ABSTRACT
Background: Multidrug-resistant tuberculosis (MDR-TB) has a socioeconomic impact and threatens global public health. We assessed treatment outcomes of MDR-TB and predictors of poor treatment outcomes in Sudan given current high prevalence rates.
Methods: Combined retrospective and prospective cohort study at Abu-Anga hospital (TB specialized hospital in Sudan). All patients with MDR-TB between 2013 and 2017 were targeted.
Results: A total of 156 patients were recruited as having good records, 117 (75%) were male, and 152 (97.4%) had pulmonary TB. Patients were followed for a median of 18 months and a total of 2108 person-months. The overall success rate was 63.5% and the mortality rate was 14.1%. Rural residency (P < 0.05) and relapsing on previous treatments (P < 0.05) were determinants of time to poor MDR-TB treatment outcomes.
Conclusion: Overall, more attention needs to be given to special MDR-TB groups that are highly susceptible to poor outcomes, i.e. rural patients. As a result, it is highly recommended to maintain total coverage of medicines for all MDR-TB patients for the entire period of treatment in Sudan. It is also recommended to instigate more treatment centers in rural areas in Sudan together with programs to enhance adherence to treatments including patient counseling to improve future outcomes.
1. Introduction
The World Health Organization (WHO) believes over 10 million people globally fell ill with tuberculosis (TB) in 2017 and 2018, although the number actually reported is only 7 million [Citation1,Citation2]. Drug-resistant TB continues to be a global public health concern with approximately 580,000 cases worldwide and mortality worse than most cancers [Citation1,Citation3–Citation6]. Overall, TB is the leading cause of death among patients with infectious diseases [Citation7,Citation8]. TB is also costly to treat [Citation4,Citation6,Citation9]. In 2015, approximately 480,000 multidrug-resistant tuberculosis (MDR-TB) new cases were notified with 100,000 incidents registered as rifampicin resistant (RR) world-wide, with 250,000 deaths due to MDR/RR-TB [Citation6]. In 2018, there were approximately 500,000 new cases of rifampicin-resistant TB of which 78% were MDR-TB [Citation1]. Previously, the WHO believed that only approximately 25–30% of MDR-TB cases were detected and only approximately 25% of patients accessed second-line medications globally [Citation10]. More recently, progress has been made in testing, detecting and treating MDR/RR-TB resulting in 51% of patients with bacteriologically confirmed TB tested for rifampicin resistance [Citation1]. Despite this progress though, the number of patients actually treated in 2017 and 2018 was only one in three (32%) of approximately 500,000 patients who developed MDR/RR-TB [Citation1,Citation2]. Furthermore, only approximately 50% of those who had received treatment were declared successfully treated [Citation10]. This has risen to 56% with more recent data [Citation1]. In 2017 in Sudan, it was estimated there were 600 MDR/RR-TB among notified pulmonary TB patients. Moreover, it was estimated that 3.5% of new TB cases and 18% of previously treated cases are MDR/RR-TB cases [Citation11].
TB-drug resistance generally occurs due to prescribing malpractice and poor adherence to anti-TB medications, with the spread of resistance enhanced by HIV co-infection [Citation3,Citation12,Citation13]. The consequences of primary infection can also result in drug resistance. Consequently, intensive interventions are typically needed to address this. MDR-TB is characterized by the high cost of treatment, longer duration of therapy, low efficacy compared to susceptible medications, and greater side-effects of treatment [Citation14]. MDR-TB can be prevented bearing in mind that an appreciable number of controlled trials have shown that a 6-month regimen of rifampicin, pyrazinamide, isoniazid, and streptomycin or ethambutol is capable of combating TB with more than 95% of cases reported cured [Citation15].
Overall, the treatment of MDR-TB takes a long time when compared with susceptible TB, and demands administration of at least four second-line anti-TB drugs (SLDs), including parenteral medicines plus pyrazinamide in the intensive phase [Citation16]. However, in view of the costs involved and concerns with adherence, the management of MDR-TB needs both financial and human resources [Citation2,Citation17]. More recently though, the WHO has advocated oral-only treatment regimens to improve adherence rates along with continued patient centered support programs [Citation18]. Fluoroquinolones, bedaquiline, and linezolid are also strongly recommended for use in longer regimens, with tailored treatments including shorter regimens also recommended in some patients to help improve adherence and reduce costs [Citation8,Citation18].
Treatment success of MDR-TB relies on the conversion of the sputum smear of the acid-fast bacilli. The status of mycobacterial cultures is needed for follow-up of treatment in limited resource areas as the findings are considered a robust interim measure for effective treatment [Citation19]. Overall, sputum culture conversion plays a crucial role in the treatment success of MDR-TB [Citation20]. The inability of sputum conversion to negative by the end of the intensive phase of treatment tends to yield poor treatment outcomes, namely failure and death [Citation21,Citation22].
The prescribing of SLDs began for MDR-TB patients in Sudan in 2008, where the program was adopted for presumptive diagnosis and empirical treatment of such cases. The Green Light Committee (GLC) Initiative Document was signed in 2010 to control MDR-TB by affording access to high-quality SLDs [Citation23]. In Sudan, the treatment of MDR-TB patients is provided at Abu-Anga hospital, Khartoum, for at least 18 months. Medicines including ciprofloxacin, ofloxacin, cycloserine, ethionamide, and amikacin are available free of charge to patients to assist with effective treatment [Citation24]. This compares with high levels of patient co-payment for medicines that is typically the case in developing countries [Citation25].
