ABSTRACT
Introduction
Hepatitis B Virus (HBV) infection can progress to chronic HBV (CHB) disease, thereby increasing the risk of severe forms of liver disease (i.e. liver cirrhosis and hepatocellular carcinoma) and resulting in a high global burden of morbidity, mortality, and health-care utilization.
Areas covered
We discuss how future therapeutic strategies and treatment guidelines may address the large unmet medical needs among patients with CHB.
Expert opinion
Complexity and a lack of consensus in current CHB treatment guidelines may limit their effective implementation. To minimize poor outcomes in patients not currently receiving treatment (including immune-tolerant and inactive carriers), a simplified harmonized treatment approach is needed across guidelines. Current treatment recommendations focus on nucleot(s)ide analogs (NAs) and pegylated interferon (Peg-IFN), both of which have limitations. NAs provide clinical benefits, but treatment is prolonged and has little impact on functional cure rates. Peg-IFN offers the potential for functional cure but has notable safety and tolerability issues. A shift toward finite treatments with acceptable safety and tolerability profiles is needed.
Conclusion
The key to achieving World Health Organization targets for the global eradication of HBV involves enhanced diagnosis with new treatments and/or combinations of existing treatments alongside globally aligned and simplified treatment guidelines for untreated/inadequately treated populations.
1. Introduction
Chronic hepatitis B (CHB) virus infection, defined by the chronic (≥6 months) expression of the hepatitis B surface antigen (HBsAg), is transmitted from blood and other bodily fluids and presents a global health problem [Citation1]. The progression of CHB to severe forms of liver disease, including liver cirrhosis and hepatocellular carcinoma (HCC), imposes a high healthcare burden, especially for low-income countries [Citation2]. CHB infection is a dynamic disease, and the status of the patients is typically characterized by the presence/absence of HBeAg, levels of serum HBV DNA, and alanine transaminase (ALT) values ().
Table 1. The Different Phases of Chronic Hepatitis B.
These three laboratory parameters allow assignment to different phases of CHB, each with a differing natural history and prognosis and, by extension, differing indications for antiviral therapy. The different phases of CHB infection include: 1) immune-tolerant CHB or hepatitis B e-antigen (HBeAg)-positive chronic infection characterized by HBeAg positivity, high levels of HBV DNA (>107 IU/mL), and normal ALT levels; 2) immune-active CHB infection in which patients are either HBeAg positive (or immune-reactive phase) or negative (or low-replicative phase) with high HBV DNA levels (>20,000 and >2,000 IU/mL, respectively) and 3) elevated ALT levels; and inactive CHB infection or HBeAg-negative chronic infection characterized by HBeAg negativity, presence of serum antibodies to HBeAg (anti-HBe), undetectable or low levels of HBV DNA (<2,000 IU/ml) and normal ALT levels [Citation4,Citation5,Citation7].
The overall prevalence of CHB was estimated at 296 million in 2019, with the highest prevalence in the Western Pacific, Africa, and Southeast Asia regions [Citation8]. Overall, only 10% of patients chronically infected were diagnosed and 5% of those eligible for treatment received antiviral therapy in 2016, with the lowest rates in Central, South, and Southeast Asia, the Caribbean, and sub-Saharan Africa, while the highest rates (>25%) were noted in some European countries (Finland, Germany, Greece, Italy, The Netherlands, and Sweden), high-income Asian countries (Japan, South Korea, and Taiwan), New-Zealand, and the US [Citation9].
In 2017, the World Health Organization (WHO) called for the elimination of HBV and hepatitis C virus (HCV) as major public health threats by aiming to reduce new infections (incidence) by 90%, and deaths (mortality) by 65% by the year 2030, compared with the 2015 baseline [Citation10]. For HBV, this means that new cases and deaths should be reduced from 1.5 million to 170,000 and from 820,000 to 310,000 from 2020 to 2030, respectively [Citation11]. In terms of vaccination targets, the WHO elimination agenda includes 3-dose vaccine coverage and vaccine birth dose for prevention of mother-to-child transmission (MTCT) of HBV in 90% of individuals by 2030 [Citation10]. One worldwide initiative that helps in reaching these goals for HBV is the treatment of pregnant women with antivirals, thus preventing MTCT of HBV. By December 2020, almost all (98%) of the WHO member countries had introduced universal infant vaccination with HBV vaccine, while 57% provided birth dose to all newborns [Citation12]. Coverage of three-dose HBV for children aged ≤5 years ranged from 82% to 85% across countries, and overall coverage of the birth dose increased from 37% to 43% during 2016–2020 [Citation12]. To meet the WHO targets for HBV infection of 90% diagnosed and 80% of those eligible treated by 2030, national screening programs for individuals other than pregnant women will need to be implemented in many countries. Low treatment rates among those diagnosed highlight the gaps in the cascade of care (i.e. the continuum of services that patients require throughout the progression of HBV infection, from diagnosis to treatment to chronic care) and are of particular importance given that treatment remains a key strategy to controlling HBV globally [Citation10].
Here we explore the gaps in the screening, diagnosis/staging, and treatment of CHB, and identify the unmet needs among patients with CHB in order to inform considerations regarding future therapeutic or curative modalities.
2. Selection of evidence
A targeted literature review was conducted to identify key evidence on epidemiological, clinical, humanistic, and economic outcomes in patients with CHB. Extensive searches (from 2010 up to April 2021) of Medline (OvidSP) and Embase (OvidSP) were conducted for treatment guidelines, systematic reviews/meta-analyses, and primary studies (economic and humanistic burdens only) on CHB infection in adults and children published in English. The search strategies included a combination of indexing terms (Medical Subject Headings terms in MEDLINE and Emtree terms in Embase) as well as free-text keywords (see Supplemental Files). In addition, the Cochrane Library, EconLit database, key websites (POLARIS Observatory, Institute for Health Metrics and Evaluation [IHME], Centers for Disease Control and Prevention [CDC], and European Centre for Disease Prevention and Control (ECDC]), and conference abstracts indexed in Embase were searched for further data. Additional ad-hoc searches for more recently published data were conducted up to May 2023.
Articles were systematically selected for inclusion by two independent reviewers based on pre-defined criteria for the population, intervention, comparators, outcomes, and study designs (PICOS criteria) of interest (see Supplemental Files); discrepancies were resolved through consensus.
A core set of 61 key articles were selected for final inclusion with priority given to those articles representing the highest levels of evidence, largest sample size, and/or reporting on the most relevant data to current clinical practice (see ). Data were extracted by one reviewer and validated by a second reviewer with discrepancies resolved through consensus.
3. Treatment goals
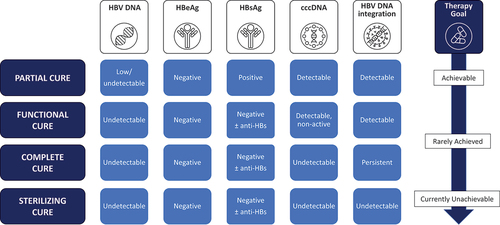
Current CHB treatment goals focus on sustained HBV DNA suppression, achieving HBeAg seroclearance or seroconversion and HBsAg seroclearance (). Lifelong nucleot(s)ide analog (NA) therapies successfully achieve HBV DNA suppression on-treatment and HBeAg seroconversion with accompanying improvements in key clinical endpoints including reduced rates of cirrhosis, HCC, and mortality in a majority of infected persons. However, sustained off-treatment HBsAg seroclearance with HBV DNA suppression (or functional cure) is only rarely achieved with the current antiviral treatments () but is considered the optimal endpoint for trials of CHB therapies by the US Food and Drug Administration (FDA) [Citation13]. A working group from the European Association for the Study of the Liver (EASL) and the American Association for the Study of Liver Diseases (AASLD) defined functional cure as sustained HBsAg loss in addition to undetectable HBV DNA 6 months post-treatment [Citation14]. When closed covalent circular DNA (cccDNA) becomes undetectable complete cure is said to be achieved, but the complete elimination of HBV (known as sterilizing cure) is elusive and defined as the elimination of cccDNA together with undetectable integrated HBV DNA () [Citation7]. Given that sterilizing cure is currently considered difficult to achieve due to the integration of HBV DNA into the host genome, functional cure is the best attainable outcome for existing therapies and is associated with clinical benefits to patients by decreasing liver disease-related mortality and improving survival by preventing the progression of liver fibrosis to cirrhosis and HCC [Citation5,Citation7].
