ABSTRACT
Objectives
We evaluated the efficacy and safety of dalbavancin in ABSSSI and ‘other sites’ infections’ (OTA).
Methods
Observational study involving 11 Italian hospitals including patients that received ≥1 dose of dalbavancin in 2016–2019. The outcome was end-of-treatment efficacy and safety in ABSSSI and OTA in a real-life setting.
Results
206 patients enrolled (males 50%, median age 62 [IQR 50–76] years), 60.2% ABSSSI, 39.8% OTA. 69.7% ABSSSI vs 90.7% OTA (p = 0.003) and 46.3% ABSSSI vs 37.2% OTA (p = 0.786) received previous and concomitant antibiotics, respectively. 82.5% reached clinical cure . Eleven (5.4%) patients had non-serious adverse events (AE). OTA patients showed longer hospitalization (13.5 days, 5.5–22 vs 3, 0–11.7; p<0.0001) and received longer previous (18 days, 9–30 vs 11, 7–19; p = 0.007)/concomitant antibiotic treatments (21 days, 14–52 vs 11, 8–14; p < 0.0001), compared to ABSSSI. ABSSSI and OTA showed similar efficacy (85.5% vs 75%, p = 0.459) and safety (no AE: 81.5% vs 64.3%, p = 0.258); efficacy was independent of previous/concomitant therapies.
Conclusions
Dalbavancin demonstrated a success rate of >80%, with similar efficacy/safety in ABSSSI and off-label indications. The preferential use of dalbavancin as second-line or combination therapy would seem to suggest the need for in-depth studies focused on its off-label use.
1. Introduction
1.1. Dalbavancin for the treatment of acute bacterial skin and skin-structure infections and other ‘difficult-to-treat infections’
Dalbavancin is a lipoglycopeptide antibiotic with unique pharmacokinetic features. It demonstrated potent activity against several gram-positive bacteria, including methicillin-resistant Staphylococcus aureus (MRSA) [Citation1].
Dalbavancin is approved for the treatment of ABSSSI [Citation2], but it stands as an interesting option for ‘difficult-to-treat infections’ caused by susceptible gram-positive microorganisms due to its half-life of 14.4 days, high bone penetration [Citation3–6] and optimal safety [Citation4,Citation7–9].
Only one dose of dalbavancin is needed for ABSSSI [Citation10]. Off-label use in ‘difficult-to-treat infections’ is an option for outpatient parenteral antimicrobial therapy (OPAT) that could reduce the need for hospitalization [Citation11]. Studies about dalbavancin use in endocarditis and osteomyelitis [Citation12–17] have been recently published, showing contrasting results. Interestingly, retrospective data show a high success rate with ≥2 doses of dalbavancin in bone infections [Citation13], and a recent randomized clinical trial confirmed that two 1500 mg weekly doses were effective as standard care in osteomyelitis [Citation15]. Likewise, in a retrospective study, dalbavancin has proven high success rate as OPAT in 27 patients with endocarditis, even if only used after clearance of blood cultures and with another antimicrobial agent [Citation14]; conversely, the emergence of glycopeptide/lipoglycopeptide non-susceptible S. aureus strains has been reported in small case series with unfavorable outcome [Citation18–20].
1.2. Dalbavancin efficacy and safety in real-life settings
While dalbavancin efficacy and safety for ABSSSI have been demonstrated by clinical trials [Citation5,Citation6,Citation8,Citation10,Citation19,Citation20], we still miss data from real-life settings in Italy [Citation16,Citation21,Citation22], where the prevalence of MRSA is one of the five highest in Europe (33.9%) [Citation23] and an appropriate empirical therapeutic approach is crucial.
We hereby conducted an observational multicentric study aimed to evaluate the end-of-treatment efficacy and safety of dalbavancin in ABSSSI and other sites’ infections (OTA) in a real-life setting in Italy. We besides explored previous and concomitant antimicrobial treatments.
2. Methods
2.1. Study design and population
This is a retrospective observational study in 11 hospitals (8 Italian cities), approved by the local Ethic Committees. We included patients ≥18 years treated with ≥1 dalbavancin dose (01/05/2016-30/06/2019). Patients were excluded if clinical information was not available.
