ABSTRACT
Objective
Tuberculosis (TB) remains a global health problem, with medications having adverse effects including drug-induced hepatotoxicity. We determined the prevalence of anti-tuberculosis drug-induced hepatotoxicity and associated risk factors.
Methods
Retrospective cross-sectional study in Botswana including TB patients admitted from 1 June 2017 to 30 June 2018. Anti-TB drug-induced hepatotoxicity was categorized according to WHO criteria whereas causality assessment was made according to the updated Roussel Uclaf Causality Assessment Method (RUCAM) scale. The association between hepatotoxicity and included variables was undertaken by binary logistic regression.
Results
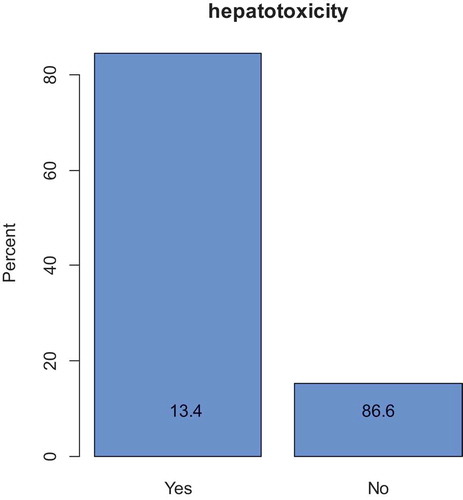
Out of 112 patient files, 15 (13.4%) developed hepatotoxicity after an average of 20.4 days from the start of treatment. Grade 3 and 4 hepatotoxicity was found in 66.7% of the cases. According to the updated RUCAM tool, 86.7% of patients were categorized as having possible anti-TB-associated hepatotoxicity. Patients with elevated baseline alanine transaminase (ALT) were more likely to develop hepatotoxicity (OR = 3.484, 95% CI = 1.02–11.90). Patients with normal hemoglobin (Hb ≥ 12 g/dl) were also more likely to develop hepatotoxicity (OR = 4.413, 95% CI = 1.160–14.8).
Conclusion
Overall, normal hemoglobin and elevated baseline ALT levels were significantly associated with anti-TB drug-induced hepatotoxicity. Additional research is needed to explore this association further.
1. Introduction
Tuberculosis (TB), is a communicable disease caused by Mycobacterium tuberculosis, and remains an important public health problem affecting millions of people worldwide [Citation1]. Despite the fact that the incidence of TB has been declining at an average rate of 1.6% per year between 2000 and 2018, and 2.0% between 2017 and 2018, globally an estimated 10.0 million people fell ill with TB in 2018 [Citation1]. Two-thirds of these cases were found in eight of the 30 TB high burden countries including Nigeria and South Africa among Sub-Saharan African countries [Citation1]. People living with human immunodeficiency virus (PLWH) accounted for 8.6% of these cases; however, the proportion of TB cases co-infected with human immunodeficiency virus (HIV) was highest in the WHO African Region, exceeding 50% or more in parts of Southern Africa [Citation1,Citation2]. This reflects the fact that patients with HIV are at a far greater risk of developing TB in view of their weakened immune systems and difficulties responding to harmful immune responses [Citation3–6]. Of the current 37.9 million people worldwide living with HIV, 25.7million are living in Africa especially Eastern and Southern Africa [Citation7,Citation8]. An appreciable proportion of patients with TB also currently live in Sub-Saharan Africa [Citation1]. This is a concern as TB remains the leading cause of death among HIV patients, accounting for a third of all AIDS-related deaths [Citation7].
Significant strides have been made between 2000 and 2018 to reduce TB mortality worldwide as seen by a 27% decrease in TB mortality among HIV negative patients, and a 60% reduction among HIV co-infected patients, during this period [Citation1]. This is important in Sub-Saharan African countries such as Botswana with a high burden of TB-HIV co-infection versus other countries with just a high TB burden [Citation1]. This high burden is exacerbated by high prevalence rates of patients with HIV within Botswana, with treatment of these patients helped by free anti-retroviral treatment (ART) as part of universal access to health care in Botswana [Citation9–12].
Botswana, like many Sub-Saharan African countries, currently has a high incidence and mortality from TB; however, the downward trend observed in the global incidence has also been noted in Botswana [Citation2]. The notification rate was high at 506 per 100,000 people in 1975 decreasing to 199 cases per 100,000 people in 1989 due to successful TB control efforts [Citation9]. However, this decline was reversed with 623 new cases per 100,000 people in 2002, one of the highest in the world [Citation9]. This increase was likely driven by the increase in the number of people infected with HIV at the time resulting in the twin epidemics of TB and HIV [Citation9,Citation13]. After 2002, there was typically a downward trend with 361 cases per 100,000 in 2010 and 272 cases per 100,000 in 2016 [Citation2,Citation13,Citation14]. It is believed that the combined concerted efforts of the Botswana national TB and HIV programs facilitated this appreciable reduction [Citation2,Citation9].