Improving MDR-TB treatment outcomes is one of the five priority actions suggested by the World Health Organization (WHO) to address the global threat of MDR-TB [Citation2], with a goal of a 75% success rate by the end of 2015 [Citation7,Citation17]. By 2030, the goal is a 90% reduction in the absolute number of deaths due to TB versus 2015 levels achieved for instance by improved identification and management of MDR/RR-TB cases helped by new treatment guidelines from the WHO [Citation2,Citation18]. This includes the provision of only orally administered medicines and more tailored treatments to improve compliance [Citation8,Citation18]. In the literature, there are several factors that affect treatment outcomes. For example, early culture conversion by the end of the first 2 months is associated with better MDR-TB treatment outcomes and vice versa [Citation26]. Moreover, a recent study from China reported that MDR-TB patients who drink, smoke, have ofloxacin resistance, or a high smear grade, were significantly more prone to poor treatment outcomes [Citation27]. Furthermore, it has been reported that male gender, urban residency, aged between 35 and 44 years, and persistence of culture positivity at 2 months were predictors of poor MDR-TB treatment outcomes in Ethiopia [Citation28]. Additionally, extensive drug-resistant TB (XDR), male gender, and a positive smear at the beginning of treatment predicted poor treatment outcomes among Korean patients [Citation29].
However, to date, there have only been a few studies on the outcomes of treatment of patients with MDR-TB in limited resource settings with high prevalence rates such as Sudan [Citation30–Citation33]. We are aware that there have been recent studies researching the incidence of TB as well as success rates for smear-positive TB between different parts of Sudan [Citation34], reasons why TB patients default on their treatment including rural areas, adverse effects of treatment and previous history of TB [Citation35], and that treatment outcomes in Sudan appear to be lagging behind current WHO targets [Citation33]. However, we believe to date that treatment outcomes of MDR-TB, as well as possible factors related with poor treatment outcomes of MDR-TB, have not been reported in Sudan. This study aimed to address this deficit to provide future guidance in this high priority area in Sudan.
2. Method
2.1. Study setting
A hospital-based study was conducted at Abu-Anga hospital, which is the specialized hospital in Sudan to which suspected TB cases are referred to as well as providing health-care services to the population of Khartoum and neighboring states.
Abu-Anga hospital is also the main MDR-TB reference hospital where all recording and reporting processes are gathered and analyzed in Sudan. The hospital in collaboration with the medical colleges in Sudan and the Ministry of Health also facilitates training in the management of patients with TB and provides access to data for research purposes.
2.2. Study design
A combined retrospective and prospective cohort study design was employed. All MDR-TB patients notified between January 2013 and September 2017 attending the hospital were consecutively targeted. Cohorts of 2013, 2014, 2015, 2016, and 2017 were followed up until the end of the treatment period. The cases from 2013, 2014 and 2015 were reviewed retrospectively. Some cases from 2016 and the cases from 2017 were followed up prospectively until the final outcomes were reported.
Consequently, data collection was started in August 2017 and ended in April 2019. This study design was adopted as the MDR-TB population is a relatively small population and needs a long period for follow-up (i.e. 18 months). Patients were enrolled in the study if they had bacteriologically proven resistance to rifampicin and isoniazid or had clinically evident MDR-TB based on a history of treatment failure or MDR-TB contact defined according to WHO guidance [Citation36,Citation37]. (). The success rate was defined as the sum of cured and completed patients*100/total cases.
Table 1. Definition of TB types, resistance, and final treatment outcomes according to WHO
2.3. Molecular screening, treatment regimen, and monitoring
In 2012, the Sudan national tuberculosis control program (NTP) started a molecular screening for TB patients by using Hain MTBDRplus for RIF/INH resistance and MTBDRsl VER 1.0 for fluoroquinolones and injectable second-line anti-TB drugs to screen XDR-TB. In 2014, GeneXpert was launched while MTBDRsl version 2 was brought in during 2017. GeneXpert simultaneously provides rapid detection of TB and resistance to RIF in less than 2 h. In terms of molecular screening, previously the national MDR-TB diagnostic algorithm divided TB presumptive cases into two groups. The first group was the high-risk group, and patients must be screened by GeneXpert. This group includes retreatment TB cases, MDR contacts, HIV-positive patients, health-care workers, and seriously ill patients. The second group includes the new cases of TB for which screening has not been routinely undertaken. However, currently, all TB presumptive cases are screened by GeneXpert including new cases of TB, extra-pulmonary and childhood TB. Sputum smear microscopy was dedicated to the monitoring of treatment of first-line anti-TB drugs (FLDs). Currently, both conventional and molecular DST are used for both FLDs and SLDs as per the NTP guidance. In practice, molecular screening and DST are not routinely performed for SLDs unless XDR-TB is suspected based on the clinical and microbiological findings. As per the Sudan National TB Management Guideline, the use of sputum smear microscopy and culture to monitor response to treatment are both recommended for the monitoring of patients with MDR-TB [Citation38].
The treatment regimen is selected based on the recommendations of the NTP, which is based on previous WHO guidelines [Citation38,Citation39]. All confirmed MDR-TB cases received an 18-month standardized regimen in two phases: an 8-month intensive and 10-month continuation phase. The medications encompassed a combination of first and second-line anti-TB medicines including kanamycin (Km), levofloxacin (Lev), cycloserine (Cs), ethionamide (Eth), and pyrazinamide (Z). All these medicines are given in the intensive phase, while aminoglycoside is withdrawn during the continuation phase [Citation39]. The enrolled MDR-TB patients are treated under two models of treatment including hospital-based and community-based; both are directly observed treatment (DOT) regimens.
