ABSTRACT
Objective: There is an urgent need to undertake Point Prevalence Surveys (PPS) across Africa to document antimicrobial utilisation rates given high rates of infectious diseases and growing resistance rates. This is the case in Botswana along with high empiric use and extended prophylaxis to prevent surgical site infections (SSIs)
Method: PPS was conducted among all hospital sectors in Botswana using forms based on Global and European PPS studies adapted for Botswana, including rates of HIV, TB, malaria, and malnutrition. Quantitative study to assess the capacity to promote appropriate antibiotic prescribing.
Results: 711 patients were enrolled with high antimicrobial use (70.6%) reflecting an appreciable number transferred from other hospitals (42.9%), high HIV rates (40.04% among those with known HIV) and TB (25.4%), and high use of catheters. Most infections were community acquired (61.7%). Cefotaxime and metronidazole were the most prescribed in public hospitals with ceftriaxone the most prescribed antimicrobial in private hospitals. Concerns with missed antibiotic doses (1.96 per patient), high empiric use, extended use to prevent SSIs, high use of IV antibiotics, and variable infrastructures in hospitals to improve future antibiotic use.
Conclusion: High antibiotic use reflects high rates of infectious diseases observed in Botswana. A number of concerns have been identified, which are being addressed.
1. Introduction
The irrational use of antibiotics increases antimicrobial resistance (AMR), increasing morbidity, mortality, and costs [Citation1–Citation6]. Concerns with rising AMR rates and its implications have resulted in a range of activities globally, regionally, and nationally to enhance appropriate antibiotic prescribing including the development and agreement of national action plans [Citation7–Citation17]. This is illustrated by colistin where its increasing use in recent years due the emergence of carbapenem-resistant gram-negative bacteria has resulted in initiatives in sub-Sahara Africa to conserve its use to preserve its effectiveness [Citation18–Citation20].
We are aware of the high and often inappropriate prescribing and dispensing of antibiotics in ambulatory care [Citation1,Citation21–Citation25] driving up AMR rates. However, there is also high and inappropriate use of antibiotics in hospitals in both high income [Citation26–Citation33] as well as in low and middle income countries (LMICs), driven in some countries by high rates of sexually transmitted infections, HIV, TB and malaria among in-patients [Citation34–Citation41]. A key step to improving antimicrobial use in hospitals is to monitor current utilization patterns and instigate activities to improve this where pertinent via Drugs and Therapeutics Committees (DTCs) and antimicrobial stewardship programmes (ASPs) [Citation11,Citation35,Citation39,Citation42–Citation46]. This includes the monitoring of antimicrobial prescribing against agreed essential medicine lists or national guidelines [Citation32,Citation46–Citation48]. Recent studies have shown adherence to guidelines is a better judgment of the quality of prescribing than the current WHO/INRUD criteria [Citation49].
However, to date, there have been limited studies documenting current antimicrobial use within hospitals in Botswana compared for instance to studies describing utilization patterns in ambulatory care [Citation50]. This is perhaps not surprising due to a lack of aggregated patient level data typically limited research into antimicrobial prescribing in hospitals across Africa as part of global and more local point prevalence surveys (PPS) compared to European countries [Citation35,Citation46,Citation51–Citation54]. There have though been studies assessing adherence to guidelines and dosing recommendations among hospitals in sub-Sahara Africa [Citation47,Citation55,Citation56]. This needs to be rectified given the high prevalence rates of infectious diseases generally in sub-Saharan Africa [Citation57,Citation58], which has led to a tripling of antibiotic consumption in Africa in recent years [Citation59,Citation60].
HIV is particularly prevalent in sub-Sahara Africa as seen in Botswana where at one stage nearly 50% of women aged between 30 and 34 years were infected with HIV; although prevalence rates have now started to fall [Citation61,Citation62]. In addition, a retrospective study carried out among HIV-infected patients at a tertiary care facility in Botswana revealed a high degree of empiric antimicrobial prescribing with very limited ordering of culture and sensitivity tests (CSTs), which is a concern. There has also been extensive use of IV antibiotics with only a limited number of patients switched to oral medicines in a recent study in Botswana [Citation63]. This is a concern as less expensive oral antibiotics can be prescribed in their place to qualifying patients, and moreover, there are issues with unsafe needle use, catheter-related complications and extended length of hospital stay with high injectable use [Citation46,Citation52].
National antimicrobial guidelines have been developed in Botswana to help address a number of issues with current antimicrobial prescribing. For instance, restrictions were placed on some antibiotics to reduce their inappropriate use such as amikacin and fluoroquinolones for use only in MDR-TB. However, there is a need to study antimicrobial utilization generally, as well as adherence to national antimicrobial guidelines, to help optimize the use of antimicrobials in Botswana [Citation63,Citation64].
Concerns with current antimicrobial prescribing have resulted in initiatives among both public and private sector hospitals in Botswana to document current activities, including current resistance patterns and ASPs, as a basis for developing quality improvement programs as part of the national action plan (NAP) to contain AMR [Citation20]. This started with an agreement at a meeting in Botswana in February 2016 involving key personnel from both public and private sectors to instigate co-ordinated activities to collect hospital antimicrobial data using a common methodology considering the unavailability of aggregated patient-level data [Citation20]. This led to the development of a protocol with a PPS design based on ECDC (European Centre for Disease Prevention and Control) and Global-PPS studies with the help of those involved with their content [Citation20,Citation46,Citation65].
The data collection form was adapted to include HIV, TB, malaria, and malnutrition as key modifiers of antimicrobial use given the wide prevalence of these conditions in sub-Sahara Africa [Citation20,Citation57,Citation66,Citation67]. A set of instructions were produced to undertake the PPS in hospitals including information on diagnoses and antibiotic history, data to assess current microbiology services and capacity, as well as the extent of any ongoing programs to enhance the appropriateness of antibiotic use [Citation20]. A pilot study was undertaken with the results presented in Botswana in July 2016 along with suggestions for improving the data collection forms [Citation68]. This resulted in the data collection forms being amended prior to undertaking the full PPS study among hospitals in Botswana. The finalized data collection forms also formed the basis for similar PPS studies in other sub-Saharan African countries including South Africa and Zimbabwe [Citation40,Citation69,Citation70].
Other ongoing activities during this time in Botswana to improve hospital antibiotic use included documenting their use as part of prophylaxis to prevent surgical site infections (SSIs), assessing the burden of healthcare-associated infections and neonatal bloodstream infections within hospitals, determining current sensitivity rates to guide future empiric use and introducing ASPs to improve future antibiotic use in hospitals [Citation71–Citation75].
The objective of this study was to describe the current antimicrobial use among hospitals throughout Botswana building on the previous discussions and presentations [Citation38,Citation76]. The findings will be used to inform initiatives included in the Botswana antimicrobial NAP to contain AMR as well as pertinent quality improvement programs within participating hospitals and throughout Botswana. In addition, provide guidance to other sub-Sahara African countries to carry out PPS and help improve their antibiotic use within hospitals.