The current treatment regimen for drug-sensitive TB in Botswana includes an initial phase for 2 months with a fixed-dose combination (FDC) containing Rifampicin (RIF), Isoniazid (INH), Pyrazinamide (PZA) and Ethambutol (EMB) – HREZ, which is similar to other countries [Citation15–17], and a continuation phase with a FDC containing HRE [Citation13]. Adherence to anti-tuberculosis treatment (ATT) is crucial in obtaining good TB treatment outcomes. Several factors can lead to poor adherence to treatment, with one of the key factors being the side effects from ATT [Citation18–22]. Other factors include a lack of a support network, long waiting times to see healthcare professionals as well as HIV co-infection [Citation19,Citation20,Citation23]. In addition, long distances to the hospital with associated costs especially if patients use public transport, lack of repeated sputum spears during follow-up, being transferred to a different facility after the intensive phase and poor knowledge about TB treatment [Citation19,Citation21,Citation24]. The adverse effects from ATT range from minor to more severe effects, and can negatively impact on treatment outcomes [Citation25,Citation26]. Rates of adverse effects from ATT can be as high as 16.6% and 4.46% among HIV infected and uninfected patients, respectively [Citation27], with published studies also reporting high rates of adverse effects in patients treated with both ATT and anti-retroviral treatments (ART) [Citation28,Citation29]. The most frequently reported adverse effects from ATT include gastrointestinal tract side-effects and drug-induced hepatitis [Citation17,Citation30,Citation31]. Other common side-effects include anorexia and abdominal pain followed by peripheral neuropathy [Citation27,Citation30,Citation32]. In their study, Gholami et al. [Citation32] found that hepatobiliary side-effects accounting for 37% of the reported side-effects of patients prescribed ATT (25% hepatitis, 11.2% increased liver transaminases).
Despite the fact that hepatotoxicity is frequently encountered in patients prescribed ATT, its prevalence is currently unknown in Botswana. This is a concern given high rates of HIV and TB in Botswana, high rates of co-morbidity and patients with combined HIV and TB having an increased risk of adverse events. Consequently, we sought to address this by investigating the prevalence of ATT-induced hepatotoxicity, and eliciting associated factors, among hospitalized patients in Gaborone, Botswana. We believe this is important alongside initiatives to improve the Tuberculosis Register Data Quality in Botswana, address concerns with undetected TB among hospitalized patients in Botswana, increase the number of patients with TB with known HIV being started on ART and ongoing policies generally to improve the management of patients with both HIV and TB in Botswana [Citation6,Citation33,Citation34]. The findings can be used to help guide future management strategies in Botswana for patients with TB including those with HIV.
2. Methods
2.1. Study design, setting and participants
A retrospective cross-sectional study was undertaken at Princess Marina Hospital (PMH), a tertiary level care hospital in Gaborone, the capital city of Botswana, treating patients with HIV and TB [Citation35]. All the medical and specific TB registries for patients who were admitted onto the medical wards from 1 June 2017 to 30 June 2018 were reviewed to identify patients meeting the inclusion criteria for the study population. The inclusion criteria included patients with confirmed or presumed TB on anti-tuberculosis treatment for 7 or more days on the day of admission, and exclusion criteria included patients with confirmed multi-drug resistant (MDR) and extensively drug-resistant (XDR) TB drug-resistant TB as well as those patients being treated with second-line TB drugs. The principal reasons for these inclusion/exclusion criteria were firstly to provide a platform for comparison with previous similar studies [Citation27,Citation36,Citation37]. Secondly, patients fitting the exclusion criteria are typically followed up at a specialized facility in Botswana other than the site for this study.
2.2. Sample size calculations
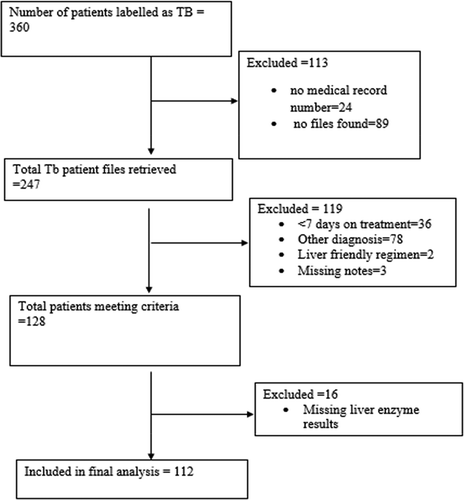
Based on the prevalence of anti-tuberculosis drug hepatotoxicity of 5.7% and 1.3% among HIV infected and uninfected patients, respectively, in a study in Kenya [Citation27], the sample size was calculated using a previous formula for prevalence in a cross-sectional study [Citation38], i.e. N = (Z α/22 pq)/d2 where N is the sample size, Z is the statistic corresponding to the level of confidence, p is expected prevalence, and d is degree of freedom. Based on a prevalence of 5.7%, p = 0.057, q = 1-p = 0.943, d = 5%, Z α/2 = Z 0.025 = 1.96. N = ((1.96)2 × (0.057) (0.943/(0.05)2 = 82.59 ≈ 83. With intention to recruit a minimal sample size of 83 patients; given the patients’ charts assessed, we ended up with 112 patients as depicted in
2.3. Data collection procedures
Data were collected by means of a structured case report form by the first author (BK). Staff from the medical records department assisted with tracing the files. The first step in data collection was to identify the study population, which was performed by preparing a list after revisiting the admission registers and the TB registers in the medical wards. Information not available from the files such as laboratory results was sought from the hospital’s Integrated Patient Management System (IPMS).
Files of patients meeting the inclusion criteria were identified to obtain the denominator when calculating prevalence rates. Patients meeting the case definition were identified. The flowchart of patients is shown in the results section (). Efforts were made to go through the patients admission notes to identify other independent variables including age, gender, whether pulmonary or disseminated TB, duration of TB treatment, previous history of TB, preexisting liver disease, HIV status (CD4 count when applicable), co-morbid conditions, concomitant drugs prescribed, and any work up undertaken such as hepatitis serology and autoimmune screening.
