ABSTRACT
Introduction
Single-dose 2-g oral secnidazole (SEC), newly approved by the U.S. Food and Drug administration (FDA) for treatment of trichomoniasis, is a potent 5-nitroimidazole with selective toxicity against various micro-organisms. It has been used internationally to treat trichomoniasis, bacterial vaginosis, and other infections for decades. Trichomoniasis is the most common non-viral sexually transmitted infection worldwide and is associated with significant morbidity. In comparison to the only other FDA-approved treatments for trichomoniasis in the United States – metronidazole and tinidazole – SEC has favorable pharmacokinetics, including a longer half-life and a lower minimal lethal concentration.
Areas covered
This work summarizes the chemistry and pharmacology of SEC and reviews the evidence on its efficacy, tolerability, and safety for the treatment of trichomoniasis.
Expert opinion
SEC is an efficacious, well tolerated, and safe treatment for patients aged ≥12 years with trichomoniasis. Single-dose administration makes it a favorable treatment option, especially in cases where adherence to multi-dose treatment regimens may be low.
1. Introduction
Trichomoniasis is the most prevalent non-viral sexually transmitted infection (STI) worldwide, affecting approximately 156 million people globally, including 5.3% of women and 0.6% of men aged 15–49 years [Citation1] and 3.7 million people in the United States [Citation2]. As a non-reportable STI, reliable prevalence estimates are limited, but a recent population-based epidemiological study found US-based prevalence rates of 2.1% in women and 0.5% in men [Citation3,Citation4]. Significant health disparities exist for T. vaginalis infection: prevalence rates are 9.6% in African American women, 1.4% in Hispanic women, and 0.8% in non-Hispanic White women. Additionally, unlike rates of chlamydia and gonorrhea, T. vaginalis prevalence rates are as high or higher among women aged >24 years as they are for women aged <24 years [Citation5]. Risk factors for trichomoniasis include multiple recent sexual partners and lower socioeconomic status [Citation6]. Women with bacterial vaginosis (BV) have an increased risk for T. vaginalis [Citation7]. Male sexual partners of T. vaginalis-infected women also are likely to be infected [Citation8].
Trichomoniasis is associated with many adverse gynecologic outcomes, including vaginitis, cervicitis, pelvic inflammatory disease, cervical cancer, and infertility [Citation9–11,Citation12,Citation13]. Women with trichomoniasis are also at higher risk for adverse birth outcomes such as preterm birth, premature rupture of membranes, and low birth weight infants [Citation14,Citation15]. Coinfection with BV and T. vaginalis is common [Citation16]. In men, trichomoniasis is associated with epididymitis, non-gonococcal urethritis, and prostatitis [Citation8] and may also lead to infertility [Citation17]. In both men and women, trichomoniasis significantly increases the risk of transmission and acquisition of human immunodeficiency virus (HIV) and other STIs (e.g. chlamydia, gonorrhea, herpes simplex virus, and syphilis) [Citation2,Citation9,Citation18].
As many as 85% of patients with trichomoniasis are asymptomatic [Citation2]. Symptomatic patients generally present with abnormal vaginal discharge, in addition to vaginal and cervical inflammation [Citation2]. Diagnostic testing for trichomoniasis in women seeking care for vaginal discharge is recommended by the Centers for Disease Control and Prevention (CDC). Annual screening for all women with HIV, including those who are pregnant [Citation2] is also recommended. Additionally, annual screening is a consideration for men and women seeking care in high-prevalence settings (e.g. STD clinics and correctional facilities) and for asymptomatic women at high risk for infection (e.g. multiple sexual partners, drug misuse, transactional sex, and/or a history of STIs or incarceration) [Citation2].
Treatment of trichomoniasis is recommended to alleviate symptoms, reduce the risk for adverse reproductive outcomes, and prevent the acquisition and transmission of HIV and other STIs. Recently updated national guidelines published by the American College of Gynecologists and Obstetricians (ACOG) in 2020 and the CDC in 2021 now recommend multi-dose metronidazole (MTZ) 500 mg orally twice daily for 7 days for the treatment of all infected women, although a single, oral dose of MTZ 2-g remains the recommended treatment for men [Citation2,Citation9]. This change in national guideline recommendations in women is based on a recent meta-analysis [Citation19] and clinical trial data [Citation20] in infected women, showing that single-dose MTZ is not as efficacious as is multi-dose MTZ for the treatment of T. vaginalis. However, single-dose MTZ continues to be the recommended regimen for men, as there are no rigorous head-to-head data comparing the 2 MTZ dosing regimens in men. For the first time ever, the recommended treatment for an STI (e.g. T. vaginalis) differs based on gender, which can present unique management challenges [Citation21]. 5-nitroimidazoles (MTZ, tinidazole [TDZ], and secnidazole [SEC]) are the only class of antimicrobial medications known to be effective against T. vaginalis.
Drug resistance to 5-nitroimidazoles, particularly MTZ, in T. vaginalis was documented as early as 1962 [Citation22]. T. vaginalis isolates with minimal lethal concentrations (MLCs) ≤25 μg/mL are MTZ sensitive. In contrast, T. vaginalis isolates with MLCs >50 μg/mL, 100–200 μg/mL, and ≥400 μg/mL have low-level, moderate-level, and high-level resistance, respectively [Citation23].
Prevalence estimates of 5-nitroimidazole resistance in T. vaginalis vary but are higher with MTZ (2.2–9.6%) than TDZ (0–2%) [Citation23,Citation24]. A recent systematic review of the literature on mechanisms of 5-nitroimidazole resistance in T. vaginalis found that resistance can be aerobic or anaerobic [Citation23]. Resistance is relative rather than absolute (i.e. it may be overcome with higher doses of drug for longer periods of time) [Citation23,Citation25]. Resistance is facilitated by altered expression and activation of enzymes and proteins involved in T. vaginalis energy production and oxygen scavenging. T. vaginalis infection with Mycoplasma hominis or T. vaginalis virus also has been associated with resistance [Citation23].
