ABSTRACT
Background
The coronavirus disease (COVID-19) led to a global health crisis. Inappropriate use of antibiotics in COVID-19 patients has been a concern, leading to antimicrobial resistance. This study evaluated the patterns and predictors of empirical antibiotic therapy in COVID-19 patients and associated outcomes.
Methods
A hospital-based retrospective study was conducted with 525 patients admitted to Kasturba Hospital, Manipal, India, with moderate and severe COVID-19 from 1 March to 1 August 2021. They were divided based on empirical therapy, and predictors of antibiotic usage were assessed by logistic regression.
Results
Four hundred and eighty (91.4%) COVID-19 patients received at least one course of antibiotics, with 440 (83.8%) initiating empirical therapy. Patients with severe COVID-19 manifestations were more likely to be prescribed empirical antibiotics. Multivariable analysis showed that patients initiated on empirical antibiotics had significantly elevated levels of procalcitonin [OR: 3.91 (95% CI: 1.66–9.16) (p = 0.001)], invasive ventilation [OR: 3.93 (95% CI: 1.70–9.09) (p = 0.001)], shortness of breath [OR: 2.25 (95% CI: 1.30–3.89) (p = 0.003)] and higher CRP levels [OR: 1.01 (95% CI: 1.00–1.01) (p = 0.005)]. Most antibiotics (65.9%) were prescribed from the ‘Watch’ group, the highest being ceftriaxone. Only 23.8% of the patients had microbiologically confirmed infections.
Conclusion
The study identified predictors for initiating empirical antibacterial therapy in our setting.
1. Introduction
Coronavirus disease (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has resulted in an unprecedented global health crisis, affecting millions worldwide. Although the World Health Organization (WHO) declared COVID-19 no longer a public health crisis of international concern on 5 May 2023, it remains a significant global health threat (Citation1). Particularly in low- and middle-income countries (LMICs) such as India (Citation2), which has recorded nearly 45 million cases and 531,872 deaths, the disease continues to pose challenges, demanding special attention and resources.
While most COVID-19 cases present with mild, self-limiting infections that require no additional treatment, a subset of patients can develop severe illness, necessitating hospitalization (Citation3). Interestingly, COVID-19 can exhibit clinical manifestations like bacterial pneumonia, with patients commonly experiencing febrile illness and respiratory symptoms, including cough, dyspnea, and bilateral X-ray changes. Consequently, healthcare professionals often face the challenge of distinguishing viral from bacterial infections, leading to the overprescription of empiric antibiotic therapy (Citation4,Citation5). However, the clinical management guidelines by WHO and other organizations (Citation6–8) discourage broad-spectrum antibiotics in patients with mild-to-moderate disease presentations. Empirical therapy is only recommended in those with severe disease presentation, supported by appropriate evidence and clinical judgment.
Despite all these recommendations, Langford et al. (Citation9) reported antibiotic use to be more than 70.0% in COVID-19 cases, with usage even going up to 98.0% in least developed countries such as Nepal (Citation10). Inappropriate antibiotic utilization can have un-avoidable implications, including increased healthcare costs, adverse events, Clostridioides difficile infection (Citation11), renal impairment, and the alarming rise of antimicrobial resistance (AMR). Notably, previous antibiotic exposure has been linked to COVID-19 severity (Citation12), raising concerns about the potential exacerbation of AMR in the post-pandemic period, especially in LMICs with limited healthcare resources and reduced surveillance of resistant organisms (Citation13,Citation14).
Reports from the first wave of the pandemic in India reported an antibiotic utilization of more than 80% with only 12.0% of the patients having positive bacterial or fungal growth, predominantly as secondary infections (Citation15,Citation16). Notably, a high prevalence of multi-drug resistant organisms was also observed with Klebsiella pneumoniae and Acinetobacter baumannii among the most isolated organisms (Citation15,Citation17). To address these issues, it is crucial to identify interventions and focus on antibiotic stewardship initiatives by evaluating patterns and predictors of antibiotic prescribing in COVID-19 patients. Unfortunately, there is a paucity of data on antibiotic usage, associated factors, and outcomes specifically in lower and lower-middle income countries.
