ABSTRACT
Introduction
Acute Bacterial Skin and Skin Structure Infections (ABSSSIs) are a common reason of Emergency Department (ED) access and account for a considerable number of hospital admissions and a high economic burden for the healthcare system. The long-acting lipoglycopeptides (LALs) allow for an outpatient management of subjects with ABSSSIs, still requiring parenteral therapy, but who do not need hospitalization.
Areas covered
The following topics were addressed: i) microbiological activity, efficacy, and safety of dalbavancin, ii) critical steps for the management of ABSSSIs in the ED (decision to hospitalize, risk of bacteremia and infection recurrence), iii) feasibility of direct/early discharge from the ED and potential advantage of dalbavancin.
Expert opinion
Authors’ expert opinion was focused on drawing the profiles of patients who could benefit most from an antimicrobial therapy with dalbavancin in the ED and positioning this drug as a direct or early discharge strategy from the ED in order to avoid hospitalization and its complications. We have provided a therapeutic and diagnostic algorithm based on evidence from the literature and authors’ expert opinion and suggest the use of dalbavancin in patients with ABSSSIs who are not eligible for oral therapies or Outpatient Parenteral Antibiotic Therapy (OPAT) programs and who would have otherwise been hospitalized only for antibiotic therapy.
1. Introduction
Acute Bacterial Skin and Skin Structure Infections (ABSSSIs) are a common reason of Emergency Department (ED) access, accounting for a considerable number of hospital admissions and translating into a high financial burden for the health care system [Citation1–5].
The most common causative pathogens of ABSSSI are β-hemolytic streptococci and Staphylococcus aureus; methicillin resistance in S. aureus characterizes 20–44% of the isolates, according to the epidemiological setting [Citation6–11]. Although being especially prevalent among patients with previous health-care contacts, methicillin resistance is no longer an exclusive feature of these patients [Citation12]. In Italy, where the prevalence of methicillin-resistant S. aureus (MRSA) is among the highest in Europe, MRSA was the most represented cause of ABSSSI when a microbial isolate was available [Citation7,Citation11,Citation13].
While hospitalization is generally required for unstable or severely infected patients, or those with significant and active comorbidities, the need for intravenous antibiotics was the sole reason for admission in approximately 40% of the patients in one study [Citation4,Citation14–17].
In this setting, the advent of long-acting lipoglycopeptides (LALs) potentially allows for an outpatient management of subjects with ABSSSI, still requiring parenteral therapy, but who do not need hospitalization [Citation18–20].
Dalbavancin is a semisynthetic lipoglycopeptide antibiotic with activity against Gram-positive pathogens including MRSA, which has been approved by the Food and Drug Administration (FDA) and European Medicines Agency (EMA) for the treatment of ABSSSIs [Citation21]. Its favorable pharmacokinetic profile and the long elimination half-life represent a key advantage over other intravenous drugs requiring multiple daily doses or oral antibiotics, which require patients’ adherence and may be encumbered by adverse events [Citation22].
This article aims to review the current literature on dalbavancin for ABSSSIs and draw the profiles of patients who could benefit most from an antimicrobial therapy with dalbavancin in the ED, allowing an early discharge. Furthermore, we propose a diagnostic-therapeutic algorithm for the management of patients presenting to the ED with ABSSSI.
2. Methods
In the first round of discussion among the authors, the following topics were identified to be addressed in this review: i) classification of ABSSSIs and LALs, with a main focus on dalbavancin and its microbiological activity, efficacy and safety profile, ii) assessment of critical steps for the management of ABSSSI in the ED (decision to hospitalize, risk of bacteremia and infection recurrence), iii) feasibility of direct/early discharge from the ED and potential advantage of dalbavancin in this setting and iv) the economic impact of dalbavancin in the ED for the treatment of ABSSSIs.
Afterwards, publications were searched through the MEDLINE/PubMed database using the following terms, used alone or combined with each other, as appropriate: ‘ABSSSIs,’ ‘Acute Bacterial Skin and Skin Structure Infections,’ ‘LALs,’ ‘long-acting lipoglycopeptides,’ ‘Dalbavancin,’ ‘Emergency Department,’ ‘Early Discharge,’ ‘Microbiological activity,’ ‘In vitro activity,’ ‘Efficacy,’ ‘Effectiveness,’ ‘Safety,’ ‘Blood Cultures,’ ‘Bacteremia,’ ‘Bacteremia Risk Score,’ ‘Bacteremia Score of Cellulitis,’ ‘OPAT,’ ‘Economic evaluation,’ ‘Budget Impact Analysis,’ ‘Telehealth medicine.’
We excluded abstracts or articles not written in English. Publications regarding the use of dalbavancin in the pediatric population were also excluded, since it is the topic of a specific publication [Citation23].
No limitations to publication dates were considered, although the literature search was completed at the end of October, 2022.
All the found articles were then selected for their relevance during subsequent rounds of discussion among the authors.
The preliminary draft was shared among authors and then organized in the final version, which was finally reviewed and approved by all the authors.
3. Classification of ABSSSIs
ABSSSIs represent a special subgroup of complicated skin and soft tissue infections (SSTIs), and have been defined by the FDA as a bacterial infection of the skin with a lesion size of at least 75 cm2 (lesion size measured by the area of redness, edema, or induration) to facilitate evaluation of new molecules in randomized clinical trials (RCT) [Citation11,Citation24]. ABSSSIs include cellulitis/erysipelas, wound infections, and major cutaneous abscesses () [Citation24]. Despite this classification, ABSSSIs represent an extremely heterogenous group of diseases in terms of prognosis, ranging from mild to potentially life-threatening conditions [Citation22,Citation23].
Table 1. Classification of acute bacterial skin and skin structure infections (ABSSSIs) according to the FDA definitions [Citation25].
Cellulitis is one of the most common ABSSSI, followed by erysipela, surgical site and diabetic foot infections, and abscesses [Citation11,Citation25–28].
Due to the prevalence of Gram-positives as causative agents of ABSSSIs, empiric antibiotic treatment is mainly targeted at covering Staphylococci and Streptococci, including MRSA in the presence of risk factors or if the prevalence of MRSA in the community is high [Citation22]. However, conditions possibly complicating the efficacy of antimicrobials are the involvement of adjacent structures, such as bone and/or joints and biofilm formation on implants [Citation29].
ABSSSIs may also require surgical interventions such as debridement, if necrotic tissue is present, or incision and drainage, if an abscess is detected [Citation30]. In the latter condition, point-of-care ultrasound (POCUS) represents a rapid, noninvasive, painless, and easy-to-repeat method able to distinguish between abscess and cellulitis [Citation31,Citation32]. When the presence of an abscess is clinically irrefutable, the role of POCUS may be questionable; however, in cases where differentiating abscess from cellulitis by means of clinical examination alone is difficult, POCUS exhibited a crucial role and resulted in management changes – to perform or not perform a drainage – in approximately 25% of the patients with suspected, but not clinically obvious, abscesses [Citation33–36].
4. Long-acting lipoglycopeptides
4.1. Types of LALs
Three LALs are currently available, telavancin, dalbavancin, and oritavancin. While telavancin is not approved in Europe, dalbavancin and oritavancin obtained approval by the FDA and EMA for the treatment of ABSSSIs caused by Gram-positive cocci in 2014 and 2015, respectively [Citation21,Citation37].
Dalbavancin is a lipoglycopeptide antibiotic with a chemical structure similar to teicoplanin; however, compared to teicoplanin, the presence of an extended lipophilic side chain allows for a better anchorage to the bacterial cell membrane and therefore enhances its potency and prolongs its terminal half-life up to 14.4 days [Citation38,Citation39]. The amidated carboxyl side group enhances the agent’s anti-staphylococcal activity [Citation39–41]. The distribution of the drug allows to reach adequate concentrations in skin, synovial fluid, and bone, which are higher than the MICs90 of the principal pathogens causing ABSSSIs [Citation42,Citation43]. In a pharmacokinetic study carried out among healthy volunteers, the penetration rate into skin blister fluid after administration of a single 30-min 1000 mg iv dose was approximately 60% [Citation43,Citation44]. Dalbavancin does not interact with cytochrome P450 enzymes, showing a low likelihood for drug – drug interactions with other drugs [Citation44–46].
Principal features and main differences among the three LALs are depicted in .
Table 2. Main features of the long acting lipoglycopeptides for the treatment of Acute Bacterial Skin and Skin Structure Infection (ABSSSI).