The direct observation for treatment and monitoring of SLDs adverse effects is facilitated by a treatment supporters’ network among community-based enrolled MDR-TB patients which provides MDR-TB nurses and specialists with weekly reports and a monthly evaluation regarding second-line anti-TB drug safety. The patients are routinely monitored for adverse effects especially for the most common adverse effects associated with SLDs including gastrointestinal adverse effects, e.g. nausea, vomiting, etc., psychosis, neurotoxicity, nephrotoxicity, thyroid dysfunction, or gouty arthritis. To reduce the incidence of these adverse effects, doses are escalated for SLDs in a period of 4 weeks. Subsequently, the full doses are gradually built to avoid toxicity and increase patient tolerance. If toxicity is reported by a treatment supporter, it is evaluated by the medical panel. Either the concerned medicine is discontinued till symptoms disappear and subsequently escalated. Alternatively, the medicines are replaced by a backup medicine para-aminosalicylic acid (PAS). The backup regimen is composed of PAS with omitting the incriminating medicines, either Cs or Eth (they are the most likely SLDs associated with adverse effects). In the case of Z, the patient with elevated uric acid is treated from gouty arthritis without omitting it.
2.4. Treatment outcomes
Treatment outcomes were assigned based on the definition of WHO as cured, treatment completed, treatment failed, died, and lost to follow-up (). Treatment success refers to the proportion of patients who were taking their full treatment course for the entire period of treatment and declared cured or completed, whereas poor treatment outcomes were defined as the proportion of death, treatment failure, or treatment default out of the total enrolled patients.
The outcome of interest for the survival analysis was poor treatment outcomes, which included death, treatment failure, or treatment default whichever occurred first. Consequently, patients were assigned censored if declared as a cure, treatment complete, or transferred out. In cases of censoring, we considered the survival time of this category starting from the start of treatment (T0) till the date of being transferred out (T1), and the date of announcing a cure or treatment complete (T2). On the other hand, time to event survival time was computed from T0 up to the date the patient developed the outcome of interest (i.e. the poor treatment outcome) (Ti) [Citation40].
2.5. Sample size
All patients registered in the hospital between 2013 and 2017 of both genders and with different ages were targeted. A total of 200 MDR-TB patients were registered during this period; however, only those with complete records were enrolled in the study (n = 156 patients; 78%). There was no sampling of patients because the total number of patients was limited and thus all patients were recruited.
2.6. Statistical analysis
Data were processed by IBM-SPSS version 24. Statistical tests such as unpaired t-test, chi-square, and Fischer exact test were run to summarize continuous and categorical variables of the sociodemographic and clinical factors. Moreover, the Kaplan–Meier curve was adopted to specify the cumulative survival probability. The log-rank test was processed to assess the influence of different covariates on the survival time of patients.
Cases with event outcome (poor treatment outcomes) were coded as 1, whereas censored cases were coded as 0 (reference category).
A bivariate Cox proportional hazard was first processed, and the significant variables were fitted in the final multivariable Cox proportional hazard model. The ninety-five percent confidence interval (95% CI), crude and adjusted hazard ratios (HR) were computed to estimate the predictors of time to poor treatment outcomes. The transferred out category was excluded from the bivariate and multivariate Cox proportional hazard model. Ultimately, the predictors were identified and considered significant at a P-value less than 0.05.
2.7. Ethical approval
Ethical approval number fmoh/nhrc/rd/ec was granted by the research directorate, Federal Ministry of Health, Sudan (FMOH) dated 29/07/2017. There was no patient consent form with this FMOH approval as the source of information was the TB patient card and TB registry book, with no direct contact with patients.
3. Results
3.1. Socio-demographic characteristics
As mentioned, 156 cases of MDR-TB (78%) were included in the analysis. The average age of enrolled patients was 35 ± 14 years, ranging from 15 to 90. Three-quarters of the patients (n = 117, 75%) were male and just over one third (37.5%) lived in rural locations (). No statistically significant (P > 0.05) differences in the socio-demographic characteristics were observed among the study variables except for residency ().
Table 2. Sociodemographics of MDR-TB patients grouped by treatment outcome.
3.2. Clinical characteristics
Most of the study sample (n = 152; 97.4%) were smear-positive pulmonary TB (PTB) whereas there were only four extrapulmonary TB (ETB) cases (2.6%). The number of primary and secondary resistant TB was 22 (14.1%) and 134 (85.9%), respectively. The mortality rate among PTB versus ETB patients was 13.8% versus 25%, respectively. The mean hemoglobin concentration of the event category was 15.4 mg/dl ± 1.3, whereas among the censored group this was found to be 14.0 ± 2.1.
The mean level of serum creatinine was assessed to be almost the same in both event and censored categories (0.7 mg/dl). All HIV/AIDS cases (n = 3) included in the study were in the censored group and all were on antiretroviral therapy (ART) (). Ultimately, there were no statistically significant differences between the two groups in terms of clinical factors except for previous treatment outcome (P < 0.05) and previous exposure to SLDs (P = 0.04) ().
Table 3. Clinical characteristics of MDR-TB patients grouped by treatment outcome
3.3. Treatment outcomes
The treatment outcomes were broken down into successful treatment (cure and treatment complete), poor outcomes (died, treatment failure or defaulters) and the transferred out-group – . Of the 156 MDR-TB patients, 26 (16.7%) were cured, 73 (44.2%) completed the treatment, 22 (14.1%) died, 30 (19.2%) defaulted on treatment, three (1.9%) were treatment failures and two (1.3%) were transferred out ().
Table 4. Treatment outcomes of patients with multidrug-resistant tuberculosis grouped by residency status and previous treatment outcome.
Consequently, the number of patients with successful treatment outcomes was 99, giving an overall success rate of 63.5%. Poor treatment outcomes were observed to be significantly associated with rural residency as compared with those living in urban facilities (P < 0.05) ().