2.4. Operation definitions
A case of anti-tuberculosis drug-induced hepatotoxicity was defined as ‘An increase in transaminases of >3 times upper limit of normal (ULN) with any symptoms of nausea, vomiting, anorexia, abdominal pain, and jaundice OR increase in transaminases >5 times ULN’ [Citation39]. The reference ranges for transaminases, alanine transaminase (ALT), and aspartate transaminase (AST), to determine ULN were adopted from specific reference ranges of PMH chemistry laboratory and are quoted in the results section. Results of transaminases considered in this study were those performed on the day of admission and initial bloods on TB treatment initiation if available on patients’ charts or the hospital’s IPMS. Hepatotoxicity severity based on ALT was graded according to World Health Organization (WHO) scoring system [Citation40] as shown in .
Table 1. Definition of hepatotoxicity according to the WHO adverse drug reaction terminology
2.5. Causality assessment
Proving that a certain medicine is the cause of drug-induced hepatotoxicity is challenging and as a result, there are several causality assessment methods that have been developed to assess the probability of a medicine being responsible for hepatotoxicity [Citation41,Citation42]. For the purpose of this study, a liver-specific probability scale called the Roussel Uclaf Causality Assessment Method (RUCAM) (updated) was used. RUCAM, which is also known as the Council of International Organization on Medical Science (CIOMS) scale, was introduced in 1993. RUCAM assigns points to important features of liver injury, with a resultant overall assessment score generated that reflects the likelihood that hepatic injury is due to the medication(s) in question [Citation41,Citation43,Citation44]. The overall grading leads to causality assessment categorized as highly probable (score of 9 or more), probable (6–8), possible (3–5), unlikely (1–2) or excluded for scores of 0 [Citation44], with RUCAM remaining the most widely used method for prospective and retrospective studies worldwide [Citation41]. Improvements in RUCAM lead to the development of the updated RUCAM, which was used to categorize patients with hepatotoxicity in this study. The updated RUCAM has separate scales that takes into consideration the classification of liver injury into hepatocellular injury, cholestatic, and mixed liver injury [Citation44].
2.6. Data analysis and statistics
All analyzes were conducted using Stata v.13 (Stata Statistical Software: Release 13. StataCorp). Since the study was evaluating the prevalence of anti-TB drug-induced hepatotoxicity, the prevalence was calculated as a ratio of the number of patients who had anti-TB drug-induced hepatotoxicity divided by the total number of patients on TB treatment during the study period. The results were presented as means (standard deviation), median (interquartile range), or frequencies of given characteristics. Chi-square test or Fisher’s exact test whenever appropriate were used to compare patients with and without hepatotoxicity. Bivariate logistic regression was performed on all socio-demographic, clinical, co-morbid, and laboratory parameters to determine associations for hepatotoxicity. However, since the bivariate analysis computed several variables with Odds ratios of almost zero, it was not possible to perform a multivariate logistic regression. A p-value of <0.05 was considered statistically significant.
2.7. Ethics approval
Ethical approval for research was sought and obtained from the Ministry of Health Botswana, Princess Marina Hospital and University of Botswana Institutional Review Boards. The waiver of patient consent was obtained as this was a retrospective study, with patient confidentiality waivered throughout.
3. Results
In this study, 360 patients were labeled as having a diagnosis of TB disease in either the nursing or TB registers in medical wards during the study period. Out of patients with a diagnosis of TB, 247/360 (68.6%) files were retrieved from medical records. The missing files either had old identifiers (PM numbers) and had been moved away from the hospital for storage (89 files) or their identifier numbers (PM) were not recorded (24 files). From 247 files that were retrieved, 78 patients had other diagnoses (were never on TB treatment) and 128 met the inclusion criteria. Sixteen (16) files among the patients meeting inclusion criteria were further excluded as they had missing results of liver enzymes (transaminases). Hence, the final analysis comprised data extracted from 112 files ().
3.1. Socio-demographic, clinical characteristics and co-morbidity conditions of study participants
The median age (interquartile range) of study participants was 41 (IQR = 29–46) years with the majority of patients being female, 72/112 (64.3%). Diagnosis of TB was made either by symptoms or symptoms and radiological evidence combined in 64/112 (57.1%) and 33/112 (29.5%) of patients, respectively. Disseminated and extrapulmonary TB put together accounted for 59/112 (52.7%) cases, with the majority of patients on the initiation phase of TB treatment, 100/112 (89.3%). Of note, 49/112 (43.8%) and 21/112 (18.8%) of patients had been on TB treatment for less or equal to 15 days and more or equal to 45 days, respectively. The majority of study participants were HIV positive, comprising 91/112 (81.2%); and over half of them (53.9%) had a CD4 count of less or equal to 200 umol/l. Among patients on ART, 40/57 (70.2%) were on a dolutegravir-based regimen. None of the patients had a history of liver disease; however, 14/112 (12.5%) of the patients had a history of renal disease and 20/112 (18.2%) had other chronic medical conditions. The remaining of sociodemographic, clinical, and co-morbid characteristics is summarized in .