4.2. Microbiological activity of dalbavancin
Dalbavancin exhibits an in vitro activity higher than vancomycin or teicoplanin against several Gram-positive pathogens () [Citation54,Citation55]. Dalbavancin regularly shows MIC values against S. aureus and MRSA isolates at ≤0.06 mg/L [Citation47,Citation56]. Large comparative studies on S. aureus, including isolates with decreased susceptibility to vancomycin, telavancin, teicoplanin, and linezolid showed that only 0.01% of the isolates were categorized as dalbavancin nonsusceptible [Citation48,Citation49,Citation57]. Notably, against vancomycin-intermediate S. aureus (VISA), with a vancomycin MIC of 4–8 µg/ml and heteroresistant VISA (hVISA), dalbavancin MICs were typically 4- to 8-fold lower than vancomycin and 16 to 32-fold lower than linezolid [Citation48]. Moreover, population data from US medical centers reported a low percentage of staphylococcal (0.3%) and streptococcal isolates (4%) with dalbavancin MIC values above the currently proposed FDA breakpoint [Citation50,Citation51]. Thus, nonsusceptible staphylococci are rare, reported in less than 1% of the cases. In support of these in-vitro findings, several studies have confirmed that dalbavancin exerts a potent in vivo activity against S. aureus strains, including those with reduced susceptibility to vancomycin [Citation58,Citation59]. Notably, dalbavancin activity against enterococci largely depends on vancomycin activity. In vancomycin-susceptible enterococci (VSE), dalbavancin is active (MIC90, 0.06 μg/mL) against both E. faecalis and E. faecium (MIC ≤0.125 µg/ml). Conversely, vancomycin-resistant enterococci (VRE) isolates are less susceptible to dalbavancin, regardless of species [Citation50,Citation51].
Table 3. Antimicrobial activity of dalbavancin against Gram-positive pathogens.
Dalbavancin showed in-vitro activity against the anaerobic Gram-positive and Corynebacterium species [Citation52]. Moreover, previous in vitro studies revealed that dalbavancin had significantly lower MICs against Clostridioides difficile than vancomycin [Citation52,Citation53].
It is worth noting that dalbavancin has demonstrated in vitro synergistic activity in combination with oxacillin and ceftaroline against staphylococci, including MRSA, VISA, and enterococci [Citation60–62]. The combination of dalbavancin plus linezolid was highly synergistic in vitro against MRSA, with no antagonistic effect [Citation63]. Therefore, combination therapy may represent an effective option in difficult-to-treat MRSA or Enterococcus spp, particularly in patients with device-associated infections [Citation64]. As device-associated infections are at high risk of developing biofilm, the observed dalbavancin activity on biofilm eradication appears as a promising feature of this drug [Citation30,Citation65,Citation66].
4.3. Efficacy and safety of dalbavancin for ABSSSIs: evidence from clinical studies
The two identically designed non-inferiority phase 3 trials DISCOVER-1 and DISCOVER-2 included 652 individuals with ABSSSIs receiving two doses of dalbavancin (1000 mg on day 1, followed by 500 mg on day 8), compared with 651 receiving intravenous vancomycin for at least 3 days (1 g every 12 h), with the option to switch to oral linezolid to complete 10–14 days of treatment [Citation21]. The primary endpoint was clinical success measured at 48–72 h of therapy. Non-inferiority of dalbavancin was demonstrated in both trials [Citation21].
Afterwards, a randomized clinical trial including 698 ABSSSI patients compared the classic two doses regimen with a 1500 mg single-dose. The authors found non-inferiority of the single dose regimen and no significant increase in the adverse events rate [Citation67]. Accordingly, the regulatory agencies FDA and EMA also expanded their approval to the single-dose schedule.
In a sub-analysis of the phase III trial, dalbavancin showed similar efficacy as a single dose and a two-dose regimen in the outpatient and inpatient subgroups, with outpatients reporting significantly greater satisfaction with antibiotic treatment and care setting compared with inpatients [Citation68].
In another post-hoc analysis specific on people who inject drug (PWID) (n = 212), dalbavancin efficacy was similar between the single and two-dose therapy groups in the PWID and non-PWID populations at all time points [Citation69].
Nadipelly et al conducted an open-labeled prospective randomized study including 200 patients with ABSSSIs who were randomized to receive either a single dose of 1500 mg intravenous dalbavancin (Group I) or intravenous telavancin 10 mg/kg every 24 h for 6 days (Group II). Clinical success, defined as a complete resolution of clinically meaningful signs and symptoms of infection was observed in 86.6% of the patients receiving dalbavancin and in 81.5% of the patients receiving telavancin () [Citation70].
Table 4. Registration and observational studies of dalbavancin for the treatment of acute bacterial skin and skin structure infections (ABSSSI).
In the subsequent years, several observational studies evaluated the real-life effectiveness of dalbavancin for the treatment of ABSSSIs () [Citation1,Citation5,Citation7,Citation15,Citation71–77]. Although the majority of the studies also included patients with infections different from ABSSSIs, the clinical efficacy of dalbavancin was overall demonstrated, with clinical cure rates ranging from 80% to 98%.
Interestingly, dalbavancin showed a remarkably good safety profile [Citation78]. In the DISCOVER trials, adverse events, including nephrotoxicity, were reported in fewer patients treated with dalbavancin than in those treated with vancomycin or linezolid [Citation21]. Similar findings were found in a safety data analysis including 1778 patients treated with dalbavancin and 1224 patients receiving a comparator agent [Citation79]. The duration of adverse events was similar for dalbavancin and the comparator regimens; therefore, the long half-life of dalbavancin did not lead to safety concerns [Citation79].
Observational studies confirmed the low incidence of adverse events, ranging from 2% to 13% of the study populations. Most of these adverse events were of mild entity [Citation1,Citation5,Citation7,Citation15,Citation71–77].
5. ABSSSIs at the emergency department: decision to hospitalize, risk of bacteremia and infection recurrence
In the ED setting, crucial importance is represented by the appropriateness of hospitalization. An analysis of more than 600,000 patients with ABSSSIs found that 60% of those hospitalized could potentially have been treated as outpatients [Citation80]. Of note, the need for intravenous antibiotics administration represented the unique reason for hospitalization in approximately half of patients [Citation14]. Therefore, the current hospitalization rate appears undue, leading to high costs for the health-care system and possible related iatrogenic complications, especially in older patients [Citation81–84].
In one study, hospitalization rates were higher in the presence of a history of fever, extension of infection, history of failed treatment and age >65 years [Citation14]. Also, abnormal imaging results, systemic inflammatory response syndrome, diabetes, previous infection at the same location and an infection involving the hand were associated with worse outcomes [Citation85,Citation86].
Identification of these criteria may therefore enable clinicians to better assess the need for hospital admission and, at the same time, identify patients who receive only little benefit from hospitalization and would otherwise be better treated in an outpatient setting [Citation85].
Another crucial step is the assessment of the risk of bacteremia, by evaluating patients who should have blood cultures (BCs) done in the ED [Citation87]. Indeed, patients with bacteremia experience a longer duration of hospitalization and a higher rate of infection recurrence [Citation88–90]. However, the role of BCs in the management of ABSSSIs remains still controversial, since performing BCs in all patients seems to be not cost effective and have only marginal clinical advantage [Citation91–94]. Furthermore, a significant rate of BC contamination may occur [Citation95]. The incidence of bacteremia during ABSSSIs widely varies, ranging from 2% to 21.3% among patients for whom BCs are performed [Citation25,Citation87,Citation96].
Risk factors for the development of bacteremia include advanced age, fever, elevated White Blood Cells (WBC), signs of Systemic Inflammatory Response Syndrome (SIRS), length of illness, lymphedema, and comorbidities, such as chronic renal disease and liver cirrhosis [Citation25,Citation95]. In particular, age (≥65 years), involvement of non-lower extremities, liver cirrhosis, and SIRS were included in the Bacteremia Score of Cellulitis: a cutoff value of 2 was able to discriminate patients with cellulitis at low or high risk of bacteremia [Citation25]. Besides, the extension and severity of cellulitis emerged as risk factors for bacteremia, suggesting that the size of the infection area should be measured in the ED, and that patients with large cellulitis should be more carefully monitored [Citation95–97]. Likewise, the presence of a device or prosthesis accounted for the highest risk of bacteremia [Citation95–98]. Furthermore, it seems reasonable to perform BCs in patients with malignancy, neutropenia, and/or immunosuppression [Citation99].
Recurrence is a common phenomenon in patients with cellulitis, especially in countries with a high prevalence of MRSA [Citation100,Citation101]. Lymphedema, chronic venous insufficiency, peripheral vascular disease, and deep vein thrombosis, which contributed to the creation of the recently proposed Cellulitis Recurrence Score (CRS), were risk factors of recurrence [Citation102].
The 30-day ED return rate after the first ED visit ranges between 8.3% and 28% [Citation71,Citation77,Citation103], and is higher in patients with abnormal WBC count at initial presentation, congestive heart failure, hypertension, and diabetes mellitus [Citation77].