Since publication of updated national guidelines [Citation2], a single, 2-g oral dose of SEC, a second-generation 5-nitroimidazole, has been approved by the U.S. Food and Drug Administration (FDA) for the treatment of trichomoniasis in both women and men. This drug offers an additional therapeutic option for T. vaginalis infection with the same dosing regimen in both women and men. It also offers the advantage of single-dose treatment, eliminating issues with non-adherence that could occur when using multi-dose MTZ regimens. SEC is also FDA approved for the treatment of BV, making it an effective, single-dose treatment for concurrent BV and T. vaginalis infections. This review presents an overview of the chemistry, pharmacology, tolerability, safety, efficacy, and drug resistance of SEC for the treatment of trichomoniasis in women and men. It also compares SEC to other 5-nitroimidazoles, MTZ and TDZ, in terms of efficacy, safety, and other attributes. Finally, it outlines unmet therapeutic needs and provides expert commentary on the use of SEC for the treatment of trichomoniasis based on recently updated treatment guidelines.
2. Overview of secnidazole
SEC has been used since the late 1960s [Citation26] to treat various bacterial and parasitic infections in many countries. It was FDA approved in 2017 to treat BV in the US [Citation27,Citation28]. Dating back to 1976, studies have shown that SEC is an effective treatment for trichomoniasis [Citation29]. Recent data have also shown the efficacy of SEC to treat trichomoniasis as well as its safety and favorable pharmacokinetic and pharmacodynamic properties (PK/PD) [Citation30,Citation31].
2.1. Chemistry and microbiology
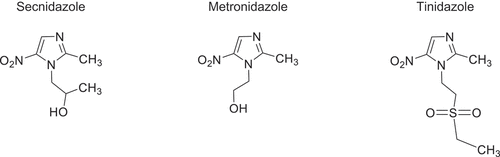
SEC is in the 5-nitroimidazole class of drugs and is a potent antimicrobial [Citation32]. Structurally, SEC is an analogue of MTZ and TDZ, the other 5-nitroimidazoles used to treat T. vaginalis () [Citation33]. The slight variation in the molecular structure of SEC is likely responsible for its longer half-life compared with MTZ and TDZ [Citation32]. SEC has selective toxicity against numerous anaerobic, Gram-positive, and Gram-negative bacteria as well as protozoa. It enters the pathogen in an inactive form through passive diffusion and is subsequently activated through reduction of the 5-nitroimidazole group by nitroreductase enzymes, ultimately damaging DNA and causing cell death [Citation23, Citation33–35].
2.2. Pharmacodynamics
As part of the clinical trial program, a thorough phase 1, four-period, crossover study was conducted to evaluate the effect of SEC on the QTc interval. Healthy adults (N = 52) were randomized to four treatment arms: 2-g proposed therapeutic dose, 6-g supratherapeutic dose, placebo, or positive control (moxifloxacin 400 mg) [Citation36]. Results of the study demonstrated that neither dose of SEC caused a clinically meaningful effect on the QTc interval [Citation36].
2.3. Pharmacokinetics and metabolism
In vitro and PK studies showed that the half-life of SEC ranges from 17 to 19 hours [Citation37], which is considerably longer than MTZ (7–8 h) [Citation38] and TDZ (12–13 h) [Citation39]. These studies also demonstrated that the half-life of SEC is longer in men versus women (20 vs 14 h) [Citation39]. Other PK data showed no clinically meaningful differences in SEC exposure (maximum serum concentration and area under the concentration-time curve [AUC]) between men and women [Citation39].
SEC has similar antimicrobial properties compared with other 5-nitroimidazoles. Both SEC and MTZ have equipotent activity against T. vaginalis, as measured by their minimal inhibitory concentration (MIC) and minimal trichomonacidal concentration (MTC) levels [Citation30]. In one study, SEC had a minimal lethal concentration (MLC) 56% lower than that of MTZ against T. vaginalis, suggesting that SEC may be more toxic to this pathogen [Citation30].
The mean peak plasma concentration (Cmax) after a SEC 2-g dose is 45.4 mcg/mL. The mean systemic exposure (AUC0-inf) is 1331.6 mcg•hr/mL. SEC reaches peak plasma concentration (Tmax) in 4 hours and remains above the MIC for several days ().
Figure 2. A single 2-g oral dose of secnidazole reaches peak plasma concentrations at 4 h, and with its long half-life, remains above the minimum inhibitory concentration for several days as opposed to oral metronidazole 500 mg, which requires repeat dosing. (Adapted from Lupin Pharmaceuticals [data on file] and Ashiq B, et al. 2011 [Citation40]).
![Figure 2. A single 2-g oral dose of secnidazole reaches peak plasma concentrations at 4 h, and with its long half-life, remains above the minimum inhibitory concentration for several days as opposed to oral metronidazole 500 mg, which requires repeat dosing. (Adapted from Lupin Pharmaceuticals [data on file] and Ashiq B, et al. 2011 [Citation40]).](/cms/asset/00432384-de48-4591-b5b8-5a9761364fd1/ierz_a_2080656_f0002_oc.jpg)
SEC can be taken without regard to timing of meals. There was no significant difference in Cmax or AUC when SEC was taken with a high-fat meal compared to applesauce under fasting conditions. The type of food (pudding, applesauce, yogurt) also did not affect absorption.
SEC has a half-life of approximately 17 hours. SEC has a total body clearance of approximately 25 mL/min and a renal clearance of approximately 3.9 mL/min. SEC is metabolized in vitro via oxidation by the human hepatic CYP450 enzyme. SEC excretion in urine is approximately 15% of a 2-g oral dose.
