ABSTRACT
Introduction
Since the onset of the pandemic, prescribing antimicrobials has become a common practice to treat patients infected with COVID-19.
Areas covered
A systematic literature search was performed in the electronic databases MEDLINE, CINAHL, WHO COVID-19 database, including EMBASE, Scopus, WHO-COVID, LILACS, and Google Scholar to identify original articles published up to 31 July 2021. A random-effects model was used to estimate the pooled prevalence or proportion of antimicrobial consumption among COVID-19 patients.
Expert opinion
We identified 43 original articles, 33 studies from high-income countries, six from upper-middle-income countries, and four from lower-middle-income countries. Most of the studies presented data from hospital or secondary health-care settings (n = 34). Included studies measured antimicrobial consumption as Daily Defined Doses (DDD) or day of therapy (DOT) or percentage. A total of 19 studies measured antimicrobial consumption as DDDs or DOT. Meta-analysis revealed an overall high antimicrobial consumption of 68% (95% CI: 60% to 75%). The subgroup analysis found a lower consumption in high-income countries (58%, 95% CI: 48% to 67%), compared with lower and middle-income countries (89%, 95% CI: 82% to 94%). High antimicrobial consumption found in COVID-19 patients demands implementation of appropriate antimicrobial stewardship interventions.
1. Introduction
The outbreak of infection caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2; COVID-19), from Wuhan, China [Citation1] in December 2019, escalated rapidly to become a global pandemic [Citation2,Citation3]. As of 9 July 2021, about 222 million people were infected with COVID-19, and 4.6 million deaths occurred worldwide [Citation4]. Moreover, COVID-19 overshadowed most other aspects of the health-care systems in every region of the world [Citation5].
The primary concern of excessive antimicrobial prescribing in a pandemic is the emergence of antimicrobial resistance (AMR), which is already a global issue aggravated by the inappropriate use of antimicrobial therapy [Citation6,Citation7]. It has been estimated that by 2050, the number of deaths due to antimicrobial-resistant infections will reach 10 million annually [Citation8]. During the COVID-19 pandemic, trends of antimicrobial consumption fluctuated in different waves. High consumption of azithromycin, doxycycline, hydroxychloroquine, and other antibiotics was observed in the community during the first wave, followed by a decrease in the consumption with the introduction of a more clear treatment guidelines [Citation9–11]. There is growing evidence suggesting that high antimicrobial consumption reported in the first wave or first few months into the pandemic was [Citation4,Citation9] due to suspected or confirmed secondary co-infection in COVID-19 patients [Citation12,Citation13]. Several assumptions were put forward to describe a drastic increase in AMR infections in a single-center study [Citation14]; however, there was no convincing data worldwide to support the evidence of increased risk of AMR during the COVID-19 pandemic. Inappropriate exposure to antimicrobials remains a crucial factor for AMR [Citation15].
Despite COVID-19 being a viral disease, antimicrobials in these patients have been common practice, especially at the beginning of the pandemic [Citation2]. According to Abelenda-Alonso et al., during the first wave of the COVID-19 pandemic, antimicrobial use drastically rose in April 2020 despite the use of antimicrobials being similar in January and February 2019 and 2020 [Citation12]. Even though the prevalence of co-infection (3.5%) and secondary infection (14.3%) in hospitalized COVID-19 patients were relatively low, antimicrobial were frequently prescribed [Citation5,Citation13,Citation16]. A rapid review and meta-analysis of studies published in the first six months of the pandemic (up to June 2020) revealed high antibiotic consumption of 75% among patients with COVID-19, which were far higher than estimated co-infection rates among patients with COVID-19 [Citation17]. Additionally, an observational cross-sectional study in Spain at the peak of the first wave found that antimicrobial consumption was raised from 79.94 in 2019 to 141.10 Defined Daily Doses (DDD)/100 bed days in 2020 [Citation18].
The increased use of antimicrobials and the associated contribution to AMR risk during the global pandemic collectively pose a major public health threat. In this systematic review, we assessed the consumption of antimicrobials amid the COVID-19 pandemic in different parts of the world, with a meta-analysis aimed to estimate the proportion of COVID-19 patients receiving antibiotics.
2. Materials and methods
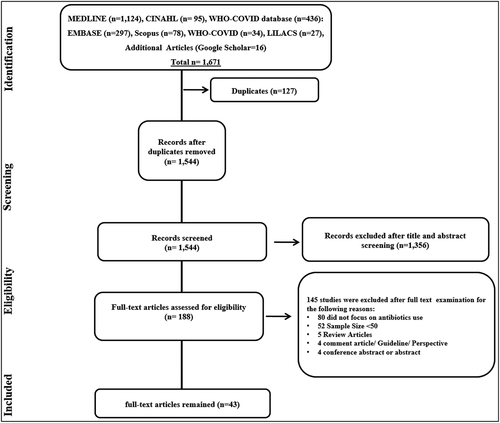
We conducted a systematic review and meta-analysis followed Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for reporting [Citation19] in . The primary investigator (SK) developed and applied eligibility criteria to examine original articles independently reviewed by another author (SSH). Preprints and original studies that reported on other aspects of antimicrobials, such as antimicrobial stewardship (AMS), were not included. Also, studies where the focus was not on antibiotics, use among COVD-19 patients or pre-COVID-19 pandemic were excluded. This was done to have meaningful results and discussion on antibiotics consumption amid COVID-19. The meta-analysis was limited to studies involving patients having confirmed COVID-19 by polymerase chain reaction (PCR) testing. Titles and abstracts were screened to remove studies that did not include the pandemic period for antimicrobial consumption.
Figure 1. PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) flow diagram of the process of study selection.
2.1. Searching
The defined full search strategy in identified original articles on antimicrobial consumption associated with the COVID-19 patients. The following search terms were used in different combinations to extract the relevant articles: ‘ antibiotics, antibacterial, antimicrobials, antibiotic use, antimicrobial use and antibiotic, antimicrobial prescribing, COVID-19 or coronavirus or 2019-ncov or SARS-CoV-2 or CoV-19.’