6. Early discharge in the ED: potential advantage of dalbavancin
Patients may be considered suitable for an early discharge from the ED or the ED short stay/observation unit by means of a reassessment at 48–72 h after treatment initiation. Indeed, culture results may be available and patients’ clinical conditions may have improved or become stable during that time [Citation104,Citation105].
Oral options such as trimethoprim/sulfamethoxazole or linezolid/tedizolid may present limitations, such as myelotoxicity, potential for drug–drug interactions or risk of drug over-exposure, leading to the need for close laboratory follow-up [Citation106–110]. Furthermore, patient’s adherence to the therapy is needed. OPAT regimen is an alternative to oral therapy; however, this strategy may be limited by the need for daily use of an intravenous line, therapeutic drug monitoring and the low diffusion of OPAT programs [Citation8,Citation111,Citation112].
Therefore, in the case of contraindications to oral therapy or unavailability/unfeasibility of OPAT programs, dalbavancin may represent the optimal choice for the treatment of selected patients with ABSSSIs, allowing patients’ early discharge [Citation113–116].
Patients’ categories, which may benefit most from the use of dalbavancin in the ED are those with expected poor oral adherence or with limited access to/contact with healthcare systems, such as homeless, elderly, prisoners, military personnel, people who inject drugs, people living in rural areas far away from hospitals, people with psychiatric disorders or alcohol abuse, frail categories including severely burned or oncologic patients [Citation22,Citation105,Citation110,Citation116–118].
Given their higher risk of recurrent ABSSSIs and worse outcomes, along with their noncompliant behavior, PWID represents one ideal candidate subgroup for LALs [Citation69,Citation118–120]. In PWID, dalbavancin efficacy was high and well tolerated, with similar rates of adverse events compared to the non-PWID population [Citation69]. With a short-duration and single intravenous infusion, dalbavancin represents an optimal alternative to the placement of a permanent venous access or a central line, thereby reducing catheters’ complications, such as line occlusion, venous thrombosis, infections or hematomas [Citation15,Citation121,Citation122]. Patients’ satisfaction may also be improved with dalbavancin: indeed, the majority of patients prefer a single-dose 30-min intravenous antibiotic over other antibiotic treatment options [Citation15,Citation68]. Avoiding prolonged hospitalization may further prevent complications usually associated with hospitalization itself, such as hospital-acquired infections or Multi-Drug Resistant Organisms (MDROs) colonization/infection [Citation1,Citation15].
7. Economic impact of dalbavancin in the ED for the treatment of ABSSSIs
A systematic review, network meta-analysis and cost analysis compared the newer lipoglycopeptides to standard care and to each other for the treatment of complicated SSTIs, estimating that using dalbavancin could save third-party payers $ 1,442 to $ 4,803 per case [Citation123].
A recent study aimed to evaluate the direct costs associated with the management of severe ABSSSI patients from a national healthcare provider’s perspective in Italy, Romania, and Spain. The hypothetical administration of dalbavancin rather than the Standard of Care (SoC) therapy (based on either vancomycin, teicoplanin, or linezolid) resulted in an estimated mean reduction in hospital stay of 3.3 days per ABSSSI patient, with no significant incremental costs from a National Health System perspective [Citation124].
A budget impact analysis considered national administrative databases of patients with non-severe ABSSSIs who accessed the ED in Italy, Spain, and Austria between 2006 and 2014, with an average calculated annual number of patients equal to 5,396, 7,884, and 1,788, respectively [Citation125]. The model estimated that a hypothetical scenario in which an early single dose of dalbavancin (1500 mg) would have been administered rather than the SoC therapy actually prescribed would have allowed in the first year of its introduction a reduction in the total financial burden in Italy and Spain (− € 352,252 and − € 233,991, respectively), while it increased the total economic burden in Austria (€ 80769); in the third year of its introduction, dalbavancin use would have reduced the total economic burden in all Countries (− € 1.1 million, − € 810,650, and − € 70269, respectively). This cost saving was mainly driven by the estimated increase in patients discharged directly by the ED combined to the reduced in-hospital length of stay for those who were hospitalized, following the hypothetical dalbavancin use rather than the actual SoC antimicrobial therapy: −1,332 days over 3 years in Italy, −1,187 in Spain, −1,537 in Austria.
Although providing remarkable insights, the above-mentioned studies present some limitations, including: the frequent lack of sufficient information; the estimation of costs for a whole, although large, study population rather than the calculation of actual costs paid for individual patients; the possible variation of tariffs between different regions of the same Country and even between different hospitals in a same region.
A real-life, individual patients-based study calculated that an early discharge strategy following the use of dalbavancin would have saved a median of € 5,034 (IQR 3,647–6,590) for each ABSSSI case compared to the actual antimicrobial therapy administered [Citation15]. Other real-life studies estimated the cumulative cost saving driven by dalbavancin use of in ABSSSI along with other sites of infection, thus not allowing to extrapolate the saving quota attributable to ABSSSI only [Citation6,Citation126].
The reduced in-hospital length of stay represents a major driver for the cost-effectiveness of dalbavancin use across studies. Indeed, this parameter is associated with a high-cost burden, although it may differ between geographical areas. For instance, the cost of one day of hospitalization in an internal medicine ward in Spain in 2014 was € 325.01 and in an infectious disease ward in Italy € 361; the average cost estimated in 2020 of a day in a U.S. State-local government hospital is $ 2,606 but varies from $ 671 in Montana to $ 5,557 in Connecticut [Citation6,Citation15,Citation127].
Several indirect costs should be considered which could as well be saved using long-acting antibiotics. For instance, daily infusion of antimicrobials often requires indwelling middle-term venous access such as peripherally inserted central catheter (PICC), which poses an average per patient cost of $ 873 for placement and $ 205 for complications (e.g., infection, thrombophlebitis, malposition, malfunction); if systemic and serious complications occur (e.g., bacteremia, endocarditis, sepsis) PICC-related costs markedly increase [Citation128]. Furthermore, glycopeptides require a therapeutic drug monitoring, which may cost from 24 to 56 euros and can be complicated by a transient nephrotoxicity, which determines additional costs for its management and the prolonged in-hospital stay [Citation124]. Moreover, daily intravenous antibiotics require nursing time related to drug dilution, positioning, and removing infusion line, and patient observation, which has variable costs across different hospitals/regions. Finally, in a single-center, real-life study, the introduction of dalbavancin use compared to usual antibiotics yielded significant improvements in work productivity and ability to complete daily activities, in addition to patient satisfaction, thus saving social costs as well [Citation129].
8. Conclusions
Early discharge from ED of eligible patients with the use of dalbavancin could represent an effective and advantageous strategy for the management of patients with ABSSSIs who are not eligible for oral therapies or OPAT programs and who would have otherwise been hospitalized only for antibiotic therapy.
A rigorous assessment of patient characteristics at admission as well as the stratification of the existing risks of bacteremia, recurrence, and ED return could provide valuable information to discriminate the need for hospitalization and select patients who may, instead, benefit from a 48–72 h observation before being discharged on ongoing dalbavancin.
9. Expert opinion: proposal of a diagnostic and therapeutic algorithm for the management of ABSSSIs at the ED
ABSSSIs represent an important reason for hospital admission worldwide; a significant rate of admissions is due to the administration of intravenous antimicrobial therapy alone, accounting for an overall excess of hospitalizations [Citation1–4,Citation14]. Dalbavancin has proven to be highly effective and safe for the treatment of ABSSSIs in registration and observational studies and represents a potential attractive option for the direct or early discharge from the ED of patients with ABSSSIs who are not candidate for oral therapy or OPAT programs [Citation4,Citation21,Citation71–77]. Indeed, dalbavancin’s favorable pharmacokinetic profile and its long elimination half-life provide advantage over other intravenous drugs requiring multiple daily doses or oral antibiotics, which need patients’ adherence and may be encumbered by adverse events [Citation8]. Furthermore, in light of the increase of multi-drug resistance in Gram-positive pathogens causing ABSSSIs, the activity of dalbavancin against MRSA represents an additional advantage, which renders dalbavancin a feasible option for selected patients for direct or early discharge from the ED [Citation7]. However, evidence is still limited and highlights the need for further research in order to optimize this treatment strategy.
shows the study panel proposal for the diagnostic and therapeutic approaches of patients presenting at the ED with ABSSSI.
Figure 1. Proposal of a diagnostic and therapeutic algorithm for the management of Acute Bacterial Skin and Skin Structure Infections (ABSSSIs) at the Emergency Department (ED).