2.2. Study procedures
The following data were collected reviewing patients’ clinical records through an electronic case report form: demographics, comorbidities, Charlson comorbidity index (CCI), baseline and end-of-treatment (EOT) blood tests, type of infection (ABSSSI and OTA), community- or hospital-acquired infection and isolated pathogens, prior/concomitant antimicrobial therapies, reasons for dalbavancin use, dosages, length, clinical outcomes at EOT and during the follow-up, relapses, and adverse events (AEs).
Previous antibiotic treatments were antibiotics used before dalbavancin for the same infection; concomitant antibiotic treatments were antibiotics used in association with dalbavancin. Among previous antibiotics, we collected monotherapies (the prescription of a single antibiotic before dalbavancin), combination therapies (the administration of two or more antibiotics before dalbavancin), and mixed mono-combo treatments (monotherapies and combination regimens that were sequentially prescribed before dalbavancin).
Reasons for dalbavancin use were indicated in clinical records, were reported by physicians who prescribed dalbavancin, and were study investigators or were obtained after chart review.
Follow-up was 30–180 days after the last dose of dalbavancin. Dosages were decided upon clinical evaluations. Microbiological tests were performed in each hospital according to their own policy.
2.3. Site of infections
The population was divided into patients with ABSSSI and OTA to evaluate in-label and off-label use of dalbavancin. ABSSSI was defined as a skin bacterial infection with a lesion size area of ≥75 cm2 [Citation16]. OTA included osteomyelitis, prosthetic joint infections, endocarditis, septic arthritis, sepsis, central venous line (CVC)-related bloodstream infections (BSI), endovascular stent infections, relapsing staphylococcal furunculosis, cutaneous patch, or breast implant infections.
2.4. Primary and secondary outcome
The primary outcome was clinical cure as evaluated by physicians at EOT defined as improvement of lesions and resolution of signs and symptoms of infection [Citation16]. Failure was defined as persistent signs/symptoms, discontinuation for toxicity, or death. Relapse of signs/symptoms of infection within 7 days after EOT was also recorded. Dalbavancin efficacy was also recorded during the follow-up visit. Secondary outcomes were: i) safety and tolerability; ii) comparison between ABSSSI and OTA; iii) comparison between patients with or without previous/concomitant antibiotics. AEs were considered as indicated by the WHO (20), tolerability was defined as not discontinuation for toxicity and prevalence of AEs <20%.
2.5. Statistical analysis
Categorical variables were presented as absolute numbers, percentages, while continuous variables as median, interquartile range. The percentage of patients who reached clinical cure, as previously defined, at EOT (efficacy) and the proportion of subjects who experienced an AE (safety) were compared between ABSSSI and OTA by Pearson’s Chi-square test. Differences between ABSSSI and OTA and according to the use of previous/concomitant antibiotics were explored by Pearson’s Chi-square/Fisher’s exact test or Mann–Whitney test. Logistic regression analyses were used to investigate whether previous (model 1) and concomitant antibiotics (model 2) were associated with clinical cure, adjusting for age, comorbidities, and infection’s site. Statistical analyses were performed with SPSS software.
3. Results
We enrolled 206 patients treated with ≥1 dose of dalbavancin: 124 (60.2%) ABSSSI, 82 (39.8%) OTA.
3.1. Patients’ demographics and baseline characteristics
Characteristics of the study population are shown in (a). The median age was 62 (IQR 50–76) years. 140/206 (68%) patients presented ≥1 comorbidity. The most common comorbidity was cardiovascular disease (72, 35%), followed by diabetes (43, 29.9%) (supplementary Figure 1). Despite no difference in the prevalence of comorbidities, ABSSSI had lower median CCI (p = 0.002) and less frequently cirrhosis (p = 0.005). Furthermore, ABSSSI were characterized by higher White Blood Cell (WBC) count (p = 0.015) and C-reactive protein (CRP), compared to OTA (p = 0.009) ((a)).