3.4. Survival time and treatment outcomes during the follow-up period
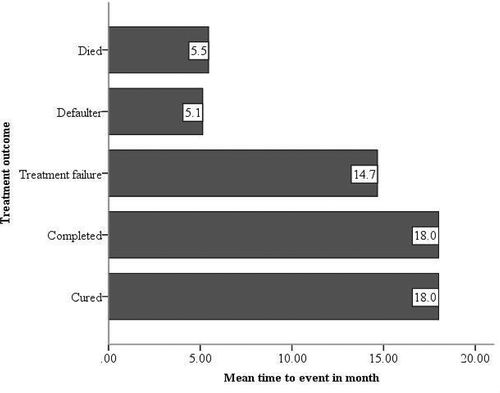
The mean survival time for the different categories (i.e. treatment outcomes) during the study period was noted to be significantly shorter among dead and defaulted cases (5 months), whereas relatively longer among treatment failures (15 months) (P < 0.05) ().
3.5. The pattern of success rate over the years
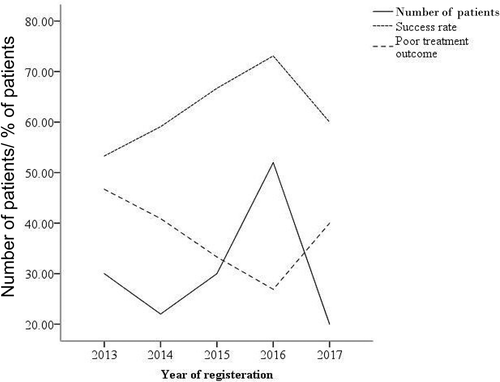
The pattern of the success rate was seen to be increasing between 2013 and 2016; however, decreasing after that. Overall, the rate of success rates was 53.3%, 59.1%, 66.7%, and 73.1% in 2013, 2014, 2015, and 2016, respectively, before decreasing to 60% in 2017 ().
3.6. The probability survival of MDR-TB patients
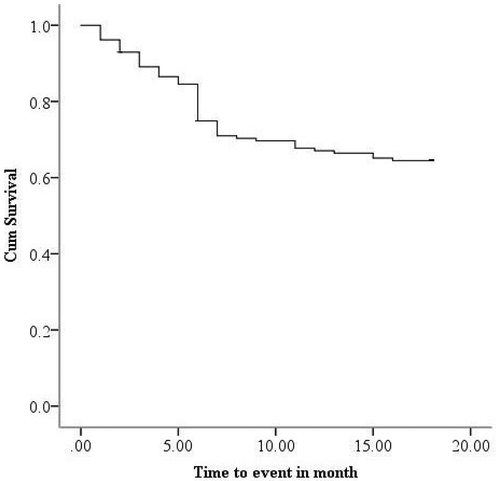
All the study participants were followed for a median of 18 months [Inter quartile range (IQR): 6 to 18 months] and a total of 2108 person-months. A total of 55 poor treatment events were reported during the study period. These included 22 deaths, 30 defaulters, and three failures that yield 26 poor outcomes per 1000 person-months ().
Figure 3. Kaplan–Meier curve showing the probability survival of MDR-TB patients since the commencement of treatment to end of the study period.
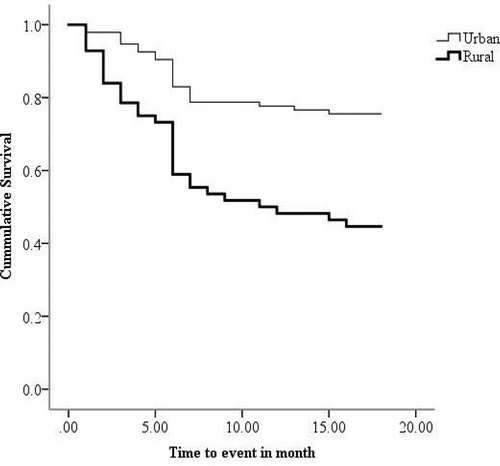
MDR-TB patients living in rural areas had significantly shorter survival times compared with those living in urban facilities (11 and 15 months, P < 0.05, respectively). The cumulative probability of survival at the end of the study period among rural residents and urban was noted to be 45% and 76%, respectively ().
3.7. Predictors of poor MDR-TB treatment outcomes
On bivariate analysis, rural residency and being a relapse patient from previous treatments were significantly associated with poor MDR-TB treatment outcomes ().
Table 5. Bivariate–Cox regression analysis of determinants for time to poor treatment outcome among 154 patients.
Furthermore, multivariate analysis showed that the same factors, i.e. being rural and relapses from previous treatments, were significantly associated with a poor outcome from treating MDR-TB. Rural residents had more than twice the risk of a poor treatment outcome (AHR = 2.5, 95 CI: 1.4–4.58). Similarly, a relapsed patient from the previous treatments was five times more likely to have a poor treatment outcome (AHR = 4.9, 95% CI: 1.8–12.9) ().
Table 6. Multivariate Cox regression analysis of determinants for time to poor treatment outcome among 154 patients.
4. Discussion
Our study aimed to assess the treatment outcomes of MDR-TB patients at the leading TB hospital in Sudan and to assess the determinants of poor treatment outcomes. Identifying these determinants in a high prevalence country such as Sudan should help improve the overall performance of the TB program. As a result, reducing future morbidity and mortality associated with MDR-TB. This could be achieved by the instigation of pertinent interventions and strategies that will help to successfully improve treatment outcomes in the future building on the recent changes in suggested regimens by the WHO [Citation18].
