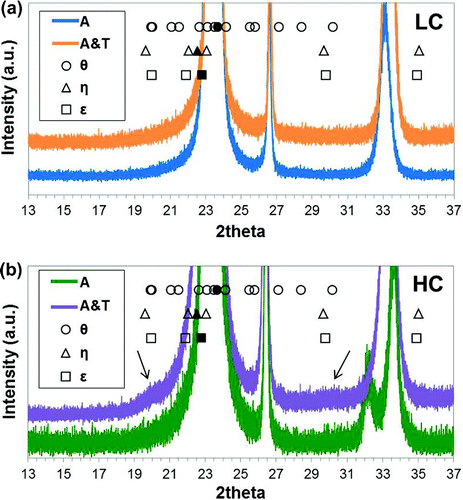
In order to check whether carbides have been formed, the region of 13–37 2
is represented in Figure . The peak positions expected for
-carbide (space group P6
22),
-carbide (space group Pnna) and
-carbide (space group Pnma) are marked. In the HC A&T steel there are a couple of broad peaks, which have been marked with arrows in Figure (b). The locations of these broad peaks correspond to the expected peak positions of the iron carbides considered. However, it is not possible to index individual peaks due to the broadness and overlapping of the peaks. Which carbide phase forms still remains unknown.
Alternatively, one could argue that the small bumps observed at 20
and
30
2
can be attributed to a weak reflection of the martensite doublet arising from the Fe
C
ordered superstructure. The lattice parameters in tetragonal martensite are known to depend on the carbon concentration according to [Citation25]:
(1a) where
is the atomic percentage of carbon in solid solution. Equations (1a) and (1b) were applied to the a and c lattice parameters obtained from XRD, where the
values used for each sample have been obtained from Table . In this work, it was found that
0.00003) nm, in close agreement with literature values [Citation26] for similar Fe–Ni compositions. Therefore, the lattice parameters expected for Fe
C
, where
at.%, are
nm, and
nm (c/
). The expected peak positions are summarised in Table .
Table 3. Expected peak position for superlattice FeC
.
It is clear that the broad peaks appearing at 20
2
does not correspond to the expected martensite doublet owing to Fe
C
. The broad peak at
30
2
could be interpreted as the (0 0 2) peak. However, the rest of peaks shown in Table are not found in the XRD pattern. Therefore, the presence of Fe
C
in the HC alloy (both A and A&T) is unlikely. The results also show that ageing at 100
C for 90 min does not have the equivalent effects as prolonged room temperature ageing, often applied to speed up room temperature ageing processes. As observed by means of XRD, the process of ageing at a higher temperature reduces the fraction of solid solution carbon in ferrite (
0.08 wt.% in LC, and
0.75 wt.% in HC). Such reductions in carbon content could be attributed to carbide formation, which has been experimentally observed by Nagakura et al. [Citation27], where transitional carbides have been observed on ageing above 97
C, and due to possible partitioning of carbon to the retained austenite, given that after quenching, the
volume fraction was found to be 0.05 and 0.15 for LC and HC, respectively.
4.2. APT on aged LC
The atom probe measurements on LC samples aged at room temperature for 5 and 10 weeks are presented here. The carbon distribution within the tips are shown in Figures and under voltage and laser mode, respectively. The images show two planes of observation, side view and top view of the reconstruction. Representative maps are shown. In this study, it is found that the mode of measurement (laser or voltage) does not have a significant impact on the carbon maps and measured concentrations. Thus for the present discussion purposes, the carbon maps and concentrations remain independent of the measurement mode.
It is also necessary to point out that the measurements were carried out at 20 K for laser mode and 50 K for voltage mode. Under such cryogenic conditions, the austenite may have transformed into martensite. However, given the limited diffusivity of carbon at such low temperatures, it is assumed that no appreciable carbon diffusion takes place within the chamber, and so any carbon redistribution within the sample comes from the ageing time at room temperature.
Figure 6. (colour online) Carbon maps of the reconstructed tips for steel LC (Fe–25Ni–0.19C wt.%) aged for five weeks showing (a) side view and (b) top view, and after 10 weeks of ageing showing (c) side view and (d) top view. Measurements were carried out under voltage pulse mode at 50 K using a 200 kHz pulse rate and 20 % pulse fraction. The arrows indicate visible carbon enrichment patterns in the reconstruction.