2. Methods
2.1. Study design
This is a quantitative observational descriptive study involving a structured PPS design [Citation46,Citation65] to describe the extent of antimicrobial use within hospitals using a structured data collection tool. The study also used a structured questionnaire to assess infrastructural capacities in hospitals for promoting appropriate antibiotic use. The structural capacity means the ability of hospitals to provide the necessary means to promote appropriate antimicrobial use to help achieve improved outcomes including access to a lab and microbiologists, the availability of commodities as well as the establishment of DTCs and ASPs.
As mentioned, the developed structured data collection tool based on the ECDC and Global-PPS study designs [Citation20,Citation46,Citation65] was assessed in a pilot study by two of the co-authors (FS and BAP) and subsequently refined before being disseminated among participating hospitals along with an instruction manual.
The study had multiple sites involving both public and private sector hospitals representing the various levels of care. Details of the 10 hospitals that took part in the study included:
Public primary hospitals: Bobonong Hospital, Gweta Hospital, Lethlakane Hospital, Goodhope Hospital.
Public district hospitals: Lethsolathebe-II Memorial Hospital Maun, DRM Hospital Mochudi, Mahalapye Hospital, Scottish Livingstone Memorial Hospital Molepolole.
Public referral hospital: Nyangabgwe Hospital, Francistown.
Private for profit specialized hospital: Lenmed-Bokamoso Private Hospital, Gaborone.
The 10 hospitals equate to 28.6% of the hospitals in Botswana covering specialized, referral, district, and primary level hospitals across both public and private sectors and 15.5% of the overall of hospital beds (773 out of a total of 4991 beds) [Citation77]. The identified hospitals cover all levels of healthcare and the most populated geographical regions in Botswana, i.e. the eastern corridor with the western corridor being principally the desert area. The specialized hospital chosen is situated in the capital city (Gaborone) and the referral hospital in the second largest city (Francistown); the remaining hospitals cover both semi-urban and rural areas in Botswana to give a geographical spread.
The inclusion criteria were all inpatient records of consenting patients that remained admitted overnight on the day of survey at 7am before any discharges were made. The surveys typically took place only on working days and there was no sampling of patients. The exclusion criteria were:
Patients or authorized persons who did not grant consent
Accident and emergency outpatients
Consulted outpatients
Patients who were kept in for observation after chemotherapy or minor procedures who did not stay overnight
Discharged patients lodging inwards due to lack of transport to their facility or homes
Patients in the labor ward
Psychiatric inpatients
Inpatients who were exclusively on TB treatment in order not to distort the findings.
2.2. Data collection
Data were collected via hospital employees who expressed a willingness to volunteer for research work released by their employer. Approximately 40 health-care professionals were trained on the principles of research ethics, PPS methodology and the structured data collection tool in a group workshop by one of the co-researchers to undertake the nationwide PPS. They were given the instructions and sample sheets to practice data input with also opportunities to ask questions and clarify most doubts before starting the PPS. The tool had seperate forms to collect hospital, ward and patient-level data with a patient coding sheet to ensure anonymity.
Data were collected on hard copies of the data collection tool from medical records of consenting inpatients or by their authorized personnel. Authorized personnel for children, mentally incompetent and critically ill patients were either the lodging parent, next of kin or the treating clinician. A brief discussion of the purpose and scope of the study was undertaken either individually or as groups in the ward cubicles giving them the opportunity to ask questions and clarify any doubts. There was neither questioning of the patient nor the treating clinicians regarding the condition being treated, prescriptions, missing data or illegible entries on records, as any such concerns could form the basis for future initiatives to improve the quality of patient care documentation in the hospitals. However, there were contacts with other hospital staff members during the study to complete the questionnaire to assess the existing institutional capacity for promoting appropriate antibiotic use (Table 1 Supplementary Information). There was constant communication support offered to data collectors by a co-researcher throughout the study through telephonic and email communications to clarify any doubts or queries when they arose.
Table 1. Patients surveyed among the 10 participating hospitals.
Collected data were subsequently captured on a standardized Excel template with drop-down menus for further analysis. It took 30 working days among the 10 hospitals (from 3rd May to 14 June 2017), with each ward completed in a single day. Typically, data collection took 1 day for primary and specialized hospitals using 1 and 5 data collectors respectively; between 3 and 5 days in the district hospitals with 1 to 2 data collectors each, and 10 days in the large referral hospital with two data collectors.
2.3. Study variables
The data collection tool used standardized codes for differentiating wards in the hospitals. Variables at the hospital level included the name of the hospital and the level of care. For ward level variables the name, code and specialty of the wards, patient census data and the total number of consenting patients were recorded. At the patient level, variables were divided into two sections with the first section containing variables for all included patients and the second for only those patients on antimicrobial therapy during admission. The variables of the first section included patient’s consent, if yes additional demographic details were included such as their age (broken down into centiles) and employment status, risk factors for infections and antibiotic use namely catheter use, intubations, re-admissions, diagnosis of HIV, TB, malaria and malnourishment, previous hospitalization and past antibiotic use in the last 90 days using ATC codes [Citation78]. Readmission was defined as a repeat admission for the same condition or a related complication arising from the previous admission. For readmitted patients who underwent surgery of deep organ or cavity, history of previous admissions was explored up to past 90 days while for other surgeries up to 30 days due to the risk of possible post-surgical infections [Citation46,Citation74,Citation79].
Section two of the patient level variables included prescriber designations, if the patient was readmitted, if the prescribed antibiotics were for treatment or prophylaxis, and if for prophylaxis, are they for medical or surgical prophylaxis. In addition, the duration of surgical prophylaxis either as one dose, one day or longer, admission diagnosis with ECDC codes, and type of infection – if acquired from the community, healthcare-associated or home-based care. The variables to assess the therapeutic part of this section included the name and ATC code of antibiotics prescribed, if prescribed on the drug sheet, whether by INN (International nonproprietary name) and whether from the Botswana essential medicines list, their indication/diagnosis codes, start date, dose, frequency of administration, injectable or oral, if injectable – bolus or continuous, stability of the gastrointestinal tract to evaluate suitability for oral switching, missed doses, CST orders, results, name of any isolate, review of therapy to sensitive antibiotics and any surgery performed that was unrelated to the admission diagnosis. No attempt was made to convert the prescribed antibiotic doses into defined daily doses (DDDs) [Citation80] in order to understand actual use including missed doses.
Antibiotics used for medical prophylaxis were identified using the documented diagnosis as those prescribed to prevent opportunistic infections in patients with medical conditions, e.g. co-trimoxazole or dapsone for the prevention of respiratory tract and gastrointestinal infections in HIV patients with low CD4 counts, broad-spectrum antibiotic prophylaxis in neutropenic patients and patients with endocarditis. Similarly, antibiotics prescribed for surgical prophylaxis were identified by the documented indications for antibiotics to prevent infections at surgical sites of the given anatomical sites.