4. Current therapies
Currently available treatments for CHB include first-line chronic therapy with NAs or their combination and, although infrequently used, a finite duration of pegylated interferon (Peg-IFN) (usually 48 weeks). Successful treatment offers the possibility of sustained viral suppression and/or normalization of ALT and improved liver histology with a reduction in the risk of HCC (especially in those with cirrhosis) [Citation4,Citation5,Citation7].
The NAs with high barrier to resistance include entecavir (ETV), tenofovir disoproxil fumarate (TDF), and tenofovir alafenamide (TAF) [Citation15]. Systematic review and meta-analysis of NA efficacy in patients with CHB showed higher efficacy of NAs compared to placebo (between 7 and 12 months of treatment in 47% of the included studies) for HBeAg seroclearance (RR from 2.54 to 11.86) and seroconversion (RR from 2.21 to 3.60), undetectable HBV DNA (RR from 36.35 to 475.3), and, at least for NAs with a high-barrier to HBV resistance, ALT normalization (RR from 4.36 to 13.39) [Citation16]. Combination treatments of a 48-week-long course of pegylated (Peg)-IFN with NAs were shown to be the most efficacious treatment strategies to achieve HBsAg seroclearance (p-score >0.75) followed by sequential treatments and monotherapy [Citation17]. However, Peg-IFN is limited by its unfavorable tolerability profile in comparison with NAs and a greater number of restrictions in terms of eligibility for treatment. For example, Peg-IFN is not recommended in patients with compensated cirrhosis (per AASLD and EASL guidelines) and is contraindicated in patients with decompensated liver disease because of safety concerns [Citation4,Citation7]. Frequently reported adverse events include flu-like syndrome, myalgia, headache, fatigue, weight loss, depression, hair loss, and local reactions at the site of injection. More serious long term (though rare) on treatment events include psychiatric, neurological, and endocrinological events [Citation4,Citation7]. Tolerance is better in patients who are younger and have fewer comorbidities [Citation7]. All patients with CHB treated with Peg-IFN require monitoring with periodical assessments of at least complete blood count, ALT, thyroid-stimulating hormone (TSH), and serum HBV DNA [Citation7]. As a consequence of the tolerability profile and treatment eligibility restrictions, there are a significant number of patients who are ineligible or unwilling to receive Peg-IFN treatment (e.g. patients with comorbidities) [Citation4,Citation7].
Several NAs are available, and those preferred by clinical guidelines are ETV, TDF, and TAF [Citation4,Citation5,Citation7]. In comparison to Peg-IFN, NA therapy offers a more favorable safety profile and improved tolerability, and is applicable to a broader array of patients, including those with cirrhosis, including decompensated cirrhosis. Across the different recommended NAs, the most commonly reported adverse events include gastrointestinal disorders (up to 22.7% of events on TAF), respiratory tract infections (up to 5.4% of events on TAF), fatigue (up to 6.5% of events on ETV), and headache (up to 17.0% of events on ETV) [Citation18]. These are infrequently treatment-limiting but can lead to a change in the type of NA used. In patients with preexisting renal and bone conditions, the most important long-term risks are associated with the use of TDF and include renal dysfunction (occurring in an estimated 6.8% of patients [Citation19]) and osteopenia/osteoporosis [Citation18,Citation20]. All patients on NA therapy are recommended to undergo assessment of estimated glomerular filtration rate (eGFR) for dose adjustment, and those on TDF require additional renal monitoring which may include serum phosphate levels, serum creatinine, urine glucose, and urine protein. Bone density monitoring whilst on TDF treatment is not considered necessary in US guidelines in the absence of other risk factors for osteoporosis or osteomalacia, but guidelines from Asia recommend that the bone profile of patients on TDF is monitored at least every 3 months [Citation4]. While viral resistance is a risk with any NA, the frequency is very low with currently preferred therapies (ETV, TDF, and TAF) in treatment naive patients [Citation4,Citation7]. Although differences in efficacy based on serum parameters have been reported between the available NAs with TDF better than ETV for viral suppression and ETV better than TDF and TAF better than TDF for ALT normalization, mixed findings have been reported on the incidence of HCC under NA treatment with 20% lower HCC rates for TDF versus ETV or no difference between these two NAs in two recent meta-analyses [Citation21,Citation22,Citation23,Citation24–26]. Ultimately, there is an unmet need for CHB treatments that are of finite duration, achieve high rates of sustained viral suppression and/or HBsAg seroclearance, and are safe and tolerated to the same extent as NAs [Citation27].
5. Treatment guidelines
Key clinical guidelines for the management of patients with CHB are available in the U.S.A. (American Association for the Study of Liver Diseases [AASLD]), Europe (European Association for the Study of the Liver [EASL]), and Asia (Asia Pacific Association for the Study of the Liver [APASL]). Guidelines from the WHO targeting low- and middle-income countries were also published in 2015 [Citation4–7]. The understanding of HBV infection is constantly evolving, and therefore, some of the older guidelines may no longer reflect the most up-to-date evidence.
Across treatment guidelines from the U.S.A., Europe, and Asia, there is general alignment that therapy should be initiated in those patients with elevated HBV DNA levels and ALT levels higher than normal, and/or histological evidence of significant liver disease [Citation4,Citation5,Citation7]. However, some differences exist in the thresholds of HBV DNA and ALT/aspartate transaminase (AST) required for treatment initiation, treatment goals, treatment implementation, and disease surveillance (see ).
Table 2. Summary of Key Treatment Guidelines Across Geographies.
Table 3. Summary of Key Populations in Treatment Guidelines Across Geographies.
AASLD and APASL guidelines recommend treatment of patients with immune active disease, where ALT levels are >2 ULN and HBV DNA ≥ 2,000 IU/mL in HBeAg negative patients or ≥20,000 IU/mL in those who are HBeAg positive, whereas EASL guidelines recommend treatment is initiated at HBV DNA > 2,000 IU/mL and ALT > ULN if at least moderate fibrosis is present or at HBV DNA > 20,000 IU/mL and ALT > 2× ULN, regardless of the degree of fibrosis [Citation4,Citation5,Citation7].
Cirrhosis is also generally accepted as an indicator for treatment across treatment guidelines globally, with EASL, AASLD, and APASL guidelines all agreeing that patients with decompensated cirrhosis should undergo treatment with NAs [Citation4,Citation5,Citation7]. However, although EASL and AASLD guidelines recommend that patients with compensated cirrhosis also undergo treatment regardless of HBV DNA and ALT levels, guidelines from APASL suggest applying a threshold of HBV DNA > 2000 IU/mL [Citation5]. Treatment with NA is suggested, but peg-IFN is also an option for selected compensated cirrhosis patients in guidelines from EASL and APASL but is not recommended by ASSLD [Citation4,Citation5,Citation7].
The endpoint of treatment varies across the guidelines. HBsAg seroclearance is suggested as the ideal primary endpoint of therapy in guidelines from EASL, with HBeAg seroconversion considered as a satisfactory endpoint for patients who are HBeAg positive in guidelines from AASLD and APASL given the rarity of HBsAg seroclearance [Citation4,Citation5,Citation7].