Figure 1. Type of infections and microbiological isolations in study population
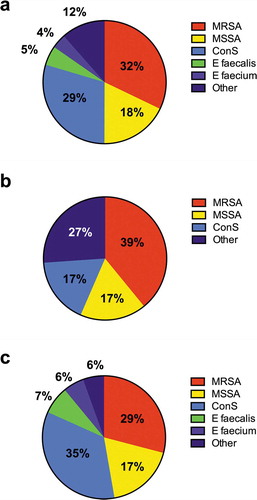
Table 1. Characteristics of study population according to on- and off-label use of dalbavancin
3.2. Infections and microorganisms
OTA included bone infections (29, 14.1% in detail: osteomyelitis 25/29, 86% – spondylodiscitis 4/29, 14%), prosthetic joint infections (17, 8.3%), endocarditis (6, 2.9%), septic arthritis (4, 1.9%), CVC-related BSI (4, 1.9%), sepsis (1, 0.5%), other infections (21, 10.2%).
Most infections were community-acquired with a higher proportion in ABSSSI (104/121, 85.9%) than in OTA (44/74, 59.5% – unknown data for 3/124, 2.4% ABSSSI, and 8/82, 9% OTA; p < 0.0001).
Microbiological isolations are listed in –). Overall, 128/206, 62% patients had no microbiological isolation (no cultural examination in 122 and negative culture in 6 subjects); the most frequent were MRSA (25/78, 32%), coagulase-negative Staphylococci (CoNS, 23/78, 29%) and methicillin-susceptible S. aureus (MSSA, 14/78, 18%).
In ABSSSI no microbiological isolation was available in the majority of patients (101/124, 81.4%); MRSA was the most represented pathogen (9/23, 39%).
The proportion of microbiological isolation was higher in OTA (no isolation only in 27/82, 33%) (p < 0.0001), and the most frequent microorganisms were CoNS (19/55, 35%), MRSA (16/55, 29%), and MSSA (10/55, 17%).
3.3. Use of dalbavancin
Data on patients’ hospitalization are shown in (b).
Most patients (119/206, 57.8%) were treated in outpatient services, mainly within ABSSSI (76/124, 61%), with no differences compared to OTA (p = 0.321). Accordingly, OTA underwent longer hospitalization versus ABSSSI (p < 0.0001).
Dalbavancin was administered at a standard dosage (1500 mg in single dose in 124/206, 60.2% patients, with no difference between ABSSSI and OTA); the maximum number of weekly repetition was 7 in a patient with osteomyelitis. While dalbavancin was used mainly empirically in ABSSSI (98/124, 79%), 58/82, 71% (p < 0.0001) of OTA patients received dalbavancin as a targeted therapy. In both ABSSSI and OTA, the most common reason to choose dalbavancin was an easier way of administration (116/206, 56.3%) and failure to previous antibiotics (62/206, 30.1%); other less common reasons were antimicrobial resistance to previous regimens in 2/206 (1%), allergy in 8/206 (3.9%), poor compliance in 4/206 (1.9%), and other reasons or missing data in 14/206 (6.8%) of patients.
3.4. Outcomes
Clinical cure was obtained in 170/206 (82.5%) patients with no significant differences between groups: 106/170 (85.5%) and 63/170 (75%) in ABSSSI and OTA, respectively ()).
Figure 2. Efficacy of dalbavancin at end of treatment according to groups of infections and according to previous and concomitant antibiotic treatment
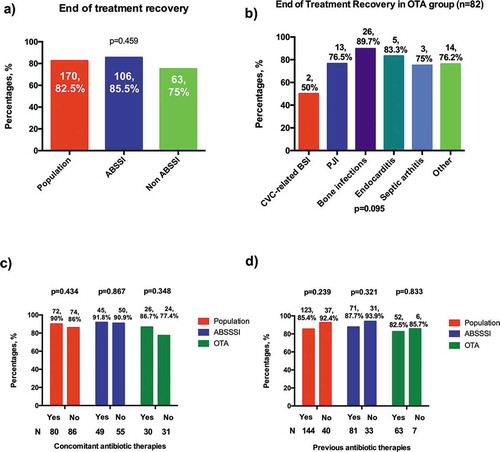
In OTA, the success rate was >75% in all infections ()), except in CVC-related BSI (50%), but the number of patients in this group was only 4. Interestingly, dalbavancin displayed a favorable clinical outcome in 26/29 (89.7%) bone infections ()).