Therefore, the objective of this study was to investigate the patterns and predictors for empirical antibiotic therapy in COVID-19 patients, as well as examine the usage of empirical antibiotic therapy and its associated outcomes. The findings from this study will inform appropriateness of antibiotic therapy in COVID-19 patients and strengthen antimicrobial stewardship programs.
2. Methodology
2.1. Study design
We conducted a retrospective, observational study at Kasturba Hospital, Manipal, a 2032 bedded tertiary care hospital in Udupi district of Karnataka state, South India. The hospital has been recognized as a dedicated COVID-19 care center in the district. The study examined the utilization patterns of antibiotics and its associated outcomes in patients with confirmed COVID-19 disease. All patients ≥18 years presenting with moderate-to-severe COVID-19 infection based on guidelines from the Ministry of Health and Family Welfare criteria (Citation18) and WHO during a 5-month period (1 March to 1 August 2021) were included. Patients who were initiated on empirical therapy for indications other than COVID-19 related manifestations or had a hospital admission of less than 24 hours were excluded. In addition, patients with mild COVID-19 were also excluded, as they were typically not admitted due to the tertiary care nature of the facility.
2.2. Materials and methods
We retrospectively identified patients from the medical records department of Kasturba Hospital, Manipal after obtaining institutional ethics approval of Kasturba Medical College and Kasturba Hospital (KMC&KH), Manipal (Reference No: IEC 693/2021, dated 8 January 2022). The study was registered in the Clinical Trial Registry of India (CTRI), Reg No: CTRI/2022/01/039784. Initially, all COVID-19 patients under U07.1 according to the International Statistical Classification of Diseases, 10th Revision (ICD-10) and related health problems were included for the study. Supplemental data such as laboratory parameters and culture reports were obtained from the Kasturba Hospital management system’s online database, specifically the Lab Report Viewer.
The patients were divided into two groups based on whether antibiotics were started within the first 48 hours of admission (empirical therapy) or if antibiotics were not initiated or initiated later (>48 hrs). The antibiotics used were classified based on the Anatomical Therapeutic Chemical (ATC) classification (Citation19) and the WHO Access, Watch, Reserve (AWaRe) criteria 2021 (Citation20).
2.3. Data collection
We collected the baseline data using a specifically designed case record form for the study. The recorded information included age, gender, comorbidities, length of hospital stay, severity of COVID-19, duration and type of antibiotic therapy, supportive therapy, and ventilatory support. Additionally, we collected biochemical and inflammatory parameters such as C-reactive protein (CRP), ferritin, lactate dehydrogenase (LDH), and D-dimer. Microbiological data, imaging, and outcomes of the patient were also collected.
2.4. Outcomes
The primary objective of this study was to analyze the patterns and predictors of early empirical antibiotic therapy in COVID-19 patients. Early empirical antibiotic therapy was defined as the initiation of any intravenous or oral antibacterial treatment within 48 hours of hospital admission. Antibacterial therapy administered after 48 hours was considered separate from empirical therapy, as it might have been intended for hospital-onset infections or secondary bacterial infections.
Azithromycin was not included as antibacterial therapy as it was also utilized as a COVID-19 specific therapy during the pandemic (Citation21). Secondary outcomes of interest included the total duration of the antibiotic therapy, differences in antibiotic consumption pattern based on severity of COVID-19 and patterns of microbiologically confirmed infections. Furthermore, associated outcomes such as length of hospital stay, days of invasive ventilation, and 45-day all cause in-hospital mortality were also analyzed.
2.5. Statistical analysis
Data analysis was performed using Microsoft Excel and EZR v1.56, an openly available statistical software based on R and R commander (Citation22). Descriptive statistics such as percentage and medians with inter-quartile range (IQR) were used to characterize the groups. Categorical data were analyzed with a Chi-square test (p < 0.05). Continuous data were analyzed using the Kolmogorov–Smirnov and Shapiro–Wilk test to check the normality. Continuous data were analyzed using Mann–Whitney U-test and tested for significance for p-value less than 0.05. A predictor model was developed by considering the possible factors based on literature and clinical inputs, and the initiation of empirical therapy was assessed. A univariable analysis of all the associated factors was initially performed at a significance of p < 0.2, and a multivariable logistic regression model was developed from them at a significance of p < 0.05. The receiver operating characteristic (ROC) curve was developed to determine the goodness of fit for the multivariable model.