While all the guidelines indicate that persons with immune-tolerant CHB not be treated, there are some important caveats. Age may influence decisions to treat, though the exact age threshold differs across guidelines with EASL suggesting a range from 30 to 40 years, WHO guidelines suggesting 30 years and AASLD 40 years [Citation4,Citation6,Citation7]. Additionally, family history of cirrhosis or HCC or presence of significant inflammation/fibrosis are considered reasons to undertake antiviral therapy in immune-tolerant CHB.
Despite the availability of global treatment guidelines for CHB, in practice there are several groups of patients who remain untreated and are a significant source of unmet need (), including undiagnosed CHB, those who meet treatment criteria but are not receiving treatment and those who are not covered under current criteria but may benefit from treatment.
Figure 3. Populations with Unmet Need.
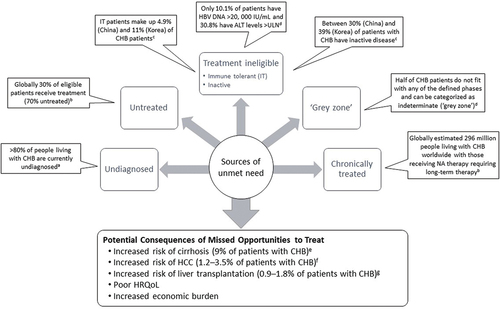
Globally differences across the guidelines may lead to confusion among clinicians suggesting the benefits of harmonization need to be considered going forward. However, ultimately guidelines are only advisory and not mandatory, and more flexibility regarding the decision to treat needs to be conveyed to clinicians in order to address the current unmet need.
6. Patients with unmet need
6.1. Undiagnosed CHB
Undiagnosed CHB is the major reason why treatment benefits have not been realized. Of the people living with CHB, only 10% globally in 2016 had been diagnosed and were aware of their infection, and this proportion varied by region from ≤1% in South Asia, and central and southern sub-Saharan Africa up to 74% in high-income Asia Pacific [Citation9]. According to a 2022 WHO report, >80% of people living with CHB are currently undiagnosed [Citation11]. Most of those with CHB infection are adults born before the availability of widespread HBV vaccination programs and vaccine use in infancy [Citation6,Citation28]. Even within high-income countries such as the US, estimates of undiagnosed CHB remain high (81.4%), suggesting only about one in five persons with CHB with private medical insurance have been diagnosed [Citation29]. Consequently, in 2020, the US Preventative Services Task Force (USPSTF) recommended that all at-risk adults and adolescents undergo HBV screening, including ‘all asymptomatic, nonpregnant adolescents and adults at increased risk for HBV infection, including those who were vaccinated before being screened for HBV infection’ [Citation30]. A proposal to conduct one-off screening of all US adults aged ≥18 years is currently under review, and other countries, particularly those with a high HBV prevalence, may need to consider similar universal screening programs to successfully identify and manage those who are already infected [Citation31]. A lack of diagnosis means that many patients may go untreated or fail to receive prompt treatment, thereby increasing their risk of adverse liver-related consequences. Looking ahead, the potential availability of curative treatment options may be associated with increases in the rates of detection and diagnoses of CHB infection.
6.2. Untreated CHB
Global estimates suggest that 6.6 million (22.7%) of those diagnosed with HBV received treatment in 2019 [Citation32]. Recent estimates from the WHO in 2022 suggest that the overall proportion of people diagnosed with CHB who receive treatment is 30% [Citation11]. This estimate aligns with a US database study among medically insured patients with a diagnosis of CHB conducted using data gathered from the Truven database (2017–2020) where 30.7% of the population received treatment (34.8% and 48.6% of patients with cirrhosis and HCC, respectively) [Citation29].
Even for those patients who undergo screening for HBV, many are unaware of their results, which subsequently affect treatment-seeking behavior. A survey of 50,909 respondents taking part in a nationwide community outreach program in Taiwan found that 41.1% of participants confirmed having undergone HBV or HCV screening, but 40% of those did not know their results, and of those who tested positive for HBV, 65.5% correctly reported their infection status as positive, but 12.8% reported they were not infected [Citation33]. Notably, 43% and 27% of those who correctly reported their positivity for HBV or HCV did not follow up for monitoring or treatment, respectively [Citation33].
Even among those diagnosed and aware of their infection, a number of potential barriers to treatment have been identified, including costs of diagnostics especially in low- and lower-middle-income countries, limited access to affordable treatment in some countries, and lack of symptoms, time, and knowledge regarding how to seek further medical help [Citation33,Citation34]. Challenges with cost and treatment access were also recently highlighted in a study reporting on patient’s queries sent to the Hepatitis B Foundation [Citation35]. In its priority actions for countries, the WHO highlighted that the removal of legal, regulatory and policy barriers that hinder hepatitis services, including treatment of treatment-eligible patients, is necessary to efforts to eliminate viral hepatitis as a major public health threat by 2030 [Citation34]. Additionally, there is an increased focus on simplification of treatment guidelines, as the complexity of guideline recommendations may be a contributing factor to the lack of treatment among eligible persons [Citation36].
Patients with CHB who remain untreated regardless of the reason have an increased risk of developing liver cirrhosis, HCC, and undergoing liver transplantation, leading to an overall increased risk of morbidity and mortality. In a long-term follow-up study, patients with CHB and cirrhosis, and treated only with liver protection and anti-inflammatory therapy, had higher incidence rates of HCC than control CHB and cirrhosis patients treated with nucleoside antiviral therapy (53.1% vs 31.9%, p = 0.026) [Citation37]. In a further large multinational study of HCC risk among patients with indeterminate CHB without cirrhosis, a similar higher cumulative incidence of HCC was observed in untreated versus treated patients (15% versus 4% after 5 years of treatment) [Citation38].
6.3. Not recommended for treatment
Current guidelines recommend treatment initiation by levels of serum HBV DNA, ALT, and/or AST levels and disease severity (presence of significant fibrosis – typically assessed by noninvasive markers). Despite some variation between treatment guidelines, patients with normal ALT levels and absence of significant fibrosis are not recommended for treatment. This includes inactive CHB with low levels of HBV DNA (<2000 IU/mL typically) as well as immune-tolerant patients with very high levels of HBV DNA (>10 million IU/ml). A recent systematic review examining the proportion of participants eligible for treatment in studies based on the WHO and other treatment guidelines (APASL, AASLD, EASL 2012 and 2017, other or mixed guidelines) reported that only 10.1% of patients had HBV DNA exceeding 20,000 IU/mL and 30.8% had ALT levels above the upper limit of normal (ULN) [Citation4,Citation5,Citation7,Citation39]. Overall, only 19% (95% confidence interval [CI]: 18, 20) of HBsAg-positive patients met the criteria for treatment, with estimates ranging from 12% (95% CI: 6, 18) in community settings to 25% (95% CI: 19, 30) in clinic settings [Citation39]. In studies using treatment eligibility criteria from different guidelines, the highest proportion of HBsAg-positive patients who met the criteria were from the European region (22–32%) followed by American (19–36%), Eastern Mediterranean (16–19%), Western Pacific (11–25%), Southeast Asia (6–22%), and African regions (3–18%) [Citation39].