Previous or concomitant therapy did not affect EOT outcome (–)): success rates, however, seem higher in absence of previous therapy in ABSSSI (93.9% vs 87.7%) and in presence of concomitant therapy in OTA (86.7% vs 77.4%), without reaching statistical significance.
By fitting two models of univariable and multivariable logistic regression, adjusted for age, comorbidities, and infection’s site, previous and concomitant antibiotics were not associated with a higher probability of clinical cure (model 1 previous antibiotics: AOR 0.557 vs no previous therapies, 95%CI 0.151–2.061, p = 0.381; model 2 concomitant antibiotics: AOR 1.387 vs no concomitant therapies, 95%CI 0.523–3.677, p = 0.51).
A sensitivity analysis comparing outcome according to different reasons of dalbavancin choice showed that dalbavancin use after other antibiotics’ failure was not associated with a different outcome, compared to other reasons of dalbavancin prescription: clinical cure was obtained in 52/59, 88.1% patients with failure to previous antibiotics (missing data in 3 patients) and in 108/123, 87.8% subjects with other dalbavancin prescription’s reasons (missing data in 21 patients), p = 0.949.
A small proportion of patients presented a clinical relapse: 12/206 (5.8%) within 7 days and 27/206 (13.1%) 7 days after EOT with no differences between ABSSSI and OTA. Median follow-up was 80 (IQR 43–121) days; at the follow-up visit, 126/155 (81.3%) patients recovered ().
Table 2. Outcome and safety profile of dalbavancin
3.5. Safety
An excellent tolerability profile of dalbavancin was confirmed in our study. AEs are listed in ; 11/206 (5.4%) patients presented an AE. Only one patient had a serious dermatologic AE (Stevens–Johnson syndrome), all other AEs were nonserious. The most common AEs were dermatologic reactions. Dalbavancin was discontinued due to AE in three patients: two for a gastrointestinal and one for a dermatologic side effect.
3.6. Previous and concomitant antibiotic therapies
Previous and concomitant antibiotics are shown in .
Table 3. Previous and concomitant treatment characteristics
3.7. Previous antibiotics
Data on antibiotics used prior to dalbavancin were available in 194/206 (94.2%) patients (119/124, 95.9% ABSSSI and 75/82, 91.4% OTA, (a)).
Patients with or without previous antibiotics did not differ in any baseline characteristics (data not shown). Overall, 151/194 (77.8%) patients received prior antibiotics for a median length of 15 (IQR 8–25) days. OTA more frequently received previous antibiotics than ABSSSI (p = 0.003) and for a longer duration (p = 0.007).
75/151 (50%) were treated with a previous monotherapy: the most used antibiotics were aminopenicillin (35/75, 47%), followed by fluoroquinolones (FQs), lipopeptides (7/75, 9% each) and glycopeptides (6/75, 8%).
Among those who underwent previous combination therapy (50/151, 33%), the most frequent regimens were penicillin plus FQs (6/50, 12%) and cephalosporin or carbapenem plus lipopeptide (3/50, 6%). Mixed regimens (previous monotherapies and combination regimens that were sequentially prescribed) were used in 17/151 (11%) subjects.
Within ABSSSI, monotherapy (50/83, 60%; penicillin plus beta-lactamase inhibitors in 28/50, 56%) was more commonly used than combination therapy (26/83, 31%) [penicillin plus FQs or lipopeptide-based regimen (6/26, 23% each), glycopeptides (5/26, 19%)].
In OTA, 25/68 (36.7%) patients had a monotherapy, with similar regimens to those used in the study population, and 24/68 (35.4%) received a combination therapy. Compared to ABSSSI, the proportion of patients that received a previous mixed regimen (both monotherapies and combination regimens before dalbavancin) was higher in OTA (p = 0.001).
3.8. Concomitant antibiotics
Data about antibiotics used in association with dalbavancin were available in 172/206 (83.5%) patients (108/124, 87% ABSSSI and 64/82, 78% OTA; (b)). Concomitant treatment was used in 82/172 (47%) patients (50/108, 46.3% ABSSSI and 31/64, 37.2% OTA, p = 0.786). Patients with or without concomitant antibiotics did not differ in any baseline characteristics (data not shown). While the proportion of concomitant treatment was similar between ABSSSI and OTA (p = 0.786), OTA received concomitant antibiotics for a longer period (p < 0.0001).
