1. Introduction
The world is facing recurrent episodic outbreaks of COVID-19 due to the emerging mutated and recombinant variants of SARS-CoV-2 [Citation1]. In 2022, a worldwide outbreak of monkeypox surfaced simultaneously in various countries, further exacerbating the complexity of the situation [Citation2,Citation3]. Meanwhile, a new species of Langya virus, also referred to as Langya henipavirus (LayV), was detected in throat swab samples from individuals experiencing fever symptoms in the eastern regions of China, including Shandong and Henan provinces [Citation4]. According to Taiwan’s Centres for Disease Control (CDC), there were 35 positive cases detected up until 10 August . Despite the presence of cough, fever, and fatigue among the symptoms, a significant portion of cases were represented by nonspecific signs, such as anorexia, headache, nausea and vomiting; no deaths have been reported to date () [Citation5].
Figure 1. Structure of Langya henipavirus and its associated symptoms (created using Biorender.com). Abbreviations: M, protein-matrix protein; F, protein-phosphorus protein; NLP, nucleocapsid (N) large protein.
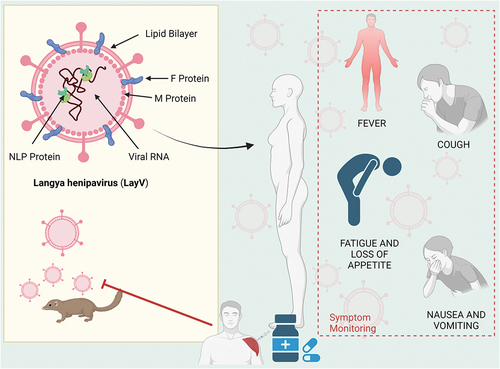
Infection with henipavirus produces severe respiratory disease and encephalitis. These viruses have been detected in bats, rodents, and shrews. Langya henipavirus is a single-stranded negative-sense RNA virus of the genus Henipavirus in the family Paramyxoviridae [Citation6]. The World Health Organization classifies Henipavirus as a biosafety Level 4 pathogen, with case fatality rates ranging between 40 and 75% [Citation7]. The results of tests conducted on over 20 different wild species suggest that the Common Shrew, scientifically known as Sorex Araneus, has the potential to serve as a vector for Langya henipavirus transmission [Citation4].
In fact viral RNA was detected in 27% of the 262 investigated shrews and presence of anti Langya henipavirus antibodies in specific goats and dogs suggests that shrews potentially act as a reservoir for the virus [Citation8,Citation9].
2. Discussion
To grasp the evolution of this outbreak, comprehensive monitoring and surveillance are imperative, especially within China and its neighboring countries. Additionally, there is a pressing demand for research into potential treatments for this ailment and the creation of corresponding diagnostic tools. The clinical presentations may bear similarity to those of COVID-19, but they differ from those of monkeypox. This similarity can lead to the inadvertent neglect of this disease in regions with high COVID-19 transmission rates. Therefore, it is of utmost importance to screen individuals in China and the surrounding regions who have a history of contact with animals and display symptoms like fever and respiratory issues, even if tested negative for COVID-19 [Citation10].
The viral genomic structure closely resembles that of other henipaviruses, comprising 18,402 nucleotides. In vivo studies suggest that while the precise human exposure route remains uncertain, epidemiological evidence points to the respiratory system as a crucial site for viral replication [Citation4]. Patients present with a decrease in white blood cell counts as well as reduced platelet counts [Citation11]. Until now, there have been no reported instances of direct human-to-human transmission [Citation5]. There is a necessity for the development of inactivation methods to enhance containment measures. Among the identified Henipaviruses, Hendra virus and Nipah virus (NiV) are highly virulent, while other variants such as cedar virus, Mòjiāng virus (MojV), and Ghanaian bat virus, are notably less virulent [Citation12].
Examining cell-cell fusion and the release of free virions within an integrated and quantitative framework, it is expected to lead to substantial progress in research related to pathogenesis [Citation13]. As per prior research findings on Henipaviruses, the advancement of the disease is gauged by the levels of critical cytokines crucial for immune cell attraction, including interleukin 6 (IL-6), IL-8, IL-1, monocyte chemoattractant protein 1 (MCP-1), and colony-stimulating factors [Citation6,Citation11,Citation14]. Understanding the pathogenesis [Citation15] of the virus, and identification of immunogenic viral proteins, vaccines and drugs could be designed against Langya henipavirus. There is currently no vaccine or treatment for Henipavirus, although a HeV-G subunit vaccine has shown promise in preclinical testing and is being translated to human clinical trials [Citation16,Citation17]. At present, the only active treatment is supportive care to manage complications. Further, the current Henipavirus vaccine (Equivac® HeV) is a vaccine for horses that is projected to deliver a significant health benefit to humans, and fits well into the idea of a ‘One Health’ strategy for the human-animal interface and environmental health [Citation6]. Currently, it is believed that Langya henipavirus does not exhibit human-to-human transmission, but there is a possibility that the virus could undergo evolutionary changes that would enable it to transmit among humans in the future [Citation10].