Patients’ eligibility for treatment varies over the course of their disease, meaning that patients who are initially ineligible for treatment may subsequently meet the guideline criteria for treatment. Evidence from a recent North American cohort study of patients with HBeAg negative (HBV DNA ≤ 10,000 IU/mL) disease suggests that continued monitoring and surveillance are key to avoiding missed opportunities for timely treatment initiation [Citation40]. However, due to a lack of surveillance or follow-up evaluation, many patients may not receive treatment or treatment may be delayed, increasing the risk of disease progression to HCC [Citation41]. A recent study suggested that untreated patients had threefold higher risks of HCC (HR 3.485, 95% CI: 1.234 to 9.846) but similar risks of cirrhotic complications (HR 0.649, 95% CI: 0.227 to 1.854), compared to the patients who received NA treatment [Citation42]. All treatment guidelines recognize the importance of monitoring levels of important clinical markers (HBV DNA, HB antigens/antibodies, ALT, etc.) although there may be minor differences in the specifics of test types and time intervals (see ).
6.3.1. Immune-tolerant CHB
Estimates of the proportion of patients with immune-tolerant CHB vary across geographies and population groups, but recent studies from Asia suggest that between 4.9% (China) [Citation43] and 11% (Korea) [Citation44] of patients with CHB are immune-tolerant. Patients with immune-tolerant CHB are highly contagious due to the high levels of HBV DNA, which is of particular concern in those groups at most risk of transmission/acquisition of HBV infection, e.g. younger individuals who are more likely to engage in high-risk sexual behaviors and intravenous drug use [Citation43–46]. However, the AASLD recommends against antiviral therapy for immune-tolerant patients, but recommends testing ALT levels every 6 months to monitor for potential transition to immune-active CHB [Citation4]. However, where patients remain immune-tolerant for extended periods of time into older age, the lack of treatment can lead to a risk of poorer outcomes such as HCC [Citation47]. Across a broad group of untreated immune-tolerant patients with varying HBV DNA levels and platelet counts, one study suggested that patients have a significantly greater risk of developing HCC and requiring liver transplantation than treated immune active patients over a 10-year period [Citation47]. However, in contrast, a more recent study in more stringently defined immune-tolerant population (low fibrosis 4 index [FIB-4] <1.45) suggests that the HCC risk is negligible [Citation48].
Individuals aged 30–40 years with immune-tolerant disease are more likely to have active disease, even if ALT levels do not meet thresholds. AASLD recommends antiviral therapy in adults >40 years of age and with immune-tolerant CHB and moderate-severe inflammation or fibrosis on liver biopsy specimen or noninvasive tests after other causes of liver disease have been excluded [Citation4]. Both European and Asian guidelines used a lower threshold (>30 years of age) in their recommendation of antiviral therapy for patients with immune-tolerant CHB [Citation5,Citation7]. This is supported by the increased potential for viral integration into the host genome due to prolonged duration of viremia with increasing age [Citation5,Citation7].
6.3.2. Inactive CHB
Estimates of the proportion of patients with inactive CHB also vary across geographies and population groups, with recent studies from Asia suggesting that between 30% (China) [Citation43] and 39% (Korea) [Citation44] of patients with CHB have inactive disease. Patients described as inactive (HBeAg negative and Anti-HBe positive with HBV DNA < 2,000 IU/mL with ALT < 1× ULN, and no significant detectable inflammation or fibrosis) can still be at risk of poor outcomes (10-year cumulative incidence of cirrhosis 3.5%) and show benefit from treatment [Citation44,Citation49]. Alternatively, it may be possible that increasing prevalence of coexisting fatty or alcohol-associated liver disease may contribute to an increased risk of HCC in inactive disease [Citation50]. However, currently across guidelines, patients with inactive CHB are not recommended for treatment but monitored for ALT levels every 3 months in the first year and every 6–12 months thereafter, and for HBsAg and HBV DNA levels when ALT remains elevated to differentiate them from immune-active HBeAg-negative CHB [Citation4,Citation5,Citation7].
6.3.3. Other patient groups
A significant proportion of patients with CHB do not fit with any of the defined phases from the guidelines and can be categorized as indeterminate (‘gray zone’). Such patients are often HBeAg-negative as in a recent Chinese study, where most (78.3%) of the patients in the ‘gray zone’ (i.e. those who did not meet the AASLD 2018 treatment criteria) were HBeAg-negative [Citation43]. In a recent longitudinal study, half (52.7%) of the patients remained indeterminate after a 10-year follow-up and had a near 10-fold increased cumulative incidence of HCC compared with those with inactive disease (4.6% vs 0.5%; p < 0.0001) [Citation50]. In another large cohort Asian study, 7-year cumulative incidence of HCC was 1% in ‘gray zone’ patients treated with antiviral therapy, compared with 4% in untreated ‘gray zone’ patients (p = 0.016) [Citation51]. A review recently suggested to treat HBeAg-negative patients with persistently normal ALT and detectable HBV DNA (≥ or <2,000 IU/mL) or undetectable HBV DNA but with the presence of moderate or severe inflammation and/or significant hepatic fibrosis (≥G2/F2) [Citation52]. However, the authors highlighted that the benefits of antiviral therapy in patients with undetectable HBV DNA should be confirmed by further studies [Citation52]. This has called into question the recommended approach for such patients, which is to monitor and withhold treatment until transition to immune active disease occurs. There is concern that this may represent a missed opportunity to prevent disease progression.
7. Challenges with chronic NA suppressive therapy
Treatment using NAs achieves sustained HBV DNA suppression but very rarely leads to functional cure as defined by HBsAg seroclearance [Citation4,Citation7]. Thus, NAs typically necessitate long-term or indefinite treatment in order to prevent viral relapse and reduce the long-term risk of HCC, though the risk of HCC is not totally eliminated as low‐level viremia during NA treatment may still be associated with a risk of HCC [Citation53]. Long-term NA therapy is also not without challenges, including issues with suboptimal responses, due to resistance and adherence issues. Resistance to NA therapy may occur in some patients through the selection of antiviral resistance mutations. The risk of resistance is also increased by poor adherence [Citation4,Citation7]. Some antiviral resistance mutations can result in cross-resistance with other NAs, which reduces future treatment options, and provides the rationale for the use of NA treatments with a higher barrier to resistance. EASL and AASLD guidelines provide detailed recommendations on the choice and sequence of different NA treatments to avoid and minimize the risk of resistance and cross-resistance, including treatment switching and add-on treatments [Citation4,Citation7]. EASL recommends switching to an alternative, higher resistant NA option or the use of an add-on treatment, especially in those patients with more advanced disease [Citation7]. Guidelines from AASLD suggest drug switching in favor of add-on therapy due to the lack of comparative evidence [Citation4].
Although NA therapies are relatively safe, some patients may nevertheless be at greater risk of adverse events (e.g. those with renal disease on TDF therapy) requiring close monitoring and potential treatment switch [Citation7]. Furthermore, consistent with long-term therapies for other chronic conditions, patients on long-term NA therapy experience issues with treatment adherence and compliance, which consequently reduces the effectiveness of treatment. A recent study of data in the Korean National Health Insurance Service claims database suggested that risk of mortality/transplantation was significantly greater in those patients that demonstrated low adherence or intermediate adherence in comparison to those who were classed as having high adherence (p for trend <0.001) [Citation54]. In patients with CHB and cirrhosis in Taiwan, the likelihood of complicated cirrhosis and mortality was higher in patients with poor adherence to NA therapy [Citation55]. Guidelines recommend that for patients who fail NA therapy (less than one log10 decrease in serum HBV DNA after 3 months of therapy), treatment compliance should be investigated as a possible cause as primary nonresponse to NA therapy is extremely rare [Citation4,Citation7]. A recent systematic review reported an overall 75% adherence rate to long-term NAs and cited forgetting, limited understanding of the importance of adherence, and change to routine as the main barriers to treatment adherence [Citation56].