Table 2. Sociodemographic and clinical characteristics of study participants (N = 112)
Table 3. Clinical characteristics of study participants (N = 112)
3.2. Prevalence of TB-associated hepatotoxicity
Hepatotoxicity occurred in 15/112 (13.4%) of the study patients ( and ). According to the updated RUCAM tool, 13/15 (86.7%) of the patients were categorized as having possible anti-TB-associated hepatotoxicity while 2/15 (13.3%) were categorized as probably due to ATT associated hepatotoxicity.
3.3. Laboratory parameters of study participants
Overall, 89 (76.4%) and 83 (74.1%) of the patients had a documented baseline ALT and AST, respectively. There were 21 (23.6%) patients with an abnormal baseline ALT and 41(49.4%) with an abnormal baseline AST. Alkaline phosphatase (ALP) was abnormal at baseline in 38 (48.1%) and GGT in 43 (55.8%) of the patients with available results, respectively. Of the 80 patients with a documented baseline albumin, 69 (86.3%) had hypoalbuminemia. On the other hand, 52 (61.9%) and 47(56.6%) of patients had a normal baseline white cell count and platelet count, respectively, whereas a majority of the patients were anemic, 64/84 (76.2%) ().
Table 4. Laboratory characteristics of study participants
3.4. Grading of hepatotoxicity
Using the WHO grading system for degree of severity, Grade 2, 3, and 4 hepatotoxicity accounted for 5/15 (33.3%), 2/15 (40.0%), and 4/15 (26.7%) of patients, respectively.
3.5. Association between hepatotoxicity and hepatitis serology/Autoimmune screen
Only 2/15 (13.3%) patients with hepatotoxicity had serology results for HbsAg; the results being negative in both cases. Hepatitis C serology was not performed for any of the patients with hepatotoxicity; this was also true for autoimmune screening.
3.6. Bivariate logistic regression of association between hepatotoxicity and socio-demographic, clinical and comorbid conditions
Patients who developed hepatotoxicity had a median age of 36 (IQR = 27–38) years, which was lower compared to those who did not develop hepatotoxicity who had a median age of 41 years (IQR = 30–47) years; however, the difference was not statistically significant. Male patients had higher rates of hepatotoxicity (15.3%) compared to female patients (10.0%); however again, the difference was not statistically significant (p-value = 0.435). Other analyzed variables including the mode of diagnosis, site of TB, history of previous TB, duration of TB treatment, HIV status, CD4 count for HIV positive patients, history of other co-morbid conditions, alcohol consumption, and the use of concomitant hepatotoxic drugs were not significantly associated with anti-TB drug-induced hepatotoxicity. Of note is the fact that the antiretroviral regimen type was also not associated with the occurrence of anti-TB-associated hepatotoxicity ().
Table 5. Association between hepatotoxicity and socio-demographic, and clinical characteristics
Table 6. Association between hepatotoxicity and clinical characteristics
Table 7. Association between hepatotoxicity and clinical characteristics/co-morbidity conditions
3.7. Bivariate logistic regression of association between hepatotoxicity and laboratory parameters
Patients who had elevated baseline ALT, 6/21 (28.6%) were more likely to develop hepatotoxicity compared to those with normal baseline ALT, 7/61 (10.3%). The association was statistically significant (p-value = 0.046; OR = 3.484, 95% CI = 1.020–11.900).
As regards hemoglobin levels, patients categorized as having normal hemoglobin (Hb ≥12 g/dl) were more likely to develop hepatotoxicity compared to patients with anemia (Hb <12 g/dl) at baseline (30.0% versus 9.4%). The association was statistically significant (p-value = 0.029; OR = 4.413, 95% CI = 1.160–14.8). The rest of studied laboratory parameters was not associated with hepatotoxicity ().
Table 8. Association between hepatotoxicity and laboratory parameters
4. Discussion
We believe this is the first study to investigate the prevalence of ATT-induced hepatotoxicity, and associated factors, among patients with TB in Botswana. The prevalence of hepatotoxicity among patients on ATT in this study was 13.4%, similar to the findings from studies undertaken outside of Africa where the prevalence ranged from 2% to 28% [Citation39,Citation45–48]. The prevalence of hepatotoxicity in this study also compares with studies performed in Ethiopia and South Africa [Citation49–51]; however, our prevalence rates were lower than a study conducted in Morocco which found the prevalence of ATT hepatotoxicity at 24.6% [Citation52]. The reason for these differences could be differences in phenotype and genotype among the populations as well as case definitions. In addition, a previous study has shown discrepancies among the different causality scales in assessing drug-induced liver injury which could also explain differences in the findings [Citation53].
4.1. Causality assessment
The majority of study participants (86.7%) scored as possible ATT using the updated RUCAM. The lower scores in the RUCAM scale leading to this categorization can be explained by several underlying factors. These include the fact that almost all of the studied patients had missing serology results for hepatitis B and C and other causes of viral hepatitis in the files and the IPMS.
While autoimmune hepatitis has been implicated to contribute to ATT associated hepatotoxicity especially in women, none of the participants in this study had results of autoimmune screening. The other big confounder is the concomitant use of other known hepatotoxic drugs such as cotrimoxazole, which is usually started concurrently with ATT in all HIV positive patients. With the majority of study participants, 90 (81.2%), in this study being HIV positive it is not surprising that two-thirds of those with hepatotoxicity 10/15 (66.7%) were on concurrent treatment with cotrimoxazole.
4.2. Association between hepatotoxicity and socio-demographic, clinical and co-morbid conditions
As mentioned, the variables studied included age, gender, mode of TB diagnosis, site/extent of disease, duration of TB treatment, and previous history of TB and HIV status. All these variables were found not to be associated with the occurrence of hepatotoxicity, with similar findings found in other studies [Citation51,Citation54–56].