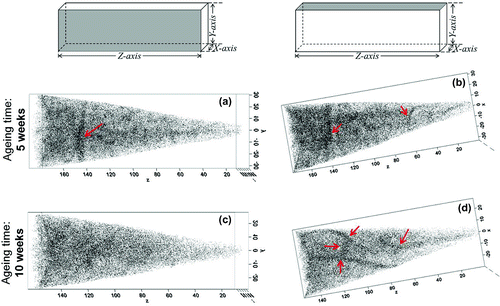
Figure 7. (colour online) Carbon maps of the reconstructed tips for steel LC (Fe–25Ni–0.19C wt.%) aged for five weeks showing (a) side view and (b) top view, and after 10 weeks of ageing showing (c) side view and (d) top view. Measurements were carried out under laser mode at 20 K using a 30 pJ laser energy and a 200 kHz pulse rate. The arrows indicate visible carbon enrichment patterns in the reconstruction.
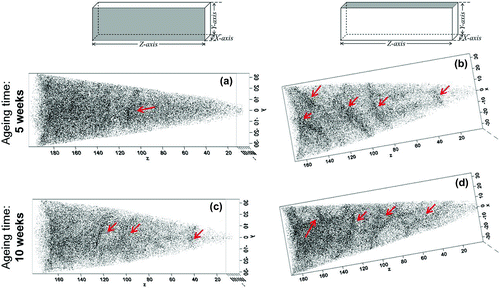
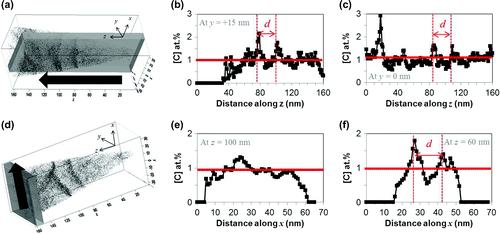
Figure 8. (colour online) Quantitative carbon analysis in Fe–25Ni–0.19 wt.% after 10 weeks of ageing at room temperature.
After five weeks of ageing, redistribution of carbon atoms is visible, which becomes more obvious after 10 weeks of ageing. Some patterns are only visible in a particular plane of observation (Figure (a) and (b)), whereas others appear present even when the reconstruction is rotated (Figure (a) and (b)). The fact that such patterns are visible in a specific plane of observation could indicate that carbon atoms are decorating line defects (e.g. dislocations), whereas a planar defect (e.g. lath/grain boundary) would be visible in all planes of observations. Therefore, it is likely that the carbon patterns observed by APT owe to carbon segregation to defects, although a more robust method is needed in order to distinguish the defect features.
The carbon concentrations have also been quantified (delocalisation 2 nm, voxel 0.7 nm). As an illustrative example, the sample that underwent 10 weeks of ageing is shown in Figure . An analysis volume has been defined as shown in Figure (a) and (d), and positioned at 5 nm intervals along the analysis axis. For example, Figure (b) and (c) show the carbon profile measured within the analysis volume (685
162 nm
) positioned at 5 nm intervals along the y axis. Similarly, Figure (e) and (f) show the carbon profiles measured within the analysis volume (68
65
5 nm
) positioned at 5 nm intervals along the z axis. The red line at around 1 at.% C represents the average carbon content of the alloy. As shown in the carbon profile map, there are distinct fluctuations in the carbon content. The spacing between these alternating carbon-rich and carbon-poor areas is indicated as d. This spacing was found to be similar for samples aged at 5 and 10 weeks, regardless of measurement mode, and on average was found to be 21
2 nm. This is significantly larger than the 4 nm spacing expected if spinodal decomposition was to occur [Citation5].
Attributing the carbon patterns to carbon segregation to line defects such as dislocations is not unprecedented. The dislocation density, , is known to be a function of carbon content, where
(0.7+3.5
)
10
m
[Citation28]. The addition of nickel is reported to increase
slightly. The expected dislocation density for LC is thus
2.3
10
m
. The spacing between dislocations can be estimated by
27 nm. This corresponds well to the spacing d obtained from APT measurements.
5. Discussion
5.1. HR-XRD on aged and aged & tempered LC and HC
According to the results by Taylor et al. [Citation1], two weeks of ageing the HC alloy (sample HC - A) should lead to the -Fe
C
product. The present experiments were initially designed to further investigate its nature, primarily whether it is carbide or a continuous phase of the ferrite. Based on the XRD patterns of HC - A and LC - A (Figure ) it is unlikely that a third phase exists other than ferrite and austenite, thus the possibility of a Fe
C
-carbide is discarded. Instead, the ferrite phase shows tetragonality due to the presence of carbon in solid solution. Nevertheless, the tetragonality observed experimentally (summarised in Table ) is significantly lower than for a Fe
C
tetragonal structure (expected values in Table ). Still, it is possible that two weeks of ageing of Fe–10Ni–0.9C and Fe–25Ni–0.2C wt.% alloys is not sufficient for the formation of the Fe
C
structure.