2.4. Drug interaction studies
SEC does not affect the efficacy of oral contraceptives. In a phase 2 study, healthy women received concomitant administration of SEC 2-g with an oral contraceptive containing ethinyl estradiol (EE) and norethindrone (NE). The effect on EE was a 29% decrease in the Cmax and no significant effect on the AUC [Citation41]. The effect on NE was not significant, showing a 13% and 16% increase in Cmax and AUC, respectively. There was no effect on the Cmax or AUC of EE or NE when SEC 2-g was administered 1 day before the oral contraceptive.
Certain antibiotics may reduce the efficacy of bowel preparation for colonoscopy by direct drug chelation with magnesium (sodium picosulfate, magnesium hydroxide, anhydrous citric acid). SEC should be administered either 2 hours before or 6 hours after bowel preparation.
2.5. Administration and dosage
SEC is administered as a single oral dose of 2-g, off-white to slightly yellowish granules with 4.8 g net weight, packed in a unit-of-use, child-resistant foil packet. The entire contents of the SEC packet should be sprinkled onto a standard serving of applesauce, yogurt, or pudding. The granules will not dissolve. The entire mixture should be consumed within 30 minutes without chewing or crunching the granules. A glass of water may be taken after the administration of SEC to aid in swallowing.
The recommended dosage of SEC for the treatment of BV in adult women is a single 2-g packet of granules taken once orally, without regard to the timing of meals. The recommended dosage of SEC for the treatment of trichomoniasis in adult women and men (aged ≥18 years) is a single 2-g packet of granules taken once orally, without regard to the timing of meals. Because trichomoniasis is a STI, ideally, sexual partners should be treated with the same dose and at the same time. There is no guidance on dosing of SEC in patients with renal or hepatic impairment, but it appears no adjustments are necessary.
3. Clinical efficacy
3.1. Summary of efficacy in women
A multicenter, randomized, double-blind, placebo-controlled, delayed-treatment trial was conducted to evaluate the efficacy of single, oral dose of SEC for the treatment of trichomoniasis in women [Citation31]. A total of 147 women (aged 15–65 years) were enrolled across 10 centers in the US. The women were randomly assigned (1:1) to receive either SEC 2-g or placebo. The modified intent-to-treat (mITT) population consisted of all randomized patients who tested positive for T. vaginalis by culture and negative for other STIs. In the mITT population of 131 patients, 90.8% were African American, and the median age was 36 years. A majority of women (84.7% [111/131]) reported baseline clinical symptoms of vaginal itching, discharge, or odor. A test-of-cure (TOC) visit occurred 6–12 days after the initial dosing at baseline. At the TOC visit, patients were given the opposite treatment from baseline (SEC patients received placebo and vice versa) with a return visit 7–12 days later to ensure all patients received appropriate treatment according to the standard of care. To minimize the risk of reinfection, participants were counseled to abstain from sexual activity during the study.
In the mITT population, microbiological cure (negative for T. vaginalis by culture) at the TOC visit was significantly higher in the SEC (92.2%) versus placebo group (1.5%) ().
Table 1. Microbiological cure rate in women with trichomoniasis at the TOC visit (mITT*)
3.2. Summary of efficacy in men
A single oral 2-g dose of SEC was assessed in four open-label trials in men (one comparative study with MTZ and ornidazole [ORZ] in men only [Citation42] and three single-arm studies in men and women [Citation38,Citation43,Citation44]) (). ORZ is another 5-nitroimidazole used to treat trichomoniasis, amebiasis, and giardiasis in other countries, but it is not approved in the US.
Table 2. Studies of SEC in men
Parasitological evaluation was performed pre- and post-treatment and reported cure rates ranged from 91.7% (165/180) to 100% (30/30) at time points ranging from 2 to 20 days after treatment (n = 437; 211 men and 226 women).
3.3. Summary of efficacy in adolescents
Secnidazole is FDA approved for BV in postmenarchal adolescent girls aged ≥12 years and for trichomoniasis in adolescent girls and boys aged ≥12 years.
The safety and efficacy of SEC for the treatment of BV have been established in female patients aged 12–17 years based on data from a multicenter, open-label safety study of 40 female pediatric patients with BV [Citation45] and on data from controlled studies in adult women [Citation27,Citation28,Citation46].
The safety and efficacy of SEC for the treatment of trichomoniasis have been established in male and female patients aged 12–17 years based on the extrapolation of clinical trial data from adult women with trichomoniasis [Citation31], four open-label studies in men with trichomoniasis [Citation38 ,Citation42–44], and an open-label safety study of female patients with BV [Citation45].
4. Post-marketing surveillance
4.1. Safety
In the US-based clinical trial, SEC was safe and well tolerated [Citation31]. Eleven patients (14.9%) in the SEC group and 16 patients (21.9%) in the placebo group reported adverse reactions (). Symptomatic vulvovaginal candidiasis was reported by two patients (2.7%) in the SEC group and none of the patients in the placebo group.
Table 3. Summary of TEAEs* (SAF)
In studies outside the US, dysgeusia was reported during the use of SEC and other 2-g formulations of SEC [Citation44,Citation47]. Because these reactions were reported voluntarily from a population of uncertain size, it is not possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
5. Drug resistance
Compared to MTZ and TDZ [Citation23,Citation24], there are minimal data on SEC resistance in T. vaginalis. The prevalence of in vitro aerobic MTZ and SEC resistance in archived T. vaginalis isolates from 100 women was recently determined [Citation30]. The mean (SD) MLC for SEC was 5.9 (13.2) µg/mL; the mean (SD) MLC for MTZ was 13.5 (26.9) µg/mL. The median MLC for SEC was significantly lower (1.6 µg/mL) than that for MTZ (6.3 µg/mL). Of the 100 T. vaginalis isolates, 96% had a lower MLC for SEC than for MTZ, while three isolates had similar MLCs for both medications (p < 0.0001). Only one T. vaginalis isolate had a higher MLC for SEC (3.1 µg/mL) than for MTZ (1.6 µg/mL). The prevalence of low-level resistance to SEC was 4%, while that for MTZ was 7%. None of the isolates demonstrated moderate- or high-level SEC resistance, while 1% of the isolates demonstrated moderate MTZ resistance. Overall, these data suggest that SEC has superior efficacy in vitro compared to MTZ against T. vaginalis. Given that drug resistance in T. vaginalis has been found to be relative and not absolute [Citation25], additional in vivo data on clinical outcomes of treatment in the setting of drug resistance, particularly for infections with low-level in vitro resistance, are needed.