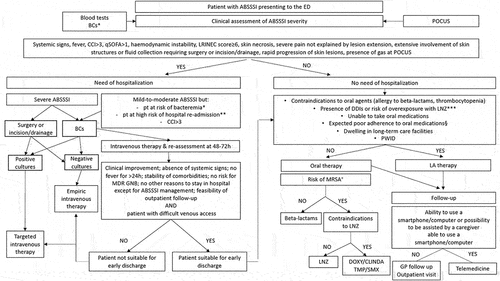
The first step is assessing infection severity and the need for hospitalization through clinical evaluation, laboratory tests and, when applicable, POCUS assessment [Citation14,Citation25,Citation31,Citation87,Citation130]. Blood cultures should be reserved to patients considered at high risk of bacteremia, while surgical procedures and empirical intravenous therapy should not be delayed in the case of hospitalization [Citation87]. If the patient meets the early discharge criteria at the 48–72 h clinical re-assessment, possible options include oral therapy or, if feasible, OPAT programs [Citation105,Citation131–134].
The use of dalbavancin as an early discharge strategy is suggested in the presence of contraindications to oral agents, inability to take oral medications, conditions leading to expected poor adherence to oral medications, such as PWID, homeless, residents in Long-Term Care Facility (LTCF), dependent people, patients with psychiatric illnesses [Citation115].
After discharge, a close follow-up should be guaranteed, in order to early identify signs and symptoms indicating that the infection is not responding to therapy and that there is the need of further care [Citation5,Citation22]. Although its use is increasing and is currently proposed as a possible follow-up strategy, the telehealth approach is feasible for patients or caregivers who are able to use a smartphone/computer (i.e., to provide images of the involved skin area and/or wound) [Citation4,Citation18,Citation19]. Otherwise, a more traditional follow-up with outpatient visits and/or the involvement of the general practitioner is suggested.
The panel believes that a similar approach to patients with ABSSSIs presenting at the ED may be easily implemented into clinical practice, following a strict collaboration between ED physicians, Infectious Diseases specialists and general practitioners for patients’ follow-up. Further studies should be promoted to address the role of dalbavancin as a direct or early discharge strategy from the ED in order to avoid hospitalization and its complications by, at the same time, ensuring high efficacy and safety. Furthermore, the additional potential advantages of using dalbavancin in the ED rely on its cost-effectiveness by reducing in-hospital length, providing indirect economic savings and, eventually, contributing to the reduction of ED overcrowding.
Article highlights
Acute Bacterial Skin and Skin Structure Infections (ABSSSIs) are a common reason of access to the Emergency Department (ED), account for a considerable number of hospital admissions and exhibit a high financial burden for the health care system
Long-acting lipoglycopeptides (LALs) allow for an outpatient management of subjects with ABSSSI, still requiring parenteral therapy, but who do not need hospitalization
Dalbavancin is a LAL with activity against Gram-positive pathogens including MRSA and has been approved by the FDA and EMA for the treatment of ABSSSIs
In registration and observational studies, dalbavancin showed high efficacy and a remarkably good safety profile for the treatment of ABSSSIs
Crucial steps in the management of patients with ABSSSIs at the ED include the decision to hospitalize and the risk of bacteremia and/or infection recurrence
Dalbavancin may be considered as a strategy for a direct or early discharge from the ED of eligible patients with ABSSSIs
Dalbavancin’s favorable pharmacokinetic profile and its long elimination half-life represent a key advantage over other intravenous drugs requiring multiple daily doses or oral antibiotics, which require patients’ adherence and may be encumbered by adverse events
Dalbavancin use is cost-effective by reducing in-hospital length of stay and saving additional indirect costs related to the need of multiple daily infusion of antimicrobials, laboratory monitoring of potential toxicities and nurse assistance
A diagnostic and therapeutic algorithm for the management of ABSSSI at the ED is proposed
List of abbreviations
| (ABSSSIs) | = | Acute Bacterial Skin and Skin Structure Infections |
| (ED) | = | Emergency Department |
| (LALs) | = | long-acting lipoglycopeptides |
| (MRSA) | = | methicillin-resistant S. aureus |
| (FDA) | = | Food and Drug Administration |
| (EMA) | = | European Medicines Agency |
| (RCT) | = | randomized clinical trials |
| (POCUS) | = | point-of-care ultrasound |
| (VISA) | = | vancomycin-intermediate S. aureus |
| (hVISA) | = | heteroresistant VISA |
| (VSE) | = | vancomycin-susceptible enterococci |
| (VRE) | = | vancomycin-resistant enterococci |
| (PWID) | = | people who inject drug |
| (BCs) | = | blood cultures |
| (WBC) | = | White Blood Cells |
| (SIRS) | = | Systemic Inflammatory Response Syndrome |
| (CRS) | = | Cellulitis Recurrence Score |
| (OPAT) | = | Outpatient Parenteral Antibiotic Therapy |
| (MDROs) | = | Multi-Drug Resistant Organisms |
| (SoC) | = | Standard of Care |
| (PICC) | = | peripherally inserted central catheter |
| (LTCF). | = | Long-Term Care Facility |
Declaration of interest
A. Oliva participated to advisory boards or speaker’s bureau for MSD, Zambon and Angelini. E Durante-Mangoni reports research funding to his Institution from MSD, Pfizer, Angelini, Infectopharm, Advanz pharma, and personal fees or participation to advisory boards or speaker’s bureau of Roche, Genentech, Pfizer, MSD, Angelini, Advanz Pharma, Bio-Merieux, Shionogi, Menarini, Abbvie, Sanofi-Aventis, Medtronic, Trx and DiaSorin. M. Venditti participated to advisory boards for MSD, Angelini, Mundipharma and received personal fee for speaking bureau from Shionogi, Menarini, Angelini, Pfizer.
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Author contribution
Conceptualization of the study: MV, EDM; literature revision: AO, SC, VC, MC, EDD, MF, GG, EDM, MV; manuscript draft writing: AO, EDD, SC; supervision: MV, MF, EDM.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Durante-Mangoni E, Gambardella M, Iula VD, et al. Current trends in the real-life use of dalbavancin: report of a study panel. Int J Antimicrob Agents. 2020 Oct;56(4):106107. Epub 2020 Jul 25. PMID: 32721599. DOI:10.1016/j.ijantimicag.2020.106107.
- Weng QY, Raff AB, Cohen JM, et al. Costs and consequences associated with misdiagnosed lower extremity cellulitis. JAMA Dermatol. 2017;153(2):141–146. DOI:10.1001/jamadermatol.2016.3816.
- Jensen IS, Wu E, Fan W, et al. Use of oritavancin in moderate-to-severe absssi patients requiring iv antibiotics: a u.s. payer budget impact analysis. J Manag Care Spec Pharm. 2016 Jun;22(6):752–764. PMID: 27231802. DOI:10.18553/jmcp.2016.22.6.752.
- Durante-Mangoni E, Riccardi A, Guarino M, et al. Emergency department care of ABSSSI with dalbavancin infusion, direct discharge, and outpatient telemedicine follow up: a study protocol. J Chemother. 2022 Oct;20:1–7. Epub ahead of print. PMID: 36264157. DOI:10.1080/1120009X.2022.2134616.
- Bosso JA, Casapao AM, Edwards J, et al. Clinical pathway for moderate to severe acute bacterial skin and skin structure infections from a US perspective: a roundtable discussion. Hosp Pract (1995). 2016 Oct;44(4):183–189. Epub 2016 Sep 12. PMID: 27598313. DOI:10.1080/21548331.2016.1230466.
- Bouza E, Valerio M, Soriano A, et al. DALBUSE Study Group (Dalbavancina: Estudio de su uso clinico en España), Dalbavancin in the treatment of different gram-positive infections: a real-life experience. Int J Antimicrob Agents. 2018 Apr;51(4):571–577. Epub 2017 Nov 24. PMID: 29180276. DOI:10.1016/j.ijantimicag.2017.11.008.
- Bai F, Aldieri C, Cattelan A, et al. Efficacy and safety of dalbavancin in the treatment of acute bacterial skin and skin structure infections (ABSSSIs) and other infections in a real-life setting: data from an Italian observational multicentric study (DALBITA study). Expert Rev Anti Infect Ther. 2020 Dec;18(12):1271–1279. Epub 2020 Aug 14. PMID: 32797758. DOI:10.1080/14787210.2020.1798227.
- Soriano A, Rossolini GM, Pea F. The role of dalbavancin in the treatment of acute bacterial skin and skin structure infections (ABSSSIs). Expert Rev Anti Infect Ther. 2020 May;18(5):415–422. Epub 2020 Mar 29. PMID: 32223465. DOI:10.1080/14787210.2020.1746643.
- Moet GJ, Jones RN, Biedenbach DJ, et al. Contemporary causes of skin and soft tissue infections in North America, Latin America, and Europe: report from the SENTRY antimicrobial surveillance program (1998–2004). Diagn Microbiol Infect Dis. 2007;57(1):7–13. DOI:10.1016/j.diagmicrobio.2006.05.009.