By 2015, the Stop TB Partnership Global Plan stated that one million MDR-TB patients worldwide were to be targeted with respect to detection and treatment coverage. Moreover, success rates of at least 75% need to be achieved as the global target [Citation17,Citation41]. Our study shows that the overall success rate of 63.5% was lower than the 2015 global target [Citation17]. Having said this, the treatment success rate of MDR-TB patients in our study was comparable to other low-resource countries such as Egypt (69.3%) [Citation42] and Pakistan (71.6%) [Citation43], although lower than high-income countries such as the United States (78%) [Citation44], and Switzerland (76%) [Citation45]. There was an improvement in the pattern of success rates from 2013 up to 2016; however, unfortunately, this declined to only 60% in 2017 (). This decline could be due to recent political and economic issues in Sudan that severely affected the healthcare system [Citation46]. However, further research is needed before we can say this with certainty. In view of this decline in this high priority disease area, we believe there is an urgent need for the health authorities in Sudan to take the initiative and maximize efforts and resources to achieve the global target of a 75% success rate for MDR-TB, and we will be making a number of suggestions under recommendations.
Our findings that patients living in rural areas had poorer treatment outcomes are similar to the findings of Ali and Prins [Citation35]. Poorer outcomes could potentially be attributed to the distance to the treatment center and the cost of transportation despite the medicines being provided free of charge. We have seen this phenomenon in studies assessing factors impacting on adherence to antihypertensive medicines [Citation47,Citation48]. However, few studies to date have described the association between residency and poor treatment outcomes among patients with MDR-TB. Similar to our findings, a case–control study undertaken in Khartoum state investigating the factors associated with treatment interruption among TB patients also reported that rural residency was significantly associated with treatment default in Sudanese patients [Citation49]. In this study, patients recorded as relapses were five times as likely to have poor treatment outcomes. Ahmed et al. also concluded that a poor previous treatment outcome was also significantly correlated with treatment default among Sudanese patients [Citation49]. Chen et al. also found that 65% of MDR-TB cases had poor treatment outcomes including relapses before becoming MDR-TB. Moreover, unlike new cases, chest radiograph findings of re-treated cases revealed significantly more cavitation when compared to new cases [Citation50]. In contrast, our regression model did not predict any association between most of the sociodemographic factors and the time to poor MDR-TB treatment outcomes (). These findings though are consistent with a number of other studies that confirmed no association for instance with gender or age and poor MDR-TB treatment outcomes [Citation51–Citation53].
On the other hand, anemia was not found to impact on poor treatment outcomes in our study. This is unlike Alene et al. who found that anemic patients were more than twice as likely to have poor treatment outcomes from their MDR-TB [Citation40]. The positive association between anemia and death among MDR-TB patients has also been reported in a number of other published studies [Citation54–Citation56]. This association could be attributed to delayed presentation [Citation40]. Despite this, the National TB program (NTP) in Sudan has recently introduced investigations including complete blood count (CBC) as a routine before treatment to help improve the outcomes in these patients, and we will be investigating this further in future studies.
Previous exposure to SLDs including fluoroquinolones and aminoglycosides was not associated with poor treatment outcomes in our study. However, the findings of a prospective cohort study undertaken in eight countries suggested that the rate of resistance to SLDs and the precipitation of extensive drug resistance-TB (XDR-TB) were seen among those patients who were previously prescribed SLDs [Citation57]. Consequently, increased prescribing of SLD such as amikacin and ofloxacin for infectious diseases other than TB might increase the incidence of XDR-TB. As a result, we will be researching potential ways to reduce their prescribing in future studies in Sudan.
We know that the rate of treatment failure and death of patients with MDR-TB are associated with clinical factors as well as sociodemographic factors, which include unemployment, imprisonment, and alcoholism [Citation58]. Our findings suggest that 19 out of 30 defaulters were rural residents (). Moreover, 65.5% of poor MDR-TB outcome events were seen in unemployed patients (). Consequently, we will be looking at a number of different measures to improve patient adherence in the future including home visits, disability stipends, monthly incentives, and assistance with transportation costs. In addition, looking at potential changes in treatment regimens following changes in WHO recommendations [Citation18]. This will be the subject of future research projects. We will also be investigating issues of education as this can impact on issues of adherence and treatment ourtcomes in these patients [Citation59,Citation60].
The current study included only three patients with HIV/AIDS (). However, we are aware that HIV/AIDS contributes to poor treatment outcomes [Citation3,Citation13,Citation54], and that patients taking ART have a lower risk of dying [Citation5]. Girum et al. found that HIV/AIDS positive patients with MDR-TB were three times more liable to die compared with seronegative patients [Citation61]. This is most probably due to severe interactions between SLDs and ART, and the accompanying adverse effects which in turn might affect patient’s adherence to treatment. Moreover, independent predictors of failure and death such as age, HIV/AIDS, comorbidities, and persistent positive cultures by the third month of treatment should draw the attention of clinicians to examine the potential for additional interventions. Older age, HIV/AIDS, and the frequency of XDR-TB increase the death rate among TB patients [Citation62]. SLDs are more toxic when compared with first-line anti-TB treatments, and this might also affect patient adherence [Citation63]. The adverse effects comprise gastrointestinal (GI) disturbances, psychosis, peripheral neuropsychiatric disorders, and hearing disturbance [Citation54]. Consequently, adverse effects need to be considered by clinicians when prescribing medicines for patients with MDR-TB, and necessary interventions should also be considered to improve adherence rates. Interventions could include greater education of patients regarding the different regimens and the need to complete the course of treatment, particularly among those patients with more limited education.