6. Comparison to other 5-nitroimidazoles
In 2010, an open-label study compared MTZ 2-g single oral dose and MTZ 500 mg twice-daily oral dose for 7 days for the treatment of trichomoniasis in HIV-infected women [Citation48]. Overall, the efficacy rates were 83.2% in the single-dose MTZ group and 91.5% the multi-dose group. Of the 244 women with HIV and trichomoniasis in this study, 66.8% also had BV coinfection at baseline. In the single-dose MTZ group, women with BV were 4.2 times more likely to be positive for T. vaginalis at TOC compared to women without BV [Citation49].
A similar open-label study was conducted in 2018 comparing MTZ 2-g single oral dose and MTZ 500 mg twice-daily oral dose for 7 days for the treatment of trichomoniasis in HIV-negative women [Citation20]. Efficacy rates were 81.4% in the single-dose MTZ group and 89.4% in the multi-dose group. This study also showed a high rate of BV co-infection at baseline (48.3%). With regard to efficacy and in contrast to the 2010 study of HIV-infected women, the 2018 study did not find a statistically significant difference in cure rates based on BV presence or absence in either treatment group [Citation20,Citation48]. It is possible that the 2018 study did not have enough power to detect a difference by BV status; however, the relative risks by BV status did not indicate a trend, so it is unlikely that a larger sample size would have led to a different conclusion [Citation20].
The overall efficacy of a single 2-g oral dose of SEC for women with trichomoniasis in the recent US-based trial was 92.2%. For the nine HIV-positive women in this study, cure rates were 100% (4/4) for SEC and 0% (0/5) for placebo [Citation31]. For the 79 women co-infected with BV at baseline (defined as meeting ≥3 Amsel criteria), cure rates were 97.7% (42/43) for SEC and 0% (0/36) for placebo (unpublished data).
No US-based studies have been conducted using the TDZ 2-g oral dose for the treatment of trichomoniasis in women and men. Approval for TDZ was based on nine studies [Citation50–58] conducted outside of the US that were considered by the FDA to be of poor quality due to methods of randomization and blinding, lack of clarity on withdrawals and dropouts, and statistical analyses that may have falsely elevated efficacy rates [Citation59]. Four studies [Citation50,Citation52,Citation56,Citation58] submitted to support the TDZ label used wet mount for T. vaginalis diagnosis at TOC, which has a low sensitivity of 44–68% [Citation60]. Reported T. vaginalis cure rates for these studies ranged from 80% (8/10) to 100% (16/16). Four additional studies [Citation51,Citation53,Citation54,Citation57] used culture at TOC at time points post-treatment ranging from 1 week to 1 month. Reported cure rates ranged from 92% (37/40) to 100% (65/65). The single oral 2-g TDZ dose also was assessed in four open-label trials in men (one comparative to MTZ and 3 single-arm studies) [Citation59,Citation61–63]. Parasitological evaluation of urine (by culture or other unspecified methods) was performed both pre- and post-treatment, and reported cure rates ranged from 83% (25/30) to 100% (80/80).
The most common adverse events in these studies were gastrointestinal complaints. In the 2010 MTZ trial [Citation48], the most common adverse events reported for the single- and multi-dose MTZ groups were nausea and upset stomach (22.4% and 28.5%), headache (7.2% and 10%), dizziness (8% and 9.2%), and vomiting (4% and 5.4%). In the 2018 MTZ trial, the most common adverse events reported in the single- and multi-dose MTZ groups were nausea (22.6% and 23.3%), vomiting (2.2% and 4.8%), and headache (5.6% and 8.5%) [Citation20]. In the TDZ trials [Citation50–58], combined adverse events for single- and multi-dose regimens were metallic/bitter taste (3.7% and 6.3%) and nausea (3.2% and 4.5%). Adverse events reported in the SEC trial were nausea (2.7%) and vulvovaginal candidiasis (2.7%) [Citation31]. compares the characteristics of SEC, MTZ, and TDZ for the treatment of trichomoniasis.
Table 4. Characteristics of secnidazole, metronidazole, and tinidazole
7. Regulatory status of secnidazole
On 18 September 2017, the FDA approved SEC (2-g oral granules) for the treatment of BV in adult women. On 30 June 2021, the FDA approved SEC (2-g oral granules) for the treatment of T. vaginalis in adult women and men. SEC also is approved for the treatment of BV and trichomoniasis in France and is used in other countries to treat various bacterial and parasitic infections including amoebiasis and giardiasis.
8. Conclusion
Cure rates for single-dose oral SEC 2-g in treating trichomoniasis in women (including women with HIV or BV coinfection) and men are high (92.2–100%). These rates are comparable or even slightly numerically better to multi-dose MTZ, which has recently become the recommended regimen for the treatment of T. vaginalis in all women. SEC has a longer half-life compared with MTZ and TDZ. It also has a lower MLC and similar MIC and MTC compared to MTZ. SEC has a favorable safety profile and is the only FDA-approved single-dose oral treatment for both trichomoniasis and BV.