- Morrissey I, Leakey A, Northwood JB. In vitro activity of ceftaroline and comparator antimicrobials against European and Middle East isolates from complicated skin and skin-structure infections collected in 2008–2009. Int J Antimicrob Agents. 2012;40(3):227–234.
- Esposito S, De Simone G, Pan A, et al. Italian society of infectious and tropical diseases. epidemiology and microbiology of skin and soft tissue infections: preliminary results of a national registry. J Chemother. 2019 Feb;31(1):9–14. Epub 2018 Dec 3. PMID: 30508410. DOI:10.1080/1120009X.2018.1536320.
- Lakhundia S, Zhang K. Methicillin-resistant staphylococcus aureus: molecular characterization, evolution, and epidemiology. Clin Microbiol Rev. 2018 Oct;31(4). e00020–18. DOI:10.1128/CMR.00020-18
- Kock R, Friedrich A. On behalf of the original author Group C. Systematic literature analysis and review of targeted preventive measures to limit healthcare-associated infections by meticillin-resistant Staphylococcus aureus. Euro Surveill. 2014 Jul 24;19(29):20860. DOI: 10.2807/1560-7917.ES2014.19.37.20902.
- Talan DA, Salhi BA, Moran GJ, et al. Factors associated with decision to hospitalize emergency department patients with skin and soft tissue infection. West J Emerg Med. 2015;16(1):89–97. DOI:10.5811/westjem.2014.11.24133.
- Poliseno M, Bavaro DF, Brindicci G, et al. Dalbavancin efficacy and impact on hospital length-of-stay and treatment costs in different gram-positive bacterial infections. Clin Drug Investig. 2021 May;41(5):437–448.
- Aubert CE, Rodondi N, Terman SW, et al. HOSPITAL score and LACE index to predict mortality in multimorbid older patients. Drugs Aging. 2022 Mar;39(3):223–234. Epub 2022 Mar 9. PMID: 35260994; PMCID: PMC8934762. DOI:10.1007/s40266-022-00927-0.
- Falcone M, Paul M, Tiseo G, et al. ESCMID Study Group for Infections in the Elderly (ESGIE). Considerations for the optimal management of antibiotic therapy in elderly patients. J Glob Antimicrob Resist. 2020 Sep;22:325–333. Epub 2020 Mar 9. PMID: 32165285. DOI:10.1016/j.jgar.2020.02.022
- Koziatek C, Klein N, Mohan S, et al. Use of a telehealth follow-up system to facilitate treatment and discharge of emergency department patients with severe cellulitis. Am J Emerg Med. 2021;41:184–189.
- Patel M, Smalley S, Dubrovskaya Y, et al. Dalbavancin use in the emergency department setting. Ann Pharmacother. 2019;53(11):1093–1101. DOI:10.1177/1060028019855159
- Falcone M, Concia E, Giusti M, et al. Acute bacterial skin and skin structure infections in internal medicine wards: old and new drugs. Intern Emerg Med. 2016 Aug;11(5):637–648. Epub 2016 Apr 15. PMID: 27084183. DOI:10.1007/s11739-016-1450-6.
- Boucher HW, Wilcox M, Talbot GH, et al. Once- weekly dalbavancin versus daily conventional therapy for skin infection. N Engl J Med. 2014;370(23):2169–2179. DOI:10.1056/NEJMoa1310480.
- Andreoni M, Bassetti M, Corrao S, et al. The role of dalbavancin for Gram positive infections in the COVID-19 era: state of the art and future perspectives. Expert Rev Anti Infect Ther. 2021 Sep;19(9):1125–1134. Epub 2021 Mar 16. PMID: 33682593. DOI:10.1080/14787210.2021.1894130.
- Volpicelli L, Venditti M, Oliva A. Acute bacterial skin and skin structure infections in pediatric patients: potential role of dalbavancin. Expert Rev Anti Infect Ther. 2023 Apr;21(4):329–341. Epub 2023 Feb 27. PMID: 36803139. DOI:10.1080/14787210.2023.2182769.
- Guidance for industry acute bacterial skin and skin structure infections: developing drugs for treatment [Internet]. U S dep heal hum serv food drug adm cent drug eval res. 2013. Available from: https://www.fda.gov/files/drugs/published/Acute-Bacterial-Skin-and-Skin-Structure-Infections—Developing-Drugs-for-Treatment.pdf
- Lee CY, Kunin CM, Chang C, et al. Development of a prediction model for bacteremia in hospitalized adults with cellulitis to aid in the efficient use of blood cultures: a retrospective cohort study. BMC Infect Dis. 2016; Oct 19;16(1):581. DOI: 10.1186/s12879-016-1907-2. PMID: 27756213; PMCID: PMC5070006.
- Christensen KLY, Holman RC, Steiner CA, et al. Infectious disease hospitalizations in the United States. Clin Infect Dis. 2009;49(7):1025–1035. DOI:10.1086/605562
- Goettsch WG, Bouwes Bavinck JN, Herings RM. Burden of illness of bacterial cellulitis and erysipelas of the leg in the Netherlands. J Eur Acad Dermatol Venereol. 2006;20:834–839.
- Cranendonk DR, Lavrijsen APM, Prins JM, et al. Cellulitis: current insights into pathophysiology and clinical management. Neth J Med. 2017;75(9):366–378.
- Oliva A, Stefani S, Venditti M, et al. Biofilm-Related Infections in Gram-Positive Bacteria and the Potential Role of the Long-Acting Agent Dalbavancin. Front Microbiol. 2021;12:749685.
- Sartelli M, Guirao X, Hardcastle TC, et al. WSES/SIS-E consensus conference: recommendations for the management of skin and soft-tissue infections. World J Emerg Surg. [Internet]. 2018;13:p. 58. Available from 2018: https://wjes.biomedcentral.com/articles/10.1186/s13017-018-0219–9
- Barbic D, Chenkin J, Cho DD, et al. In patients presenting to the emergency department with skin and soft tissue infections what is the diagnostic accuracy of point-of-care ultrasonography for the diagnosis of abscess compared to the current standard of care? A systematic review and meta-analysis. BMJ Open. 2017 Jan 10;7(1):e013688. Erratum in: BMJ Open. 2017 Sep 14;7(9):e013688corr1. PMID: 28073795; PMCID: PMC5253602. DOI:10.1136/bmjopen-2016-013688
- Counselman FL, Sanders A, Slovis CM, et al. The status of bedside ultrasonography training in emergency medicine residency programs. Acad Emerg Med. 2003;10(1):37–42. DOI:10.1197/aemj.10.1.37
- Tayal VS, Hasan N, Norton HJ, et al. The effect of soft-tissue ultrasound on the management of cellulitis in the emergency department. Acad Emerg Med. 2006 Apr;13(4):384–388. Epub 2006 Mar 10. PMID: 16531602. DOI:10.1197/j.aem.2005.11.074.
- Mower WR, Crisp JG, Krishnadasan A, et al. Effect of initial bedside ultrasonography on emergency department skin and soft tissue infection management. Ann Emerg Med. 2019 Sep;74(3):372–380. Epub 2019 Mar 27. PMID: 30926187. DOI:10.1016/j.annemergmed.2019.02.002.
- Marin JR, Dean AJ, Bilker WB, et al. Emergency ultrasound assisted examination of skin and soft tissue infections in the pediatric emergency department. Acad Emerg Med. 2013;20(6):545–553. DOI:10.1111/acem.12148
- Marin JR, Bilker W, Launternbach E, et al. Reliability of clinical examinations for pediatric skin and soft-tissue infections. Pediatrics. 2010;126(5):925–930. DOI:10.1542/peds.2010-1039
- Rubino CM, Bhavnani SM, Moeck G, et al. Population pharmacokinetic analysis for a single 1200-milligram dose of oritavancin using data from two pivotal phase 3 clinical trials. Antimicrob Agents Chemother. 2015;59(6):3365–3372. DOI:10.1128/AAC.00176-15
- Zhanel GG, Calic D, Schweizer F, et al. New lipoglycopeptides: a comparative review of dalbavancin, oritavancin and telavancin. Drugs. 2010;70(7):859–886. DOI:10.2165/11534440-000000000-00000
- Smith JR, Roberts KD, Rybak MJ. Dalbavancin: a novel lipoglycopeptide antibiotic with extended activity against Gram-positive infections. Infect Dis Ther. 2015;4(3):245–258.
- Bailey J, Summers KM. Dalbavancin: a new lipoglycopeptide antibiotic. Am J Health Syst Pharm. 2008;65(7):599–610.
- Carrothers TJ, Chittenden JT, Critchley I. Dalbavancin population pharmacokinetic modeling and target attainment analysis. Clin Pharmacol Drug Dev. 2020 Jan;9(1):21–31.