Previous studies have shown that patients with the habit of heavy alcohol consumption tend to be significantly associated with ATT hepatotoxicity [Citation50,Citation57,Citation58]. However, alcohol consumption was not associated with hepatotoxicity in our study. This could be due to the retrospective nature of our study where it was not possible to quantify alcohol intake at the individual level. It should however be noted that the lack of association between alcohol consumption and hepatotoxicity has been documented in other previous studies [Citation52,Citation56,Citation59,Citation60].
A previous study in area with high burden of liver disease showed that preexisting liver disease is a risk factor of anti-TB-associated hepatotoxicity [Citation58]. There was though no documented preexisting liver disease in this study. However, this is unlikely to be the case in reality underlining the limitations of retrospective studies and the fact that patients were not thoroughly worked up for possible underlying chronic liver diseases such as viral hepatitis and autoimmune diseases at the time of admission onto the wards.
A history of chronic medical conditions such as chronic pulmonary disease and chronic kidney disease (CKD) is also associated with higher rates of ATT-associated hepatotoxicity [Citation50,Citation55,Citation61]. However, chronic medical conditions grouped together and individually were not associated with hepatotoxicity in this study. This may be explained in different ways; firstly, a small sample size not primed to pick up this difference. However, as noted 12/15 (80%) of patients in this study had no history of CKD. Secondly, since this was a retrospective study, objective ways of evaluating for CKD such as creatinine clearance, proteinuria, and radiology were not utilized. This is again a weakness of retrospective studies.
Malnutrition as indicated by either hypoalbuminemia or low BMI has been found to be associated to anti-TB drug-induced hepatotoxicity [Citation50,Citation54,Citation57,Citation58]. Given the nature of this study, it was not possible to calculate the BMI because weight and height of the patients are not routinely measured in medical wards in Botswana and calculation of the BMI requires these two variables. For patients with available serum albumin results, 9/10 (90%) of patients with hepatotoxicity had hypoalbuminemia compared to 60/70 (85.7%) of patients without hepatotoxicity who had hypoalbuminemia. The difference was not statistically significantly similar to previous studies [Citation51,Citation56]. The lack of association can also possibly be explained by the fact that the majority of patients in this study, 69/80 (86.3%), had hypoalbuminemia and low serum albumin as a negative reactive protein is not unexpected in patients with tuberculosis [Citation62].
4.3. Association between hepatotoxicity and laboratory parameters
Among the liver enzymes parameters studied, baseline elevated ALT was significantly associated with the occurrence of ATT hepatotoxicity. This is similar to the findings from previous studies [Citation37,Citation58]. However, the lack of association between baseline ALP and AST in this study contrasts with findings from previous studies [Citation37,Citation57,Citation58]. The possible reason for the lack of association is that 28.6% of patients had missing ALP results. The presence of normal hemoglobin appeared to be more associated with the occurrence of ATT hepatotoxicity in our study, which contrasts with the findings of a study in Peru that found anemia to be a risk for hepatotoxicity [Citation63]. However, the protective role of anemia has not been reported elsewhere. The possible explanations for this are that patients with hepatotoxicity might be adherent to TB treatment with resulting clinical improvement unlike those without hepatotoxicity who could have been admitted for other conditions such as opportunistic infections that cause anemia. In addition, studies undertaken in Ethiopia and Iran have also revealed that patients’ hemoglobin levels have improved as result of ATT [Citation64,Citation65]. Consequently, it is possible that significantly better hemoglobin levels in patients with hepatotoxicity in this study further indicates better adherence to treatment as well as improvement of TB disease status. In view of these findings, there is a need to conduct prospective studies in the future to help understand the possible role of hemoglobin levels as a risk factor for ATT associated hepatotoxicity, and we will be following this up.
4.4. ART and hepatotoxicity
TB/HIV co-infection rate in Botswana is as high as 59.2% [Citation13]. The different classes of ARTs have the potential to cause hepatotoxicity; however, this is more commonly associated with non-nucleoside reverse transcriptase inhibitors (NNRTIs). Botswana has now moved away from an NNRTI-based regimen to a dolutegravir (DTG) first-line regimen [Citation66]. The majority of HIV positive patients on ART in this study (70.2%) were on a DTG-based regimen that is rarely associated with hepatotoxicity [Citation67,Citation68]. The different types of ART regimen were not significantly associated with hepatotoxicity in our study, which may be partly due to a small sample size that was not powered to show any difference. On the other hand, the switch of guidelines to replace NNRTIs such as efavirenz, which has a high propensity to cause hepatotoxicity as compared to DTG, might also have played a part.
4.5. Limitations
We are aware that there were several limitations noted in our study. Firstly, the retrospective study design. As a result, there were some important information that was missing, which included socio-demographic, clinical, and comorbid aspects. BMIs were also not routinely documented in patient’s files; consequently, it was not possible to determine its role in hepatotoxicity. Due to the retrospective nature of our study, it was also difficult to elicit causality other than by use of scores. Missing serology results for other causes of hepatitis also occurred as most of these investigations are not routinely undertaken at Princess Marina Hospital. This meant that we had to rely on scores in our analysis. Prospectively, it would have been possible to stop the treatment and assess responses and possibly re-challenge and observe further associations. Furthermore, this was a single-center study with a small sample size, which means it is likely to be underpowered to determine the significance of some variables. In addition, this study being hospital based in a tertiary setting is bound to have selection bias; hence, our findings may not be representative of all patients starting TB treatment in Botswana. Despite the limitations, this is the first study of its kind in Botswana, a country with a high TB and HIV burden. Future studies will aid our understanding in Botswana and other similar settings in sub-Saharan Africa.