The treatment of infections were classified into community acquired infections (CAIs) and healthcare-associated infections (HAIs) as well as home-based care infections (HBCIs). Infections were considered as CAIs if patients presented with infections or symptoms appeared <48 h from admission to hospital, as HAIs if symptoms appeared >48 h after admission to hospital based on chart review [Citation41,Citation46,Citation53], and as HBCIs if a terminally ill patient was transferred in from a long-term care facility due to an infection.
Table 1 (Supplementary Information) contains details of the structured questionnaire to assess the capacity of the hospitals to promote appropriate antibiotic use, i.e. their institutional capacity to improve future antibiotic utilization. This was again based on the ECDC and Global-PPS experiences and piloted before use. Key respondents in the hospital were approached by the data collectors to help complete the questionnaire in all participating hospitals.
The findings will be compared with those from the Global-PPS study as well as other African PPS studies to benchmark the findings in Botswana [Citation35,Citation46,Citation52–Citation54].
2.4. Data analysis
Data validation was undertaken prior to data analysis through data exploration to identify any typographical errors, extreme values, as well as incomplete, missing and incoherent responses in order to eliminate errors and prepare for the analysis.
All concerned entries were subsequently discussed and verified by the principal investigator (BAP) with pertinent data collectors to agree suitable amendments where pertinent prior to full analysis of the results. Data were analyzed using MS-Excel-2013 and presented as frequencies and percentages, with mean and standard deviations or median and interquartile ranges.
2.5. Ethical considerations
Ethical consent to undertake the PPS study throughout Botswana was granted by the Health Research and Development Division (13/18/1 X560) of the Ministry of Health in Botswana and by all participating hospital’s research and ethics committees or senior hospital management personnel in the hospital. Patients or authorized personnel were explained about the purpose and scope of the study, given assurance of the confidentiality of their personal health information by anonymizing the collected data by use of codes, and clarifying any doubts, prior to granting of informed consent. As mentioned, all captured data were de-identified by data collectors in their respective hospitals and only anonymized data were emailed to investigators.
3. Results
3.1. Sample demography and consent
Altogether 773 inpatients were admitted among the participating hospitals during the days of the surveys, and the medical records of 711 (92%) consenting patients were further analyzed ().
88.18% (627) of the surveyed patients were adults with a median age of 34 years (IQR 24–52 years), 2.95% (21) of patients were children (median age of 10 years – IQR 9–12 years), 3.09% (22) were infants (median age of 4 months, IQR of 2–6 months) and 5.76% (41) were neonates (median age of 1 day, IQR of 1–6 days). 60.9% (433) of patients were females and 39.1% (278) males. 33.4% (171) of patients where documented were unemployed. Most admissions occurred in the obstetrics, gynecology, adult medical and surgical wards (). The total number of patient days was 8553, and the median length of stay was 5 days (IQR 2–12days).
Table 2. Admissions of surveyed patients in different wards.
3.2. Risk factors for potential antibiotic use
Among the documented risk factors for infections that may potentially increase antibiotic prescribing, 42.9% of patients were found to be transferred in from other hospitals and 12.7% had a previous history of hospitalization in the last 90 days. Among those patients tested for various conditions, 2.54% had confirmed malaria during admission, 25.4% were TB positive, and 5% were diagnosed as malnourished ().
Table 3. Potential risk factors influencing antibiotic use in participating hospitals broken down by patient’s age groups.
HIV is also a risk factor for opportunistic infections. Among 462 (64.94%) patients where their HIV test results were documented, 40.04% (185) were known to be positive with 85.4% (158) receiving HAART. Catheter use is also a risk factor for contracting healthcare-associated infections (HAIs) increasing antibiotic use. Overall, 53.02% (377) of patients had a peripheral IV cannula, 7.45% (53) had a urinary catheter, 1.41% (10) had a hemodialysis catheter and in 1.27% (9) a central venous catheter was inserted. Intubation is also a risk factor for infection with 6.89% (49) of patients having nasogastric tubes, 3.94% (28) having endotracheal intubation and the use of suction tubes, and 0.28% (2) having tracheostomy tubes.
3.3. Types of diagnosed infections
Of the 711 patients, nearly three quarters 70.6% (502) had a documented bacterial infection, of which 61.7% (439) were CAIs, 60 (8.44%) were HAIs and three (0.42%) were HBCIs (). Of the 60 HAIs, 51.7% (31) occurred among the neonates and children under five years, 23.3% (14) occurred among the elderly patients >61 years of age. 29.4% (209) of admissions were for non-infectious conditions.
Table 4. Types of documented infection broken down by patients’ age groups.
3.4. Previous antibiotic exposure
The records of 134 patients (18.85%) showed prior antibiotic exposure in the previous 90 days. Cefotaxime and amoxicillin were the most commonly prescribed antibiotics, being prescribed in 28.36% and 26.12% patients, respectively, with a mean duration of 5.53 and 6 days, respectively. This was followed by metronidazole, erythromycin, co-trimoxazole tablets, and metronidazole injections among 17.91%, 17.16%, 14.18% and 10.45% patients, respectively, with a mean duration of 10.22, 7.35, 16.25, and 5.56 days, respectively. Exposure to amoxicillin clavulanic acid was 3.73% (mean duration 3.8 days), vancomycin 2.24% (mean duration 3.4 days), and carbapenem and fluoroquinolones at 1.49% (mean duration 10.5 and 4 days respectively).
3.5. Common diagnosis
The most commonly diagnosed conditions at admission were related to obstetrics and gynecology in 94 (13.22%) patients, followed by pneumonia in 62 patients (8.72%) and skin and soft tissue infections in 54 patients (7.59%), reflected in on ward admissions. The remaining infectious conditions diagnosed were all below 3.6%.
3.6. Antibiotic use during admission
A total of 982 antibiotics were prescribed for the 711 patients suggesting a national antibiotic prescribing ratio of 1.38. lists the prescribed antibiotics by ATC code of which cefotaxime followed by parenteral metronidazole were the most prescribed in all levels of public hospitals, while ceftriaxone use was the second highest antimicrobial prescribed in the specialist private hospital. Carbapenem antibiotics and piperacillin with enzyme inhibitor were prescribed mostly at specialist and tertiary hospital levels, while vancomycin is prescribed also at the district level.
Table 5. Antibiotics prescriptions broken down by ATC code and hospital type.
The highest antibiotic prescription ratio (calculated as the number of antibiotics prescribed per patient) was seen in the tertiary hospital (1.76) followed by the specialist hospital (1.42), primary hospitals at 1.15 and lowest in the district hospitals at 1.01. The ratio of injectable antibiotic use was highest in the specialist hospital (1.37) followed by the tertiary hospital (1.22), and lowest in primary hospitals (0.67) and district hospitals (0.60). The commonly prescribed classes were the penicillins (28.2%), cephalosporins (26.1%) and nitroimidazole (20.7%) compounds ().