3. Results
We screened a total of 900 COVID-19 patients admitted to the hospital during the study period. Among them, 375 patients were excluded from the analysis due to mild presentation of COVID, hospital admission of less than 24 hours or antibiotics being initiated for causes other than COVID-related manifestations. The remaining 525 patients were included in the final analysis ().
Table 1. Characteristics of the study population – demographic, clinical, laboratory, and treatment details (n = 525).
3.1. Clinical and demographic details
The study population had a median age of 60 (IQR: 48–68) years, with males being the most predominant (66.9%). The patients in whom empirical therapy was initiated, the median age was 60 (49–68) years, while for those in whom empirical therapy was not initiated, the median age was 55 (45–68) years.
The major comorbid conditions observed were diabetes [n = 284 (54.0%)], hypertension [n = 258 (49.0%)] and cardiovascular diseases [n = 106 (20.1%)]. The most common presenting symptoms were fever [n = 360 (68.4%)], cough [n = 341 (64.9%)] and shortness of breath (SOB) [n = 356 (67.8%)]. Acute Respiratory Distress Syndrome (ARDS) was recorded in 27.0% (n = 142) of the patients ().
In our study, a significant proportion of patients [n = 480 (91.4%)] received at least one course of antibiotics during their hospital stay. Among them, 83.8% (n = 440) were initiated on empirical therapy, while 7.6% (n = 40) and 8.6% (n = 45) were initiated on antibiotics at a later stage or not initiated on antibiotics, respectively. The patients with severe manifestations of COVID-19 [n = 359 (68.4%)] were more likely to be prescribed empirical antibiotic therapy [OR: 3.34 (CI: 2.07–5.37) (p < 0.001)]. Details on other comorbidities, presenting symptoms, radiographic and laboratory findings on admission and co-medications are shown in .
3.2. Predictors of initiation of empirical therapy
Univariable analysis revealed that patients initiated on empirical antibiotics had higher levels of absolute neutrophil count [OR: 1.08 (95% CI: 1.02–1.13) (p = 0.001)], D-dimer [OR: 1.13 (95% CI: 1.02–1.25) (p = 0.004)], LDH [OR: 1.002 (95% CI: 1.001–1.003) (p = <0.001)], ferritin [OR: 1.0006 (95% CI: 1.0002–1.001) (p < 0.001)] and WBC count [OR: 1.0000 (95% CI: 1.0000–1.0001) (p = 0.001)] on initial presentation than those who were not initiated on antibiotics (). Additionally, 189 (36.0%) patients who underwent invasive ventilation were more likely to be prescribed empirical antibiotics than those who were not [n = 336 (64.0%)] [OR: 5.83 (95% CI: 2.81–13.58) p < 0.001].
Multivariable logistic regression revealed that patients who had an elevated procalcitonin value at the time of admission were 3.91 times [(95% CI: 1.66–9.16) (p = 0.001)] more likely to be prescribed empirical antibiotics. Similarly, invasive ventilation was also a significant factor for empirical therapy initiation [OR: 3.93 (95% CI: 1.70–9.09) (p = 0.001)]. Patients presenting with SOB [OR: 2.25 (95% CI: 1.30–3.89) (p = 0.003)] and higher CRP level [OR: 1.01 (95% CI: 1.00–1.01) (p = 0.005)] were also likely to be prescribed empirical antibiotic therapy (). Among those initiated on empirical antibiotics, 29.1% (n = 128) of the patients were stopped within 5 days as part of de-escalation measures. The parameters considered for the multivariable regression model have been shown in the Supplementary Table S1. The ROC curve for the model is depicted in Supplementary Figure S1.