In ABSSSI, a single antibiotic in association with dalbavancin was more frequently used than the association of ≥2 antibiotics (37/50, 74%) and FQs were used in 17/37 (46%) regimens.
In OTA, the proportion of a single antibiotic in association with dalbavancin was 20/31 (62.5%) and FQs were the most frequent antimicrobials (6/20, 30%), followed by rifampin and tetracycline (3/20, 15% each). The association of ≥2 antibiotics with dalbavancin was prescribed in 7/31 (21.9%) patients and always included FQs.
4. Discussion
In a multicenter real-life Italian setting, dalbavancin demonstrated: i) an overall success rate in the treatment of both ABSSSI and non-ABSSSI infections >80%, with an optimal safety profile; ii) a frequent use as in-patient treatment after previous lines of antibiotic and in associations with other antibiotics also in ABSSSI; iii) equal efficacy when used alone and as a combination/sequential therapy.
Dalbavancin is the first long-acting anti-infective approved by FDA, that has been recently investigated with encouraging results for in- and off-label indications [Citation13,Citation14,Citation16,Citation17,Citation22,Citation24–32]; clinical trials on osteomyelitis and complicated BSI are still ongoing (NCT03091439-NCT03426761-NCT03148756-NCT02940730).
We hereby show results of an observational study including 206 patients that to our knowledge is the largest real-life setting of dalbavancin. Our population had a wide range of age and more than half presented ≥1 comorbidity, well reflecting the variety of Gram-positive infections and the clinical context.
4.1. Dalbavancin’s efficacy in ABSSSI and OTA
Dalbavancin demonstrated a success rate >80%, up to 85.5% in ABSSSI, comparable to clinical trials [Citation10,Citation19,Citation33] and previous real-life studies [Citation6,Citation16,Citation17,Citation22,Citation28,Citation30,Citation31,Citation34], coupled to optimal safety and tolerability in both ABSSSI and OTA, better than other real-life studies [Citation16,Citation17].
In our study dalbavancin has been primarily used for ABSSSI; contrarily, other European retrospective studies reported the use of this antibiotic mainly in non-ABSSSI [Citation16,Citation17,Citation22]. Our clinical cure rates in OTA are worse than data by Wunsch et al. (89% of clinical cure in osteomyelitis, prosthetic joint infections, and endocarditis) [Citation22]: we achieved also a 83.3% success in endocarditis, lower than 92.6% reported by Toboudic et al. [Citation21]. Conversely, we confirmed positive outcomes in bone and prosthetic joint infections, probably reflecting the good bone concentrations and activity against biofilms that were reported by previous works [Citation3,Citation13,Citation15,Citation16,Citation35–37]. Lastly, despite the small sample size, we show that 3/4 (75%) patients diagnosed with arthritis obtained the clinical cure, in line with encouraging previous PK studies [Citation3].
4.2. Main dalbavancin’s reasons of use
Aside from clinical efficacy, our study provides a deeper insight into the mode of dalbavancin use in our setting. The main reason for dalbavancin use was easier administration; it is well known that dalbavancin prescription could improve patients’ compliance and avoid hospitalization [Citation13,Citation16,Citation17]. However, despite most ABSSSI patients were treated in outpatient services, nearly 40% were still hospitalized, with a median 3-day hospitalization, all possibly suggesting that despite the indisputable advantage of dalbavancin pharmacokinetic, the clinical setting and/or physician approach often involve brief hospital admissions even in ABSSSI. A possible reason likely stems from Italian economic/reimbursement policies and should be addressed in specifically designed studies [Citation16,Citation34,Citation38–40].
4.3. Previous and concomitant antibiotic therapies
Intrigued by the finding that in about 30% of our cohort dalbavancin was used secondary to previous antibiotic failure, we investigated possible differences according to previous/combination therapies. Indeed, one strength of this study was the collection of all prior/concomitant antibiotics. Overall 80% of patients received previous therapies, with a significantly higher proportion of OTA, that also had a longer previous antibiotic therapy. While the use of previous antibiotics in OTA could be considered reasonable, a different reasoning could probably apply to ABSSSI. Indeed, up to 70%, ABSSSI received the previous antibiotic for a median of 11 days, in spite of dalbavancin specific approval for skin infections. Moreover, almost 50% of patients received dalbavancin together with other antibiotics. ABSSSI were receiving long concomitant therapies (median of 11 days), mostly with FQs, that are not even included in ABSSSI guidelines [Citation41]. Taken together, we could speculate that the high propensity to long previous/concomitant therapies in ABSSSI might unravel an enduring concerned attitude about the risk of relapsing infection, probably related to the residual erythema/edema in ABSSSI.