Table 6. Prescribed antibiotic classes across health facilities.
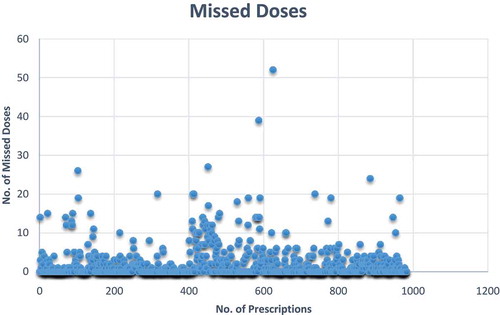
Administration of 1923 doses from 437 antibiotic prescriptions were missed, with a mean of 1.96 doses (SD 4.17) missed for administration ().
3.7. Antibiotic use in surgical prophylaxis
A wide range of antibiotics were used for surgical prophylaxis in 127 patients. The principal ones were ampicillin (26.77%), amoxicillin (24.41%), metronidazole (17.32% injectable and 6.30% oral), ceftriaxone (7.09%), cefotaxime (6.30%) and amoxicillin clavulanic acid (3.94%). The duration of surgical prophylaxis also varied. This was typically greater than one day across the surveyed facilities ().
Table 7. Duration of surgical prophylaxis across the hospitals.
3.8. Microbiological tests
Culture and sensitivity tests (CSTs) were rarely ordered, with most being requested in the specialist and primary hospitals (). There were also concerns that the results were not always reported on time, although when reported they did lead to changes in antibiotics prescribed in most situations.
Table 8. Culture and sensitivity findings.
3.9. Assessment of institutional capacity for promoting ASP
The infrastructure and capacity within hospitals to establish and support ASPs, and the policy framework to provide formal leadership and governance, currently appears to be inadequate although most hospitals have optimal capacity for providing lab microbiological support with functioning DTCs and Infection Prevention and Control (IPC) committees ().
Table 9. Existing institutional capacity to promote appropriate antimicrobial use (n = 10).
The PPS also found deficiencies in the capacity for monitoring and providing feedback on the prescribing practices and current antimicrobial utilization patterns. Though the current Botswana EML and guidelines were routinely updated, not all hospitals had them available for use and decision support tools such as the antibiogram were generally unavailable to guide empiric antibiotic use across hospitals. There were also concerns with the lack of capacity to conduct audits and generation of reports for useful feedback for effecting suitable changes in prescribing practices.
4. Discussion
There was a high prevalence of infections (70.6%) among inpatients treated in the hospitals in Botswana with antimicrobials. This prevalence was similar to one hospital in Kenya (67.7%) as well as hospitals in Pakistan (77.6%) but higher than other hospitals in Kenya (54.7%) as well as Ghana (51.4%) and South Africa (31% and 38.5%) [Citation39–Citation41,Citation45,Citation52,Citation81]. In addition, higher than the findings of the Global PPS with an overall prevalence of 34.4%, up to 50.0% among the 12 participating hospitals in five African countries [Citation46]. This high prevalence in Botswana may be due to high rates of patients transferred in from other hospitals (42.9%), readmissions (12.7%), high rates of admissions due to infectious conditions (gynaecological and obstetric) in women (13.22%), admissions with coexisting TB (25.4%) and HIV (40.04% where known), high rates of catheterization (>50%) as well as malnourishment in a minority of patients (5%). The type of infections seen had similarities with the Global PPS where pneumonia or lower respiratory tract infections, skin and soft tissue infections, intra-abdominal and urinary tract infections were the most prevalent infections [Citation46], with Kenya where gynecological sites, respiratory tract, skin, and soft tissue infections were among the most prevalent sites [Citation35,Citation39] and South Africa where bone and joint infections, gynecological sites, respiratory tract, as well as skin and soft tissue infections were among the most prevalent infections [Citation40]. In Ghana, the wards with the highest prevalence of antibiotic use were pediatric surgery, child health, orthopedics and general surgery [Citation52].
The prevalence of community-acquired infections (61.7%) was higher than the overall rate seen in the Global-PPS study (45.6%) but similar to the 12 African hospitals (57.4%) in the Global-PPS study [Citation46] and one study in Kenya (54.2%) [Citation39]. The prevalence of CAIs in Botswana was also higher than those seen in Ghana (40.1%) and another hospital in Kenya (28%) [Citation35,Citation52]. This may again be attributed to the commonly diagnosed infections in women, community-acquired pneumonia and skin and soft tissue infections. Whilst the prevalence of HAIs (8.44%) in Botswana was considerably lower than that seen in the Global-PPS Study (25.2%), one hospital in Ghana (21.1%) and Kenya (13%); it was similar to the rates among 12 African hospitals in the Global-PPS study (9.5%) as well as among 10 other hospitals in Ghana, a further hospital in Kenya (2.8% to 14.4%) and 13 hospitals in Pakistan (5.9%) [Citation35,Citation41,Citation46,Citation52,Citation53]. This could be due to the initiatives of the functioning IPC committees (100%) in the hospitals in Botswana as reported in the institutional capacity survey (). However, it should be noted that the prevalence of HAIs was high among the neonates to under five aged children (51.7%), and among the elderly (23.3%) in Botswana ().
There were also similarities and differences with the antibiotics prescribed () with PPS in other studies. The most prescribed antibiotics in the Global-PPS study were the penicillins with a ß-lactamase inhibitor, followed by the fluoroquinolones, carbapenems and glycopeptides [Citation46]. Third generation cephalosporins, imidazole derivatives, broad-spectrum penicillins, and aminoglycosides were the most prescribed antibiotics in Kenya [Citation35,Citation39]. In Ghana, the most prescribed antibiotics were the penicillins followed by the nitroimidazoles, cephalosporins (3rd and 2nd generations) and aminoglycosides [Citation52].
Perhaps not surprising, the highest ratio of antibiotic prescribing in Botswana (1.76) was in the tertiary hospital. This is similar to Ghana where the median number of antibiotics prescribed was 2 [Citation52], and in the Global-PPS study where the majority of patients were on two antibiotics [Citation46]. There was also a high use of injectable antibiotics in Botswana similar to the Global-PPS study where IV antibiotics accounted for more than 80% of patients on antibiotics [Citation46]. This is a concern due to potential rise in the cost of care and is being looked at further in ongoing research projects in Botswana [Citation72]. The number of missed doses is also a concern () that may pose a risk for emergence of drug-resistant strains of bacteria in the hospitals. This is also currently being investigated further to develop ways to reduce this.