Table 1. Search strategy
2.2. Study selection
The primary investigator (SK) screened all retrieved titles and abstracts to determine their potential relevance. The assessed abstracts were assessed independently by a second investigator (SSH) against five inclusion criteria: (i) original research studies with a minimum sample of 50 patients focusing on antibiotics use; (ii) antimicrobial consumption; (iii) patients with COVID-19 or consumption amid COVID-19 pandemic; (iv) any age group or gender; and (v) reported in the English language.
2.3. Information sources
The following databases were searched to identify original articles published between 1 December 2019, and 31 July 2021: MEDLINE (Medical Literature Analysis and Retrieval System Online, or MED-LARS Online), Cumulative Index to Nursing and Allied Health Literature (CINAHL – Elsevier, Amster-dam, Netherlands), WHO COVID-19 database including studies published in EMBASE (Excerpta Medica dataBASE), Scopus, WHO-COVID and LILACS (Latin American and Caribbean Health Sciences Literature) only. Google Scholar was searched to find additional articles not indexed in the aforementioned databases. The bibliography of each identified original article was also searched for any additional articles relevant to this review and subject to the same eligibility evaluation. All identified titles and abstracts were checked for duplication.
2.4. Data collection process
The primary investigator developed a data collection form using Microsoft Excel to collect extracted data. The data from all the retrieved studies were subsequently collected and tabulated . In addition, two reviewers (SK and SSH) screened all studies selected for this systematic review independently to validate the results. The extracted data were study design, study participants and settings, sample size, outcomes measures, and summarized results.
Table 2. Summary of studies examining antimicrobial consumption during the COVID-19 pandemic or among COVID-19 patients
The study demographics include population, either COVID-19 or non-COVID-19, age, gender, and antimicrobial consumption. This review aims to assess antimicrobial consumption during the pandemic and estimate the proportion of COVID-19 positive patients receiving antibiotics, where the medication is typically prescribed empirically due to suspected co-infection.
2.5. Classification of outcomes
The outcome measure was antimicrobial consumption (excluding repurposed drugs such as remdesivir) in hospital and community settings. Antimicrobial consumption is presented as frequency, percentage, ratios, defined daily doses (DDD; as defined by World Health Organization (WHO), or day of therapy (DOT). All relevant original articles included in this review include antimicrobial consumption and antimicrobial prescribing.
2.6. Assessment of quality/ risk of bias
The primary investigator independently assessed the quality of each of the included studies and discussed their assessments with the second author to achieve consensus. The modified version of the widely used Newcastle-Ottawa Scale (NOS) was used to measure biases in included studies [Citation22]. The primary investigator rated each paper using the NOS assessment methods to select study participants, confounding, appropriate statistical methods, and measure outcome variables.
2.7. Data analysis
Pooled prevalence or proportion with 95% confidence intervals (CIs) was used as a summary measure. The number of COVID-19 patients and the number of COVID-19 who received antibiotic therapy were used. The statistical heterogeneity was reported using I2 and Q statistics. We examined the heterogeneity between studies using the I2 statistics and the χ2 test, with significant heterogeneity at 50% and P < 0.10, respectively. Subgroup analysis was conducted to determine potential differences based on regions. All the analyses were performed using Meta XL, version 5.3 (EpiGear International, Queensland, Australia). The default option of MetaXL, double arcsine transformed prevalence, was used to run meta-analysis of prevalence with random effects model.
3. Results
3.1. Search results
The initial screening for search strategy generated 1,671 exclusive titles, as shown in the PRISMA flow diagram (). After the search records, title, abstract, and full-text screening, 43 articles have met the inclusion criteria for the review. Studies included in this review were abstracted from 19 countries, including 33 studies from high-income countries (HICs) and 10 from lower & middle-income countries (LMICs), which were classified by the World Bank [Citation23]. The 11 studies from the US, nine from the UK (including two from Scotland), four from Spain, two from China, two from Singapore and Bangladesh, and one each from France, Germany, India, Ireland, Jordan, Mexico, Netherland, Norway, Oman, Portugal, Pakistan, Kosovo, and Turkey, respectively.
Of the 43 studies, 34 studies presented data from hospital or secondary health-care services. In contrast, only six studies [Citation9,Citation10,Citation24–27] presented data from primary health-care services, in which four studies were from the UK and two from the USA. Most of the studies were retrospective [Citation6,Citation9,Citation10,Citation24–53], with only three prospective in design [Citation54–56]. Most of the studies included are observational, comparative, and surveys using the WHO Global Point Prevalence Survey-GPPS.
The majority of the studies reported antimicrobial consumption in percentage or Daily Defined Dose (DDD) or day of therapy (DOT). A total of 19 studies measured antimicrobial consumption as Daily De-fine Dose (DDD) or day of therapy (DOT) as per WHO guidelines or ratios. Majority (n = 29) of the studies were published in 2021 [Citation6,Citation24–26,Citation28,Citation29,Citation31–38,Citation42,Citation45,Citation47,Citation48,Citation50,Citation52–55,Citation57–62], while 14 were published in 2020 [Citation9,Citation10,Citation27,Citation39–41,Citation43,Citation44,Citation46,Citation56,Citation63–65], as shown in .
3.2. Quality assessment
presents the quality assessment of included studies using the Newcastle-Ottawa Scale. Our quality assessment revealed eight studies that were of unsatisfactory quality (scoring three or less); all others were either of good quality (n = 12) or of satisfactory quality (n = 23).
3.3. Antimicrobial consumption in high-income countries
In this review, 33 studied represent data from high-income countries, eleven studies from the US, nine studies from the UK, four from Spain, two from Singapore, and each study from France, Germany, Ireland, Netherland, Norway, Oman, and Portugal. Out of 33, six studies presented data from primary health-care services [Citation9,Citation10,Citation24–27]. In addition, one study from England compared the changes and trends of out-of-hours (OOH) antimicrobial prescription pre-pandemic and during the first pandemic wave [Citation26]. Other studies evaluated antimicrobial prescribing in North West London through COVID-19 waves [Citation24] and antimicrobial prescribing during the pandemic to treat RTI/UTI [Citation25].