Interestingly, as a possible response to such concern, our data showed no significant clinical advantage when dalbavancin was used alone or with previous/concomitant therapies.
4.4. Limitations of the study
Some limitations should be acknowledged that include the study’s retrospective nature and the lack of uniform criteria in dalbavancin use and of a control group. Furthermore, the limited size of OTA limits the possibility to draw definitive conclusions in these settings. Likewise, data on clinical relapse and follow-up visits were available only in about 80% of the patients, therefore hampering the speculations on the long-term dalbavancin efficacy.
5. Conclusions
To the best of our knowledge, this is the largest retrospective nation-wide study on dalbavancin. Our data suggest that dalbavancin use as first-line treatment should be implemented especially in ABSSSI, without any concomitant treatment when possible, and as an outpatient or emergency department’s regimen in order to reduce hospitalization rates and costs. In OTA, despite excellent available data for bone infections [Citation15], more experience and efficacy studies on larger populations are needed, especially in prosthetic joint infections, endocarditis, and complicated bacteremia where dalbavancin could really change the paradigm of maintenance therapy.
In conclusion, despite its well-established safety and efficacy, an optimal location for dalbavancin still remains somehow elusive in Italy. According to our data, dalbavancin is currently placed as second-line and/or association therapy also in ABSSSI, somehow thwarting its potency and favorable pharmacokinetic properties. Should dalbavancin efficacy in difficult-to-treat infections be confirmed in randomized controlled trials, the current off-label use of dalbavancin could be enhanced at the advantage of patients’ and antimicrobial stewardship’s perspective.
6. Expert opinion
Dalbavancin is lipoglycopeptide antibiotic with a long half-life, and proved safe and efficacious in the treatment of both ABSSSI and other “difficult-to-treat infections” including osteomyelitis, prosthetic joint infections, endocarditis, septic arthritis, CVC-related BSI.
Declaration of interest
G Marchetti is a member of Gilead, ViiV and Janssen advisory board, and has received travel grants to attend conferences from Gilead. F Bai has received a grant from Gilead for another research project outside the current manuscript. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Ethical approval
The study was approved by the Ethic Committee of the coordinating center (Ethic Committee 1 Milan, San Paolo Hospital, ASST Santi Paolo e Carlo, Department of Health Sciences, University of Milan, Milan, Italy; n. 1649, 31/10/2018) and by the Ethic Committees of each participating center. The research was conducted in accordance with the Declaration of Helsinki and national and institutional standards.
Supplemental Material
Download TIFF Image (621.9 KB)Acknowledgments
We are grateful to all patients and their families for their kind participation in the study.
We would like to acknowledge all the staff of the Clinic of Infectious Diseases, Department of Health Sciences, San Paolo Hospital, ASST Santi Paolo e Carlo, Milan, Italy, and all the staff of each participating centre for their precious help in the study.
Contribution: A.d.M., M.P. and G.M. developed the concept of this study. All the authors collected data on Case Report Form and C.A. performed data entry. F.B. did the statistical analyses. F.B. and C. A. wrote the manuscript; F.B., C.A., M.P., A.d.M and G.M. contributed to the final text. All the authors revised the text critically and have read and approved the final text.
Data availability statement
The data that support the findings of this study are available on request from the corresponding author (G.M.). The data are not publicly available because their containing information could compromise the privacy of research participants.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Anderson VR, Keating GM. Dalbavancin. Drugs. 2008;68(5):649–651.
- Gupta AK, Foley KA, Abramovits W, et al. Dalbavancin (Dalvance) for the treatment of acute bacterial skin infection. Skinmed. 2014;12(6):366–369.
- Dunne MW, Puttagunta S, Sprenger CR, et al. Extended-duration dosing and distribution of dalbavancin into bone and articular tissue. Antimicrob Agents Chemother. 2015;59(4):1849–1855.