Ampicillin, amoxicillin with or without clavulanic acid, metronidazole, ceftriaxone, and cefotaxime were the most prescribed antibiotics for surgical prophylaxis. This is different to antibiotics used to prevent SSIs in another leading tertiary hospital in Botswana where cefotaxime (80.7%), metronidazole (63.5%), cefradine (13.6%) and amoxicillin-clavulanic acid (11.6%) were the most commonly prescribed antibiotics [Citation74], and the Global PPS where cefazolin was the most prescribed antibiotic for surgical prophylaxis overall. However, ceftriaxone the most prescribed antibiotic for surgical prophylaxis among the 12 African hospitals taking part in the Global PPS (27.7%) similar to Kenya [Citation39,Citation46] and Pakistan [Citation41]. Third generation cephalosporins were also commonly used in the surgical wards in another hospital in Kenya (55%) where surgical prophylaxis was the most common indication (67.9%) [Citation35]. In Ghana, the most prescribed antibiotics for surgical prophylaxis were metronidazole (32.2%), amoxicillin-clavulanic acid (25.9%) and cefuroxime (13.7) [Citation52].
Extended surgical prophylaxis (>1day) was high in almost all levels of hospitals in Botswana (), and against current guidelines which advocates that antibiotic prophylaxis should be given 1–2 h before a surgical incision for adequate serum and tissue drug levels to be achieved during surgery [Citation82–Citation84]. A longer duration of prophylaxis potentially increases resistance rates, side-effects and costs [Citation46,Citation74]. However, the findings in Botswana are similar to an earlier study which found that the majority of patients were given antibiotics for a mean of five days to prevent SSIs [Citation74], as well as the Global-PPS study that found that prolonged surgical prophylaxis (>1 day) was also very common – up to 86.3% of cases [Citation46]. Extended prophylaxis was also seen among neurotrauma patients in Kenya [Citation85]. Botswana should consider inclusion of cefazolin in the EML for surgical prophylaxis in procedures that do not extend to deep organ cavity. Prescriptions of extended surgical prophylaxis were often justified citing the suboptimal operating conditions experienced by surgeons in theater, limiting the possibilities of maintaining the required aseptic conditions. This again is being addressed.
There were also concerns with wide gap in the requests made for CST, with as low as 2.61% in the tertiary hospital, higher in the specialist (29.82%) and primary hospitals (22.39%) (). While empiric antibiotic use is highly prevalent, this also needs addressing by developing appropriate guidelines in hospitals recommending cases for targeted therapy guided by CST results. Absence of CST results minimizes historical data on local prevalence and sensitivity, which subsequently limits guidance for facility-specific recommendations, continual monitoring, and education on local resistance rates and trends (20%) – .
The current infrastructure among the hospitals in Botswana to promote appropriate antimicrobial prescribing in terms of ASPs and support also appears inadequate (), with only 50% of hospitals having accessible microbiologists as well as pharmacists to help ensure appropriate antibiotic use. Additional concerns include a lack of a prescribing policy for documenting indications, audits for monitoring of the indication for antibiotic use and variable availability and use of the Botswana antimicrobial guidelines to guide prescribing (). These issues were also seen in the Global-PPS where there were concerns with guideline availability and adherence to these among the African hospitals taking part [Citation46], as well as for surgical prophylaxis in South Africa [Citation56]. Encouragingly though, most hospitals had functioning DTCs and IPC committees as well as optimal capacity for providing lab microbiological support (). These existing capacities can be used as starting points to develop pertinent ASPs among the hospitals in Botswana similar to those seen in other African countries [Citation44] as well as instigate quality improvement programmes (QIPs) to address identified concerns.
We are aware of a number of limitations with this study. These include the fact that we only surveyed 28.6% of the hospitals and 15.5% of available hospital beds. However, we believe based on the geographical spread of the hospitals and their level of care, e.g. private, public, primary, district, tertiary, specialist, rural, semi urban, and urban hospitals was a representational sample of Botswana. There was also a need to have a site coordinator to enable the data collectors to plan and arrange the necessary logistic support for data collection and capturing as well as clarify any doubts. This wasn’t always possible with verbal support in most situations. The specialty hospital used both manual and electronic records which are not harmonious with their entries and had to consult clinicians for accuracy. In addition, there was no opportunity to confirm the accuracy of events and comments in patient records, similar to other PPS. Despite these limitations, we believe the findings are robust and provide direction to other lower and middle income countries seeking to undertake PPS studies in their hospitals.
5. Conclusion and recommendations
This was the first national PPS study conducted among all hospital sectors in Botswana and shows that the PPS methodology could be used in LMICs to study antimicrobial utilization in settings where aggregate patient level data are unavailable. The methodology also helped identify the underlying prevalence of morbidities and risk factors that drives prescribing of antibiotics and their patterns to identify key areas to inform the Botswana antimicrobial NAP and other QIPs to reduce inappropriate antimicrobial use in the future.
In addition to other risk factors described, the inclusion of local co-morbidities such as HIV, TB, malaria, and malnutrition, along with the ECDC and Global-PPS variables, appeared justified given the high prevalence of infections resulting in high use of antibiotics among hospitals in Botswana. We suggest these co-morbidities are routinely included in PPS studies in sub-Sahara Africa to compare the findings across African countries.
There were concerns that antimicrobial prescriptions were typically empiric therapy, and there was high injectable use. Recommended initiatives to address these and other identified concerns include improving the number of CST requests for targeted therapy through education and other activities, auditing prescriber’s adherence to the Botswana antimicrobial guidelines, educating physicians to reduce currently high rates of extended surgical prophylaxis and monitoring antibiotic doses prescribed to reduce the extent of missed doses. These activities are now being instituted among hospitals in Botswana and supported through DTC and IPCs as part of quality improvement projects. At the national level, these committees are strengthened by the Botswana Essential Drugs Action Programme, and we will be monitoring their influence in future studies.
Whilst such issues and concerns have been seen in other African countries, identified concerns will now become part of QIPs in Botswana, building on current microbiological capacity as well as active DTCs and IPCs among hospitals in Botswana. The findings will be reported in the future.
6. Expert opinion
PPS studies are essential to enable hospitals and Ministries of Health improve their understanding of current antimicrobial use within their facilities and plan for the future. This is especially important in countries where there are high rates of infectious diseases including Botswana with its high rate of HIV along with TB. There are also high rates of malaria in a number of African countries as well as malnutrition. These combined factors need to be taken into account when comparing antimicrobial utilisation among hospitals in sub-Saharan African countries such as Botswana with those in Western countries. As a result, these four potential factors that drive antibiotic consumption were successfully included in the PPS forms in Botswana and wider, building on the data collection forms for the Global and European PPS studies.