A brief US outpatient antibiotic prescribing data report described the pandemic’s impact; data represented monthly prescription fills per 1000 person. This study determined a significant decrease in antimicrobial prescription fills in comparison with previous years. However, the consumption of azithromycin, amoxicillin/clavulanate, and levofloxacin, measured as monthly prescription fills per 1000 persons, was not changed between April and July 2020 [Citation27]. Another study from the USA also examined outpatient antimicrobial prescriptions from 2017 to 2020 and found that the number of antimicrobials dispensed from Jan to May 2020 decreased; significant declines were identified in April and May, with a drop of −39% and −42%, respectively [Citation9]. The only Norwegian study representing total national sales of systemic antimicrobials in 2019 and 2020 revealed noticeably increased sales of antimicrobials in March 2020 (+15.9% from Feb 2020 and +20.3% from Mar 2019). The sales of azithromycin (DDD/1000 inhabitants/day) increased by 86% from Feb to Mar 2020 [Citation31].
A prospective study from Spain reports increased antimicrobial consumption from +99.4 DDD/1000 OBD and +53.1 DDD/1000 OBD in the first two-quarters during the pandemic compared with the previous year [Citation56]. Santiago et al., and Ponce-Alonso, Fuente et al. also report an increase in DDD during the pandemic [Citation30,Citation41]. Over the same time period, a study focused on the appropriateness of antimicrobial therapy indicated that 23% of patients received an inappropriate prescription (3047/13932), while 44% had an appropriate prescription for antimicrobials (6116/13932) [Citation35]. The Scottish study and study from France also determined increased consumption of antimicrobials in hospitals during the first wave [Citation38,Citation58].
3.4. Antimicrobial consumption in upper-middle-income and lower-middle-income countries
The review also included studies from upper-middle-income and lower- and middle-income countries. Of the 43 studies included in this review, six studies from upper-middle-income countries include two studies from China, one each from Jordan, Kosovo, Mexico, and Turkey. In comparison, four-studies from lower- and middle-income countries included two studies from Bangladesh, each from India and Pakistan.
A study from Jordan measured antimicrobial consumption in total defined daily dose (DDD) per 1000 inhabitants per day and compared national antimicrobial use in 2019 and 2020 [Citation57]. The authors found an evident reduction of 53% in amoxicillin consumption, from 8.4 DDD per 1000 inhabitants per day in 2019 to 3.7 DDD per 1000 inhabitants per day in 2020.
A study determined that 88% of patients were prescribed antimicrobials without performing any culture testing in Pakistan and antimicrobial consumption increased during the pandemic. For example, azithromycin use increased from 11.5 DDDs per 100 occupied bed-days in 2019 to 17.0 DDDs per 100 occupied bed-days in 2020, and ceftriaxone from 20.2 DDDs per 100 occupied bed-days in 2019 to 25.1 DDDs per 100 occupied bed-days in 2020. Almost all antimicrobials prescribed were from the Watch category (WHO AWaRe category) [Citation33]. Total sales of antibiotic doses were measured in a study from India, which showed high consumption of HCQ and azithromycin during a pandemic [Citation38].
A study from Turkey published representative sample data from tertiary care hospitals comparing DDD during the pre-pandemic period (PPP) and pandemic period (PP). The total consumption of antimicrobials in PPP was 725.8 DDD/1000 inpatient days, which increased to 811.4 DDD/1000 inpatient days during the PP (p = 0.002). The most consumed antimicrobials during the pandemic period included ceftriaxone, piperacillin/tazobactam, and meropenem. Ceftriaxone increased to 224.6 from 199.6 DDD/1000 inpatient-days, meropenem 86.4 from 65.1 DDD/1000 inpatients-days, and piperacillin-tazobactam raised 63.6 from 35.5 DDD/1000 inpatients-days while increasing utilization of teicoplanin to 11.6 to 35.5 DDD/1000 inpatient-days was also noted [Citation6].
3.5. Overall antimicrobial consumption
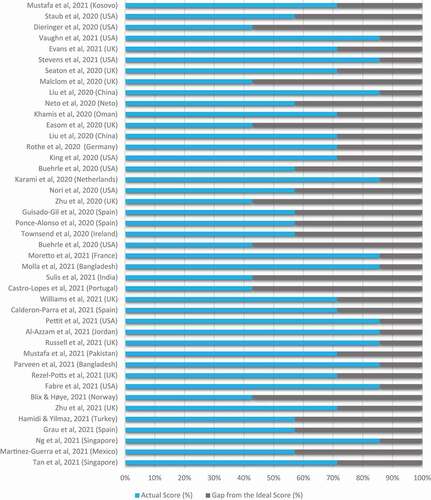
Our meta-analysis examined the overall antimicrobial consumption using data from different countries and regions of the world. A total of 27 studies presented the total number of COVID-19 patients and the number of patients who received antimicrobial therapy (). Overall, antimicrobial use occurred in 68% (95% CI: 60% to 75%) of patients with COVID-19 patients, using data from 27 studies. Subgroup analyses of studies from high-income countries (19 studies) revealed antimicrobial use in 58% (95% CI: 48% to 67%; ) in COVID-19 patients, compared with 89% (95% CI: 82% to 94%; ) in lower and middle-income countries (8 studies)
Figure 3. Pooled antimicrobial consumption estimate (%) in COVID-19 patients (Heterogeneity: I2 = 100%; p = 0.001).
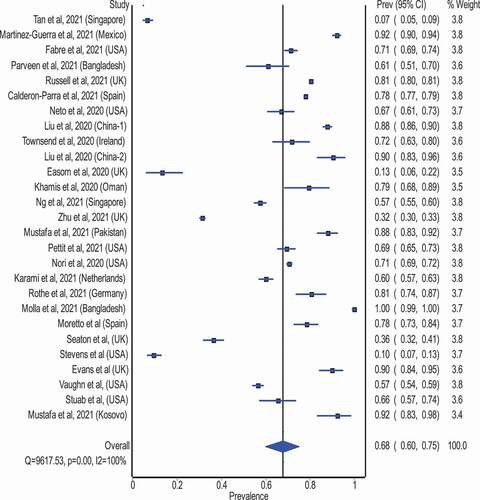
Figure 4. Pooled proportion of COVID-19 patients prescribed an antibiotic in high-income countries.