8. Withdrawal from long term treatment
Discontinuation of NA treatment is recommended in patients who achieve HBsAg seroclearance, by EASL, AASLD, and APASL, although this is an infrequent event [Citation4,Citation5,Citation7]. All three guidelines recommend indefinite therapy for patients with decompensated cirrhosis. WHO guidelines recommend lifelong NA for all cirrhosis patients and only consider treatment cessation in exceptional circumstances for those without clinical evidence of cirrhosis and who have been closely monitored for reactivation with evidence of HBeAg loss and seroconversion to anti-HBe, normal ALT levels, and persistently undetectable HBV DNA levels for a period of at least 1 year [Citation6]. In countries without access to HBV DNA testing, and in line with other guidelines, NA cessation may be considered where there is evidence of persistent HBsAg seroclearance after completion of at least one additional year of treatment, regardless of prior HBeAg status [Citation6].
Guidelines from EASL, AASLD, and APASL recommend discontinuation of NA treatment among non-cirrhotic patients with HBeAg seroconversion and HBV DNA suppression ≥12 months (preferably 3 years by the APASL) but not in patients with cirrhosis with the exception of APASL [Citation5], which considers that treatment can be discontinued in patients with compensated cirrhosis with close monitoring [Citation4,Citation5,Citation7]. For patients with HBeAg-negative immune active CHB, AASLD recommends treatment until HBsAg clearance [Citation4]; EASL not only supports this approach but also suggests NA withdrawal can be considered in select patients after at least 3 years of sustained HBV DNA suppression who are without cirrhosis and have normal ALT [Citation7]. A recent guidance from the APASL recommends discontinuation of NA treatment in HBeAg-negative CHB with normal ALT and suppressed HBV DNA who have HBsAg ≤ 100 IU/mL [Citation57]. However, following discontinuation, although HCC and decompensation events are rare, disease relapse often occurs increasing over time. A recent systematic review and pooled analysis suggest that the cumulative incidence of clinical relapse (defined as HBV DNA > 2000 IU/mL, or HBV DNA > 60 IU/mL or HBV DNA > 200 IU/mL with ALT > ULN or ALT > 2 × ULN and HBsAg loss) after stopping NA treatment with ETV/TDF was 17% after 6 months increasing up to 35% at 12 months; the incidence of virological relapse (defined as HBV DNA > 2000 IU/mL, HBV DNA > 60 IU/mL or HBV DNA > 200 IU/mL) was even greater at 44% (6 months) and 65% (12 months) [Citation58].
9. Psychosocial and economic impact of CHB disease and treatment
While treatment has clear benefits to a patient’s physical health, there are limited data regarding the impact of treatment or treatment discontinuation on a patient’s health-related quality of life (HRQoL). Patients with CHB suffer from psychological stressors, such as stigma and fears about how their diagnosis will be perceived by family, friends, and the wider society, which adversely affects their overall HRQoL [Citation59]. Improved HRQoL was reported in patients with CHB undergoing treatment compared to untreated patients, as assessed by the Hepatitis B Virus-specific Chronic Liver Disease Questionnaire (CLDQ-HBV), with the greatest magnitude of improvement recorded in the worry domain [Citation60]. In contrast, another study showed that patients who discontinued long-term therapy reported significant reductions in depression and significant improvements in HRQoL after treatment discontinuation [Citation61].
Others have reported decreases in HRQoL as CHB progresses in severity. In a large North American cohort of untreated patients with CHB (Hepatitis B Research Network), impairment in HRQoL as assessed by the SF-36 health survey was reported only in patients with more advanced disease (AST-platelet-ratio index ≥1.5) and with a higher comorbidity burden [Citation62]. Variability in the HRQoL across patients may be due to the higher symptom burden reported by some patients [Citation39,Citation62–67]. Patients with ALT levels >40 U/L had impaired HRQoL related to emotional symptoms (e.g. the fear of death and belief in cure) and social stigma (e.g. family, social activities, and employment) compared to patients with normal ALT values. Furthermore, those positive for HBeAg also had impaired HRQoL related to social stigma compared to HBeAg-negative patients [Citation59,Citation60,Citation68]. Patients with CHB were also shown to have significant impairments in the presenteeism and activity domains of the Work Productivity and Activity Impairment Specific Health Problem (WPAI-SHP) questionnaire [Citation69]. However, treatment offered no benefit on productivity and activity impairment [Citation70].
Missed opportunities to treat patients with CHB can incur considerable economic burden resulting from disease progression. U.S. patients with CHB who experienced decompensated cirrhosis, HCC, or a liver transplantation incurred the highest annual costs and utilization of health-care resources [Citation63]. For the Medicare population 2004–2015, annual total costs (medical plus pharmacy) were $69,107, $184,215, $83,499, and $142,774 for patients with compensated cirrhosis, decompensated cirrhosis, HCC, and liver transplantation, respectively, compared to $19,060 for matched controls without CHB, and a similar trend was observed for healthcare resource use [Citation63].
9.1. Cost-effectiveness of screening and treatment of CHB
With respect to the cost-effectiveness of different treatments in comparison to no treatment, treating immune-tolerant CHB with either TDF or ETV appears effective at preventing the downstream costs of disease progression and HCC (incremental cost-effectiveness ratio [ICER] less than $20,000 compared with no treatment) [Citation71]. Evidence from Asia suggests that extending treatment to a broader CHB population that currently remain untreated, i.e. initiating NA treatment in patients who are untreated with minimally active disease, would be a cost-effective strategy (ICER of $12,067/quality-adjusted life-year [QALY] over a 10-year period) [Citation71]. A recent US modeling study evaluated different combinations of HBV screening and treatment. Screening for HBV infection and treatment of active patients with tenofovir TDF-based regimens offered advantages with respect to both cost and the quality of life of patients (ICER of $17 000 to $26 000/QALY) [Citation72]. However, the most cost-effective option evaluated used an inclusive strategy, where patients were screened for both HBV infection and/or immunity and were treated or vaccinated appropriately (ICER ranged from $3,203 to $18,009/QALY across the different groups) [Citation72]. Similarly, a cost-effectiveness study from Africa (The Gambia) showed that a screening and treatment strategy was cost-effective compared to the current situation (no publicly funded screening or treatment) with an ICER of $511/QALY [Citation73].
10. Expert opinion
HBV is a significant threat to public health globally, resulting in considerable mortality and morbidity, which has substantial impact on the economic and humanistic burden of disease. Although HBV is a vaccine-preventable disease and national vaccination and screening programs exist in many countries, the WHO’s global target of eliminating viral hepatitis (including HBV and HCV) as a global health threat by 2030 remains a testing target to achieve [Citation11]. Even with calls to expand universal HBV testing strategies, evidence on the cost-effectiveness of such universal screening and follow-up strategies in low- and middle-income countries is limited and may not be affordable [Citation74]. Therefore, moving forward, we need to ensure that we have comprehensive screening strategies to identify those not yet diagnosed and to offer protection through vaccination to those who are HBV uninfected.
Current treatment guidelines are viewed by many as being too complex, which limits the willingness of non-specialists to undertake treatment. Additionally, while the societal guidelines are broadly well-aligned, there are some differences, and a lack of harmonization may lead to confusion among physicians and ultimately under-treatment [Citation34]. The high proportion of ‘gray zone’ patients is well-recognized, and a simplified treatment approach is needed to minimize these patients incurring a missed opportunity for treatment. More controversial areas regarding treatment include immune-tolerant and inactive carriers. Consensus across guidelines globally will serve to support best practice globally to maximize the benefits of current therapies, particularly in those who are at greatest need.