- Marbury T, Dowell JA, Seltzer E, et al. Pharmacokinetics of dalbavancin in patients with renal or hepatic impairment. J Clin Pharmacol. 2009;49(4):465–476.
- Nicolau DP, Sun HK, Seltzer E, et al. Pharmacokinetics of dalbavancin in plasma and skin blister fluid. J Antimicrob Chemother. 2007;60(3):681–684. DOI:10.1093/jac/dkm263.
- Pea F. Practical concept of pharmacokinetics/pharmacodynamics in the management of skin and soft tissue infections. Curr Opin Infect Dis. 2016;29(2):153–159.
- Dorr MB, Jabes D, Cavaleri M, et al. Human pharmacokinetics and rationale for once-weekly dosing of dalbavancin, a semisynthetic glycopeptide. J Antimicrob Chemother. 2005;55(Suppl 2):ii25–30. DOI:10.1093/jac/dki008
- Roberts KD, Sulaiman RM, Rybak MJ. Dalbavancin and oritavancin: an innovative approach to the treatment of Gram-positive infections. Pharmacotherapy. 2015;35(10):935–948.
- Riccobono E, Giani T, Baldi G, et al. Update on activity of dalbavancin and comparators against clinical isolates of Gram-positive pathogens from Europe and Russia (2017-2018), and on clonal distribution of MRSA. Int J Antimicrob Agents. 2022 Feb;59(2):106503. DOi:10.1016/j.ijantimicag.2021.106503
- Sader HS, Mendes RE, Duncan LR, et al. Antimicrobial activity of dalbavancin against Staphylococcus aureus with decreased susceptibility to glycopeptides, daptomycin, and/or linezolid from U.S. Medical Centers. Antimicrob Agents Chemother. 2018;62(3):e02397–17. DOi:10.1128/AAC.02397-17.
- Pfaller MA, Mendes RE, Duncan LR, et al. Activity of dalbavancin and comparator agents against Gram-positive cocci from clinical infections in the USA and Europe 2015–16. J Antimicrob Chemother. 2018;73(10):2748–2756. DOI:10.1093/jac/dky235.
- Jones RN, Flamm RK, Sader HS. Surveillance of dalbavancin potency and spectrum in the United States (2012). Diagn Microbiol Infect Dis. 2013;76(1):122–123.
- Jones RN, Sader HS, Flamm RK. Update of dalbavancin spectrum and potency in the USA: report from the SENTRY antimicrobial surveillance program (2011). Diagn Microbiol Infect Dis. 2013;75(3):304–307.
- Goldstein EJ, Citron DM, Merriam CV, et al. In vitro activities of dalbavancin and nine comparator agents against anaerobic gram-positive species and corynebacteria. Antimicrob Agents Chemother. 2003 Jun;47(6):1968–1971. doi:10.1128/AAC.47.6.1968-1971.2003.
- Binyamin D, Nitzan O, Azrad M, et al. In Vitro activity of Tedizolid, Dalbavancin, and Ceftobiprole against clostridium difficile. Front Microbiol. 2018 Jun 11;9:1256. DOI:10.3389/fmicb.2018.01256.
- Jones RN, Farrell DJ, Flamm RK, et al. Surrogate analysis of vancomycin to predict susceptible categorization of dalbavancin. Diagn Microbiol Infect Dis. 2015;82(1):73–77. DOI:10.1016/j.diagmicrobio.2015.01.017
- Citron DM, Tyrrell KL, Goldstein EJ. Comparative in vitro activities of dalbavancin and seven comparator agents against 41 Staphylococcus species cultured from osteomyelitis infections and 18 VISA and hVISA strains. Diagn Microbiol Infect Dis. 2014;79(4):438–440.
- Leuthner KD, Buechler KA, Kogan D, et al. Clinical efficacy of dalbavancin for the treatment of acute bacterial skin and skin structure infections (ABSSSI). Ther Clin Risk Manag. 2016;12:931–940.
- Biedenbach DJ, Ross JE, Fritsche TR, et al. Activity of Dalbavancin Tested against Staphylococcus spp. and β-Hemolytic Streptococcus spp. Isolated from 52 geographically diverse medical centers in the United States. J Clin Microbiol. 2007;45(3):998–1004. DOI:10.1128/JCM.02368-06
- Lepak A, Marchillo K, VanHecker J, et al. Impact of glycopeptide resistance in Staphylococcus aureus on the dalbavancin in vivo pharmacodynamic target. Antimicrob Agents Chemother. 2015;59(12):7833–7836. DOI:10.1128/AAC.01717-15
- Simonetti O, Lucarini G, Morroni G, et al. New evidence and insights on dalbavancin and wound healing in a mouse model of skin infection. Antimicrob Agents Chemother. 2020;64(4):e02062–19. DOI:10.1128/AAC.02062-19.
- Johnson DM, Fritsche TR, Sader HS, et al. Evaluation of dalbavancin in combination with nine antimicrobial agents to detect enhanced or antagonistic interactions. Int J Antimicrob Agents. 2006;27(6):557–560. DOI:10.1016/j.ijantimicag.2005.12.015
- Xhemali X, Smith JR, Kebriaei R, et al. Evaluation of dalbavancin alone and in combination with beta-lactam antibiotics against resistant phenotypes of Staphylococcus aureus. J Antimicrob Chemother. 2019;74:82–86.
- Kebriaei R, Rice SA, Stamper KC, et al. Dalbavancin alone and in combination with ceftaroline against four different phenotypes of Staphylococcus aureus in a simulated pharmacodynamic/pharmacokinetic model. Antimicrob Agents Chemother. 2019;63(4):e01743–18. DOI:10.1128/AAC.01743-18.
- Aktas G, Derbentli S. In vitro activity of daptomycin combined with dalbavancin and linezolid, and dalbavancin with linezolid against MRSA strains. J Antimicrob Chemother. 2017;72(2):441–443.
- Cacopardo B, Cattaneo D, Cortese F, et al. Role of dalbavancin as combination therapy: evidence from the literature and clinical scenarios. Exp Rev Anti-Infective Ther. 2022;20(7):997–1004. DOI:10.1080/14787210.2022.2060820
- Sivori F, Cavallo I, Kovacs D, et al. Role of extracellular DNA in dalbavancin activity against methicillin-resistant staphylococcus aureus (MRSA) biofilms in patients with skin and soft tissue infections. Microbiol Spectr. 2022 Apr 27;10(2):e0035122. DOI:10.1128/spectrum.00351-22.
- Díaz-Navarro M, Hafian R, Manzano I, et al. A dalbavancin lock solution can reduce enterococcal biofilms after freezing. Infect Dis Ther. 2022 Apr;11(2):743–755. DOI:10.1007/s40121-021-00579-4.
- Dunne MW, Puttagunta S, Giordano P, et al. A randomized clinical trial of single-dose versus weekly dalbavancin for treatment of acute bacterial skin and skin structure infection. Clin Infect Dis. 2016; Mar 1;62(5):545–551. Epub 2015 Nov 26. PMID: 26611777; PMCID: PMC4741365. DOI:10.1093/cid/civ982.
- Rappo U, Gonzalez PL, Puttagunta S, et al. Single-dose dalbavancin and patient satisfaction in an outpatient setting in the treatment of acute bacterial skin and skin structure infections. J Glob Antimicrob Resist. 2019 Jun;17:60–65. Epub 2019 Feb 20. PMID: 30797084. doi:10.1016/j.jgar.2019.02.007.
- Gonzalez PL, Rappo U, Akinapelli K, et al. Treatment of acute bacterial skin and skin structure infection with single-dose dalbavancin in persons who inject drugs. Drugs Context. 2018 Dec 11;7:212559. PMID: 30574170; PMCID: PMC6292452. DOI:10.7573/dic.212559
- Nadipelly J, Raghunath P, Kothapalli J. Efficacy of dalbavancin and telavancin in the treatment of acute bacterial skin and skin structure infections. MAEDICA – a J Clinical Med. 2018;13(3):208–212.
- Koziatek C, Mohan S, Caspers C, et al. Experience with dalbavancin for cellulitis in the emergency department and emergency observation unit. Am J Emerg Med. 2018 Jul;36(7):1312–1314. Epub 2017 Nov 20. PMID: 29157791. DOI:10.1016/j.ajem.2017.11.037.
- Wunsch S, Krause R, Valentin T, et al. Multicenter clinical experience of real life Dalbavancin use in gram-positive infections. Int J Infect Dis. 2019 Apr;81:210–214. Epub 2019 Feb 19. PMID: 30794940. DOi:10.1016/j.ijid.2019.02.013.
- Tobudic S, Forstner C, Burgmann H, et al. Real-world experience with dalbavancin therapy in gram-positive skin and soft tissue infection, bone and joint infection. Infection. 2019 Dec;47(6):1013–1020. Epub 2019 Sep 13. Erratum in: Infection. 2019 Nov 18;: PMID: 31520397. DOI:10.1007/s15010-019-01354-x.