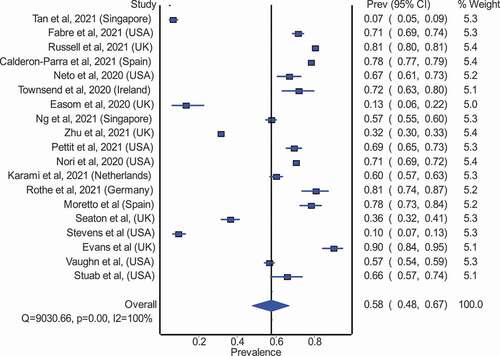
Figure 5. Pooled proportion of COVID-19 patients prescribed an antibiotic in low-middle income countries [NS: Not Specified, AMC: Antimicrobial/ antibiotic consumption, PPS: Point Prevalence Survey, PO: Per Oral, IV: Intravenous, WHO ATC: World Health Organization Anatomical Therapeutic Chemical, DDD: Defined Daily Dose, PPP: Pre-Pandemic Period, PP: Pandemic Period, ICU: Intensive Care Unit, CABP: Community-Acquired Bacterial Pneumonia, BDOC: Bed Days of Care, CAP: Community-Acquired Pneumonia, bCAP: Bacterial-Community Acquired Pneumonia, HAP/VAP: Hospital-Acquired Pneumonia/ Ventilator-Associated Pneumonia, PCT: Procalcitonin, SI: Suspected bacterial infection, NI: No evidence of bacterial infection. LRTI: Lower Respiratory Tract Infection, HCFA – CDI: Healthcare Facility-Associated Clostridioides Difficile Infection, CLABI: Central Line-Associated Bloodstream Infection, IQR: Interquartile Range, HCQ: Hydroxychloroquine, DP: Days Present].
![Figure 5. Pooled proportion of COVID-19 patients prescribed an antibiotic in low-middle income countries [NS: Not Specified, AMC: Antimicrobial/ antibiotic consumption, PPS: Point Prevalence Survey, PO: Per Oral, IV: Intravenous, WHO ATC: World Health Organization Anatomical Therapeutic Chemical, DDD: Defined Daily Dose, PPP: Pre-Pandemic Period, PP: Pandemic Period, ICU: Intensive Care Unit, CABP: Community-Acquired Bacterial Pneumonia, BDOC: Bed Days of Care, CAP: Community-Acquired Pneumonia, bCAP: Bacterial-Community Acquired Pneumonia, HAP/VAP: Hospital-Acquired Pneumonia/ Ventilator-Associated Pneumonia, PCT: Procalcitonin, SI: Suspected bacterial infection, NI: No evidence of bacterial infection. LRTI: Lower Respiratory Tract Infection, HCFA – CDI: Healthcare Facility-Associated Clostridioides Difficile Infection, CLABI: Central Line-Associated Bloodstream Infection, IQR: Interquartile Range, HCQ: Hydroxychloroquine, DP: Days Present].](/cms/asset/3ec535df-e623-438d-954a-543d89970508/ierz_a_2011719_f0005_oc.jpg)
4. Discussion
This systematic review aimed to assess antimicrobial consumption during the COVID-19 pandemic or among COVID-19 patients. Potential overuse and irrational antimicrobial prescribing have become a complex conundrum for health care. This can impact patient safety, increases the risk of adverse drug events, more resistant infections, progressive antimicrobial resistance (AMR), and an incremental economic burden to the health-care system [Citation66,Citation67]. The leading cause of respiratory tract infections, mainly upper respiratory tract infections (URTI), are viruses [Citation68]. Corresponding to the WHO stated that 71% of URTIs are usually treated with antimicrobials while fewer than 10% of URTIs are bacterial infections [Citation69].
Although COVID-19 is a viral disease, the prescribing of antimicrobials has become a more common practice in many countries since the onset of the pandemic [Citation2]. This high antimicrobial consumption in COVID-19 patients was initiated after early reports from China revealed 50% of patients died from secondary bacterial infection [Citation70]. This particular finding of increased mortality risk due to secondary bacterial infection during the early phase of the pandemic resulted in a very high antimicrobial consumption of 68% (95% CI: 60% to 75%), suggesting that 68 out 100 COVID-19 patients received antimicrobial therapy. As expected, antimicrobial consumption in high-income countries was lower compared with lower and middle-income countries (58% versus 85%). This is expected because of better health-care systems and support available in high-income countries.
High consumption of antimicrobials was observed during the first two waves of the COVID-19 pandemic, while this declined later in the pandemic. The antibiotic use was substantial during the first few months into the pandemic in several high-income countries, but this trend rapidly changed in many of these areas after the introduction of more clear guidelines recommending against the routine use of antibiotics in patients with COVID-19. For example, the ISARIC WHO CCP-UK study reported that 46,061 inpatients with COVID-19 received medical management. Of 46,061 patients, 39,258 (85%) received at least one or more antimicrobials during their hospital stay. The use of antimicrobials was highest in March–April 2020, which later declined from May 2020. A significant decline was observed during March–May 2020 in critical care units in Scotland and the South-West region of England at ward level. This multicentre study also revealed that about 18% (8,649 out of 48,902) of patients had microbial testing, and only 1,107 patients were identified as having a blood-stream infection [Citation55]. Another study by Zhu et al. analyzed prescribing antimicrobials across two pandemic waves in 351 General Practitioners (GP) practices in Northwest London (NWL). During the study period (Jan–Nov 2020), a decreasing trend was noticed during the first lockdown in March 2020. However, a long-term constant decreasing pattern in the volume of antimicrobial prescriptions was evident across NWL before the pandemic. Of 33,708 COVID-19 positive patients, 6,158 patients received at least one antimicrobial prescription during the study period [Citation24]. Similarly, Rezel-Potts et al., in a large population-based study exploring antimicrobials prescribing by GPs from 2017 up to the first eight months of the pandemic periods, found elevated antimicrobials prescribing in March 2020 followed by a reduction between April and August 2020, reaching a minimum in May 2020 [Citation25].