While current therapies, specifically NAs, have provided significant clinical benefits, long-term suppression therapy is associated with reduced rates of cirrhosis, HCC, and liver-related mortality. However, treatment with NA is lengthy – often lifelong for patients. Current treatment recommendations focus on NAs and Peg-IFN, both of which have limitations. NAs have little impact on functional cure and although Peg-IFN offers some potential benefits with respect to functional cure there are still opportunities for finite therapies that offer higher rates of HBsAg seroclearance, or at minimum long-term viral suppression (HBV DNA undetectable, but still HBsAg) off treatment – so called partial cure. Of crucial importance is the shift away from long-term (indefinite) therapy toward treatments that are finite but can maintain an acceptable safety and tolerability profile. New therapies currently in development target a broad range of novel and existing therapeutic targets aimed at preventing/disrupting viral entry, nucleic acid integration, viral protein production, and HBV DNA replication, as well as enhancing immunologic responses to the virus. Combinations of new and existing therapies may also offer further opportunities to improve patient outcomes through synergistic effects on virological and immunologic control, potentially leading to higher rates and earlier functional cure.
11. Conclusions
HBV infection, and particularly CHB, represent a significant global public health issue despite the availability of vaccines for over 30 years and attempts to introduce screening programs. Notwithstanding the current lack of a curative therapy hampers attempts to control the disease. Combining testing and screening strategies with broader treatment strategies to cover currently untreated/inadequately treated populations is key to reducing the global burden of CHB. Several potential treatment options are currently under development but in order to achieve significant impacts globally and realize the WHO’s targets for the global eradication of HBV, it is important to ensure that treatment guidelines are aligned and that new guidelines and treatments are targeted to those most at need.
Abbreviation
| AASLD | = | American Association for the Study of Liver Diseases |
| AFP | = | Alpha-fetoprotein |
| ALT | = | Alanine transaminase |
| APASL | = | Asia Pacific Association for the Study of the Liver |
| APRI | = | Aspartate aminotransferase-to-platelet ratio index |
| AST | = | Aspartate transaminase |
| BDI | = | Beck Depression Inventory |
| cccDNA | = | Closed covalent circular DNA |
| CDC | = | Centers for Disease Control and Prevention |
| CHB | = | Chronic hepatitis B |
| CHBQOL | = | Chronic hepatitis B quality of life |
| CI | = | Confidence interval |
| CLDQ-HBV | = | Hepatitis B Virus-specific Chronic Liver Disease Questionnaire |
| DNA | = | Deoxyribonucleic acid |
| EASL | = | European Association for the Study of the Liver |
| ECDC | = | European Centre for Disease Prevention and Control |
| eGFR | = | Estimated glomerular filtration rate |
| ETV | = | Entecavir |
| FDA | = | Food and Drug Administration |
| FIB-4 | = | Fibrosis 4 index |
| HBeAg | = | Hepatitis B e-antigen |
| HBsAg | = | Hepatitis B surface antigen |
| HBV | = | Hepatitis B virus |
| HCC | = | Hepatocellular carcinoma |
| HCV | = | Hepatitis C virus |
| HRQoL | = | Health-related quality of life |
| IC | = | Inactive carrier |
| ICER | = | Incremental cost-effectiveness ratio |
| ICMJE | = | International Committee of Medical Journal Editors |
| IHME | = | Institute for Health Metrics and Evaluation |
| IT | = | Immune-tolerant |
| IU | = | International units |
| mL | = | Milliliter |
| MTCT | = | Mother-to-child transmission |
| NA | = | Nucleot(s)ides analog |
| PCR | = | Polymerase chain reaction |
| Peg-IFN | = | Pegylated interferon |
| PICOS | = | Population, intervention, comparators, outcomes and study designs |
| QALY | = | Quality-adjusted life year |
| RR | = | Relative risk |
| SF-36 | = | 36-Item Short Form Health Survey |
| SLR | = | Systematic literature review |
| ULN | = | Upper limit of normal |
| US | = | United States |
| USG | = | ultrasonography |
| TAF | = | Tenofovir alafenamide |
| TDF | = | Tenofovir disoproxil fumarate |
| TSH | = | Thyroid-stimulating hormone |
| WHO | = | World Health Organization |
| WPAI-SHP | = | Work Productivity and Activity Impairment Specific Health Problem |
| WTP | = | Willingness-to-pay |
Article highlights
Burden of CHB disease, global treatment guidelines, treatment outcomes, psychosocial and economic impacts, and populations with unmet medical need.
Latest data from the WHO and recently published treatment guidelines and where these guidelines vary in recommendations on treatment.
Several patient groups remain untreated, including those with indications for treatment and at risk of developing progressive liver disease.
Considerations for future treatment strategies to increase rates of functional cure and stabilization/reversal of liver disease.
Declaration of interest
L. Shen is a stockholder of Vir Biotechnology. S. Satram, A. Arizpe, and V. Thanawala are employees and stockholders of Vir Biotechnology.
N. Terrault has received institutional grant support from Gilead Sciences, GlaxoSmithKline, Roche-Genentech, Helio Health, Eiger Pharmaceuticals, and Durect Corporation and served on the DSMB for Moderna.
C. Forbes and L. Lavoie are employed by Evidera, which provides consulting and other research services to pharmaceutical, medical device, and related organizations. In their salaried positions, they work with a variety of companies and organizations, and are precluded from receiving payment or honoraria directly from these organizations for services rendered. Evidera received funding from Vir Biotechnology to participate in the study and the development of this manuscript.
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplemental Material
Download MS Word (43.2 KB)Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14787210.2023.2225771.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Nguyen MH, Wong G, Gane E, et al. Hepatitis B virus: advances in prevention, diagnosis, and therapy. Clin Microbiol Rev. 2020;33(2):e00046–19. doi:10.1128/CMR.00046-19
- Zampino R, Boemio A, Sagnelli C, et al. Hepatitis B virus burden in developing countries world. J Gastroenterol. 2015;21(42):11941–11953. doi:10.3748/wjg.v21.i42.11941
- Lampertico P, Agarwal K, Berg T, et al. Clinical practice guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67(2):370–398. doi:10.1016/j.jhep.2017.03.021.
- Terrault NA, Lok ASF, McMahon BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: aASLD 2018 hepatitis B guidance. Clin Liver Dis. 2018;12(1):33–34. doi: 10.1002/cld.728.
- Sarin SK, Kumar M, Lau GK, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10(1):1–98. doi:10.1007/s12072-015-9675-4
- World Health Organization. Guidelines for the prevention, care and treatment of persons with chronic hepatitis B infection. France: World Heath Organization. 2015. p. 1–166.
- Lampertico P, Agarwal K, Berg T, et al. EASL 2017 clinical practice guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67(2):370–398. doi: 10.1016/j.jhep.2017.03.021.
- World Health Organization. Hepatitis B; 2021. [cited 2022 May 10]. Available from: https://www.who.int/en/news-room/fact-sheets/detail/hepatitis-b
- The Polaris Observatory Collaborators. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol. 2018;3(6):383–403.
- World Health Organization. Global Hepatitis Report 2017. France: World Health Organization. 2017.
- World Health Organization. Global health sector strategies on, respectively, HIV, viral hepatitis and sexually transmitted infections for the period 2022-2030. 2022.
- Khetsuriani N, Lesi O, Desai S, et al. Progress toward the elimination of mother-to-child transmission of hepatitis B virus — worldwide, 2016–2021. MMWR Morb Mortal Wkly Rep. 2022;71(30):958–963. doi:10.15585/mmwr.mm7130a2
- US Food and Drug Administration. Chronic hepatitis B virus infection: developing drugs for treatment guidance for industry. US: U.S. Department of Health and Human ServicesFood and Drug Administration Center for Drug Evaluation and Research (CDER); 2022.
- Cornberg M, Lok AS, Terrault NA, et al. Guidance for design and endpoints of clinical trials in chronic hepatitis B - Report from the 2019 EASL-AASLD HBV Treatment Endpoints Conference(double dagger). J Hepatol. 2020;72(3):539–557. doi:10.1016/j.jhep.2019.11.003
- Broquetas T, Carrion JA. Current perspectives on nucleos(t)ide analogue therapy for the long-term treatment of hepatitis B. Virus Hepat Med. 2022;14(87–100):87–100. doi:10.2147/HMER.S291976.