PPS studies also provide baseline data regarding the extent of hospital acquired infections and the extent of prophylaxis to prevent SSIs especially where there are concerns with extended use. PPS studies can also help assess current rates of conversion from IV to oral antibiotics as well as the extent of infrastructures within hospitals in given countries to enhance appropriate use of antimicrobials. This includes the extent of active DTCs, microbiological support and AMS programmes. The PPS in Botswana showed a high rate of antimicrobial use (70.6%), enhanced by high rates of HIV, patients being transferred from other hospitals and high use of catheters. The study also identified concerns with missed antibiotic doses, the extent of empiric use of antibiotics with limited use of CSTs to guide prescribing, extended use of antimicrobials to prevent SSIs, and limited conversion of IV to oral antibiotics. There were also concerns with current infrastructures within hospitals in Botswana to improve future antimicrobial use. Quality improvement programmes are now in place in a number of the hospitals in Botswana to address identified concerns, and this will continue. We are likely to see the growth in AMS programmes among the hospitals in Botswana in the future helping to address identified concerns. In addition, we will see further PPS studies to monitor and improve antimicrobial use to meet the goals of the Botswana National Action Plan (NAP) to reduce AMR. These current findings in Botswana can also provide baseline data to other African countries as they undertake their PPS studies as part of their NAPs to improve their antimicrobial utilisation in hospitals.
Declaration of interest
C Tiroyakgosi and M Morokotso are employed by the Ministry of Health and Wellness in Botswana. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplemental Material
Download MS Word (23.3 KB)supplementary material
supplemental data for this article can be accessed here.
Additional information
Funding
References
- Llor C, Bjerrum L. Antimicrobial resistance: risk associated with antibiotic overuse and initiatives to reduce the problem. Ther Adv Drug Saf. 2014;5(6):229–241.
- Bebell LM, Muiru AN. Antibiotic use and emerging resistance: how can resource-limited countries turn the tide? Glob Heart. 2014;9(3):347–358.
- Founou RC, Founou LL, Essack SY. Clinical and economic impact of antibiotic resistance in developing countries: A systematic review and meta-analysis. PloS One. 2017;12(12):e0189621.
- Gandra S, Barter DM, Laxminarayan R. Economic burden of antibiotic resistance: how much do we really know? Clin Microbiol Infect. 2014;20(10):973–980.
- O’Neill J Securing new drugs for future generations: the pipeline of antibiotics. The review of antimicrobial resistance. [cited 2019 Mar 10]. Available at URL: https://amr-review.org/sites/default/files/SECURING%20NEW%20DRUGS%20FOR%20FUTURE%20GENERATIONS%20FINAL%20WEB_0.pdf.
- Md Rezal RS, Hassali MA, Alrasheedy AA, et al. Physicians’ knowledge, perceptions and behaviour towards antibiotic prescribing: a systematic review of the literature. Expert Rev Anti Infect Ther. 2015;13(5):665–680.
- Seale AC, Gordon NC, Islam J, et al. AMR Surveillance in low and middle-income settings - A roadmap for participation in the Global Antimicrobial Surveillance System (GLASS). Wellcome Open Res. 2017;2:92.
- WHO. 2018. Antimicrobial resistance. Fact Sheet. [cited 2019 Mar 11]. Available at URL: http://www.who.int/mediacentre/factsheets/fs194/en/.
- Abdula N, Macharia J, Motsoaledi A, et al. National action for global gains in antimicrobial resistance. Lancet. 2016;387(10014):e3–5.
- Ashiru-Oredope D, Hopkins S. Antimicrobial resistance: moving from professional engagement to public action. J Antimicrob Chemother. 2015;70(11):2927–2930.
- Cox JA, Vlieghe E, Mendelson M, et al. Antibiotic stewardship in low-and middle-income countries: ‘same, but different’? Clin Microbiol Infect. 2017;23(11):812–818.
- Jinks T, Lee N, Sharland M, et al. A time for action: antimicrobial resistance needs global response. Bull World Health Organ. 2016;94(8):558–a.
- WHO. Global action plan on antimicrobial resistance. [cited 2019 Mar 10]. Available at URL: http://www.who.int/antimicrobial-resistance/publications/global-action-plan/en/[
- Mendelson M, Matsoso MP. The world health organization global action plan for antimicrobial resistance. South Afr Med J. 2015;105(5):325.
- Dar OA, Hasan R, Schlundt J, et al. Exploring the evidence base for national and regional policy interventions to combat resistance. Lancet. 2016;387(10015):285–295.
- Van Dijck C, Vlieghe E, Cox JA. Antibiotic stewardship interventions in hospitals in low-and middle-income countries: a systematic review. Bull World Health Organ. 2018;96(4):266–280.
- Wesangula E, Guantai A. Oluka M. KENYAN NATIONAL ACTION PLAN ON ANTIMICROBIALS. 3rd MURIA Training Workshops and Symposium. Available at URL: file:///C:/Users/mail/Downloads/Kenyan-NAP-Antimicrobials-EWesangula%252c-AGuantai%252c-MOluka.pdf.
- Mendelson M, Brink A, Gouws J, et al. The One Health stewardship of colistin as an antibiotic of last resort for human health in South Africa. Lancet Infect Dis. 2018;18(9):e288–e94.
- Falagas ME, Rafailidis PI. Re-emergence of colistin in today’s world of multidrug-resistant organisms: personal perspectives. Expert Opin Investig Drugs. 2008;17(7):973–981.
- Massele A, Tiroyakgosi C, Matome M, et al. Research activities to improve the utilization of antibiotics in Africa. Expert Rev Pharmacoecon Outcomes Res. 2017;17(1):1–4.
- Dyar OJ, Beovic B, Vlahovic-Palcevski V, et al. How can we improve antibiotic prescribing in primary care? Expert Rev Anti Infect Ther. 2016;14(4):403–413.
- Kalungia AC, Burger J, Godman B, et al. Non-prescription sale and dispensing of antibiotics in community pharmacies in Zambia. Expert Rev Anti Infect Ther. 2016;14(12):1215–1223.
- Alhomoud F, Aljamea Z, Almahasnah R, et al. Self-medication and self-prescription with antibiotics in the Middle East-do they really happen? A systematic review of the prevalence, possible reasons, and outcomes. Inter J Infect Dis. 2017;57:3–12.
- Nepal G, Bhatta S. Self-medication with antibiotics in WHO Southeast Asian Region: a systematic review. Cureus. 2018;10(4):e2428.
- Köchling A, Löffler C, Reinsch S, et al. Reduction of antibiotic prescriptions for acute respiratory tract infections in primary care: a systematic review. Implement Sci. 2018;13(1):47.
- Fridkin S, Baggs J, Fagan R, et al. Vital signs: improving antibiotic use among hospitalized patients. Morbidity Mortality Weekly Rep. 2014;63(9):194–200.
- Akhloufi H, Streefkerk RH, Melles DC, et al. Point prevalence of appropriate antimicrobial therapy in a Dutch university hospital. Eur J Clin Microbiol Infect Dis. 2015;34(8):1631–1637.