Despite the high consumption of antimicrobials in most studies, some reported significantly lower usage of antimicrobials during the pandemic. For example, a study from Jordan that measured antimicrobial consumption in total defined daily dose (DDD) per 1000 inhabitants per day found an evident reduction of 53% in amoxicillin consumption, from 8.4 DDD per 1000 inhabitants per day in 2019 to 3.7 DDD per 1000 inhabitants per day in 2020 [Citation57] Similar overall reductions in antimicrobial consumption were also reported by studies from the UK [Citation24,Citation25]. The use of high-risk antimicrobials, such as carbapenem (52%), a macrolide (57%), and azithromycin (74%) was prevalent in Jordan [Citation57]. This overuse of antimicrobials has significant long-term consequences, one of which could possibly be an increase in antimicrobial resistance [Citation16,Citation71]. This may result in outbursts of multidrug drug resistance (MDR) infections, a disruption in national stewardship programs, infection control and prevention measures, and potential effects on immunization programs for preventable diseases [Citation5,Citation16].
The use of antimicrobials without consultation and culture testing was prevalent, particularly in lower-middle-income countries. For example, in a study from Bangladesh, 45% of patients received antimicrobials without proper consultation [Citation72]. The antimicrobial consumption was mainly attributed to bacterial co-infection among patients with COVID-19 patients [Citation73], but many patients received antimicrobials without culture testing. For example, a study determined that 88% of patients were prescribed antimicrobials without performing any culture testing in Pakistan [Citation33]. Similarly, the ISARIC WHO CCP-UK study revealed that only 18% (8,649 out of 48,902) of patients had microbial testing, and only 1,107 patients were identified with blood-stream infection [Citation55]. In addition, there was widespread empirical antimicrobial utilization; more than 90% had antimicrobial therapy during hospitalization [Citation6,Citation50]. In addition, a study from India determined the sale of antibiotics, which revealed a decreasing trend in 2020 compared with 2019, except for azithromycin and HCQ that were peaked during the initial phase [Citation11]. Many COVID-19 patients received antimicrobial therapy, which included a range of antimicrobials was used. Ceftriaxone, azithromycin, and piperacillin/tazobactam were the most commonly prescribed antimicrobials [Citation63,Citation65]. Most antimicrobials prescribed during the pandemic were from the Watch category (WHO AWaRe category) [Citation33].
Almost all studies reported azithromycin as the most prescribed or consumed antimicrobial at the beginning of the pandemic. For example, two studies from the USA revealed increased use of azithromycin during the initial pandemic period [Citation9,Citation27]. In addition, many patients received azithromycin as atypical coverage for COVID-19 management [Citation6,Citation54]. Azithromycin has been touted as a potential treatment of COVID-19 along with hydroxychloroquine in the early phases of the COVID-19 pandemic. However, studies revealed no evidence of clinical improvement later in the pandemic with or without azithromycin [Citation74,Citation75].
5. Conclusion
The present review found an overall high antimicrobial consumption among COVID-19 patients. During the first few months of the COVID-19 pandemic, a substantial increase of antimicrobials was also observed in several high-income countries in primary and secondary health care. In LMIC and LIC, trends in hospitalized revealed inappropriate use of the antimicrobials therapy. The meta-analysis in our review showed 68% of patients with COVID-19 received antimicrobial therapy. The antimicrobial use in high-income countries is 58% in COVID-19 patients, compared with 89% in lower and middle-income countries.
6. Expert opinion
The contributing factor for the substantial increase in antimicrobial consumption was the risk of secondary infection in the initial days of the pandemic, which was the major contributor to the number of deaths among patients with COVID-19. Later in the pandemic, several studies reported the empirical use of antimicrobial in patients with COVID-19, especially among patients with moderate to severe disease. In addition, the second contributing fact was the absence of proper treatment guidelines, and the confirmation of suspected co-infection in hospitalized patients. The most common antimicrobial prescribed were cephalosporins, azithromycin, co-amoxiclav, and carbapenem in ICU patients. This review presents the antimicrobial consumption and identifies inappropriate antimicrobial therapy prescribed to patients with COVID-19. While decreasing trends in HICs were reported after the first wave, we observed the extensive use of azithromycin was reported in almost all studies. After primary analysis of RECOVERY trial revealed no benefits of azithromycin, decreasing trends in the hospital were also reported. This review also highlights the patient safety concern, including adverse drug events, organ damage, increased health care cost, and increased risk of AMR due to irrational or misuse of antimicrobials. Since AMR is also one of the major public health threats and inappropriate use of antimicrobial consumption may also contribute. We also observed that antimicrobial use was prevalent in Low income and LMIC in the absence of microbial culture, and no consultation was opted. All these contribute to the alarming situation to put forward antimicrobial stewardship approach and proper guidelines to combat the severity of the disease in COVID-19 patients. In our meta-analysis, 67% of COVID-19 patients who received an antibiotic are lower than 75% reported in the previous meta-analysis by Langford et al. This difference could result from using more strict inclusion criteria in our review. For example, the inclusion of studies with a sample size of more than 50 reduces the risk of overestimated antibiotic consumption. Moreover, the previous meta-analysis by Langford et al. covered studies of the first six months of the pandemic when antibiotics were prescribed to almost all COVID-19 positive patients due to its potential mortality benefits, as indicated in early reports.