- Bartoletti M, Mikus E, Pascale R, et al. Clinical experience with dalbavancin for the treatment of deep sternal wound infection. J Glob Antimicrob Resist. 2019 Sep;18:195–198. Epub 2019 Mar 27. PMID: 30926464. DOI:10.1016/j.jgar.2019.03.015.
- Dinh A, Duran C, Pavese P, et al. Dalbavancin French Study Group, French national cohort of first use of dalbavancin: a high proportion of off-label use. Int J Antimicrob Agents. 2019 Nov;54(5):668–672. Epub 2019 Aug 7. PMID: 31400471. DOI:10.1016/j.ijantimicag.2019.08.006
- Arrieta-Loitegui M, Caro-Teller JM, Ortiz-Pérez S, et al. Effectiveness, safety and cost analysis of dalbavancin in clinical practice. Eur J Hosp Pharm. 2022 Jan;29(1):55–58. Epub 2020 Oct 5. PMID: 33020060; PMCID: PMC8717798. DOI:10.1136/ejhpharm-2020-002315.
- Dolan A, Kuge E, Bremmer E, et al. Real world utilization of Dalbavancin at a rural community emergency department. Am J Emerg Med. 2022 Apr;54:253–256. Epub 2022 Feb 6. PMID: 35190304. DOI:10.1016/j.ajem.2022.02.006.
- Simonetti O, Rizzetto G, Molinelli E, et al. Review: a safety profile of dalbavancin for on- and off-label utilization. Ther Clin Risk Manag. 2021 Mar 22;17:223–232. PMID: 33790563; PMCID: PMC7997409. DOI:10.2147/TCRM.S271445
- Dunne MW, Talbot GH, Boucher HW, et al. Safety of dalbavancin in the treatment of skin and skin structure infections: a pooled analysis of randomized, comparative studies. Drug Saf. 2016 Feb;39(2):147–157. PMID: 26715497; PMCID: PMC4735234. DOI:10.1007/s40264-015-0374-9.
- Lodise TP, Fan W, Sulham KA. Hospital admission patterns in adult patients with skin and soft tissue infections: identification of potentially avoidable hospital admissions through a retrospective database analysis. Hosp Pract. 1995;2015(43):137–143.
- Sabbatini AK, Nallamothu BK, Kocher KE. Reducing variation in hospital admissions from the emergency department for low-mortality conditions may produce savings. Health Aff. 2014;33(9):1655–1663.
- Revankar N, Ward AJ, Pelligra CG, et al. Modeling economic implications of alternative treatment strategies for acute bacterial skin and skin structure infections. J Med Econ. 2014;17(10):730–740. DOI:10.3111/13696998.2014.941065
- Caminiti C, Meschi T, Braglia L, et al. Reducing unnecessary hospital days to improve quality of care through physician accountability: a cluster randomised trial. BMC Health Serv Res. 2013 Jan 10;13(1):14. PMID: 23305251; PMCID: PMC3577481. DOI:10.1186/1472-6963-13-14
- Shojania KG, Duncan BW, McDonald KM, et al. Safe but sound: patient safety meets evidence-based medicine. JAMA. 2002;288(4):508–513.
- Moran GJ, Krishnadasan A; EMERGEncy ID Net Study Group, et al. Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med 2006 Aug 17;355(7):666–674. PMID: 16914702. DOI:10.1056/NEJMoa055356.
- Falcone M, Meier JJ, Marini MG, et al. Diabetes and acute bacterial skin and skin structure infections. Diabet Res Clin Pract. 2021 Apr;174:108732. Epub 2021 Mar 5. PMID: 33676996. DOI:10.1016/j.diabres.2021.108732.
- Lipsky BA, Kollef MH, Miller LG, et al. Predicting bacteremia among patients hospitalized for skin and skin-structure infections: derivation and validation of a risk score. Infect Control Hosp Epidemiol. 2010 Aug;31(8):828–837. PMID: 20586653. DOI:10.1086/654007.
- Tay EY, Thirumoorthy T, Pang SM, et al. Clinical outcomes of bacteraemia in cellulitis of the leg. Clin Exp Dermatol. 2014 Aug;39(6):683–688. Epub 2014;Jul 1. PMID: 24985315. DOI:10.1111/ced.12366.
- Falcone M, Serra P, Venditti M. Serious infections due to methicillin-resistant Staphylococcus aureus: an evolving challenge for physicians. Eur J Intern Med. 2009 Jul;20(4):343–347. Epub 2008;Nov 1. PMID: 19524170. DOI:10.1016/j.ejim.2008.08.016.
- Venditti M, Falcone M, Micozzi A, et al. Staphylococcus aureus bacteremia in patients with hematologic malignancies: a retrospective case-control study. Haematologica. 2003 Aug;88(8):923–930. PMID: 12935981.
- Perl B, Gottehrer NP, Raveh D, et al. Cost effectiveness of blood cultures for adult patients with cellulitis. Clin Infect Dis. 1999;29(6):1483–1488. DOI:10.1086/313525
- Paolo WF, Poreda AR, Grant W, et al. Blood culture results do not affect treatment in complicated cellulitis. J Emerg Med. 2013;45(2):163–167. DOI:10.1016/j.jemermed.2013.01.016
- Sadow KB, Chamberlain JM. Blood cultures in the evaluation of children with cellulitis. Pediatrics. 1998;101(3):e4.
- Stevenson A, Hider P, Than M. The utility of blood cultures in the management of non-facial cellulitis appears to be low. 100 Years Ago In The NZMJ. 2005;118:1.
- Taniguchi T, Tsuha S, Shiiki S, et al. High yield of blood cultures in the etiologic diagnosis of cellulitis, erysipelas, and cutaneous abscess in elderly patients. Open Forum Infect Dis. 2022 Jun 24;9(7):ofac317. PMID: 35899281; PMCID: PMC9310324. DOI:10.1093/ofid/ofac317
- Gunderson CG, Martinello RA. A systematic review of bacteremias in cellulitis and erysipelas. J Infect. 2012 Feb;64(2):148–155. Epub 2011 Nov 11. PMID: 22101078. DOI:10.1016/j.jinf.2011.11.004.
- Collazos J, de la Fuente B, de la Fuente J, et al. Factors associated with sepsis development in 606 Spanish adult patients with cellulitis. BMC Infect Dis. 2020 Mar 12;20(1):211. PMID: 32164590; PMCID: PMC7066725. DOI:10.1186/s12879-020-4915-1
- Micek ST, Hoban AP, Pham V, et al. Bacteremia increases the risk of death among patients with soft-tissue infections. Surg Infect (Larchmt). 2010;11(2):169–176. DOI:10.1089/sur.2009.007
- Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the infectious diseases society of America. Clin Infect Dis. 2014;59(2):e10. DOI:10.1093/cid/ciu296
- Obaitan I, Dwyer R, Lipworth AD, et al. Failure of antibiotics in cellulitis trials: a systematic review and meta-analysis. Am J Emerg Med. 2016;34(8):1645–1652. DOI:10.1016/j.ajem.2016.05.064
- Raff AB, Kroshinsky D. Cellulitis: a review. JAMA. 2016;316(3):325–337.
- Tay EY, Fook-Chong S, Oh CC, et al. Cellulitis recurrence score: a tool for predicting recurrence of lower limb cellulitis. J Am Acad Dermatol. 2015 Jan;72(1):140–145. Epub 2014 Oct 16. PMID: 25443627. DOI:10.1016/j.jaad.2014.08.043.
- Pizzuti AG, Murray EY, Wagner JL, et al. Financial analysis of dalbavancin for acute bacterial skin and skin structure infections for self-pay patients. Infect Dis Ther. 2020 Dec;9(4):1043–1053. Epub 2020;Oct 21. PMID: 33083894; PMCID: PMC7680485. DOI:10.1007/s40121-020-00347-w.
- Edelsberg J, Berger A, Weber DJ, et al. Clinical and economic consequences of failure of initial antibiotic therapy for hospitalized patients with complicated skin and skin-structure infections. Infect Control Hosp Epidemiol. 2008;29(2):160–169. DOI:10.1086/526444
- Nathwani D, Dryden M, Garau J. Early clinical assessment of response to treatment of skin and soft-tissue infections: how can it help clinicians? Perspectives from Europe. Int J Antimicrob Agents. 2016 Aug;48(2):127–136. Epub 2016 May 25. PMID: 27283729. DOI:10.1016/j.ijantimicag.2016.04.023.