9. Expert opinion
SEC is a newly FDA-approved treatment for trichomoniasis in women and men that was found to have comparable efficacy to multi-dose MTZ and a favorable safety profile. MTZ has been used to treat bacterial and parasitic infections since the 1960s. As previously mentioned, until recently, the recommended dosing regimen for the treatment of trichomoniasis in both women and men was a single 2-g dose of MTZ. Recently updated 2020 ACOG guidelines [Citation9] and 2021 CDC STI treatment guidelines [Citation2] now recommend MTZ 500 mg orally twice daily for 7 days in all T. vaginalis−infected women based on a meta-analysis [Citation19] and updated clinical trial data [Citation20], or alternatively, TDZ 2-g single dose [Citation2,Citation9]. In men, the CDC continues to recommend MTZ 2-g single dose (as a rigorous head-to-head comparison of single- and multi-dose oral MTZ has not been conducted for men), or alternatively, TDZ 2-g single dose [Citation2].
Thus, for the first time, recommended treatment of a STI (with MTZ) is different based on gender. This presents some unique challenges for the management of sexual partnerships infected with T. vaginalis. For example, if a T. vaginalis-infected woman is treated with the multi-dose MTZ regimen but her male partner(s) is/are treated with the single-dose MTZ regimen, there could be a conflict in the timing of abstinence from sex, which could put the couple at risk for re-exposure to T. vaginalis [Citation21]. Use of single-dose 2-g oral SEC (or TDZ) in both T. vaginalis-infected women and their male sexual partner(s) eliminates any confusion that may occur when using different MTZ dosing regimens. Although single-dose TDZ 2 g is an alternative recommended treatment for T. vaginalis in both women and men, it is important to note that that single-dose TDZ is not a recommended treatment in the setting of BV coinfection [Citation2]. Both the updated 2020 ACOG [Citation9] and 2021 CDC guidelines [Citation2] were published before FDA approval of SEC for trichomoniasis. Although SEC is not yet included in national treatment guidelines, we believe SEC to be a valuable additional treatment option for trichomoniasis based on its efficacy, excellent safety profile, and the same one-time dosing regimen in both women and men. In addition, SEC is the only oral, single-dose treatment option for both BV and trichomoniasis in women.
Given the serious consequences and increased risk for transmission and acquisition of HIV and other STIs associated with BV and trichomoniasis, treatment adherence is critical. Poor treatment adherence to multi-dose treatment regimens may be a potential cause of recurrent or persistent T. vaginalis infection. Studies have shown that adherence to multi-dose antibiotics is low [Citation75,Citation76]. One study reported adherence rates of only 50–63% for patients taking multi-dose MTZ for BV [Citation77]. Duration of therapy can be a barrier and adherence can worsen as the length of therapy increases [Citation78]. Although a head-to-head study has not been conducted to evaluate single-dose SEC compared with multi-dose MTZ for women with trichomoniasis, adherence is likely to be improved with a single-dose treatment option (i.e. single-dose SEC) compared to a multi-dose treatment option (i.e. 14 pills over 7 days for oral MTZ). Improved adherence may lead to better patient outcomes by reducing the partner-to-partner cycle of reinfections.
While there are some benefits to single-dose oral SEC, there are some drawbacks. It is in the same class of 5-nitroimidazoles as MTZ and TDZ. This makes it susceptible to drug class issues such as hypersensitivity reactions and potentially drug resistance. Nevertheless, resistance to 5-nitroimidazoles in trichomoniasis has been found to be relative and not absolute (as discussed above), dating back to 1986 [Citation25]. Thus, there is a role for treating through drug resistance, particularly low-level resistance or in the setting of clinical treatment failure, with higher doses of 5-nitroimidazoles for longer periods of time, as is currently recommended in the 2021 CDC STI Treatment Guidelines [Citation2]. Although SEC is not explicitly discussed in the trichomonas section of these guidelines, in our expert opinion, its use can be considered in T. vaginalis-infected patients in the presence of known MTZ drug resistance or clinical treatment failure. Although susceptibility breakpoints are not yet clearly defined for SEC, susceptibility testing can provide useful information, particularly in the setting of a favorable MLC of one 5-nitroimidazole over another.
In developed countries like the US, cost of medications is complex and should be taken into account when considering use of SEC or other 5-nitroimidazoles for the treatment of trichomoniasis. To our knowledge, there are no published cost-effectiveness or real-world compliance studies comparing all three 5-nitroimidazoles which should be an area of future research. Thus, whether the additional direct cost of SEC is countered by an overall reduction in health-care costs, particularly compliance-related therapeutic failure, is unknown. Patient-specific factors, including cost, preferences, insurance coverage, assistance programs, pharmacy availability, and compliance considerations should guide decisions regarding the selection of therapy. In developing countries, the cost of SEC therapy or product availability may be overriding factors, with limited resources and patient assistance opportunities.
Article highlights
In the United States, trichomoniasis affects 2.1% of women and 0.5% of men aged 18–59 years and disproportionately affects African Americans [1].
Trichomoniasis is associated with significant adverse outcomes if not treated promptly and appropriately. In women, trichomoniasis can cause vaginitis, cervicitis, pelvic inflammatory disease [2-4], cervical cancer [5], infertility [6], and adverse birth outcomes, including preterm birth, premature rupture of membranes, and low birth weight [7,8]. In men, trichomoniasis can result in non-gonococcal urethritis, epididymitis, prostatitis, and infertility [1,9]. Infection can increase the risk for human immunodeficiency virus (HIV) and other sexually transmitted infections (STIs; chlamydia, gonorrhea, herpes simplex virus, and syphilis) in both sexes [1].
Coexistence of bacterial vaginosis (BV) and T. vaginalis is very common, with coinfection rates of 60–80% [10]. The risk of acquiring trichomoniasis is two-fold higher among women with BV compared to women without BV [11].
The Centers for Disease Control and Prevention (CDC) recommend diagnostic testing for T. vaginalis for all women seeking care for vaginal discharge. Screening is recommended annually for HIV-infected women and can be considered for persons receiving care in high-prevalence settings (e.g. STD clinics and correctional facilities) and for asymptomatic women at high risk for infection (e.g. multiple sexual partners, transactional sex, drug misuse, and/or a history of STIs or incarceration) [1].