The review has some limitations which should be considered while interpreting the results. Firstly, the information about the incidence of co-infection and compliance with antimicrobial stewardship strategies was not analyzed to compare infection rates and risk of antimicrobial resistance in COVID-19 and non-COVID patients. Secondly, the review did not include antimicrobial use in the immunocompromised patient, who are most vulnerable and at higher risk of resistant pathogen infections, and empirical therapy must be offered to minimize the risk of secondary infection. Non-evidence-based and empirical antimicrobial use will contribute to AMR and increase healthcare costs and healthcare burden. Therefore, the rationalized and judicious use of antimicrobial therapy should be encouraged. Various antimicrobial stewardship elements must be modified to meet the targets during the ongoing and future pandemics. The development of local and international guidelines, monitoring and documentation of national antimicrobial consumption, antimicrobial resistance, and antimicrobial stewardship strategies should be pledged to combat inappropriate use, particularly during a global pandemic.
Article highlights
Since the inception of the COVID-19 pandemic, an increased focus on antimicrobial consumption was observed.
The antimicrobial includes amoxicillin, amoxicillin/clavulanic acid, azithromycin, doxycycline, and hydroxychloroquine was frequently prescribed during the first wave in primary and secondary health-care settings.
Empirical use of broad-spectrum antimicrobial was also observed in patients with COVID-19 during hospitalization.
The most common antimicrobial prescribed in almost all included studies was azithromycin.
After the study revealed that azithromycin has no benefits on hospitalized patients, rapid decrease was observed in most studies.
An increasing trend of antimicrobials use was observed in the first few months of the COVID-19 pandemic, which decreased rapidly after the proper guideline was established.
In low-middle income and low-income countries, irrational antimicrobial use was observed in the absence of microbial cultures.
The rationalized and judicious use of antimicrobial therapy should be encouraged.
Antimicrobial stewardship policies should be assured to reduce inappropriate antimicrobial use, particularly during a global pandemic.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Funding
This paper was not funded.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
References
- Phelan AL, Katz R, Gostin LO. The novel coronavirus originating in Wuhan, China: challenges for global health governance. Jama. 2020;323(8):709–710.
- Murgadella-Sancho A, Coloma-Conde A, Oriol-Bermúdez I. Impact of the Strategies Implemented by an Antimicrobial Stewardship Program on the Antibiotic Consumption in the Covid-19 Pandemic. Infect Control Hosp Epidemiol. 2021;1–5. https://doi.org/10.1017/ice.2021.237
- Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062.
- WHO COVID-19 Dashboard. Geneva: world Health Organization; 2020 [ cited 2021 23 May]. Available from: https://covid19.who.int
- Rawson TM, Ming D, Ahmad R, et al. Antimicrobial use, drug-resistant infections and COVID-19. Nature Rev Microbiol. 2020;18(8):409–410.
- Hamidi AA, Yilmaz Ş. Antibiotic consumption in the hospital during COVID-19 pandemic, distribution of bacterial agents and antimicrobial resistance: a single-center study. J Surg Med. 2021;5(2):124–127
- Aldeyab M, López-Lozano J-M, Gould IM. Global antibiotics use and resistance. Global Pharmaceutical Policy: Springer; 2020. p. 331–344.
- O’Neill J. Review on Antimicrobial Resistance: tackling a crisis for the health and wealth of nations. UK; 2014.
- King LM, Lovegrove MC, Shehab N, et al. Trends in US outpatient antibiotic prescriptions during the COVID-19 pandemic. Clinl Infect Dis. 2020;70(3):370–377.
- Malcolm W, Seaton RA, Haddock G, et al. Impact of the COVID-19 pandemic on community antibiotic prescribing in Scotland. JAC Antimicrob Resist. 2020;2(4):dlaa105.
- Sulis G, Batomen B, Kotwani A, et al. Sales of antibiotics and hydroxychloroquine in India during the COVID-19 epidemic: an interrupted time series analysis. PLoS Med. 2021;18(7):e1003682.
- Abelenda-Alonso G, Padullés A, Rombauts A, et al. Antibiotic prescription during the COVID-19 pandemic: a biphasic pattern. Infect Control Hosp Epidemiol. 2020 07; 41(11): 1371–1372.
- Lansbury L, Lim B, Baskaran V, et al. Co-infections in people with COVID-19: a systematic review and meta-analysis. J Infect. 2020;81(2):266–275.
- Clancy CJ, Buehrle DJ, Nguyen MHPRO, et al. pandemic will result in increased antimicrobial resistance rates. JAC Antimicrob Resist. 2020;2(3):3.
- Collignon P, Beggs JJ, Walsh TR, et al. Anthropological and socioeconomic factors contributing to global antimicrobial resistance: a univariate and multivariable analysis. Lancet Planet Health. 2018;2(9):e398–e405.
- Rawson TM, Moore LSP, Zhu N, et al. Bacterial and Fungal Coinfection in Individuals With Coronavirus: a Rapid Review To Support COVID-19 Antimicrobial Prescribing. Clin Infect Dis. 2020;71(9):2459–2468.
- Langford BJ, So M, Raybardhan S, et al. Antibiotic prescribing in patients with COVID-19: rapid review and meta-analysis. In Clinical microbiology and infection: the official publication of the European Society of Clinical Microbiology and Infectious Diseases 2021; 27(4) :520–531.
- Tejedor Prado P, Lázaro Cebas A, Sánchez Artola B, et al. editors. 4CPS-246 Coronavirus first wave effect on antibiotic consumption and antimicrobial resistance. London, UK: British Medical Journal Publishing Group; 2021.
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
- Zhu NJ, McLeod M, McNulty CAM, et al. Trends in Antibiotic Prescribing in Out-of-Hours Primary Care in England from January 2016 to June 2020 to Understand Behaviours during the First Wave of COVID-19. Antibiotics (Basel). 2021 Jan 1;10:1.
- Liu H, Gao J, Wang Y, et al. Epidemiological and clinical characteristics of 2019 novel coronavirus disease (COVID-19) in Jilin, China: a descriptive study. Medicine (Baltimore). 2020;99:47.
- Wells G, Shea B, O’connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa Hospital Research Institute. Clin Epidemiol. 2016;
- Bank W. Data: country Classification 2021. [updated Jul 2021]. Available from: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups
- Zhu N, Aylin P, Rawson T, et al. Investigating the impact of COVID-19 on primary care antibiotic prescribing in North West London across two epidemic waves. In Clinical microbiology and infection: the official publication of the European Society of Clinical Microbiology and Infectious Diseases 2021;27(5) :762–768 .
