Coronavirus disease 2019 (COVID-19), is a highly contagious illness caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Since December 2019, SARS-CoV-2 has rapidly spread across the world, leading the World Health Organization to declare a global pandemic on 11 March 2020.
To date, several studies have investigated the different aspects of COVID-19 in pregnancy and there is an increasing number of scientific papers focusing on SARS-CoV-2 immunity in infected mothers and their newborns/infants [Citation1,Citation2]. Most of the studies analyzed predominantly the SARS-CoV-2 passive immunity in infants born to mothers infected during pregnancy [Citation1]. Active neonatal immune response has been poorly investigated so far given that SARS-CoV-2 vertical transmission (in utero or intrapartum) has been described as rare [Citation1,Citation2]. SARS-CoV-2 neonatal immunity after maternal infection is summarized in .
Figure 1. SARS-CoV-2 neonatal immunity after maternal infection: maternally derived passive immunity and fetal/neonatal active immunity.
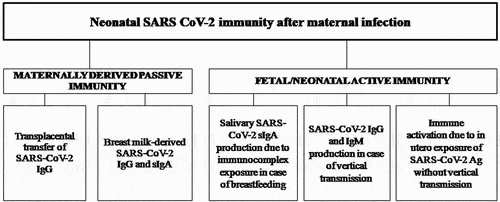
During the first weeks after birth, an important role in infant protection against infections is provided by the maternally derived passive immunity. This is almost certainly due to: i) an effective maternal pathogen-specific antibody production; ii) an efficient transfer of maternal antibodies across the placenta to the fetus, and iii) the post-natal maternal antibody persistence in the infant. Although the major target of neutralizing antibodies against SARS-CoV-2 was identified in the receptor-binding domain (RBD) of the viral spike (S) protein, some studies investigated the production of IgG against nucleocapsid and different S protein epitopes [Citation3–6]. However, the production of IgM and IgG antibodies against RBD peaks in 14–20 days and 3–4 weeks after COVID-19 symptom onset, respectively [Citation6]. Interestingly, even though in pregnant women anti-RBD IgG titers were found to be lower than those detected in non-pregnant women [Citation7], transplacental transfer of SARS-CoV-2 IgG antibodies from mother to fetus was demonstrated [Citation3–6,Citation8]. Flannery et al., analyzing 83 mother/newborn dyads, proved the transplacental transfer of maternal SARS-CoV-2 specific antibodies in 87% of infants delivered to seropositive women [Citation3]. Similar results were obtained by Song et al., detecting specific IgG antibodies in 90% of cases (69 newborns delivered to 77 IgG positive mothers) [Citation5]. Marsico et al. found 96 neonates with detectable SARS-CoV-2 IgG titers at birth, among 106 dyads with maternal positive IgG [Citation4]. A positive correlation between SARS-CoV-2 IgG levels in newborns and their mothers’ samples paired was reported [Citation3–5]. This finding was supported by the evidence that the IgG levels of women with seronegative infants were significantly lower when compared with the IgG levels of women with seropositive infants [Citation3]. In addition, Flannery et al. reported that IgG levels in cases of maternal severe/moderate illness trended higher in both mothers and newborns in comparison with mild or asymptomatic cases, although these differences were not statistically significant [Citation3]. Other Authors observed there was no significant difference in IgG levels between neonates born to asymptomatic or symptomatic mothers [Citation4]. Assessing the efficiency of transplacental antibody transfer as transfer ratio (calculated as infant IgG level divided by maternal IgG level), Song et al. found higher values in mothers with severe/critical symptoms compared to moderate, mild, or asymptomatic cases [Citation5]. However, most studies showed that the transfer ratio is affected by the time elapsed between maternal infection onset and time of delivery. Marsico et al. in a study involving 106 mother-neonate dyads, showed higher transplacental IgG transfer ratios when maternal SARS-CoV-2 infection occurred in the second trimester respect to those whose onset was later, during the third trimester of pregnancy [Citation4]. Similar results were reported by other Authors, highlighting a higher efficiency of transplacental antibody transfer when infection occurs more than 60 days before delivery [Citation3,Citation5,Citation9]. This is probably due to the IgG transplacental transfer that occurs throughout all gestation period increasing exponentially and therefore reaching a higher efficiency when there is more time between the onset of infection and the delivery. Some Authors reported a lower transfer of SARS-CoV-2 IgG than the expected and previously demonstrated for other pathogens such as B. pertussis, influenza virus, and measles virus [Citation9,Citation10]. Several mechanisms leading to this effect were considered, including a placental impairment [Citation9,Citation10]. In fact, a recent Italian study involving a large population of pregnant women with SARS-CoV-2 infection found signs of placental inflammation mostly due to virus induced immune response [Citation11]. Nevertheless, the efficient transfer of specific IgG against other pathogens among the mother-neonate dyads with maternal SARS-CoV-2 infection seems to rule out the placental dysfunction and suggest a potential alteration in SARS-CoV-2 specific antibodies behind this inefficient antibody transfer [Citation9,Citation10].