- Moran GJ, Fang E, Corey GR, et al. Tedizolid for 6 days versus linezolid for 10 days for acute bacterial skin and skin-structure infections (ESTABLISH-2): a randomised, double-blind, phase 3, non-inferiority trial. Lancet Infect Dis. 2014;14(8):696–705. DOI:10.1016/S1473-3099(14)70737-6
- Prokocimer P, De Anda C, Fang E, et al. Tedizolid phosphate vs linezolid for treatment of acute bacterial skin and skin structure infections: the ESTABLISH-1 randomized trial. JAMA. 2013;309(6):559–569. DOI:10.1001/jama.2013.241
- Cojutti PG, Merelli M, Bassetti M, et al. Proactive therapeutic drug monitoring (TDM) may be helpful in managing long-term treatment with linezolid safely: findings from a monocentric, prospective, open-label, interventional study. J Antimicrob Chemother. 2019 Dec 1;74(12):3588–3595. PMID: 31504570. DOI:10.1093/jac/dkz374
- Pea F, Furlanut M, Cojutti P, et al. Therapeutic drug monitoring of linezolid: a retrospective monocentric analysis. Antimicrob Agents Chemother. 2010 Nov;54(11):4605–4610. Epub 2010 Aug 23. PMID: 20733043; PMCID: PMC2976143. DOI:10.1128/AAC.00177-10.
- Taylor K, Williamson J, Luther V, et al. Evaluating the use of dalbavancin for off-label indications. Infect Dis Rep. 2022 Apr 11;14(2):266–272. PMID: 35447884; PMCID: PMC9026399. DOI:10.3390/idr14020032
- Falcone M, Russo A, Pompeo ME, et al. Retrospective case-control analysis of patients with staphylococcal infections receiving daptomycin or glycopeptide therapy. Int J Antimicrob Agents. 2012 Jan;39(1):64–68. Epub 2011 Nov 1. PMID: 22047703. DOI:10.1016/j.ijantimicag.2011.09.011.
- Falcone M, Russo A, Venditti M, et al. Considerations for higher doses of daptomycin in critically ill patients with methicillin-resistant Staphylococcus aureus bacteremia. Clin Infect Dis. 2013 Dec;57(11):1568–1576. Epub 2013 Sep 17. PMID: 24046298. DOI:10.1093/cid/cit582.
- Dryden M, Saeed K, Townsend R, et al. Antibiotic stewardship and early discharge from hospital: impact of a structured approach to antimicrobial management. J Antimicrob Chemother. 2012;67(9):2289–2296. DOI:10.1093/jac/dks193
- Gray A, Dryden M, Charos A. Antibiotic management and early discharge from hospital: an economic analysis. J Antimicrob Chemother. 2012;67(9):2297–2302.
- Talan DA, Mower WR, Lovecchio FA, et al. Pathway with single-dose long-acting intravenous antibiotic reduces emergency department hospitalizations of patients with skin infections. Acad Emerg Med. 2021 Oct;28(10):1108–1117. Epub 2021 May 5. PMID: 33780567; PMCID: PMC8597095. DOI:10.1111/acem.14258.
- Krsak M, Morrisette T, Miller M, et al. Advantages of outpatient treatment with long-acting lipoglycopeptides for serious gram-positive infections: a review. Pharmacotherapy. 2020 May;40(5):469–478. Epub 2020 Apr 23. PMID: 32239771. DOI:10.1002/phar.2389.
- Bork JT, Heil EL, Berry S, et al. Dalbavancin use in vulnerable patients receiving outpatient parenteral antibiotic therapy for invasive Gram-positive infections. Infect Dis Ther. 2019;8(2):171–184. Available from: http://link.springer.com/10.1007/s40121-019-0247–0
- Attwood LO, McKechnie M, Vujovic O, et al. Review of management priorities for invasive infections in people who inject drugs: highlighting the need for patient-centred multidisciplinary care. Med J Aust. 2022 Jul 18;217(2):102–109. Epub 2022 Jun 26. PMID: 35754144; PMCID: PMC9539935. DOI:10.5694/mja2.51623
- Hemmige V, McNulty M, Silverman E, et al. Recurrent skin and soft tissue infections in HIV-infected patients during a 5-year period: incidence and risk factors in a retrospective cohort study. BMC Infect Dis. 2015;15(1):455.
- Waldron C, Solon JG, O’Gorman J, et al. Necrotizing fasciitis: the need for urgent surgical intervention and the impact of intravenous drug use. Surgeon. 2015;13(4):194–199. DOI:10.1016/j.surge.2014.01.005
- Grau D, Clarivet B, Lotthé A, et al. Complications with peripherally inserted central catheters (PICCs) used in hospitalized patients and outpatients: a prospective cohort study. Antimicrob Resist Infect Control. 2017;6(1):18. DOI:10.1186/s13756-016-0161-0
- Antosz K, Al-Hasan MN, Lu ZK, et al. Clinical utility and cost effectiveness of long-acting lipoglycopeptides used in deep-seated infections among patients with social and economic barriers to care. Pharmacy. 2022;10(1):1. DOI:10.3390/pharmacy10010001
- Agarwal R, Bartsch SM, Kelly BJ, et al. Newer glycopeptide antibiotics for treatment of complicated skin and soft tissue infections: systematic review, network meta-analysis and cost analysis. Clin Microbiol Infect. 2018 Apr;24(4):361–368. Epub 2017 Sep 4. PMID: 28882727; PMCID: PMC5925741. DOI:10.1016/j.cmi.2017.08.028.
- Marcellusi A, Viti R, Sciattella P, et al. Economic evaluation of the treatment of Acute Bacterial Skin and Skin Structure Infections (ABSSSIs) from the national payer perspective: introduction of a new treatment to the patient journey. A simulation of three European countries. Expert Rev Pharmacoecon Outcomes Res. 2019 Oct;19(5):581–599. Epub 2019 Feb 4. PMID: 30714834.127. DOI:10.1080/14737167.2019.1569516.
- Marcellusi A, Bini C, Andreoni M, et al. Budget impact analysis of dalbavancin in the treatment of acute bacterial skin and skin structure infections in three European countries. Clin Drug Investig. 2020 Apr;40(4):305–318. PMID: 32034687. DOI:10.1007/s40261-020-00891-w.
- Streifel AC, Sikka MK, Bowen CD, et al. Dalbavancin use in an academic medical centre and associated cost savings. Int J Antimicrob Agents. 2019 2nd;54(5):652–654. DOI:10.1016/j.ijantimicag.2019.08.007.
- Kaiser Family Foundation. Hospital adjusted expenses per inpatient day by ownership. Accessed 2022 Dec 4 https://www.kff.org/health-costs/state-indicator/expenses-per-inpatient-day-by-ownership/?currentTimeframe=0&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22asc%22%7D.
- Ektare V, Khachatryan A, Xue M, et al. Assessing the economic value of avoiding hospital admissions by shifting the management of gram+ acute bacterial skin and skin-structure infections to an outpatient care setting. J Med Econ. 2015;18(12):1092–1101. DOI:10.3111/13696998.2015.1078339
- McCarthy MW, Keyloun KR, Gillard P, et al. Dalbavancin reduces hospital stay and improves productivity for patients with acute bacterial skin and skin structure infections: the ENHANCE trial. Infect Dis Ther. 2020 Mar; 9(1):53–67. DOI:10.1007/s40121-019-00275-4.
- Wong CH, Khin LW, Heng KS, et al. The LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) score: a tool for distinguishing necrotizing fasciitis from other soft tissue infections. Crit Care Med. 2004 Jul;32(7):1535–1541. PMID: 15241098. DOI:10.1097/01.ccm.0000129486.35458.7d.
- Lagi F, Ottino L, Mantengoli E, et al. Early discharge criteria in patients with acute bacterial skin and skin structure infections in a large tertiary-care teaching hospital in Florence, Italy. Eur J Clin Microbiol Infect Dis. 2019 Sep;38(9):1781–1785. Epub 2019 Jun 20. PMID: 31222396; PMCID: PMC6695376. DOI:10.1007/s10096-019-03609-9.
- Viallon A, Marjollet O, Berthelot P, et al. Risk factors associated with methicillin-resistant Staphylococcus aureus infection in patients admitted to the ED. Am J Emerg Med. 2007 Oct;25(8):880–886. PMID: 17920971. DOI:10.1016/j.ajem.2007.01.013.
- Karanika S, Zervou FN, Zacharioudakis IM, et al. Risk factors for meticillin-resistant Staphylococcus aureus colonization in dialysis patients: a meta-analysis. J Hosp Infect. 2015 Nov;91(3):257–263. Epub 2015 Aug 28. PMID: 26428959. DOI:10.1016/j.jhin.2015.07.014.
- Lee AS, de Lencastre H, Garau J, et al. Methicillin-resistant Staphylococcus aureus. Nat Rev Dis Primers. 2018; May 31;4(1). PMID: 29849094. 18033. DOI:10.1038/nrdp.2018.33.