5. Conclusion
This study showed that the prevalence of ATT-associated hepatotoxicity in this tertiary hospital in Botswana was 13.4%. Hepatotoxicity severity was categorized as grade 2 or worse. Elevated baseline ALT and normal hemoglobin at baseline were significantly associated with ATT hepatotoxicity. There is a need to undertake prospective studies with a large sample size to foster greater understanding on this topic in Botswana given its high rates of patients with both TB as well as TB with HIV co-morbidity, and we will be undertaking these in the future to help guide the improved management of these patients.
Article highlights
Tuberculosis (TB) remains an important public health problem affecting millions of people worldwide.
Botswana, like many Sub-Saharan African countries, currently has a high incidence and mortality from TB.
Adherence to anti-tuberculosis treatment (ATT) is crucial in getting good TB treatment outcomes. Side effects from ATT including hepatotoxicity results to poor treatment compliance.
Anti-tuberculosis-associated hepatotoxicity occurred in 13.4% of the study participants.
Elevated baseline alanine transaminase (ALT) and normal hemoglobin (≥ 12 g/dl) were the only factors associated with occurrence of ATT hepatoxicity in this study
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
BK and GR designed the concept for the study, undertook the research and the initial analysis. BG and GR undertook the literature search and the first draft of the manuscript. All authors approved the initial and revised manuscript.
Additional information
Funding
References
- World Health Organization. WHO global tuberculosis report (Full). 2019 [cited 2020 Mar 30]. Available from: https://apps.who.int/iris/bitstream/handle/10665/329368/9789241565714-eng.pdf?ua=1
- Musuka G, Teveredzi V, Busang L, et al. Community attitudes on tuberculosis in Botswana: an opportunity for improving the national tuberculosis programme outcomes, 2011. BMC Res Notes. 2018;11(1):499.
- AVERT. Global information and education on HIV and AIDS. HIV and AIDS in South Africa 2018. [cited 2020 Mar 28]. Available from: https://www.avert.org/professionals/hiv-around-world/sub-saharan-africa/south-africa
- Walker NF, Meintjes G, Wilkinson RJHIV-1. and the immune response to TB. Future Virol. 2013;8(1):57–80.
- Bell LC, Pollara G, Pascoe M, et al. In vivo molecular dissection of the effects of HIV-1 in active tuberculosis. PLoS Pathog. 2016;12(3):e1005469.
- AVERT. HIV and tuberculosis co-infection programmes. 2018 [cited 2020 Mar 28]. Available from: https://www.avert.org/professionals/hiv-programming/hiv-tb-coinfection
- UNAIDS. Global HIV & AIDS statistics — 2019 fact sheet. [cited 2020 Mar 26]. Available from: https://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf
- World Health Organisation - Global Health Observatory data repository. Number of people (all ages) living with HIV - estimates by WHO region. 2019 [cited 2020 Mar 26]. Available from: https://apps.who.int/gho/data/view.main.22100WHO?lang=en
- Ministry of Health Botswana. TB/HIV Collaborative Policy Guidelines. [cited 26 Mar 2020]. Available from: http://www.tbonline.info/media/uploads/documents/botswana_tb:hiv_policy_guidelines_%282011%29.pdf
- AVERT. HIV and AIDS in Botswana. 2018 [cited 2020 Mar 26]. Available from: https://www.avert.org/professionals/hiv-around-world/sub-saharan-africa/botswana
- Government of Botswana. Emergency plan for AIDS relief. Botswana country operational plan 2019 - strategic direction summary. [cited 2020 Mar 28]. Available from: https://www.state.gov/wp-content/uploads/2019/09/Botswana_COP19-Strategic-Directional-Summary_public.pdf
- Alwano MG, Bachanas P, Block L, et al. Increasing knowledge of HIV status in a country with high HIV testing coverage: results from the Botswana combination prevention project. PloS One. 2019;14(11):e0225076.
- Republic of Botswana Ministry of Health. National tuberculosis programme manual 2007. [cited 2020 Mar 25]. Available from: https://www.who.int/hiv/pub/guidelines/botswana_tb.pdf
- Statistics Botswana. Botswana environment statistics - human settlements report T 2018. 2020 Feb [cited 2020 Mar 25]. Available from: http://www.statsbots.org.bw/sites/default/files/Botswana%20Environment%20Statistics-%20Human%20Settlements%20Report%202018.pdf
- Godman B, McCabe H, DL T. Fixed dose drug combinations - are they pharmacoeconomically sound? Findings and implications especially for lower- and middle-income countries. Expert Rev Pharmacoecon Outcomes Res. 2020;20(1):1–26.
- Denti P, Jeremiah K, Chigutsa E, et al. Pharmacokinetics of Isoniazid, pyrazinamide, and ethambutol in newly diagnosed pulmonary TB patients in Tanzania. PloS One. 2015;10(10):e0141002.
- Fei CM, Zainal H, Ali IAH. Evaluation of adverse reactions induced by anti-tuberculosis drugs in hospital Pulau Pinang. Malays J Med Sci. 2018;25(5):103–114.