Table 2. Predictors for initiation of empirical therapy.
3.3. Microbiologically confirmed infections
Culture testing was performed in about 87.0% of the admitted patients. Among those, 125 (23.8%) patients had a microbiologically confirmed infection, with 95 (76.0%) of them having a secondary bacterial infection. Multiple infections during hospitalization were present in 23 (4.4%) patients. The most isolated infections were from blood [n = 64 (51.2%)], endotracheal aspirate [n = 53 (42.4%)] and sputum [n = 17 (13.6%)] (Supplementary Table S2). Among those initiated on empirical therapy, 25.0% of the patients (n = 110/440) had a microbiologically confirmed bacterial infection, while 17.6% (n = 17/85) of those not initiated on empirical antibiotics had bacterial infection.
The most prevalent organisms isolated from the culture were A. baumannii [n = 54 (36.5%)], K. pneumoniae [n = 38 (25.7%)], Escherichia coli [n = 15 (10.1%)], and Pseudomonas aeruginosa [n = 9 (6.1%)]. The sensitivity patterns of A. baumannii revealed that 15 (23.8%) of the isolates were sensitive to tigecycline alone. Alarmingly, 21 (33.3%) of isolates showed resistance to all tested antibiotics, indicating the high burden of AMR. Among patients who were initiated on empirical antibiotics, A. baumannii [n = 54 (41.0%)] and K. pneumoniae [n = 32 (24.4%)] were the most prevalent isolated organisms. Whereas among the non-empirical antibiotic group, K. pneumoniae [n = 6 (37.5%)] and E. coli [n = 5 (31.0%)] were the most prevalent. The patterns of all organisms isolated are depicted in Supplementary Figure S2.
3.4. Antibiotic utilization patterns
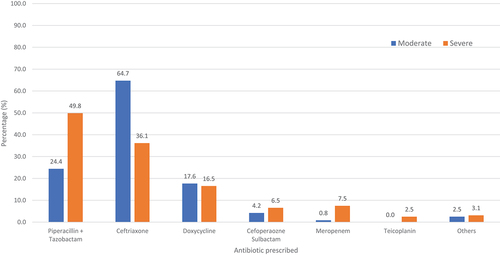
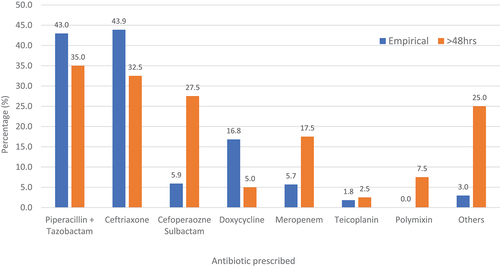
The most empirically prescribed antibiotics were ceftriaxone [n = 193 (43.9%)] and piperacillin-tazobactam [n = 189 (43.0%)] followed by doxycycline [n = 74 (16.8%)] and cefoperazone-sulbactam [n = 26 (5.9%)]. Ceftriaxone was the most empirically prescribed antibiotic in those with moderate severity of COVID-19 [n = 77 (64.7%)], while the most empirically prescribed antibiotic in patients with severe COVID-19 was piperacillin-tazobactam [n = 160 (49.8%)] (). In patients who were initiated on antibiotics after 48 hours, piperacillin-tazobactam was the most prescribed [n = 14 (35.0%)] followed by ceftriaxone [n = 13 (32.5%)] and cefoperazone-sulbactam [n = 11 (27.5%)] ().
Based on the WHO AWaRe criteria, most antibiotics prescribed were from the ‘Watch’ group [n = 677 (65.9%)] and the least from the ‘Reserve’ group [n = 102 (9.9%)]. The ‘Access group’ and ‘Not recommended’ group comprised 12.4% (n = 128) and 11.7% (n = 120), respectively. Polymyxin B was the most utilized antibiotic from the ‘Reserve’ group [n = 62 (18.0%)], mainly used in severe manifestation of COVID-19. Cefoperazone-sulbactam [n = 101 (21.0%)] and meropenem-sulbactam [n = 18 (3.8%)] which falls under ‘Not recommended’ category of WHO was also prescribed, mostly in the severe group ().