In the setting of maternally derived passive immunity, the persistence of maternal SARS-CoV-2 IgG in newborns was also investigated. Despite a wide variety in reports, the presence of SARS-CoV-2 IgG has also been documented at least until 6 months of life, with a positive correlation between a longer duration and higher maternal antibody levels as well as higher transfer ratios [Citation3–5].
Passive protection also includes the secretary immunoglobulin A (sIgA) and IgG antibodies contained in breast milk (breast milk-derived immunity). In fact, maternal sIgA and IgG specific for SARS-CoV-2 have been found in breast milk of women who recovered from COVID-19 [Citation12–14]. Fox et al. reported significant specific sIgA reactivity to the full trimeric S protein in all breast milk samples obtained from both eight COVID-19 recovered and seven COVID-19-suspected donors (previously infected mothers, 3–4 weeks after symptoms mitigation), whereas 80% exhibited significant sIgA binding activity to RBD [Citation12]. Similarly, Pace et al. reported that 76% and 80% of the repeated milk samples collected from 18 women with diagnosis of COVID-19 during pregnancy contained SARS-CoV-2-specific IgA and IgG, respectively. In addition, the same study also mentioned that 62% of the milk samples were able to neutralize SARS-CoV-2 infectivity in vitro, whereas milk samples collected prior to the COVID-19 pandemic were unable to do so, supporting the benefits and the recommendations to encourage breastfeeding [Citation13]. In a prospective cohort study including 21 pregnant women with SARS-CoV-2 infection at the time of delivery, no S-specific antibodies were detected in serum specimens of the corresponding infants, whereas S-specific IgA antibodies were found in the saliva samples of breastfed infants. In addition, comparing the neonatal saliva samples collected at 48 hours after birth with those collected at 2 months of life, a significant increase in IgA levels was found. Of note, at 2 months of life there were no S-specific antibodies detectable, whereas the IgA antibodies detected in the saliva of the infants were more concentrated than in breast milk. Authors hypothesized that these findings could be due to neonatal active immune response mediated by antigen–antibody S IgA immune complexes present in breast milk able to stimulate specific mucosal immunity in newborns [Citation14]. Further studies are needed in order to determine if breastfeeding provides not only passive protection but also elicits an active immune response in newborns.
Finally, the potential risk of SARS-CoV-2 mother-to-infant transmission via breast milk has also been investigated; Pace et al. reported that none of the milk samples collected from 18 women with diagnosis of COVID-19 during pregnancy contained detectable SARS-CoV-2 RNA [Citation13]. In addition, studies showing the presence of SARS-CoV-2 RNA in breast milk samples highlighted that the detectable viral RNA could be due to contamination of milk via skin and/or respiratory droplets and that no replication-competent SARS-CoV-2 was detected [Citation15]. These data support the safety of breast milk and encourage breastfeeding practices, although larger scale studies focusing on this important topic are needed.
To date, little is known regarding the active immune response of infants born to mothers with SARS-CoV-2 infection during pregnancy, due to the rarity of vertical transmission events and the difficulty in confirming their diagnosis. Suspected vertical transmission of SARS-CoV-2 has been described in single-case reports, small case series, and systematic reviews [Citation1,Citation5,Citation16]. In some reported cases of newborns infected in utero with SARS-CoV-2, specific IgM and IgG antibodies were detected in neonatal serum samples at birth [Citation16]. Considered that IgM antibodies do not cross the placenta due to their high molecular weight and that they represent the first constituent of the humoral immune response against infections, their detection suggests a recent exposure to SARS-CoV-2. Nevertheless, placental inflammation due to virus induced immune response could allow maternal SARS-CoV-2 IgM transfer. Additionally, transient positive IgM found in cord blood specimens, have also been described in single-case reports [Citation5], with no virological evidence of infection at birth. Hence, the presence of IgM antibodies alone in neonatal serum samples does not necessarily prove a neonatal active immune response and therefore a vertical transmission, thus the real significance of IgM antibodies recovery should be carefully considered. Finally, growing evidence suggests that exposure to maternal infection in utero may trigger the developing immune system, even in the absence of infant infection, although the mechanism of this process remains unclear [Citation17]. The mechanisms underlying the fetal immune response to such exposure in utero need to be further investigated.