We are aware that there are several limitations to this study. Firstly, the information in some patient cards was often incomplete. In addition, information was only collected once during the study period. We also did not consider other sociodemographic and clinical factors impacting on outcomes including smoking, alcohol, asthma, heart diseases, and drug addiction as the primary source of data was the patient cards. In addition, the immunovirological status of HIV-patients was not considered in this study because the test data were not recorded in TB registries. The study scope focused on the treatment outcomes of MDR-TB patients and the factors associated with poor treatment outcomes, and provided useful data for future guidance of these patients in Sudan given the limited data to date in this important area. However, other aspects such as the adverse reactions of drugs used to treat patients with MDR-TB was out of the scope of this study. Future studies will though be undertaken to explore this aspect further. Despite these limitations, we believe our findings are robust in view of the fact that this is the leading hospital treating MDR-TB patients in Sudan and we included all the patients during the period of 2013–2017.
5. Conclusion and recommendations
In conclusion, we believe based on our findings that the current situation of TB in general and MDR-TB, in particular, in Sudan is a concern especially with a decline in the success rate of MDR-TB in recent years. We also ascertained that patients living in rural areas and relapse patients from previous treatments were associated with poorer outcomes.
In view of our findings, we recommend more care to be given to MDR-TB patient groups that are highly susceptible to death and default in Sudan, which should include greater educational input especially in those patients with limited education. Moreover, it is highly recommended that the authorities in Sudan continue to maintain total coverage of the drug supply for all MDR-TB patients for the entire period of their treatment. The authorities should also look to instigate more treatment centers in rural areas alongside programs to enhance adherence to prescribed treatments including patient counseling. We will be exploring these options in the Ministry of Health in the coming months alongside their other priority areas. Moreover, in light of these findings and as Sudan is lacking behind the global target, further studies are recommended to tackle other variable aspects and barriers to successful MDR-TB treatment outcomes in Sudan. We will also be pursuing these as well with relevant personnel and authorities in Sudan and reporting on developments and their impact in the future.
Article highlights
Multidrug-resistant tuberculosis (MDR-TB) has an appreciable socioeconomic impact and threatens global public health. The current study aimed to assess treatment outcomes of MDR-TB and predictors of poor treatment outcomes in Sudan.
All MDR-TB patients with complete records admitted between 2013 and 2017 to a leading TB hospital in the capital of Sudan, Khartoum, were included in the study. Overall, 156 patients were included.
Treatment success for patients with MDR-TB (defined by WHO criteria) was 63.5%, behind the global target of 75%
Rural residency and relapsing on previous treatments were predictors of poor outcomes of MDR-TB treatment in this study.
More effort is needed to tackle this disease in Sudan. This should include instigating more treatment centers in rural areas alongside programs to enhance adherence including greater patient counseling especially for patients who have difficulties in reading.
Declaration of interest
Hamdan Mustafa Hamdan Ali works for the National Tuberculosis Control Program, Federal Ministry of Health, Sudan.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
The authors acknowledge the staff of Abu-Anga Chest Hospital, Khartoum state, Sudan for the collaboration and facilitating this work. Special thanks also go to the data collectors for their help.
Additional information
Funding
References
- WHO. Global tuberculosis report executive summary 2019. [cited 2019 Oct 28]. Available from: URL:https://www.who.int/tb/publications/global_report/tb19_Exec_Sum_15October2019.pdf?ua=1.
- WHO. Global tuberculosis report (full). 2019. [cited 2019 Oct 28]. Available from: URL:https://apps.who.int/iris/bitstream/handle/10665/329368/9789241565714-eng.pdf?ua=1
- Seaworth BJ, Griffith DE. Therapy of multidrug-resistant and extensively drug-resistant tuberculosis. Microbiol Spectr. 2017;5:2.
- Dheda K, Chang KC, Guglielmetti L, et al. Clinical management of adults and children with multidrug-resistant and extensively drug-resistant tuberculosis. Clin Microbiol Infect. 2017;23(3):131–140.
- Kapata N, Grobusch MP, Chongwe G, et al. Outcomes of multidrug-resistant tuberculosis in Zambia: a cohort analysis. Infection. 2017;45(6):831–839.
- WHO. GLOBAL TUBERCULOSIS REPORT. 2016. [cited 2019 Jun 1]. Available from URL:http://apps.who.int/iris/bitstream/10665/250441/1/9789241565394-eng.pdf?ua=1
- WHO. Global Tuberculosis Report. 2018. [cited 2019 Jun 2]. Available from: http://apps.who.int/iris/bitstream/handle/10665/274453/9789241565646-eng.pdf?ua=1
- Riccardi N, Alagna R, Saderi L, et al. Towards tailored regimens in the treatment of drug-resistant tuberculosis: a retrospective study in two Italian reference Centres. BMC Infect Dis. 2019;19(1):564.
- Pooran A, Pieterson E, Davids M, et al. What is the cost of diagnosis and management of drug resistant tuberculosis in South Africa? PloS One. 2013;8(1):e54587.
- WHO. Drug-Resistant TB Surveillance & Response. Supplement to Global Tuberculosis Report 2014. Available at URL:http://apps.who.int/medicinedocs/en/m/abstract/Js21635en/
- WHO. MULTIDRUG-RESISTANT TUBERCULOSIS (MDR-TB). 2018. [cited 2019 Oct 28]. Available from: URL:https://www.who.int/tb/areas-of-work/drug-resistant-tb/MDR-RR_TB_factsheet_2018_Apr2019.pdf?ua=1
- Dean AS, Cox H, Zignol M. Epidemiology of Drug-Resistant Tuberculosis. Adv Exp Med Biol. 2017;1019:209–220.
- Wilson JW, Tsukayama DT. Extensively drug-resistant tuberculosis: principles of resistance, diagnosis, and management. Mayo Clin Proc. 2016;91(4):482–495.
- Ahuja SD, Ashkin D, Avendano M, et al. Multidrug resistant pulmonary tuberculosis treatment regimens and patient outcomes: an individual patient data meta-analysis of 9,153 patients. PLoS Med. 2012;9(8):e1001300.