Updated guidelines now recommend multi-dose metronidazole (MTZ) 500 mg orally twice daily for 7 days for the treatment of all women with T. vaginalis, although a single 2-g dose of oral MTZ remains the recommended treatment for men. This is the first time recommended treatment regimens for a STI differ based on gender, which may present unique challenges.
Updated guidelines have removed the recommendation to refrain from alcohol use while taking 5-nitroimidazoles due to lack of data on disulfiram-like reaction [1].
Retest all sexually active T. vaginalis-infected women within 3 months of treatment due to high rates of recurrence. If retesting at 3 months is not possible, providers can retest whenever women next seek medical care [1].
A single 2-g dose of secnidazole is newly approved by the U.S. Food and Drug Administration for the treatment of trichomoniasis in adult women and men and is the only single-dose oral treatment option for both BV and trichomoniasis.
Declaration of interest
C Muzny has received research grant support from Lupin Pharmaceuticals, Gilead Sciences, Inc, and Abbott Molecular, is a consultant for Lupin Pharmaceuticals, PhagoMed, and BioFire Diagnostics, and has received honoraria from Elsevier, Abbott Molecular, Cepheid, Becton Dickinson, Roche Diagnostics, and Lupin. O Van Gerwen has received research grant support from Gilead Sciences, Inc and Abbott Molecular. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Scientific accuracy review
Lupin Pharmaceuticals provided a scientific accuracy review at the request of the journal editor.
Acknowledgments
Elizabeth Sarkar, Jespersen & Associates, LLC for communications with authors, coordination of reference retrieval and illustration of molecular structures.
Additional information
Funding
References
- Rowley J, Vander Hoorn S, Korenromp E, et al. Chlamydia, gonorrhoea, trichomoniasis and syphilis: global prevalence and incidence estimates, 2016. Bull World Health Organ. 2019;97(8):548–562.
- Workowski KA, Bachmann LH, Chan PA, et al., Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 70(4): 1–187. 2021.
- Flagg EW, Meites E, Phillips C, et al. Prevalence of trichomonas vaginalis among civilian, noninstitutionalized male and female population aged 14 to 59 years: United States, 2013 to 2016. Sex Transm Dis. 2019;46(10):e93–e96.
- Daugherty M, Glynn K, Byler T. Prevalence of trichomonas vaginalis infection among US males, 2013-2016. Clin Infect Dis. 2019;68(3):460–465.
- Meites E. Trichomoniasis: the “neglected” sexually transmitted disease. Infect Dis Clin North Am. 2013;27(4):755–764.
- Sutton M, Sternberg M, Koumans EH, et al. The prevalence of trichomonas vaginalis infection among reproductive-age women in the United States, 2001-2004. Clin Infect Dis. 2007;45(10):1319–1326.
- Seña AC, Goldstein LA, Ramirez G, et al. Bacterial vaginosis and its association with incident trichomonas vaginalis infections: a systematic review and meta-analysis. Sex Transm Dis. 2021 in press;48:e192–e201.
- Seña AC, Miller WC, Hobbs MM, et al. Trichomonas vaginalis infection in male sexual partners: implications for diagnosis, treatment, and prevention. Clin Infect Dis. 2007;44(1):13–22.
- Committee on Practice Bulletins-Gynecology. Vaginitis in nonpregnant patients: ACOG practice bulletin, number 215. Obstet Gynecol. 2020;135(1):e1–e17.
- Moodley P, Wilkinson D, Connolly C, et al. Trichomonas vaginalis is associated with pelvic inflammatory disease in women infected with human immunodeficiency virus. Clin Infect Dis. 2002;34(4):519–522.
- Yagur Y, Weitzner O, Tiosano LB, et al. Characteristics of pelvic inflammatory disease caused by sexually transmitted disease - an epidemiologic study. J Gynecol Obstet Hum Reprod. 2021;50:102176.
- Yang S, Zhao W, Wang H, et al. Trichomonas vaginalis infection-associated risk of cervical cancer: a meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2018;228:166–173.
- Mielczarek E, Blaszkowska J. Trichomonas vaginalis: pathogenicity and potential role in human reproductive failure. Infection. 2016;44(4):447–458.
- Silver BJ, Guy RJ, Kaldor JM, et al. Trichomonas vaginalis as a cause of perinatal morbidity: a systematic review and meta-analysis. Sex Transm Dis. 2014;41(6):369–376.
- Van Gerwen OT, Craig-Kuhn MC, Jones AT, et al. Trichomoniasis and adverse birth outcomes: a systematic review and meta-analysis. Bjog. 2021;128(12):1907–1915.
- Sobel JD, Subramanian C, Foxman B, et al. Mixed vaginitis-more than coinfection and with therapeutic implications. Curr Infect Dis Rep. 2013;15(2):104–108.
- Van Gerwen OT, Camino AF, Sharma J, et al. Epidemiology, natural history, diagnosis, and treatment of trichomonas vaginalis in men. Clin Infect Dis. 2021;73:1119–1124.
- Allsworth JE, Ratner JA, Peipert JF. Trichomoniasis and other sexually transmitted infections: results from the 2001-2004 national health and nutrition examination surveys. Sex Transm Dis. 2009;36(12):738–744.
- Howe K, Kissinger PJ. Single-dose compared with multidose metronidazole for the treatment of trichomoniasis in women: a meta-analysis. Sex Transm Dis. 2017;44(1):29–34.
- Kissinger P, Muzny CA, Mena LA, et al., Single-dose versus 7-day-dose metronidazole for the treatment of trichomoniasis in women: an open-label, randomised controlled trial. Lancet Infect Dis. 18(11): 1251–1259. 2018.
- Muzny CA, Richter S, Kissinger P. Is it time to stop using single-dose oral metronidazole for the treatment of trichomoniasis in women? Sex Transm Dis. 2019;46(5):e57–e59.