- Chen AY, Zervos MJ, Vazquez JA. Dalbavancin: a novel antimicrobial. Int J Clin Pract. 2007;61(5):853–863.
- Bassetti M, Peghin M, Carnelutti A, et al. The role of dalbavancin in skin and soft tissue infections. Curr Opin Infect Dis. 2018;31(2):141–147.
- Leuthner KD, Buechler KA, Kogan D, et al. Clinical efficacy of dalbavancin for the treatment of acute bacterial skin and skin structure infections (ABSSSI). Ther Clin Risk Manag. 2016;12:931–940.
- Dunne MW, Talbot GH, Boucher HW, et al. Safety of dalbavancin in the treatment of skin and skin structure infections: a pooled analysis of randomized, comparative studies. Drug Saf. 2016;39(2):147–157.
- Seltzer E, Dorr MB, Goldstein BP, et al. Once-weekly dalbavancin versus standard-of-care antimicrobial regimens for treatment of skin and soft-tissue infections. Clin Infect Dis. 2003;37(10):1298–1303.
- Leighton A, Gottlieb AB, Dorr MB, et al. Tolerability, pharmacokinetics, and serum bactericidal activity of intravenous dalbavancin in healthy volunteers. Antimicrob Agents Chemother. 2004;48(3):940–945.
- Dunne MW, Puttagunta S, Giordano P, et al. A randomized clinical trial of single-dose versus weekly dalbavancin for treatment of acute bacterial skin and skin structure infection. Clin Infect Dis. 2016;62(5):545–551.
- Krsak M, Morrisette T, Miller M, et al. Advantages of outpatient treatment with long-acting lipoglycopeptides for serious gram-positive infections: a review. Pharmacotherapy. 2020;40(5):469–478.
- Almangour TA, Perry GK, Terriff CM, et al. Dalbavancin for the management of gram-positive osteomyelitis: effectiveness and potential utility. Diagn Microbiol Infect Dis. 2019;93(3):213–218.
- Morata L, Cobo J, Fernández-Sampedro M, et al. Safety and efficacy of prolonged use of dalbavancin in bone and joint infections. Antimicrob Agents Chemother. 2019;63(5). DOI:10.1128/AAC.02280-18
- Tobudic S, Forstner C, Burgmann H, et al. Dalbavancin as primary and sequential treatment for gram-positive infective endocarditis: 2-year experience at the general hospital of Vienna. Clin Infect Dis. 2018;67(5):795–798.
- Rappo U, Puttagunta S, Shevchenko V, et al. Dalbavancin for the treatment of osteomyelitis in adult patients: a randomized clinical trial of efficacy and safety. Open Forum Infect Dis. 2019;6(1):ofy331.
- Bouza E, Valerio M, Soriano A, et al. Dalbavancin in the treatment of different gram-positive infections: a real-life experience. Int J Antimicrob Agents. 2018;51(4):571–577.
- Dinh A, Duran C, Pavese P, et al. French national cohort of first use of dalbavancin: a high proportion of off-label use. Int J Antimicrob Agents. 2019;54(5):668–672.
- Kussmann M, Karer M, Obermueller M, et al. Emergence of a dalbavancin induced glycopeptide/lipoglycopeptide non-susceptible Staphylococcus aureus during treatment of a cardiac device-related endocarditis. Emerg Microbes Infect. 2018;7(1):202.
- Boucher HW, Wilcox M, Talbot GH, et al. Once-weekly dalbavancin versus daily conventional therapy for skin infection. N Engl J Med. 2014;370(23):2169–2179.
- Jauregui LE, Babazadeh S, Seltzer E, et al. Randomized, double-blind comparison of once-weekly dalbavancin versus twice-daily linezolid therapy for the treatment of complicated skin and skin structure infections. Clin Infect Dis. 2005;41(10):1407–1415.
- Tobudic S, Forstner C, Burgmann H, et al. Real-world experience with dalbavancin therapy in gram-positive skin and soft tissue infection, bone and joint infection. Infection. 2019;47(6):1013–1020.
- Wunsch S, Krause R, Valentin T, et al. Multicenter clinical experience of real life dalbavancin use in gram-positive infections. Int J Infect Dis. 2019;81:210–214.