- Dumartin C, L’Heriteau F, Pefau M, et al. Antibiotic use in 530 French hospitals: results from a surveillance network at hospital and ward levels in 2007. J Antimicrob Chemother. 2010;65(9):2028–2036.
- Hecker MT, Aron DC, Patel NP, et al. Unnecessary use of antimicrobials in hospitalized patients: current patterns of misuse with an emphasis on the antianaerobic spectrum of activity. Arch Internal Med. 2003;163(8):972–978.
- Pollmann AS, Bailey BJG, Davis PJ, et al. Antibiotic use among older adults on an acute care general surgery service. Can J Surg. 2017;60(6):388-393.
- Baig MT, Sial AA, Huma A, et al. Irrational antibiotic prescribing practice among children in critical care of tertiary hospitals. Pak J Pharm Sci. 2017;30(4(Suppl.)):1483–1489.
- Atif M, Azeem M, Saqib A, et al. Investigation of antimicrobial use at a tertiary care hospital in Southern Punjab, Pakistan using WHO methodology. Antimicrob Resist Infect Control. 2017;6:41.
- Flanders SA, Saint S. Why does antimicrobial overuse in hospitalized patients persist? JAMA Intern Med. 2014;174(5):661–662.
- Bizo PT, Dumitras D, Popa A. Evaluation of restricted antibiotic use in a hospital in Romania. Int J Clin Pharm. 2015;37(3):452–456.
- Okoth C, Opanga S, Okalebo F, et al. Point prevalence survey of antibiotic use and resistance at a referral hospital in Kenya: findings and implications. Hosp Pract. 2018;46(3):128–136.
- Gandra S, Singh SK, Jinka DR, et al. Point prevalence surveys of antimicrobial use among hospitalized children in six hospitals in India in 2016. Antibiotics. 2017;6(3):19.
- Talaat M, Saied T, Kandeel A, et al. a point prevalence survey of antibiotic use in 18 hospitals in Egypt. Antibiotics. 2014;3(3):450–460.
- Tiroyakgosi C, Matome M, Summers E, et al. Ongoing initiatives to improve the use of antibiotics in Botswana: university of Botswana symposium meeting report. Expert Rev Anti Infect Ther. 2018;16(5):381–384.
- Momanyi L, Opanga S, Nyamu D, et al. Antibiotic prescribing patterns at a leading referral hospital in kenya: a point prevalence survey. Accepted Publ J Res Pharm Pract. [cited 2019 Jun 8]. Available at URL: https://strathprints.strath.ac.uk/66791/.
- Dlamini NN, Meyer JC, Kruger D, et al. Feasibility of using point prevalence surveys to assess antimicrobial utilisation in public hospitals in South Africa; a pilot study and implications. Hosp Pract. 2019;47(2):88–95.
- Saleem Z, Hassali MA, Versporten A, et al. A multicenter point prevalence survey of antibiotic use in Punjab, Pakistan: findings and implications. Expert Rev Anti Infect Ther. 2019; 17(4):285-293.
- Lima-Dellamora Eda C, Caetano R, Gustafsson LL, et al. An analytical framework for assessing drug and therapeutics committee structure and work processes in tertiary Brazilian hospitals. Basic Clin Pharmacol Toxicol. 2014;115(3):268–276.
- Matlala M, Gous AG, Godman B, et al. Structure and activities of pharmacy and therapeutics committees among public hospitals in South Africa; findings and implications. Expert Rev Clin Pharmacol. 2017;10(11):1273–1280.
- Schellack N, Bronkhorst E, Coetzee R, et al. SASOCP position statement on the pharmacist’s role in antibiotic stewardship 2018. S Afr J Infect Dis. 2018;33(1):28–35.
- H VA F, Whitelaw A, Goossens H, et al. The global point prevalence survey of antimicrobial consumption and resistance (Global-PPS): results of antimicrobial prescribing in a South African Tertiary Hospital. 2016. Available at URL: http://www.global-pps.com/wp-content/uploads/ECCMID-2016_South-Africa.pdf
- Versporten A, Zarb P, Caniaux I, et al. Antimicrobial consumption and resistance in adult hospital inpatients in 53 countries: results of an internet-based global point prevalence survey. Lancet Glob Health. 2018;6(6):e619–e29.
- Nakwatumbah S, Kibuule D, Godman B, et al. Compliance to guidelines for the prescribing of antibiotics in acute infections at Namibia’s national referral hospital: a pilot study and the implications. Expert Rev Anti Infect Ther. 2017;15(7):713–721.
- Matsitse TB, Helberg E, Meyer JC, et al. Compliance with the primary health care treatment guidelines and the essential medicines list in the management of sexually transmitted infections in correctional centres in South Africa: findings and implications. Expert Rev Anti Infect Ther. 2017;15(10):963–972.
- Niaz Q, Godman B, Massele A, et al. Validity of World Health Organisation prescribing indicators in Namibia’s primary healthcare: findings and implications. Int J Qual Health Care. 2019;31(5):338–345.
- Mashalla Y, Setlhare V, Massele A, et al. Assessment of prescribing practices at the primary healthcare facilities in Botswana with an emphasis on antibiotics: findings and implications. Int J Clin Pract. 2017;71:12.
- Momanyi L, Oluka M, Opanga S, et al. Antibiotic prescribing patterns at a referral hospital in Kenya: A point prevalence survey. Muria 4. 2018;7. Available at URL: file:///C:/Users/mail/Downloads/Consolidated-abstract-booklet%20(4).pdf
- Labi A-K, Obeng-Nkrumah N, Nartey ET, et al. Antibiotic use in a tertiary healthcare facility in Ghana: a point prevalence survey. Antimicrob Resist Infect Control. 2018;7:15.
- Labi AK, Obeng-Nkrumah N, Owusu E, et al. Multi-centre point-prevalence survey of hospital-acquired infections in Ghana. J Hosp Infect. 2019;101(1):60-68.
- Ahoyo TA, Bankole HS, Adeoti FM, et al. Prevalence of nosocomial infections and anti-infective therapy in Benin: results of the first nationwide survey in 2012. Antimicrob Resist Infect Control. 2014;3:17.
- Afriyie DK, Amponsah SK, Dogbey J, et al. A pilot study evaluating the prescribing of ceftriaxone in hospitals in Ghana: findings and implications. Hosp Pract (1995). 2017;45(4):143–149.
- van der Sandt N, Schellack N, Mabope LA, et al. Surgical antimicrobial prophylaxis among pediatric patients in South Africa comparing two healthcare settings. Pediatr Infect Dis J. 2019;38(2):122–126.
- Ghosn J, Taiwo B, Seedat S, et al. HIV. Lancet. 2018;392(10148):685–697.
- Wanyiri JW, Kanyi H, Maina S, et al. Infectious diarrhoea in antiretroviral therapy-naïve HIV/AIDS patients in Kenya. Trans R Soc Trop Med Hyg. 2013;107(10):631–638.