- Rezel-Potts E, Esperance V, Gulliford MC. Antimicrobial stewardship in the UK during the COVID-19 pandemic: a population-based cohort study and interrupted time-series analysis. Brjgenpract. 2021;71(706):e331–e338.
- Zhu NJ, McLeod M, McNulty CAM, et al. Trends in Antibiotic Prescribing in Out-of-Hours Primary Care in England from January 2016 to June 2020 to Understand Behaviours during the First Wave of COVID-19. Antibiotics (Basel). 2021;10:1.
- Buehrle DJ, Nguyen MH, Wagener MM, et al. editors. Impact of the Coronavirus Disease 2019 Pandemic on Outpatient Antibiotic Prescriptions in the United States. Open forum infectious diseases Oxford University Press US; 2020.
- Tan SH, Ng TM, Tay HL, et al. A point prevalence survey to assess antibiotic prescribing in patients hospitalized with confirmed and suspected coronavirus disease 2019 (COVID-19). J Glob Antimicrob Resist. 2021;24:45–47.
- Ng TM, Tan SH, Heng ST, et al. Effects of coronavirus disease 2019 (COVID-19) pandemic on antimicrobial prevalence and prescribing in a tertiary hospital in Singapore. Antimicrob Resist Infect Control. 2021;10(1):28.
- Grau S, Echeverria-Esnal D, Gómez-Zorrilla S, et al. Evolution of antimicrobial consumption during the first wave of COVID-19 pandemic. Antibiotics (Basel). 2021;10:2.
- Blix HS, Høye S. Use of antibiotics during the COVID-19 pandemic. Tidsskrift for den Norske laegeforening: tidsskrift for praktisk medicin, ny raekke. 2021;141:4.
- Fabre V, Karaba S, Amoah J, et al. The role of procalcitonin in antibiotic decision-making in Covid-19 infection. Infect Control Hosp Epidemiol. 2021 Apr;19:1–24.
- Mustafa ZU, Salman M, Aldeyab M, et al. Antimicrobial consumption among hospitalized patients with COVID-19 in Pakistan. SN Compreh Clin Med. 2021;1–5 doi:https://doi.org/10.1007/s42399-021-00966-5.
- Pettit NN, Nguyen CT, Lew AK, et al. Reducing the use of empiric antibiotic therapy in COVID-19 on hospital admission. BMC Infect Dis. 2021;21(1):1–7.
- Calderón-Parra J, Muiño-Miguez A, Bendala-Estrada AD, et al. Inappropriate antibiotic use in the COVID-19 era: factors associated with inappropriate prescribing and secondary complications Analysis of the registry SEMI-COVID. PloS One. 2021;16(5):e0251340.
- Williams EJ, Mair L, de Silva TI, et al. Evaluation of procalcitonin as a contribution to antimicrobial stewardship in SARS-CoV-2 infection: a retrospective cohort study. J Hosp Infect. 2021;110:103–107.
- Castro-Lopes A, Correia S, Leal C, et al. Increase of antimicrobial consumption in a tertiary care hospital during the first phase of the COVID-19 pandemic. Antibiotics. 2021;10(7):778.
- Sulis G, Sayood S, Gandra S. Antimicrobial resistance in low- and middle-income countries: current status and future directions. In Expert review of anti-infective therapy. 2021.
- Buehrle DJ, Decker BK, Wagener MM, et al. Antibiotic consumption and stewardship at a hospital outside of an early coronavirus disease 2019 epicenter. Antimicrob Agents Chemother. 2020;64(11):11.
- Townsend L, Hughes G, Kerr C, et al. Bacterial pneumonia coinfection and antimicrobial therapy duration in SARS-CoV-2 (COVID-19) infection. JAC Antimicrob Resist. 2020 09; 2(3): dlaa071–dlaa071.
- Ponce-Alonso M, Sáez de La Fuente J, Rincón-Carlavilla A, et al. Impact of the coronavirus disease 2019 (COVID-19) pandemic on nosocomial Clostridioides difficile infection. Infect Control Hosp Epidemiol. 2021;42(4) :406–410.
- Nori P, Cowman K, Chen V, et al. Bacterial and fungal coinfections in COVID-19 patients hospitalized during the New York City pandemic surge. Infect Control Hosp Epidemiol. 2021 Jan;42(1):84–88.
- Karami Z, Knoop BT, Dofferhoff ASM, et al. Few bacterial co-infections but frequent empiric antibiotic use in the early phase of hospitalized patients with COVID-19: results from a multicentre retrospective cohort study in The Netherlands. Infect Dis (Lond). 2021;53(2) 10:102–110.
- Rothe K, Feihl S, Schneider J, et al. Rates of bacterial co-infections and antimicrobial use in COVID-19 patients: a retrospective cohort study in light of antibiotic stewardship. Eur J Clin Microbiol Infect Dis. 2020 11; 40(4): 859–869.
- Liu C. Antibiotic stewardship challenges in an evolving health-care market in China. Lancet Infect Dis. 2021;21(6):753–754.
- Seaton RA, Gibbons CL, Cooper L, et al. Survey of antibiotic and antifungal prescribing in patients with suspected and confirmed COVID-19 in Scottish hospitals. J Infect. 2020 09; 81(6): 952–960.
- Stevens RW, Jensen K, O’Horo JC, et al. Antimicrobial prescribing practices at a tertiary-care center in patients diagnosed with COVID-19 across the continuum of care. Infect Control Hosp Epidemiol. 2021;42(1):89–92.
- Dieringer TD, Furukawa D, Graber CJ, et al. Inpatient antibiotic utilization in the Veterans’ Health Administration during the coronavirus disease 2019 (COVID-19) pandemic. Infect Control Hosp Epidemiol. 2021;42(6):751–753.
- Staub MB, Beaulieu RM, Graves J, et al. Changes in antimicrobial utilization during the coronavirus disease 2019 (COVID-19) pandemic after implementation of a multispecialty clinical guidance team. Infect Control Hosp Epidemiol. 2021;42(7) :810–816.