To date, the only protection against SARS-CoV-2 infection for the newborns derives from maternal immunity since no anti-SARS-CoV-2 immunization strategies are currently available for infants under 6 months of age. All the considerations above are referring to specific immunity deriving from naturally acquired maternal and potential neonatal infection. However, the current knowledge derives from heterogenic studies in terms of different serological assays used, detection of SARS-CoV-2-specific antibodies against various epitope/protein targets, study population characteristics (confounding factors should be taken into account, for example, co-morbidities, difficulty in dating the infection onset in asymptomatic pregnant women, weeks of gestation at the time of infection diagnosis, number of previous SARS-CoV-2 infections, fetus sex, etc.). Further studies are needed in order to better characterize the kinetics of maternal antibody responses to SARS-CoV-2 infection during pregnancy and the consequent transplacental antibody transfer which are key elements of neonatal protection. Moreover, since mutations in Spike Protein and specifically in RBD can lead to differences in the neutralization potency of SARS-CoV-2 antibodies, additional studies are needed to investigate the maternal immune response against all variants of concern and the potential impact on antibody transfer to the fetus/neonate [Citation18]. These data together with those derived by studies involving women who received anti-SARS-CoV-2 vaccination during pregnancy will provide evidence for the implementation of the most appropriate maternal and neonatal vaccination strategies.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or material discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or mending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Jamieson DJ, Rasmussen SA. An update on COVID-19 and pregnancy. Am J Obstet Gynecol. 2022;226(2):177–186.
- Barcelos IDES, Penna Ia de A, Soligo A de G, et al. Vertical transmission of SARS-CoV-2: a systematic review. Rev Bras Ginecol Obstet. 2021;43(3):207–215.
- Flannery DD, Gouma S, Dhudasia MB, et al. Assessment of maternal and neonatal cord blood SARS-CoV-2 antibodies and placental transfer ratios. JAMA Pediatr. 2021;175(6):594–600.
- Capretti MG, Marsico C, Gabrielli L, et al. Infants Born Following SARS-CoV-2 Infection in Pregnancy. Pediatrics. 2022 Nov 1;150(5):e2022056206.
- Song D, Prahl M, Gaw SL, et al. Passive and active immunity in infants born to mothers with SARS-CoV-2 infection during pregnancy: prospective cohort study. BMJ Open. 2021;11(7):e053036.
- Moore KM, Suthar MS. Comprehensive analysis of COVID-19 during pregnancy. Biochem Biophys Res Commun. 2021;538:180–186.
- Sherer ML, Lei J, Creisher P, et al. Dysregulated immunity in SARS-CoV-2 infected pregnant women. medRxiv. 2020; DOI:10.1101/2020.11.13.20231373.
- Edlow AG, Li JZ, Collier A-RY, et al. Assessment of maternal and neonatal SARS-CoV-2 viral load, transplacental antibody transfer, and placental pathology in pregnancies during the COVID-19 pandemic. JAMA Network Open. 2020;3(12):e2030455.
- Rubio R, Aguilar R, Bustamante M, et al. Maternal and neonatal immune response to SARS-CoV-2, IgG transplacental transfer and cytokine profile. Front Immunol. 2022;13:999136.
- Atyeo C, Pullen KM, Bordt EA, et al. Compromised SARS-CoV-2-specific placental antibody transfer. Cell. 2021;184(3):628–642.e10.
- Salvatore MA, Corsi Decenti E, Bonasoni MP, et al. Placental characteristics of a large Italian cohort of SARS-CoV-2-positive pregnant women. Microorganisms. 2022;10(7):1435.
- Fox A, Marino J, Amanat F, et al. Robust and specific secretory IgA Against SARS-CoV-2 detected in human milk. iScience. 2020;23(11):101735.
- Pace RM, Williams JE, Järvinen KM, et al. Characterization of SARS-CoV-2 RNA, antibodies, and neutralizing capacity in milk produced by women with COVID-19. mBio. 2021;12(1):e03192–20.
- Conti MG, Terreri S, Piano Mortari E, et al. Immune response of neonates born to mothers infected with SARS-CoV-2. JAMA Network Open. 2021;4(11):e2132563.
- Powell RLR. Safety of breast/chest-feeding by those infected by SARS-CoV-2. Curr Opin Clin Nutr Metab Care. 2022;25(2):129–132.
- Kotlyar AM, Grechukhina O, Chen A, et al. Vertical transmission of coronavirus disease 2019: a systematic review and meta-analysis. Am J Obstet Gynecol. 2021;224(1):35–53.e3.
- Wilcox CR, Jones CE. Beyond passive immunity: is there priming of the fetal immune system following vaccination in pregnancy and what are the potential clinical implications? Front Immunol. 2018;9:1548.
- Torbati E, Krause KL, Ussher JE. The immune response to SARS-CoV-2 and variants of concern. Viruses. 2021 Sep 23;13(10):1911.