- Geng J, Bao H, Chen Y, et al. Nucleos(t)ide analogues for the treatment of chronic hepatitis B: a systematic review with network meta-analysis. Expert Rev Anti Infect Ther. 2020;18(8):823–834. doi:10.1080/14787210.2020.1760843
- Sbarigia U, Vincken T, Wigfield P, et al. A comparative network meta-analysis of standard of care treatments in treatment-naive chronic hepatitis B patients. J Comp Eff Res. 2020;9(15):1051–1065. doi:10.2217/cer-2020-0068
- de Fraga RS, Van VV, Mendes LCA, et al. Adverse events of nucleos(t)ide analogues for chronic hepatitis B: a systematic review. J Gastroenterol. 2020;55(5):496–514. doi:10.1007/s00535-020-01680-0
- de FRS, Van VV, Mendes LCA, et al. Adverse events of nucleos(t)ide analogues for chronic hepatitis B: a systematic review. J Gastroenterol. 2020;55(5):496–514. doi:10.1007/s00535-020-01680-0
- Yang YM, Choi EJ. Renal safety of tenofovir and/or entecavir in patients with chronic HBV monoinfection. Ther Clin Risk. Manag. 2017;13(1273–1285):1273–1285. doi:10.2147/TCRM.S143286.
- Chen MB, Wang H, Zheng QH, et al. Comparative efficacy of tenofovir and entecavir in nucleos(t)ide analogue-naive chronic hepatitis B: a systematic review and meta-analysis. PLoS One. 2019;14(11):e0224773. doi:10.1371/journal.pone.0224773
- Con D, Goodwin T, Majeed A, et al. Comparison of 48-week efficacy of tenofovir vs entecavir for patients with chronic hepatitis B: a network meta-analysis. J Viral Hepat. 2021;28(1):40–50. doi:10.1111/jvh.13400
- Buti M, Gane E, Seto WK, et al. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of patients with HBeAg-negative chronic hepatitis B virus infection: a randomised, double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol Hepatol. 2016;1(3):196–206. doi:10.1016/S2468-1253(16)30107-8
- Choi WM, Choi J, Lim YS. Effects of Tenofovir vs Entecavir on Risk of Hepatocellular Carcinoma in Patients with Chronic HBV Infection: a Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2021;19(2):246–258 e9. doi:10.1016/j.cgh.2020.05.008.
- Tseng CH, Tseng CM, Wu JL, et al. Magnitude of and prediction for risk of hepatocellular carcinoma in patients with chronic hepatitis B taking entecavir or tenofovir therapy: a systematic review. J Gastroenterol Hepatol. 2020;35(10):1684–1693. doi:10.1111/jgh.15078
- Tseng C-H, Hsu Y-C, Chen T-H, et al. Hepatocellular carcinoma incidence with tenofovir versus entecavir in chronic hepatitis B: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5(12):1039–1052. doi:10.1016/S2468-1253(20)30249-1
- Yardeni D, Ghany MG. Review article: hepatitis B—current and emerging therapies. Aliment Pharmacol Ther. 2022;55(7):805–819. doi:10.1111/apt.16828.
- Hutin Y, Nasrullah M, Easterbrook P, et al. Access to treatment for hepatitis B virus infection — worldwide, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(28):773–777. doi:10.15585/mmwr.mm6728a2.
- Ogawa E, Yeo YH, Dang N, et al. Diagnosis rates of chronic hepatitis B in privately insured patients in the United States. JAMA Netw Open. 2020;3(4):e201844. doi:10.1001/jamanetworkopen.2020.1844
- Preventive Services Task Force US, Krist AH, Davidson KW, et al. Screening for Hepatitis B Virus Infection in Adolescents and Adults: uS Preventive Services Task Force Recommendation Statement. JAMA. 2020;324(23):2415–2422. doi: 10.1001/jama.2020.22980.
- Centers for Disease Control and Prevention. Peer review plan for recommendations for hepatitis B screening and testing 2022 [updated 6 Apr 2022; cited 2022 5 Aug]. Available from: https://www.cdc.gov/hepatitis/policy/isireview/hepbscreeningandtesting.htm#:~:text=Universal%2C%20one%2Dtime%20hepatitis%20B,to%20screen%20for%20hepatitis%20B
- Cui F, Blach S, Manzengo Mingiedi C, et al. Global reporting of progress towards elimination of hepatitis B and hepatitis C. Lancet Gastroenterol Hepatol. 2023;8(4):332–342. doi:10.1016/S2468-1253(22)00386-7
- Lin C, Clark R, Tu P, et al. The disconnect in hepatitis screening: participation rates, awareness of infection status, and treatment-seeking behavior. J Glob Health. 2019;9(1):010426–010426. doi: 10.7189/jogh.09.010426.
- World Health Organization. Global health sector strategy on viral hepatitis 2016–2021: towards ending viral hepatitis. France: World Heath Organization. 2021.
- Freeland C, Farrell S, Kumar P, et al. Common concerns, barriers to care, and the lived experience of individuals with hepatitis B: a qualitative study. BMC Public Health. 2021;21(1):1004. doi:10.1186/s12889-021-11093-0
- Wong RJ, Kaufman HW, Niles JK, et al. Simplifying Treatment Criteria in Chronic Hepatitis B: reducing Barriers to Elimination. Clin Infect Dis. 2023;76(3):e791–e800. doi:10.1093/cid/ciac385
- Jiang XY, Huang B, Huang DP, et al. Long-term follow-up of cumulative incidence of hepatocellular carcinoma in hepatitis B virus patients without antiviral therapy. World J Gastroenterol. 2021;27(11):1101–1116. doi:10.3748/wjg.v27.i11.1101
- Huang DQ, Tran A, Yeh ML, et al. Antiviral therapy substantially reduces hepatocellular carcinoma risk in chronic Hepatitis B patients in the indeterminate phase. Hepatology. 2023;Publish Ahead of Print. doi:10.1097/HEP.0000000000000459.
- Tan M, Bhadoria AS, Cui F, et al. Estimating the proportion of people with chronic hepatitis B virus infection eligible for hepatitis B antiviral treatment worldwide: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2021;6(2):106–119. doi: 10.1016/S2468-1253(20)30307-1.
- Zhou K, Wahed AS, Cooper S, et al. Phase transition is infrequent among North American adults with e-antigen-negative chronic hepatitis B and low-level viremia. Am J Gastroenterol. 2019;114(11):1753–1763. doi:10.14309/ajg.0000000000000400
- Spradling PR, Xing J, Rupp LB, et al. Infrequent clinical assessment of chronic hepatitis B patients in United States general healthcare settings. Clin Infect Dis. 2016;63(9):1205–1208. doi:10.1093/cid/ciw516
- Lee HW, Kim SU, Park JY, et al. Prognosis of untreated minimally active chronic hepatitis B patients in comparison with virological responders by antivirals. Clin Transl Gastroenterol. 2019;10(6):e00036. doi:10.14309/ctg.0000000000000036
- Yao K, Liu J, Wang J, et al. Distribution and clinical characteristics of patients with chronic hepatitis B virus infection in the grey zone. J Viral Hepat. 2021;28(7):1025–1033. doi:10.1111/jvh.13511
- Lee JS, K BK, Park JY, et al. A nationwide prospective cohort study on chronic hepatitis B in Korea: an interim analysis. Hepatol. 2021;74:443A–444A.