- Kock R, Friedrich A. On behalf of the original author Group C. Systematic literature analysis and review of targeted preventive measures to limit healthcare-associated infections by meticillin-resistant Staphylococcus aureus. Euro Surveill. 2014 Jul 24;19(29):20860. .DOI: 10.2807/1560-7917.es2014.19.29.20860
- Bork JT, Heil EL, Berry S, et al. Dalbavancin use in vulnerable patients receiving outpatient parenteral antibiotic therapy for invasive gram-positive infections. Infect Dis Ther. 2019;8(2):171–184.
- Bryson-Cahn C, Beieler AM, Chan JD, et al. Dalbavancin as secondary therapy for serious Staphylococcus aureus infections in a vulnerable patient population. Open Forum Infect Dis. 2019;6(2):ofz028.
- Patel M, Smalley S, Dubrovskaya Y, et al. Dalbavancin use in the emergency department setting. Ann Pharmacother. 2019;53(11):1093–1101.
- Ciccullo A, Giuliano G, Segala FV, et al. Dalbavancin as a second-line treatment in methicillin-resistant Staphylococcus aureus prosthetic vascular graft infection. Infection. 2020;48(2):309–310.
- Bartoletti M, Mikus E, Pascale R, et al. Clinical experience with dalbavancin for the treatment of deep sternal wound infection. J Glob Antimicrob Resist. 2019;18:195–198.
- Hakim A, Braun H, Thornton D, et al. Successful treatment of methicillin-sensitive Staphylococcus aureus tricuspid-valve endocarditis with dalbavancin as an outpatient in a person who injects drugs: a case report. Int J Infect Dis. 2020;91:202–205.
- Hidalgo-Tenorio C, Vinuesa D, Plata A, et al. DALBACEN cohort: dalbavancin as consolidation therapy in patients with endocarditis and/or bloodstream infection produced by gram-positive cocci. Ann Clin Microbiol Antimicrob. 2019;18(1):30.
- Buzón Martín L, Mora Fernández M, Perales Ruiz JM, et al. Dalbavancin for treating prosthetic joint infections caused by gram-positive bacteria: a proposal for a low dose strategy. A retrospective cohort study. Rev Esp Quimioter. 2019;32(6):532–538.
- Howard-Anderson J, Pouch SM, Sexton ME, et al. Left ventricular assist device infections and the potential role for dalbavancin: a case report. Open Forum Infect Dis. 2019;6(9):ofz235.
- Golan Y. Current treatment options for acute skin and skin-structure infections. Clin Infect Dis. 2019;68(Suppl 3):S206–S212.
- Streifel AC, Sikka MK, Bowen CD, et al. Dalbavancin use in an academic medical centre and associated cost savings. Int J Antimicrob Agents. 2019;54(5):652–654.
- Knafl D, Tobudic S, Cheng SC, et al. Dalbavancin reduces biofilms of methicillin-resistant Staphylococcus aureus (MRSA) and methicillin-resistant Staphylococcus epidermidis (MRSE). Eur J Clin Microbiol Infect Dis. 2017;36(4):677–680.
- Murillo Ó, El-Haj C. [Analysis of dalbavancin in animal models]. Enferm Infecc Microbiol Clin. 2017;35(Suppl 1):28–32.
- Barnea Y, Lerner A, Aizic A, et al. Efficacy of dalbavancin in the treatment of MRSA rat sternal osteomyelitis with mediastinitis. J Antimicrob Chemother. 2016;71(2):460–463.
- Wilke M, Worf K, Preisendörfer B, et al. Potential savings through single-dose intravenous dalbavancin in long-term MRSA infection treatment – a health economic analysis using German DRG data. GMS Infect Dis. 2019;7:Doc03.
- McCarthy MW, Keyloun KR, Gillard P, et al. Dalbavancin reduces hospital stay and improves productivity for patients with acute bacterial skin and skin structure infections: the ENHANCE trial. Infect Dis Ther. 2019;9(1):53–67.
- Azamgarhi T, Donaldson J, Shah A, et al. Dalbavancin to treat infected massive endoprostheses: a case report and cost comparison analysis. J Bone Jt Infect. 2019;4(5):234–237.
- Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59(2):e10–52.