- Reardon S. Antibiotic resistance sweeping developing world. Nature. 2014;509(7499):141–142.
- Van Boeckel TP, Gandra S, Ashok A, et al. Global antibiotic consumption 2000 to 2010: an analysis of national pharmaceutical sales data. Lancet Infect Dis. 2014;14(8):742–750.
- UNDP on Botswana HIV. [cited 2019 Mar 11]. Available at URL: http://www.bw.undp.org/content/botswana/en/home/ourwork/hiv_aids/overview.html
- UNAIDS. Prevention Gap report 2016. [cited 2019 Mar 10]. Available at URL: http://www.unaids.org/sites/default/files/media_asset/2016-prevention-gap-report_en.pdf
- Massele A, Burger J, Katende-Kyenda NL, et al. Outcome of the first medicines utilization research in Africa group meeting to promote sustainable and rational medicine use in Africa. Expert Rev Pharmacoecon Outcomes Res. 2015;15(6):885–888.
- Tafuma TA, Burnett RJ, Huis I, et al. National guidelines not always followed when diagnosing smear-negative pulmonary tuberculosis in patients with HIV in Botswana. PloS One. 2014;9(2):e88654.
- ECDC. Point prevalence survey of healthcare-associated infections and antimicrobial use in European acute care hospitals. Available at URL: http://ecdc.europa.eu/en/publications/Publications/PPS-HAI-antimicrobial-use-EU-acute-care-hospitals-V5-3.pdf
- Kibuule D, Verbeeck RK, Nunurai R, et al. Predictors of tuberculosis treatment success under the DOTS program in Namibia. Expert Rev Respir Med. 2018;12(11):979–987.
- Mwita SJM, Marwa K, Hamasaki K, et al. Medicines dispensers’ knowledge on the implementation of an artemisinin-based combination therapy policy for the treatment of uncomplicated malaria in Tanzania. J Pharm Health Serv Res. 2017;8:227–233.
- Paramadhas BDA, Tiroyakgosi C, Godman B. A point prevalence study on antibiotic utilization among public hospitals in Botswana. Muria 2. 2016;7. Available at URL: file:///C:/Users/mail/Downloads/Anti-infectives.pdf
- Schellack N, Dlamini N, Meyer JC, et al. Point prevalence survey of antimicrobial utilisation in an academic hospital in the Gauteng province, South Africa. Muria 3. 2017;7. Available at URL: http://muria.mandela.ac.za/muria/media/Store/documents/Abstract%20book%20-%20MURAI%203/MURIA3-AbstractBook-July-2017.pdf
- Mtapuri-Zinyowera S, Tarupiwa A, Manangazira P, et al. Investigation of foodborne pathogens and their antimicrobial resistance in humans, animals and the environment – a pilot project for Zimbabwe. Muria 4. 2018;11. Available at URL: file:///C:/Users/mail/Downloads/Consolidated-abstract-booklet%20(4).pdf
- Mpinda-Joseph P, Paramadhas BDA, Reyes G, et al. Point prevalence survey to estimate the true burden of healthcare associated infections and their risk factors in Nyangabgwe Hospital, Botswana. Muria 4. 2018;9. Available at URL: file:///C:/Users/mail/Downloads/Consolidated-abstract-booklet%20(4).pdf
- Molefhi L, Paramadhas BDA, Phiri HR, et al. Trends in utilization of parenteral antibiotics from 2014–2016 at Nyangabgwe Hospital, Botswana. Muria 4. 2018;8. Available at URL: file:///C:/Users/mail/Downloads/Consolidated-abstract-booklet%20(4).pdf
- Malone B, Phiri M, Kurusa G. Evolution of ESKAPE organism antibiotic sensitivity over time at a private hospital in Botswana. MURIA 2 Anti-Infectves. 2016;3. Available at URL: file:///C:/Users/mail/Downloads/Anti-infectives%20(1).pdf
- Mwita JC, Souda S, Magafu M, et al. Prophylactic antibiotics to prevent surgical site infections in Botswana: findings and implications. Hosp Pract. 2018;46(3):97–102.
- Didimalang T, Jolomba C. Antimicrobial stewardship implementation in gaborone private hospital. MURIA 2 Anti-Infectives. 2016;6. Available at URL: file:///C:/Users/mail/Downloads/Anti-infectives%20(1).pdf
- Bda P, Kgatlwane J, Tiroyakgosi C, et al. Antibiotic utilization studies using point prevalence survey in Botswana. Muria 3. 2017;8. Available at URL: http://muria.mandela.ac.za/muria/media/Store/documents/Abstract%20book%20-%20MURAI%203/MURIA3-AbstractBook-July-2017.pdf
- Motlapele D. 2017. Statistics botswana. Health statistics stats brief 2007–2015. [cited 2019 Mar 12]. Available at URL: http://www.statsbots.org.bw/sites/default/files/publications/Health%20Statistics%20Stats%20Brief%202007_2015.pdf
- WHO. WHO Collaborating Centre for Drug Statistics Methodology. ATC/DDD Index. [cited 2019 Mar 11]. Available at URL: https://www.whocc.no/
- ECDC. 2011–2012. Point prevalence survey of healthcare-associated infections and antimicrobial use in European acute care hospitals. [cited 2019 Mar 12]. Available at URL: https://ecdc.europa.eu/sites/portal/files/media/en/publications/Publications/healthcare-associated-infections-antimicrobial-use-PPS.pdf.
- WHO. 2017. WHO collaborating centre for drug statistics methodology. Guidelines for ATC Classification and DDD Assignment. Available at URL: https://www.whocc.no/filearchive/publications/2017_guidelines_web.pdf
- Saleem Z, Hassali MA, Godman B, et al. A multicenter point prevalence survey of health care-associated infections in Pakistan: findings and implications. Am J Infect Control. 2019; 47(4):421-424.
- Bratzler DW, Houck PM. Antimicrobial prophylaxis for surgery: an advisory statement from the national surgical infection prevention project. Clinl Infect Dis. 2004;38:1706-1715.
- de Jonge SW, Gans SL, Atema JJ, et al. Timing of preoperative antibiotic prophylaxis in 54,552 patients and the risk of surgical site infection: A systematic review and meta-analysis. Medicine (Baltimore). 2017;96(29):e6903.
- Ban KA, Minei JP, Laronga C, et al. Executive summary of the American College of Surgeons/surgical infection society surgical site infection guidelines-2016 update. Surg Infect (Larchmt). 2017;18(4):379–382.
- Opanga SA, Mwang’ombe NJ, Okalebo FA, et al. Determinants of the Effectiveness of Antimicrobial Prophylaxis among Neurotrauma Patients at a Referral Hospital in Kenya: findings and Implications. Infect Dis Preve Med. 2017;5(3):1-7.