- Harris A, Iqbal K, Schillie S, et al. Increases in acute hepatitis B virus infections — Kentucky, Tennessee, and West Virginia, 2006–2013. MMWR Morb Mortal Wkly Rep. 2016;65(3):47–50. doi:10.15585/mmwr.mm6503a2
- Ly KN, Xing J, Spradling PR. Trends in prevalence and characteristics of resolved and current hepatitis B among US-Born persons: national health and nutrition examination survey, 2001-2018. J Infect Dis. 2021;224(5):804–812. doi:10.1093/infdis/jiab224.
- Kim GL, Han S, Choi J, et al. High risk of hepatocellular carcinoma and death in patients with immune-tolerant-phase chronic hepatitis B. Gut. 2018;67(5):945–952. doi:10.1136/gutjnl-2017-314904.
- Jeon MY, Kim BK, Lee JS, et al. Negligible risks of hepatocellular carcinoma during biomarker-defined immune-tolerant phase for patients with chronic hepatitis B. B Clin Mol Hepatol. 2021;27(2):295–304. doi:10.3350/cmh.2020.0216
- Wu W, Zhu Y, Yu C, et al. Clinical features of treatment-naive patients with hepatitis B virus infection. Medicine. 2017;96(16):e6660. doi:10.1097/MD.0000000000006660
- Huang DQ, Li X, Le MH, et al. Natural history and hepatocellular carcinoma risk in untreated chronic hepatitis B patients with indeterminate phase. Clin Gastroenterol Hepatol. 2022;20(8):1803–1812 e5. doi: 10.1016/j.cgh.2021.01.019.
- Teng W, Chang TT, Yang HI, et al. Risk scores to predict HCC and the benefits of antiviral therapy for CHB patients in gray zone of treatment guidelines. Hepatol Int. 2021;15(6):1421–1430. doi:10.1007/s12072-021-10263-x
- Zhou J, Wang F, Li L, et al. Expanding antiviral therapy indications for HBeAg-negative chronic hepatitis B patients with normal ALT and positive HBV DNA. Precis Clin Med. 2022;5(4):bac030. doi:10.1093/pcmedi/pbac030
- Kim JH, Sinn DH, Kang W, et al. Low-level viremia and the increased risk of hepatocellular carcinoma in patients receiving entecavir treatment. Hepatology. 2017;66(2):335–343. doi:10.1002/hep.28916
- Lee J, Cho S, Kim HJ, et al. High level of medication adherence is required to lower mortality in patients with chronic hepatitis B taking entecavir: a nationwide cohort study. J Viral Hepat. 2021;28(2):353–363. doi:10.1111/jvh.13418
- Fu KY, Hsieh ML, Chen JA, et al. Association between medication adherence and disease outcomes in patients with hepatitis B-related cirrhosis: a population-based case-control study. BMJ Open. 2022;12(6):e059856. doi:10.1136/bmjopen-2021-059856
- Ford N, Scourse R, Lemoine M, et al. Adherence to nucleos(t)ide analogue therapies for chronic hepatitis B infection: a systematic review and meta-analysis. Hepatol Commun. 2018;2(10):1160–1167. doi:10.1002/hep4.1247
- Kao JH, Jeng WJ, Ning Q, et al. APASL guidance on stopping nucleos(t)ide analogues in chronic hepatitis B patients. Hepatol Int. 2021;15(4):833–851. doi:10.1007/s12072-021-10223-5
- Hall S, Vogrin S, Wawryk O, et al. Discontinuation of nucleot(s)ide analogue therapy in HBeAg-negative chronic hepatitis B: a meta-analysis. Gut. 2022;71(8):1629–1641. doi:10.1136/gutjnl-2020-323979
- Li G, Wang G, Hsu FC, et al. Effects of depression, anxiety, stigma, and disclosure on health-related quality of life among chronic hepatitis B patients in dalian. China Am J Trop Med Hyg. 2020;102(5):988–994. doi:10.4269/ajtmh.19-0007
- Younossi ZM, Stepanova M, Younossi I, et al. Development and validation of a hepatitis B-specific health-related quality-of-life instrument: cLDQ-HBV. J Viral Hepat. 2021;28(3):484–492. doi:10.1111/jvh.13451
- Xue X, Cai S, Ou H, et al. Health-related quality of life in patients with chronic hepatitis B during antiviral treatment and off-treatment. Patient Prefer Adherence. 2017;11(85–93):85–93. doi:10.2147/PPA.S127139
- Evon DM, Lin HS, Khalili M, et al. Patient-reported outcomes in a large North American cohort living with chronic hepatitis B virus: a cross-sectional analysis. Aliment Pharmacol Ther. 2020;51(4):457–468. doi: 10.1111/apt.15618.
- Nguyen MH, Burak OA, Liou I, et al. Healthcare resource utilization and costs by disease severity in an insured national sample of US patients with chronic hepatitis B. J Hepatol. 2020;70(1):24–32. doi: 10.1016/j.jhep.2018.09.021.
- Campbell C, Wang T, McNaughton AL, et al. Risk factors for the development of hepatocellular carcinoma (HCC) in chronic hepatitis B virus (HBV) infection: a systematic review and meta-analysis. J Viral Hepat. 2021;28(3):493–507. doi:10.1111/jvh.13452
- Kuang XJ, Jia RR, Huo RR, et al. Systematic review of risk factors of hepatocellular carcinoma after hepatitis B surface antigen seroclearance. J Viral Hepat. 2018;25(9):1026–1037. doi:10.1111/jvh.12905
- Raffetti E, Fattovich G, Donato F. Incidence of hepatocellular carcinoma in untreated subjects with chronic hepatitis B: a systematic review and meta-analysis. Liver Int. 2016;36(9):1239–1251. doi:10.1111/liv.13142.
- Tan Y, Wei S, Zhang W, et al. Type 2 diabetes mellitus increases the risk of hepatocellular carcinoma in subjects with chronic hepatitis B virus infection: a meta-analysis and systematic review. cancer Manag Res. 2019;11(705–713):705–713. doi:10.2147/CMAR.S188238
- Zhu L, Kong J, Zheng Y, et al. Development and initial validation of the chronic hepatitis B quality of life instrument (CHBQOL) among Chinese patients. Qual Life Res. 2019;28(11):3071–3081. doi:10.1007/s11136-019-02240-7
- Younossi ZM, Stepanova M, Younossi I, et al. Long-term effects of treatment for chronic HBV infection on patient-reported outcomes. Clin Gastroenterol Hepatol. 2019;17(8):1641–1642.e1. doi:10.1016/j.cgh.2018.09.041
- Younossi ZM, Stepanova M, Younossi I, et al. Patient-reported outcomes in patients chronic viral hepatitis without cirrhosis: the impact of hepatitis B and C viral replication. Liver Int. 2019;39(10):1837–1844. doi:10.1111/liv.14171
- Lee H, Kim BK, Jang S, et al. Cost-effectiveness analysis of antiviral therapy for untreated minimally active chronic hepatitis B to prevent liver disease progression. Clin Transl Gastroenterol. 2021;12(2):e00299. doi:10.14309/ctg.0000000000000299
- Chahal HS, Peters MG, Harris AM, et al. Cost-effectiveness of hepatitis B virus infection screening and treatment or vaccination in 6 high-risk populations in the United States. Open Forum Infect Dis. 2019;6(1):ofy353. doi:10.1093/ofid/ofy353
- Nayagam S, Conteh L, Sicuri E, et al. Cost-effectiveness of community-based screening and treatment for chronic hepatitis B in the Gambia: an economic modelling analysis. Lancet Glob Health. 2016;4(8):e568–e578. doi:10.1016/S2214-109X(16)30101-2
- Wright CM, Boudarène L, Ha NT, et al. A systematic review of hepatitis B screening economic evaluations in low- and middle-income countries BMC. BMC Public Health. 2018;18(1):373. doi:10.1186/s12889-018-5261-8