- Goncalves Mendes Neto A, Lo KB, Wattoo A, et al. Bacterial infections and patterns of antibiotic use in patients with COVID-19. J Med Virol. 2021 Mar;93(3):1489–1495.
- Pedrotti CHS, Accorsi TAD, De Amicis Lima K, et al. Antibiotic stewardship in direct-to-consumer telemedicine consultations leads to high adherence to best practice guidelines and a low prescription rate. Int J Infect Dis. 2021;105:130–134.
- Liu C, Wen Y, Wan W, et al. Clinical characteristics and antibiotics treatment in suspected bacterial infection patients with COVID-19. Int Immunopharmacol. 2021 Jan 01;90:107157.
- Molla MMA, Yeasmin M, Islam MK, et al. Antibiotic prescribing patterns at COVID-19 dedicated wards in Bangladesh: findings from a single center study. Infect Prav Pract. 2021 June 01;3(2):100134.
- Martinez-Guerra BA, Gonzalez-Lara MF, De-leon-cividanes NA, et al. Antimicrobial resistance patterns and antibiotic use during hospital conversion in the COVID-19 pandemic. 2021 03;10:2.
- Russell CD, Fairfield CJ, Drake TM, et al. Co-infections, secondary infections, and antimicrobial use in patients hospitalised with COVID-19 during the first pandemic wave from the ISARIC WHO CCP-UK study: a multicentre, prospective cohort study. In The Lancet Microbe. 2021.
- Guisado-Gil AB, Infante-Domínguez C, Peñalva G, et al. Impact of the COVID-19 pandemic on antimicrobial consumption and hospital-acquired candidemia and multidrug-resistant bloodstream infections. Antibiotics (Basel). 2020;9:11.
- Al-Azzam S, Mhaidat NM, Banat HA, et al. An assessment of the impact of coronavirus disease (COVID-19) pandemic on national antimicrobial consumption in jordan. Antibiotics. 2021;10(6):690.
- Moretto F, Sixt T, Devilliers H, et al. Is there a need to widely prescribe antibiotics in patients hospitalized with COVID-19? Inter J Infect Dis. 2021;105:256–260.
- Evans TJ, Davidson HC, Low JM, et al. Antibiotic usage and stewardship in patients with COVID-19: too much antibiotic in uncharted waters? J Infect Prev. 2021;22(3):119–125.
- Vaughn VM, Gandhi TN, Petty LA, et al. Empiric antibacterial therapy and community-onset bacterial coinfection in patients hospitalized with coronavirus disease 2019 (COVID-19): a multi-hospital cohort study. Clinl Infect Dis. 2021;72(10):e533–e541.
- Staub MB, Beaulieu RM, Graves J, et al. Changes in antimicrobial utilization during the coronavirus disease 2019 (COVID-19) pandemic after implementation of amultispecialty clinical guidance team. Infect Control Hosp Epidemiol. 2021;42(7):810–816.
- Mustafa L, Tolaj I, Baftiu N, et al. Use of antibiotics in COVID-19 ICU patients. J Infect Developing Countries. 2021;15(4):501–505.
- Monira P, Mahmuda Y M, Maruf Ahmed M. antimicrobial resistance, evidences on irrational anti-microbial prescribing and consumption during COVID-19 pandemic and possible mitigation strategies: a Bangladesh perspective. 2020.
- Easom N, Moss P, Barlow G, et al. Sixty‐eight consecutive patients assessed for COVID‐19 infection: experience from a UK regional infectious diseases unit. Influenza Other Respir Viruses. 2020;14(4):374–379.
- Khamis F, Al-Zakwani I, Al Naamani H, et al. Clinical characteristics and outcomes of the first 63 adult patients hospitalized with COVID-19: an experience from Oman. J Infect Public Health. 2020;13(7):906–913.
- Gandra S, Barter D, Laxminarayan R. Economic burden of antibiotic resistance: how much do we really know? Clin Microbiol Infect. 2014;20(10):973–980.
- Woolhouse M, Waugh C, Perry MR, et al. Global disease burden due to antibiotic resistance–state of the evidence. J Glob Health. 2016;6(1):1.
- Li J, Song X, Yang T, et al. A systematic review of antibiotic prescription associated with upper respiratory tract infections in China. Medicine (Baltimore). 2016;95:19.
- World Health Organization. Medicines use in primary care in developing and transitional countries: fact book summarizing results from studies reported between 1990 and 2006. Geneva: World Health Organization; 2009.
- Lucien MAB, Canarie MF, Kilgore PE, et al. Antibiotics and antimicrobial resistance in the COVID-19 era: perspective from resource-limited settings. Int J Infect Dis. 2021;104:250–254.
- Jirjees FJ, Al-Obaidi HJ, Sartaj M, et al. Antibiotic Use and Resistance in Hospitals: Time-Series Analysis Strategy for Determining and Prioritising Interventions. Hosp. Pharm. Eur. 2020 :13–19. Available online: https://hospitalpharmacyeurope.com/news/reviews-research/antibiotic-use-and-resistance-in-hospitals-time-series-analysis-strategy-for-determining-and-prioritising-interventions/ [(accessed on 11 July 2021)]
- Monira PMMMA, Mahmuda YTNBAA, Ghosh AK. Evidences on irrational anti-microbial prescribing and consumption among COVID-19 positive patients and possible mitigation strategies: a descriptive cross sectional study. (Special Issue: pandemic COVID19). Bangladesh J Infect Dis. 2020;7(Supplementary Issue):S3–S7.
- Chedid M, Waked R, Haddad E, et al. Antibiotics in treatment of COVID-19 complications: a review of frequency, indications, and efficacy. J Infect Public Health. 2021 May;14(5):570–576.
- Oldenburg CE, Doan T. Azithromycin for severe COVID-19. Lancet. 2020;396(10256):936–937.
- Oliver ME, Hinks TSC. Azithromycin in viral infections. Rev Med Virol. 2020;2020:e2163–e2163.