- Robinson SC. Trichomonal vaginitis resistant to metranidazole. Can Med Assoc J. 1962;86(14):665.
- Graves KJ, Novak J, Secor WE, et al. A systematic review of the literature on mechanisms of 5-nitroimidazole resistance in Trichomonas vaginalis. Parasitology. 2020;147(13):1383–1391.
- Kirkcaldy RD, Augostini P, Asbel LE, et al. Trichomonas vaginalis antimicrobial drug resistance in 6 US cities, STD surveillance network, 2009-2010. Emerg Infect Dis. 2012;18(6):939–943.
- Lossick JG, Muller M, Gorrell TE. In vitro drug susceptibility and doses of metronidazole required for cure in cases of refractory vaginal trichomoniasis. J Infect Dis. 1986;153(5):948–955.
- Faught BM, Harris K. The history of secnidazole. [cited May 16, 2022]. Available from: https://www.npwomenshealthcare.com/history-of-secnidazole/
- Hillier SL, Nyirjesy P, Waldbaum AS, et al. Secnidazole treatment of bacterial vaginosis: a randomized controlled trial. Obstet Gynecol. 2017;130(2):379–386.
- Schwebke JR, Morgan FG Jr., Koltun W, et al. A phase-3, double-blind, placebo-controlled study of the effectiveness and safety of single oral doses of secnidazole 2 g for the treatment of women with bacterial vaginosis. Am J Obstet Gynecol. 2017;217(6):678 e1–678 e9.
- Benazet F, Guillaume L. Amoebecide and trichomonacide activities of secnidazole in the laboratory. Bull Soc Pathol Exot Filiales. 1976;69(4):309–319.
- Ghosh AP, Aycock C, Schwebke JR. In vitro study of the susceptibility of clinical isolates of trichomonas vaginalis to metronidazole and secnidazole. Antimicrob Agents Chemother. 2018;62(4):e02329–17.
- Muzny CA, Schwebke JR, Nyirjesy P, et al., Efficacy and safety of single oral dosing of secnidazole for trichomoniasis in women: results of a phase 3, randomized, double-blind, placebo-controlled, delayed-treatment study. Clin Infect Dis. 73(6): e1282–e1289. 2021.
- Nyirjesy P, Schwebke JR. Secnidazole: next-generation antimicrobial agent for bacterial vaginosis treatment. Future Microbiology. 2018;13(5):507–524.
- Gillis JC, Wiseman LR, Secnidazole. A review of its antimicrobial activity, pharmacokinetic properties and therapeutic use in the management of protozoal infections and bacterial vaginosis. Drugs. 1996;51(4):621–638.
- Edwards DI. Nitroimidazole drugs–action and resistance mechanisms. I. mechanisms of action. J Antimicrob Chemother. 1993;31(1):9–20.
- Muller M. Reductive activation of nitroimidazoles in anaerobic microorganisms. Biochem Pharmacol. 1986;35(1):37–41.
- Darpo B, Xue H, Adetoro N, et al. Thorough QT/QTc evaluation of the cardiac safety of secnidazole at therapeutic and supratherapeutic doses in healthy individuals. J Clin Pharmacol. 2018;58(3):286–293.
- Pentikis HS, Adetoro N. Two phase 1, open-label, single-dose, randomized, crossover studies to assess the pharmacokinetics, safety, and tolerability of orally administered granules of secnidazole (2 g) in healthy female volunteers under different administration conditions. Clin Pharmacol Drug Dev. 2018;7(5):543–553.
- Videau D, Niel G, Siboulet A, et al., Secnidazole. A 5-nitroimidazole derivative with a long half-life. Br J Vener Dis. 54(2): 77–80. 1978.
- Populaire P, Decouvelaere B, Renard A, et al. Study of serum concentrations and urinary excretion of secnidazole after oral administration in man. Comparison with tinidazole. Pathol Biol. 1980;28(9):621–624.
- Ashiq B, Usman M, Ashraf M, et al. Comparative pharmacokinetics of metronidazole in healthy volunteers and in patients suffering from amoebiasis. Pak J Pharm. 2011;24. 41–46.
- Pentikis HS, Adetoro N, Braun CJ. Lack of a pharmacokinetic interaction between sym-1219 granules containing 2 grams of secnidazole and a combined oral contraceptive in a phase 1, randomized, open-label study in healthy female volunteers. Adv Ther. 2017;33(12):2229–2241.
- Özbilgin A, Özbel Y, Alkan MZ, et al. Trichomoniasis in non-gonococcic urethritis among male patients. J Egypt Soc Parasitol. 1994;24(3):621–625.
- Siboulet A, Catalan F, Videau D, et al. La trichomonase urogénitale. Essais d’un imidazole à demi-vie longue: le secnidazole. Médecine et Maladies Infectieuses. 1977;7(9):400–404.
- Dyudyun AD, Polyon NM, Gorbuntsov VV. Secnidazole in complex treatment of patients with urogential trichomoniasis. Dermatovenerol Cosmetol Sexopathol. 2016;1(4):287–292.
- Chavoustie S, Shaw J, Pandey B, et al. Single-dose secnidazole is effective and safe for the treatment of bacterial vaginosis in adolescent girls. 24th Nurse Practitioners in Women’s Health’s Annual Premier Women’s Healthcare VirtualConference; 2021 October 13-16; Virtual meeting.
- Chavoustie SE, Gersten JK, Samuel MJ, et al. A phase 3, multicenter, prospective, open-label study to evaluate the safety of a single dose of secnidazole 2 g for the treatment of women and postmenarchal adolescent girls with bacterial vaginosis. J Womens Health (Larchmt). 2018;27(4):492–497.
- Moraes ME, Cunha GH, Bezerra MM, et al. Efficacy of the mentha crispa in the treatment of women with trichomonas vaginalis infection. Arch Gynecol Obstet. 2012;286(1):125–130.