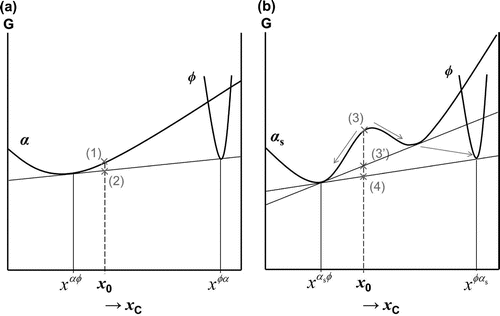
The phase transformations occurring during the transition between the ageing and tempering of ferrous martensite remain an issue. It was presented in Figure (a) that, should spinodal decomposition occur, the ferrite curve must have a miscibility gap. In this case, the set of free energy curves describing carbide precipitation from ferrite would also change. Consider the precipitation of a carbide in ferrite, where
can also be either
-,
- or
-carbides. When ferrite is in its carbon supersaturated form,
, the reduction in the system’s free energy is given by
. Let us consider the following cases: (i)
precipitates from cubic
, and (ii)
precipitates from ferrite with a miscibility gap,
. Figure (a) shows the classical curve for carbide precipitation in ferrite (
) with carbon fraction
, where the free energy is reduced from the value indicated by (1) to the value indicated by (2). Next, consider the scenario where a miscibility gap exists (
), Figure (b). In the literature of martensite ageing, it is often seen that upon tempering of the modulated structures, these structures disappear and carbide precipitation occurs, e.g. [Citation27]. When the composition is
, the overall free energy reduction would be given by (3)
(4). However, there could occur a metastable situation at (3
), where the products of spinodal decomposition first forms, before continuing further until the final equilibrium point (4) is reached. The sequence is then (3)
(3
)
(4). Although the theoretical difference is evident in Figure , it is not possible to distinguish between these modes from the experimental tempering kinetics.
5.2. APT on aged LC
The principal experimental evidence that is used for justifying spinodal decomposition in literature is formed by (i) local carbon enrichment to 11 at.%C, and (ii) alternating carbon-rich and carbon-poor regions with 1–5 nm spacing. The APT results presented in the current work for Fe–25Ni–0.2C wt.% show that (i) the local carbon enrichment does not exceed 2 at.%, and (ii) the spacing between C-rich regions is five-fold to the expected values in spinodal decomposition. Considering the debate between the interpretation given by Taylor et al. [Citation1] and Ohmori and Tamura [Citation3], the spacing is in agreement with the expected dislocation density, thus the APT results appear to support the conclusions of carbon segregation to defects.
5.3. Challenges to spinodal decomposition in Fe–X–C
As previously mentioned, the theory of spinodal decomposition requires that parent and product phases are one continuous phase, with the same crystallographic property. Consider the FeC
structure in Figure (d). In this supercell structure, the carbon atoms are ordered in such way that only specific ‘c’ octahedral interstitial sites are occupied. Such alignment of the carbon atoms distorts the lattice in a specific direction, which in this case is along the ‘c’ axis, thus exhibiting tetragonality, where a body-centred tetragonal (BCT) structure is adopted. On the other hand, carbon-depleted martensite is considered to be body-centred cubic (BCC). In spinodal decomposition, the product phases would only differ by composition (carbon-rich and carbon-poor). However, in Fe–C, there is also a discontinuity in the crystal symmetry between the carbon-rich (tetragonal) and carbon-poor (cubic) region. In such case, the process of separation into solute-rich and solute-poor regions would be considered to be a ‘pseudospinodal decomposition’ [Citation29].
Nonetheless, in light of recent literature, one alternative solution is to assume that ferrite is naturally a body-centred tetragonal structure, a topic that has attracted many researchers [Citation30–Citation33]. It is worth noting that a way to justify the tetragonal nature of ferrite is to consider the effects of magnetism in iron. There has been renewed interest in the scientific community to distinguish the ferromagnetic form of ferrite (the so-called -ferrite), which consists of a BCT structure with space group I4/mmm, from the cubic paramagnetic ferrite with space group Im
m above 770
C [Citation34]. In the earlier days, the
-ferrite was discarded as a distinct phase since the tetragonality could not be resolved experimentally using X-ray diffraction [Citation35]. However, the solutes also interact with magnetic moments. Therefore, there is the possibility that carbon atoms exert additional tetragonal distortion to
-ferromagnetic structure, increasing its tetragonality and hence its carbon solubility.