- Ormerod LP. Multidrug-resistant tuberculosis (MDR-TB): epidemiology, prevention and treatment. Br Med Bull. 2005;73–74(1):17–24.
- World Health Organization. Guidelines for the programmatic management of drug-resistant tuberculosis 2011. [cited 2019 Jun 2]. Available from: URL:https://apps.who.int/iris/bitstream/handle/10665/44597/9789241501583_eng.pdf;jsessionid=A09557FE3108BD00C5B0C8C604DE4127?sequence=1
- Stop TB partnership. The global plan to stop TB 2011–2015. [cited 2019 Oct 29]. Available from: URL:http://www.stoptb.org/assets/documents/global/plan/TB_GlobalPlanToStopTB2011-2015.pdf
- WHO. WHO updates its treatment guidelines for multidrug- and rifampicin-resistant tuberculosis. 2018. [cited 2019 Oct 29]. Available from: URL:https://www.who.int/tb/features_archive/updated-treatment-guidelines-multigrug-rifampicin-resistant-TB/en/
- World Health Organization. Guidelines for establishing DOTS-Plus pilot projects for the management of multidrug-resistant tuberculosis (MDR-TB). 2000. [cited 2019 May 30]. Available from: URL:https://apps.who.int/iris/bitstream/handle/10665/66368/WHO_CDS_TB_2000.279.pdf;jsessionid=883E3D89810A0FEC391F729F6062DC4F?sequence=1
- Nagaraja C, Shashibhushan BL, Asif M, et al. Pattern of drug-resistance and treatment outcome in multidrug-resistant pulmonary tuberculosis. Indian J Chest Dis Allied Sci. 2012;54(1):23–26.
- Kurbatova EV, Cegielski JP, Lienhardt C, et al. Sputum culture conversion as a prognostic marker for end-of-treatment outcome in patients with multidrug-resistant tuberculosis: a secondary analysis of data from two observational cohort studies. Lancet Respir Med. 2015;3(3):201–209.
- Kurbatova EV, Gammino VM, Bayona J, et al. Predictors of sputum culture conversion among patients treated for multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2012;16(10):1335–1343.
- Adam MAM, Ali HMH, Khalil EAG. Initial second-line drug resistance of Mycobacterium tuberculosis isolates from Sudanese retreatment-patients. J Clin Tuberculosis Other Mycobacterial Dis. 2017;9:21–23.
- Sharaf Eldin GS, Fadl-Elmula I, Ali MS, et al. Tuberculosis in Sudan: a study of Mycobacterium tuberculosis strain genotype and susceptibility to anti-tuberculosis drugs. BMC Infect Dis. 2011;11(1):219.
- Cameron A, Ewen M, Ross-Degnan D, et al. Medicine prices, availability, and affordability in 36 developing and middle-income countries: a secondary analysis. Lancet. 2009;373(9659):240–249.
- Basit A, Ahmad N, Khan AH, et al. Predictors of two months culture conversion in multidrug-resistant tuberculosis: findings from a retrospective cohort study. Plos One. 2014;9(4):e93206.
- Liu Q, Lu P, Martinez L, Yang H, Lu W, Ding X, et al. Factors affecting time to sputum culture conversion and treatment outcome of patients with multidrug-resistant tuberculosis in China. BMC Infect Dis. 2018;18(1):114.
- Melese A, Zeleke B. Factors associated with poor treatment outcome of tuberculosis in Debre Tabor, northwest Ethiopia. BMC Res Notes. 2018;11(1):25.
- Jeon DS, Shin DO, Park SK, et al. Treatment outcome and mortality among patients with multidrug-resistant tuberculosis in tuberculosis hospitals of the public sector. J Korean Med Sci. 2011;26(1):33–41.
- Meressa D, Hurtado RM, Andrews JR, et al. Achieving high treatment success for multidrug-resistant TB in Africa: initiation and scale-up of MDR TB care in Ethiopia–an observational cohort study. Thorax. 2015;70(12):1181–1188.
- Getachew T, Bayray A, Weldearegay B. Survival and predictors of mortality among patients under multi-drug resistant tuberculosis treatment in Ethiopia: St. Peter’s Specialized Tuberculosis Hospital, Ethiopia. Int J Pharm Sci Res. 2013;4(2):776–787.
- IAMAT. Sudan for specific travellers: tuberculosis. 2017. [cited 2019 May 28]. Available from: URL:https://www.iamat.org/country/sudan/risk/tuberculosis
- Elmadhoun WM, Noor SK, Bushara SO, et al. Epidemiology of tuberculosis and evaluation of treatment outcomes in the national tuberculosis control programme, River Nile state, Sudan, 2011–2013. East Mediterr Health J. 2016;22(2):95–102.
- Hassanain SA, Edwards JK, Venables E, et al. Conflict and tuberculosis in Sudan: a 10-year review of the national tuberculosis programme, 2004–2014. Confl Health. 2018;12:18.
- Ali AOA, Prins MH. Disease and treatment-related factors associated with tuberculosis treatment default in Khartoum State, Sudan: a case-control study. East Mediterr Health J. 2017;23(6):408–414.
- Lange C, Abubakar I, Alffenaar J-WC, et al. Management of patients with multidrug-resistant/extensively drug-resistant tuberculosis in Europe: a TBNET consensus statement. Eur Respir J. 2014;44(1):23–63.
- Geissler PW, Nokes K, Prince RJ, et al. Children and medicines: self-treatment of common illnesses among Luo schoolchildren in western Kenya. Soc Sci Med. 2000;50(12):1771–1783.