Further, of 26 patients infected with Langya Henipavirus being under investigation, episodes of mental confusion and impairment in neurological balance, were noted in 38.5% of cases [Citation18]. Between severe and non-severe cases, the rate of confusion in the severe cases of Langya Henipavirus infection is significantly higher than that of the non-severe ones. According to the latest study, confusion can serve as an indicator of severe illness. Patients exhibited increased antibody titers during both the acute and convalescent stages of infection, with titters being notably higher during the convalescent phase compared to the acute phase [Citation19].
Recently, the structure of the fusion protein was determined by cryogenic electron microscopy [Citation20]. In the prefusion state, the LayV-F fusion peptide is buried within a highly conserved, hydrophobic interprotomer pocket and is noticeably less flexible than the rest of the protein, highlighting its ‘spring-loaded’ state and suggesting that the mechanism of pre-to-post transition must involve pocket perturbations and fusion peptide release [Citation21]. In fact, both LayV fusion protein and MojV fusion proteins contain a ‘tree-like’ prefusion F structure. This has ramifications for vaccine development as well as treatments using immunotherapeutic approaches. This shows that a single HNV immunogen would be ineffective as a broad-spectrum vaccination and that a trivalent or tetravalent strategy is necessary [Citation22]. This data contributes to our comprehension of virus biology and allows our readiness for epidemic vaccine development. In a recent study, an immunoinformatics-driven approach was used to identify potential epitopes to develop a multi-epitope-based vaccine targeting Langya henipavirus [Citation23]. The projected epitopes were linked together with appropriate linkers and adjuvants to enhance their immunogenicity. Additionally, comprehensive assessments, including allergenicity, antigenicity, physicochemical characteristics, and molecular docking, were conducted to assess the binding affinity and stability of the complexes formed between toll-like receptors and the vaccine. Lastly, in silico cloning was utilized to confirm the stability and efficacy of expression of the vaccine construct.
3. Expert opinion
In our ever more interconnected and globalized world, it is crucial to remain vigilant over emerging viruses and infectious diseases, given their potential for rapid and global transmission. The identification of Langya henipavirus in Eastern China highlights the importance of ongoing local epidemiological surveillance, particularly in non-human hosts. The presence of Langya henipavirus in several animal species and humans, coupled with limited documented cases of infection, underscores the need for further investigation into both the short-term and long-term aspects of its transmission. Further research is required to determine the biological attributes of Langya henipavirus and host immune responses, with the goal of achieving a more comprehensive understanding of this newly emerging virus-induced human disease. Finally, the new illnesses highlight the critical need of global zoonotic surveillance to detect the introduction and spread of novel zoonotic organisms in humans [Citation24]. Additionally, it is crucial to understand the transmission mechanisms of Langya henipavirus and pinpoint potential host traits that could hinder viral replication. Such efforts will play a crucial role in the development of effective prevention and treatment strategies. The key strategy for addressing this viral challenge is prevention. It is highly recommended to establish a targeted and all-encompassing framework to tackle this viral outbreak, while simultaneously allocating resources to implement effective health policies aimed at diminishing the viral threat stemming from recognized zoonotic reservoirs. To alleviate the socioeconomic impact, it is essential to disseminate information and raise awareness. This includes improving contact tracing, enhancing understanding of transmission routes, implementing proper hygiene measures, advancing diagnostic tools, and effectively developing antiviral medications and vaccines.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
V.P.C. is grateful to the L.M. College of Pharmacy, Ahmedabad, India, for providing necessary support in carrying out the literature search. V.A. would like to thank Victoria University, Australia, VU Research for their support.
Additional information
Funding
References
- Chavda VP, Patel AB, Vaghasiya DD. SARS-CoV-2 variants and vulnerability at the global level. J med virol. 2022;94(7):2986–3005. doi: 10.1002/jmv.27717
- Chavda VP, Apostolopoulos V. Rare monkeypox: is it really a threat to the elderly? Maturitas. 2022;163:90–91.