- Muture BN, Keraka MN, Kimuu PK, et al. Factors associated with default from treatment among tuberculosis patients in Nairobi province, Kenya: a case control study. BMC Public Health. 2011;11:696.
- Castelnuovo B. A review of compliance to anti tuberculosis treatment and risk factors for defaulting treatment in sub Saharan Africa. Afr Health Sci. 2010;10(4):320–324.
- Zegeye A, Dessie G, Wagnew F, et al. Prevalence and determinants of anti-tuberculosis treatment non-adherence in Ethiopia: a systematic review and meta-analysis. PloS One. 2019;14(1):e0210422.
- Tekle B, Mariam DH, Ali A. Defaulting from DOTS and its determinants in three districts of Arsi zone in Ethiopia. Int J Tuberc Lung Dis. 2002;6(7):573–579.
- Awofeso N. Anti-tuberculosis medication side-effects constitute major factor for poor adherence to tuberculosis treatment. Bull World Health Organ. 2008;86(3):B–d.
- Kibuule D, Verbeeck RK, Nunurai R, et al. Predictors of tuberculosis treatment success under the DOTS program in Namibia. Expert Rev Respir Med. 2018;12(11):979–987.
- Nuwaha F. Control of tuberculosis in Uganda: a tale of two districts. Int J Tuberc Lung Dis. 1999;3(3):224–230.
- Alipanah N, Jarlsberg L, Miller C, et al. Adherence interventions and outcomes of tuberculosis treatment: a systematic review and meta-analysis of trials and observational studies. PLoS Med. 2018;15(7):e1002595.
- Munro SA, Lewin SA, Smith HJ, et al. Patient adherence to tuberculosis treatment: a systematic review of qualitative research. PLoS Med. 2007;4(7):e238.
- Masese JO, Nyamu GD, Ombenga JN, et al. Adverse drug reactions among HIV infected and uninfected adults receiving anti-tuberculosis therapy at Kenyata national hospital. East Afr Med J. 2011;88(10):327–331.
- Sadiq S, Khajuria V, Tandon VR, et al. Adverse drug reaction profile in patients on anti-tubercular treatment alone and in combination with highly active antiretroviral therapy. J Clin Diagn Res. 2015;9(10):FC01–FC4.
- Michael OS, Sogaolu OM, Fehintola FA, et al. Adverse events to first line anti-tuberculosis drugs in patients co-infected with HIV and tuberculosis. Ann Ib Postgrad Med. 2016;14(1):21–29.
- Prasad R, Singh A, Gupta N. Adverse drug reactions in tuberculosis and management. Indian J Tuberc. 2019;66(4):520–532.
- El Hamdouni M, Ahid S, Bourkadi JE, et al. Incidence of adverse reactions caused by first-line anti-tuberculosis drugs and treatment outcome of pulmonary tuberculosis patients in Morocco. Infection. 2020;48(1):43–50.
- Gholami K, Kamali E, Hajiabdolbaghi M, et al. Evaluation of anti-tuberculosis induced adverse reactions in hospitalized patients. Pharm Pract (Granada). 2006;4(3):134–138.
- Muyaya LM, Musanda EM, Tamuzi JL. Human immunodeficiency virus-associated tuberculosis care in Botswana: evidence from a real-world setting. BMC Infect Dis. 2019;19(1):767.
- Kleinman NJ, Mawandia S, Kgwaadira B, et al. Increasing tuberculosis register data quality in Botswana with continuous quality improvement activities. Qual Primary Care. 2017;26(2):45–48.
- Baik Y, Fane O, Wang Q, et al. Undetected tuberculosis at enrollment and after hospitalization in medical and oncology wards in Botswana. PloS One. 2019;14(7):e0219678.
- Jeong I, Park JS, Cho YJ, et al. Drug-induced hepatotoxicity of anti-tuberculosis drugs and their serum levels. J Korean Med Sci. 2015;30(2):167–172.
- Khalili H, Dashti-Khavidaki S, Rasoolinejad M, et al. Anti-tuberculosis drugs related hepatotoxicity; incidence, risk factors, pattern of changes in liver enzymes and outcome. DARU. 2009;17(3):163–167.
- Hsieh FY, Liu AA. Adequacy of sample size in health studies. Stanley Lemeshow, David W. Hosmer Jr., Janelle Klar and Stephen K. Lwanga published on behalf of WHO by Wiley, Chichester, 1990. No. of pages: xii + 233. Price:£D17.50. Stat Med. 1990;9(11):1382.
- Tostmann A, Boeree MJ, Aarnoutse RE, et al. Antituberculosis drug-induced hepatotoxicity: concise up-to-date review. J Gastroenterol Hepatol. 2008;23(2):192–202.
- WHO Collaborating Centre for International Drug Monitoring Uppsala. International monitoring of adverse reactions to drugs: adverse reaction terminology. 1992 [cited 2020 Mar 26]. Available from: https://apps.who.int/iris/handle/10665/61056
- Danan G, Teschke R. Roussel Uclaf causality assessment method for drug-induced liver injury: present and future. Front Pharmacol. 2019;10:853.
- García-Cortés M, Stephens C, Lucena MI, et al. Causality assessment methods in drug induced liver injury: strengths and weaknesses. J Hepatol. 2011;55(3):683–691.