- Sudan national TB management guideline 2019. [cited 2019 Oct 28]. Available from: URL:https://www.humanitarianresponse.info/sites/www.humanitarianresponse.info/files/2019/07/Sudan-National-TB-management-Guideline-March.2019-1.pdf
- WHO Guidelines approved by the guidelines review committee. Companion Handbook to the WHO Guidelines for the Programmatic Management of Drug-Resistant Tuberculosis. Geneva: World Health Organization; 2014.
- Alene KA, Viney K, McBryde ES, et al. Treatment outcomes in patients with multidrug-resistant tuberculosis in north-west Ethiopia. Trop Med Int Health. 2017;22(3):351–362.
- Dhavan P, Dias HM, Creswell J, et al. An overview of tuberculosis and migration. Int J Tuberculosis Lung Dis. 2017;21(6):610–623.
- Gadallah MA, Mokhtar A, Rady M, et al. Prognostic factors of treatment among patients with multidrug-resistant tuberculosis in Egypt. J Formosan Med Assoc. 2016;115(11):997–1003.
- Khan I, Ahmad N, Khan S, et al. Evaluation of treatment outcomes and factors associated with unsuccessful outcomes in multidrug resistant tuberculosis patients in Baluchistan province of Pakistan. J Infect Public Health. 2019;12:809–815.
- Marks SM, Flood J, Seaworth B, et al. Treatment practices, outcomes, and costs of multidrug-resistant and extensively drug-resistant tuberculosis, United States, 2005–2007. Emerg Infect Dis. 2014;20(5):812–821.
- Peter Helblinga EA, Eggerb J-M, Zellweger, J-P. Treatment outcomes of multidrug-resistant tuberculosis in Switzerland. Swiss Med Wkly. 2014;144:w14053.
- Ebrahim EM, Ghebrehiwot L, Abdalgfar T, et al. Health care system in Sudan: review and analysis of strength, weakness, opportunity, and threats (SWOT Analysis). Sudan J Med Sci. 2017;12(3):133–150.
- Nashilongo MM, Singu B, Kalemeera F, et al. Assessing adherence to antihypertensive therapy in primary health care in Namibia: findings and implications. Cardiovasc Drugs Ther. 2017;31(5–6):565–578.
- Ambaw AD, Alemie GA, Yohannes SM, et al. Adherence to antihypertensive treatment and associated factors among patients on follow up at University of Gondar Hospital, Northwest Ethiopia. BMC Public Health. 2012;12:282.
- Ahmed OA, Ali MHP. Disease and treatment-related factors associated with tuberculosis treatment default in Khartoum State, Sudan: a case–control study. East Mediterr Health J. 2017;23(6):408–414.
- Chen M-Y, Lo Y-C, Chen W-C, et al. Recurrence after successful treatment of multidrug-resistant tuberculosis in Taiwan. PloS One. 2017;12(1):e0170980–e.
- Espinal MA, Laserson K, Camacho M, et al. Determinants of drug-resistant tuberculosis: analysis of 11 countries. Int J Tuberc Lung Dis. 2001;5(10):887–893.
- Mirsaeidi SM, Tabarsi P, Khoshnood K, et al. Treatment of multiple drug-resistant tuberculosis (MDR-TB) in Iran. Inter J Infect Dis. 2005;9(6):317–322.
- Sampurna Kakchapati BNG, Rakesh Kumar J. Chamnein choonpradub. Treatment outcome of multidrug-resistant mycobacterium tuberculosis in Nepal. Asia-Pac J Public Health. 2012;24(4):631–640.
- Charles M, Vilbrun SC, Koenig SP, et al. Treatment outcomes for patients with multidrug-resistant tuberculosis in post-earthquake Port-au-Prince, Haiti. Am J Trop Med Hyg. 2014;91(4):715–721.
- Isanaka S, Mugusi F, Urassa W, et al. Iron deficiency and anemia predict mortality in patients with tuberculosis. J Nutr. 2012;142(2):350–357.
- Kourbatova EV, Borodulin BE, Borodulina EA, et al. Risk factors for mortality among adult patients with newly diagnosed tuberculosis in Samara, Russia. Int J Tuberc Lung Dis. 2006;10(11):1224–1230.
- Dalton T, Cegielski P, Akksilp S, et al. Prevalence of and risk factors for resistance to second-line drugs in people with multidrug-resistant tuberculosis in eight countries: a prospective cohort study. Lancet. 2012;380(9851):1406–1417.
- Kurbatova EV, Taylor A, Gammino VM, et al. Predictors of poor outcomes among patients treated for multidrug-resistant tuberculosis at DOTS-plus projects. Tuberculosis.(2012);92:397–403.
- Patel SV, Nimavat KB, Alpesh PB, et al. Treatment outcome among cases of multidrug-resistant tuberculosis (MDR TB) in Western India: A prospective study. J Infect Public Health. 2016;9(4):478–484.
- MdS G, Penna ML, Perez-Porcuna TM, et al. Factors associated with tuberculosis treatment default in an endemic area of the Brazilian Amazon: A case control-study. Plos One. 2012;7(6):e39134.
- Girum T, Tariku Y, Survival Status DS. Treatment outcome of Multidrug Resistant Tuberculosis (MDR-TB) among patients treated in Treatment Initiation Centers (TIC) in South Ethiopia: A retrospective cohort study. Ann Med Health Sci Res. 2017;7(5):331–336.
- Waitt CJ, Squire SB. A systematic review of risk factors for death in adults during and after tuberculosis treatment. [Review article]. Int J Tuberc Lung Dis. 2011;15(7):871–885.
- Ramachandran G, Swaminathan S. Safety and tolerability profile of second-line anti-tuberculosis medications. Drug Saf. 2015;38(3):253–269.