- Kissinger P, Mena L, Levison J, et al., A randomized treatment trial: single versus 7-day dose of metronidazole for the treatment of Trichomonas vaginalis among HIV-infected women. J Acquir Immune Defic Syndr. 55(5): 565–571. 2010.
- Gatski M, Martin DH, Levison J, et al. The influence of bacterial vaginosis on the response to trichomonas vaginalis treatment among HIV-infected women. Sex Transm Infect. 2011;87(3):205–208.
- Aimakhu VE. Vaginal trichomoniasis: one stat dose of tinidazole compared with a seven-day course of metronidazole. West Afr Med J. 1975;23:97–100.
- Chaisilwattana P, Bhiraleus P, Patanaparnich P, et al. Double blind comparative study of tinidazole and ornidazole as a single dose treatment of vaginal trichomoniasis. J Med Assoc Thai. 1980;63(8):448–453.
- Chaudhuri P, Drogendijk AC. A double-blind controlled clinical trial of carnidazole and tinidazole in the treatment of vaginal trichomoniasis. Eur J Obstet Gynecol Reprod Biol. 1980;10(5):325–328.
- Gabriel G, Robertson E, Thin RN. Single dose treatment of trichomoniasis. J Int Med Res. 1982;10(2):129–130.
- Hillstrom L, Pettersson L, Palsson E, et al. Comparison of ornidazole and tinidazole in single-dose treatment of trichomoniasis in women. Br J Vener Dis. 1977;53(3):193–194.
- Lyng J, Christensen J. A double-blind study of the value of treatment with a single dose tinidazole of partners to females with trichomoniasis. Acta Obstet Gynecol Scand. 1981;60(2):199–201.
- Mati JK, Wallace RJ. The treatment of trichomonal vaginitis using a single dose of tinidazole by mouth. East Afr Med J. 1974;51(12):883–888.
- Prasertsawat PO, Jetsawangsri T. Split-dose metronidazole or single-dose tinidazole for the treatment of vaginal trichomoniasis. Sex Transm Dis. 1992;19(5):295–297.
- Rees PH, McGlashan HE, Mwega V. Single-dose treatment of vaginal trichomoniasis with tinidazole. East Afr Med J. 1974;51(11 SPEC NO):782–785.
- U.S. Food and Drug Administration. Drug Approval Package: tindamax (Tinidazole) Tablets 2004. Accesssed on December 4, 2021 at https://www.accessdata.fda.gov/drugsatfda_docs/nda/ 2004/21-618_21-681_21-682_Tindamax.cfm
- Hobbs MM, Sena AC. Modern diagnosis of Trichomonas vaginalis infection. Sex Transm Infect. 2013;89(6):434–438.
- Beric B, Pribicevic V, Djordjevic M, et al. Clinical studies on the therapeutic effect of tinidazole (“Fasigyn”) during treatment of urogenital trichomonas infections in women and men (with comparative laboratory studies on the effect of metronidazole and tinidazole). Zentralbl Gynakol. 1978;100(24): 1594–1599
- Massa M, Arias B, Subiabre V, et al. Therapeutic trial of Trichomonas vaginalis in male by using a single dose of tinidazole (author’s transl). Bol Chil Parasitol. 1976;31(1–2):46–47.
- Wallin J, Forsgren A. Tinidazole–a new preparation for T. vaginalis infections. II. clinical evaluation of treatment with a single oral dose. Br J Vener Dis. 1974;50(2):148–150.
- Lupin Pharmaceuticals Inc. SOLOSEC® (secnidazole): full Prescribing Information. 2022.
- Pfizer Inc. FLAGYL® (metronidazole): full prescribing Information. 2021.
- Mission Pharmacal Inc. TINDAMAX® (tinidazole): full prescribing Information. 2019.
- ClinicalKey® [Internet]. Elsevier. 2022. [cited May 1, 2022]. Available from: https://www.elsevier.com/solutions/clinicalkey.
- Fjeld H, Raknes G. Is combining metronidazole and alcohol really hazardous? Tidsskr Nor Laegeforen. 2014;134(17):1661–1663.
- Pentikis H, Eder S, Kaufman G, et al. Secnidazole, an approved single dose drug for bacterial vaginosis, does not cause reproductive toxicity in animals. American Academy of Obstetricians and Gynecologists: Seattle WA. 2020 April 24-27.
- Burtin P, Taddio A, Ariburnu O, et al. Safety of metronidazole in pregnancy: a meta-analysis. Am J Obstet Gynecol. 1995;172(2 Pt 1):525–529.
- Piper JM, Mitchel EF, Ray WA. Prenatal use of metronidazole and birth defects: no association. Obstet Gynecol. 1993;82(3):348–352.
- Sheehy O, Santos F, Ferreira E, et al. The use of metronidazole during pregnancy: a review of evidence. Curr Drug Saf. 2015;10(2):170–179.
- Czeizel AE, Kazy Z, Vargha P. Oral tinidazole treatment during pregnancy and teratogenesis. Int J Gynaecol Obstet. 2003;83(3):305–306.
- Subramanian C, Sobel JD. A case of high-level metronidazole-resistant trichomoniasis in pregnancy successfully treated. J Low Genit Tract Dis. 2011;15(3):248–249.
- Muzny CA, Kardas P. A narrative review of current challenges in the diagnosis and management of bacterial vaginosis. Sex Transm Dis. 2020;47(7):441–446.
- Kissinger P. Trichomonas vaginalis: a review of epidemiologic, clinical and treatment issues. BMC Infect Dis. 2015;15:307.
- Bartley JB, Ferris DG, Allmond LM, et al. Personal digital assistants used to document compliance of bacterial vaginosis treatment. Sex Transm Dis. 2004;31(8):488–491.
- Srinivasan S, et al. An antimicrobial bioassay to assess adherence to metronidazole treatment for bacterial vaginosis. Infectious Diseases Society of Gynecology Annual Meeting (Virtual).; 2021 July 29-30.