- Uwishema O, Shariff S, Rai A, et al. Is the new Langya virus in China a threat to global health? A short communication. Ann Med Surg (Lond) Internet. 2023;85(4):1348–1351. http://europepmc.org/abstract/MED/37113840
- Zhang X-A, Li H, Jiang F-C, et al. A zoonotic henipavirus in febrile Patients in China. N Engl J Med. 2022;387(5):470–472. doi: 10.1056/NEJMc2202705
- Henipavirus Infections | CDC Yellow Book 2024 [Internet]. [cited 2023 May 14]. Available from: https://wwwnc.cdc.gov/travel/yellowbook/2024/infections-diseases/henipavirus-infections
- Broder CC, Wong KT. Henipaviruses. In: Reiss C, editor. Neurotropic Viral Infections. Springer, Cham; 2016. doi: 10.1007/978-3-319-33133-1_3
- Gazal S, Sharma N, Gazal S, et al. Nipah and Hendra Viruses: deadly zoonotic paramyxoviruses with the potential to cause the next pandemic. Pathogens. 2022;11(12):11. doi: 10.3390/pathogens11121419
- Choudhary OP, Priyanka, Fahrni ML, et al. Spillover zoonotic ‘Langya virus’: is it a matter of concern? Veterinary Quarterly. 2022;42(1):172–174. doi: 10.1080/01652176.2022.2117874
- Tabassum S, Naeem A, Rehan ST, et al. Langya virus outbreak in China, 2022: are we on the verge of a new pandemic? J Virus Erad. 2022;8(3):100087. doi: 10.1016/j.jve.2022.100087
- Thakur CK, Adhikari JB, Gupta N, et al. Is the emergence of the zoonotic Langya virus amidst COVID-19 and monkeypox a cause for concern? Future Virol. 2023;18(1):5–7. doi: 10.2217/fvl-2022-0175
- Kummer S, Kranz D-C, Holbrook MR. Henipaviruses—A constant threat to livestock and humans. PLoS negl trop dis. 2022;16(2):e0010157. doi: 10.1371/journal.pntd.0010157
- Sah R, Mohanty A, Chakraborty S, et al. Langya virus: a newly identified zoonotic henipavirus. J Med Virol United States. 2022;94(12):5621–5622. doi: 10.1002/jmv.28095.
- Gamble A, Yeo YY, Butler AA, et al. Drivers and distribution of henipavirus-induced syncytia: what do we know? Viruses. 2021;13(9):1755. doi: 10.3390/v13091755
- Patel SK, Sharma K, Agrawal A, et al. Viral spillover to humans: could Langya (LayV) virus cause a pandemic? QJM Internet. 2023 [[cited 2023 Jul 17]];116:332–334. doi:10.1093/qjmed/hcac278
- Chuang J-H. Langya virus found in China causing liver, kidney failure [Internet]. Live Mint. 2022 [cited 2022 Aug 10]. Available from: https://www.livemint.com/news/india/langya-virus-found-in-china-causing-liver-kidney-failure-know-the-symptoms-about-this-new-virus-11660052226437.html
- Geisbert TW, Bobb K, Borisevich V, et al. A single dose investigational subunit vaccine for human use against Nipah virus and Hendra virus. npj vaccines. Internet. 2021;6(1):23. doi:10.1038/s41541-021-00284-w
- Bossart KN, Rockx B, Feldmann F, et al. A Hendra virus G glycoprotein subunit vaccine protects African green monkeys from Nipah virus challenge. Sci, trans med Internet. 2012 [[cited 2023 Jul 17]];4:ra146107–ra146107. doi:10.1126/scitranslmed.3004241
- Mungmunpunipantip R, Wiwanitkit V. Confusion in Langya henipavirus and severity of infection: a preliminary observation. Neurol India. 2023;71(1):176. doi: 10.4103/0028-3886.370477
- Agrawal A, Patel SK, Niranjan AK, et al. Queued viral emergence and risk of global exposure: langya virus. IJS Global Health Internet. 2023;6(3):e134–e134. https://journals.lww.com/ijsgh/Fulltext/2023/05010/Queued_viral_emergence_and_risk_of_global.3.aspx
- Release P. Langya virus: University of Queensland researchers uncover the structure of the fusion protein [Internet]. Outbreak News Today. 2023 [cited 2023 Jul 17]. Available from: http://outbreaknewstoday.com/langya-virus-university-of-queensland-researchers-uncover-the-structure-of-the-fusion-protein-63206/
- May Aaron J, Pothula KR, Janowska K, et al. Structures of Langya virus fusion protein ectodomain in pre- and postfusion conformation. J Virol Internet. 2023 [cited 2023 Jul 17]. ;97(6):e00433–23. doi:10.1128/jvi.00433-23
- Isaacs A, Low YS, Macauslane KL, et al. Structure and antigenicity of divergent henipavirus fusion glycoproteins. Nat Commun. 2023;14(1):3577. Internet. doi: 10.1038/s41467-023-39278-8
- Fahira A, Amin RS, Arshad U, et al. Chimeric vaccine design against the epidemic Langya henipavirus using immunoinformatics and validation via immune simulation approaches. Heliyon. 2023;9(6):e17376. Internet. doi: 10.1016/j.heliyon.2023.e17376
- Kadir AKMS, Umar TP, Rabbi AA, et al. Preparedness of South Asian countries regarding Langya virus emergence: a view on the current situation. Health Care Science Internet. 2023 [cited 2023 Jul 17]. ;2(3):194–197. doi:https://doi.org/10.1002/hcs2.42