- LiverTox: clinical and research information on drug-induced liver injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-. Adverse Drug Reaction Probability Scale (Naranjo) in Drug Induced Liver Injury; 2019 [cited 2020 Mar 26]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK548069/
- Danan G, Teschke R. RUCAM in drug and herb induced liver injury: the update. Int J Mol Sci. 2015;17:1.
- Shu CC, Lee CH, Lee MC, et al. Hepatotoxicity due to first-line anti-tuberculosis drugs: a five-year experience in a Taiwan medical centre. Int J Tuberc Lung Dis. 2013;17(7):934–939.
- Golemba AS, Ferreyra FG, Martearena RE, et al. Drug-induced hepatotoxicity and tuberculosis in a hospital from the Argentinian northeast: cross-sectional study. Medwave. 2015;15(4):e6135.
- Marzuki OA, Fauzi AR, Ayoub S, et al. Prevalence and risk factors of anti-tuberculosis drug-induced hepatitis in Malaysia. Singapore Med J. 2008;49(9):688–693.
- Sun Q, Zhang Q, Gu J, et al. Prevalence, risk factors, management, and treatment outcomes of first-line antituberculous drug-induced liver injury: a prospective cohort study. Pharmacoepidemiol Drug Saf. 2016;25(8):908–917.
- Pande JN, Singh SP, Khilnani GC, et al. Risk factors for hepatotoxicity from antituberculosis drugs: a case-control study. Thorax. 1996;51(2):132–136.
- Jong E, Conradie F, Berhanu R, et al. Consensus statement: management of drug-induced liver injury in HIV- positive patients treated for TB. S Afr J HIV Med. 2013;14(3):113–119.
- Wondwossen A, Waqtola C, Gemeda A. Incidence of antituberculosis-drug-induced hepatotoxicity and associated risk factors among tuberculosis patients in Dawro zone, South Ethiopia: a cohort study. Int J Mycobacteriol. 2016;5(1):14–20.
- Bouazzi OE, Hammi S, Bourkadi JE, et al. First line anti-tuberculosis induced hepatotoxicity: incidence and risk factors. Pan Afr Med J. 2016;25:167.
- Das S, Behera SK, Xavier AS, et al. Agreement among different scales for causality assessment in drug-induced liver injury. Clin Drug Investig. 2018;38(3):211–218.
- Khoharo HK, Ansari S, Siddiqui AA, et al. Standard anti-tuberculosis drug induced hepatotoxicity: do the risk factors matter? JLUMHS. 2010;9(2):84–87.
- Gaude GS, Chaudhury A, Hattiholi J. Drug-induced hepatitis and the risk factors for liver injury in pulmonary tuberculosis patients. J Family Med Prim Care. 2015;4(2):238–243.
- Isa SE, Ebonyi AO, Shehu NY, et al. Antituberculosis drugs and hepatotoxicity among hospitalized patients in Jos, Nigeria. Int J Mycobacteriol. 2016;5(1):21–26.
- Abbara A, Chitty S, Roe JK, et al. Drug-induced liver injury from antituberculous treatment: a retrospective study from a large TB centre in the UK. BMC Infect Dis. 2017;17(1):231.
- Makhlouf HA, Helmy A, Fawzy E, et al. A prospective study of antituberculous drug-induced hepatotoxicity in an area endemic for liver diseases. Hepatol Int. 2008;2(3):353–360.
- Singh J, Arora A, Garg PK, et al. Antituberculosis treatment-induced hepatotoxicity: role of predictive factors. Postgrad Med J. 1995;71(836):359–362.
- Saha A, Shanthi FXM, Winston AB, et al. Prevalence of hepatotoxicity from antituberculosis therapy: a five-year experience from South India. J Prim Care Community Health. 2016;7(3):171–174.
- Wang JY, Liu CH, Hu FC, et al. Risk factors of hepatitis during anti-tuberculous treatment and implications of hepatitis virus load. J Infect. 2011;62(6):448–455.
- Moshage HJ, Janssen JA, Franssen JH, et al. Study of the molecular mechanism of decreased liver synthesis of albumin in inflammation.. J Clin Invest. 1987;79(6):1635–1641.
- Chung-Delgado K, Revilla-Montag A, Guillen-Bravo S, et al. Factors associated with anti-tuberculosis medication adverse effects: a case-control study in Lima, Peru. PloS One. 2011;6(11):e27610.
- Kassa E, Enawgaw B, Gelaw A, et al. Effect of anti-tuberculosis drugs on hematological profiles of tuberculosis patients attending at University of Gondar hospital, Northwest Ethiopia. BMC Hematol. 2016;16:1.
- Mirlohi MS, Ekrami A, Shirali S, et al. Hematological and liver toxicity of anti-tuberculosis drugs. Electron Physician. 2016;8(9):3005–3010.
- Ministry of Health of Botswana. Handbook of the Botswana 2016 integrated hiv clinical care guidelines. [cited 2020 Mar 26]. Available from: https://aidsfree.usaid.gov/sites/default/files/botswana_art_2016.pdf
- Castagna A, Maggiolo F, Penco G, et al. Dolutegravir in antiretroviral-experienced patients with raltegravir- and/or elvitegravir-resistant HIV-1: 24-week results of the phase III VIKING-3 study. J Infect Dis. 2014;210(3):354–362.
- Raffi F, Rachlis A, Stellbrink HJ, et al. Once-daily dolutegravir versus raltegravir in antiretroviral-naive adults with HIV-1 infection: 48 week results from the randomised, double-blind, non-inferiority SPRING-2 study. Lancet. 2013;381(9868):735–743.