6. Concluding remarks
Two competitive mechanisms for carbon redistribution during room temperature martensite ageing have been reviewed in the context of spinodal decomposition vs. carbon segregation to defects. In the set of experimental evidence presented in the current work, no hint of FeC
, formed by spinodal decomposition, was observed, where by means of synchrotron radiation XRD and APT, the redistribution of carbon atoms was studied during room temperature ageing and tempering at 100
C. Instead, carbon segregation to defects is more likely to occur. Nevertheless, the absence of Fe
C
can also be attributed to much slower kinetics of carbon-ordering reactions than reported in the literature.
Whether the free energy curve for the ferrite phase has a miscibility gap or not, it will influence the thermodynamic description of ferrite in equilibrium with other phases, such as austenite or carbides in steels. This will have an impact on the description of the thermodynamics and kinetics of phase transformations involving carbon partitioning. Therefore, when modelling carbon diffusion related processes, the determination of the thermodynamic curve is essential which remains a challenge and requires further investigation. The current knowledge, based on both theoretical and experimental evidence, is not sufficient to decide on the thermodynamic description.
Acknowledgements
The authors would like to thank the Diamond Light Source for access to Beamline I11 and the beamline team for the measurements under Rapid Access mode. Author BK would like to express her gratitude to Mr Hans Hofman for his help in carrying out the heat treatments. The authors would like to thank the National Atom Probe Facility at TU Eindhoven, particularly Dr Sebastian Koelling for the sample preparation and measurements on the LEAP.
Additional information
Funding
Notes
No potential conflict of interest was reported by the authors.
References
- K.A. Taylor, L. Chang, G.B. Olson, G.D.W. Smith, M. Cohen, and J.B. Vander Sande, Spinodal decomposition during aging of Fe-Ni-C martensites, Metall Trans. 20A (1989), pp. 2717–2737.
- S.B. Ren, T. Tadaki, K. Shimizu, and X.T. Wang, Electron diffraction study of aging processes in Fe-1.83 wt pct C martensite at room temperature, Metall. Mater. Trans. 26A (1995), pp. 2001–2005.
- Y. Ohmori and I. Tamura, An interpretation of the carbon redistribution process during aging of high carbon martensite, Metall. Trans. 23A (1992), pp. 2147–2158.
- Y. Ohmori and I. Tamura, Authors’ reply, Metall. Mater. Trans. 24A (1993), pp. 2588–2589.
- K.A. Taylor and M. Cohen, Aging of ferrous martensites, Prog. Mater. Sci. 36 (1992), pp. 225–272.
- F.E. Fujita, On the lattice deformation in martensite transformation in steel, Metall. Trans. 8A (1977), pp. 1727–1736.
- D.E. Jiang and E.A. Carter, Carbon dissolution and diffusion in ferrite and austenite from first principles, Phys. Rev. B. 67 (2003), pp. 1–11.
- C. Zener, Theory of strain interaction of solute atoms, Phys. Rev. 74 (1948), pp. 639–647.
- R. Naraghi, M. Selleby, and J. Ågren, Thermodynamics of stable and metastable structures in Fe-C system, CALPHAD. 46 (2014), pp. 148–158.
- C.W. Sinclair, M. Perez, R.G.A. Veiga, and A. Week, Molecular dynamics study of the ordering of carbon in highly supersaturated α-Fe, Phys. Rev. B. 81 (2010), pp. 1–9.
- M. van Genderen, A. Böttger, R. Cernik, and E. Mittemeijer, Early stages of decomposition in iron-carbon and iron-nitrogen martensites: Diffraction analysis using synchrotron radiation, Metall. Trans. A. 24 (1993), pp. 1965–1973.
- D. Kalish and M. Cohen, Structural changes and strengthening in the strain tempering of martensite, Mater. Sci. Eng. 6 (1970), pp. 156–166.
- E. Clouet, S. Garruchet, H. Nguyen, M. Perez, and C. Becquart, Dislocation interaction with C in α-Fe: A comparison between atomistic simulations and elasticity theory, Acta Mater. 56 (2008), pp. 3450–3460.
- R.G.A. Veiga, M. Perez, C.S. Becquart, E. Clouet, and C. Domain, Comparison of atomistic and elasticity approaches for carbon diffusion near line defects in α--iron, Acta Mater. 59 (2011), pp. 6963–6974.
- R.G. Veiga, M. Perez, C.S. Becquart, and C. Domain, Atomistic modeling of carbon Cottrell atmospheres in bcc iron, J. Phys.: Condens. Matter. 25 (2013), p. 025401.
- C.J. Först, J. Slycke, K.J. van Vliet, and S. Yip, Point defect concentrations in metastable Fe-C alloys, Phys. Rev. Lett. 96 (2006), pp. 1–4.
- C. Domain, C.S. Becquart, and J. Foct, Ab initio study of foreign interstitial atom (C, N) iinteraction with intrinstic point defects in α-Fe, Phys. Rev. B. 69 (2004), pp. 1–16.
- B. Kim, J. Sietsma, and M. Santofimia, Spinodal decomposition and the carbon solubility in BCC Fe-C, in Proceedings of the International Conference on Solid-solid Phase Transformations in Inorganic Materials (PTM), Whistler, BC, 2015, pp. 537–538.
- J. Wilde, A. Cerezo, and G.D.W. Smith, Three-dimensional atomic-scale mapping of a Cottrell atmospheres around a dislocation in iron, Scripta Mater. 43 (2000), pp. 39–48.
- F. Danoix, H. Zapolsky, S. Allain, and M. Goune, Atom probe tomogaphy investigation of carbon segregation and redistribution from supersaturated virgin Fe-C martensites, in Proceedings of the international conference on solid-solid phase transformations in inorganic materials (PTM), Whistler, BC, 2015, pp. 537–538.
- M. Herbig, R. Marceau, L. Morsdorf, and D. Raabe, Spinodal decomposotion of Fe-Ni-C martensite by room temperature redistribution of carbon investigated by correlative ECCI/TEM/APT, in Proceedings of the international conference on solid-solid phase transformations in inorganic materials (PTM), Whistler, BC, 2015, pp. 537–538.
- S.P. Thompson, J.E. Parker, J. Potter, T.P. Hill, A. Birt, T.M. Cobb, F. Yuan, and C.C. Tang, Beamline I11 at Diamond: A new instrument for high resolution powder diffraction, Rev. Sci. Instrum. 80 (2009), pp. 1–9.
- L. Lutterotti, S. Matties, H.R. Wenk, A.S. Schultz, and J.W. Richardson, Combined texture and structure analysis of deformed limestone from time-of-flight neutron diffraction spectra, J. Appl. Phys. 71 (1997), pp. 594–600.
- J.W. Christian, Tetragonal martensites in ferrous alloys -- A critique, Mater. T. JIM. 33 (1992), pp. 208–214.
- L. Cheng, A. Böttger, T. de Keijser, and E. Mittemeijer, Lattice parameters of iron-carbon and iron-nitrogen martensites and austenites, Scripta Mater. Metall. 24 (1990), pp. 509–514.
- R. Reed, and R. Schramm, Lattice parameters of martensite and austenite in Fe–Ni alloys, J. Appl. Phys. 40 (1969), pp. 3453–3458.
- S. Nagakura, Crystallographic study of the tempering of martensitic carbon steel by electron microscopy and diffraction, Metall Trans. 14A (1983), pp. 1025–1031.
- S. Morito, J. Nishikawa, and T. Maki, Dislocation density within lath martensite in Fe-C and Fe-Ni alloys, ISIJ Int. 43 (2003), pp. 1475–1477.
- Y. Ni, W. Rao, and A.G. Khachaturyan, Pseudospinodal mode of decomposition in films and formation of chessboard-like nanosructure, Nano Lett. 9 (2009), pp. 3275–3281.
- J.H. Jang, H.K.D.H. Bhadeshia, and D.W. Suh, Solubility of carbon in tetragonal ferrite in equilibrium with austenite, Scripta Mater. 68 (2013), pp. 195–198.
- C.N. Hulme-Smith, I. Lonardelli, A.C. Dippel, and H.K.D.H. Bhadeshia, Experimental evidence for non-cubic bainitic ferrite, Scripta Mater. 69 (2013), pp. 409–412.
- C. Garcia-Mateo, J. Jimenez, H.W. Yen, M. Miller, L. Morales-Rivas, M. Kuntz, S. Ringer, J.R. Yang, and F. Caballero, Low temperature bainitic ferrite: Evidence of carbon super-saturation and tetragonality, Acta Mater. 91 (2015), pp. 162–173.
- E.V. Pereloma, Critical assessment: On carbon excess in bainitic ferrite, Mater. Sci. Tech. 32 (2015), pp. 99–103. Available at http://dx.doi.org/10.1179/1743284715Y.0000000139.
- T. Massalski and D. Laughlin, The surprising role of magnetism on the phase stability of Fe (Ferro), CALPHAD. 33 (2009), pp. 3–7.
- A. Westgren and G. Phagmn, X-ray studies on the crystal structure of steel, J. Iron Steel I. 105 (1922), pp. 241–273.