Table 3. Prescription pattern of antibiotics based on WHO AWaRe criteria.
3.5. Clinical and treatment outcomes
The median duration of antibiotic therapy among the study patients was 7 days (IQR: 5–12) with higher days of antibiotic therapy in the empirical group than those not initiated on empirical antibiotics [OR 1.09 (95% CI: 1.01–1.18) (p = 0.04)] (). Furthermore, patients with severe manifestations of COVID-19 had significantly higher days of antibiotic therapy compared to those with moderate disease [OR 1.12 (95% CI: 1.07–1.17) (p < 0.001)] (Supplementary Table S3). However, there were no significant differences observed in length of stay, invasive ventilation days, or the development of microbiologically confirmed bacterial infection between patients initiated and not initiated on empirical antibiotic therapy ().
Table 4. Association of empirical therapy with outcomes.
Severe COVID-19 cases had an increased length of stay with a median of 12 days (IQR: 8–17) [OR 1.09 (95% CI: 1.05–1.13) (p < 0.001)]. These patients also had a higher prescription rate of steroids and anticoagulants, with a median duration of use of 8 (6–10) days [OR 1.15 (95% CI: 1.08–1.23)] and 11 (7–15) days [OR 1.12 (95% CI: 1.07–1.17)], respectively (p < 0.001). Additionally, there was an increased occurrence of microbiologically confirmed secondary infections [OR: 3.16 (95% CI: 1.71–6.24) (p < 0.001)] (Supplementary Table S3). Notably, a higher mortality rate was also observed among patients with severe COVID-19 [OR: 61.43 (95% CI: 20.02–309.13) (p < 0.001)].
4. Discussion
The COVID-19 pandemic has led to an increase in the global antibiotic utilization (Citation23), thereby causing an unprecedented impact on the rise of AMR and associated mortality, especially in LMICs (Citation24). This study was carried out at a major Indian tertiary care center and assessed the usage of empirical antibiotics in patients with moderate and severe COVID-19 infection. It also assessed the occurrence of bacterial infections among hospitalized COVID-19 patients during the second wave of the pandemic in India. In our study, despite the high utilization of antibiotics, a relatively low rate of secondary bacterial infections was observed. Among these, the majority of infections identified were secondary in nature, having drug-resistant organisms, hence indicating a significant burden of AMR.
The study found that empirical therapy initiation was more common among older individuals and predominantly among males which is consistent with previous studies on hospitalized COVID-19 patients (Citation15,Citation25,Citation26). Approximately one-fourth of the study population was presented with ARDS, especially among those with severe COVID-19 manifestations. Invasive ventilation was undertaken in 36.0% of the admitted patients, and the majority of them were initiated on empirical therapy. Invasive ventilation has been identified as a predictor for the initiation of empirical antibiotics in previous studies and guidelines (Citation6,Citation25). In addition, higher CRP values, patients experiencing SOB and elevated procalcitonin levels were identified as predictors for initiating empirical anti-bacterial therapy. These findings are consistent with previous studies (Citation16,Citation27) which reported CRP and procalcitonin as predictors for the initiation of empirical antibiotic therapy. However, there is conflicting evidence on using these markers as a guide for empirical antibiotic therapy in COVID-19 (Citation28,Citation29).
Antibiotics were prescribed in more than 90.0% of the patients, while only a quarter had a confirmed bacterial infection. Secondary infections developed in around 18.0% of the patients admitted to our hospital. The rates of bacterial and secondary infections were higher in the study than many studies which reported an overall bacterial infection rate of approximately 7.0% (Citation9,Citation25,Citation30–33). Baghdadi et al. (Citation34) reported a rate of 21.7% of bacterial infection, like our findings. However, the rate of secondary infections was nearly 4.5 times higher in our study. In the Indian context, an infection rate of 3.9–13.0% for secondary infections has been reported (Citation15,Citation17). The high rate of bacterial infections in our patients could be attributed to our study focusing on moderate-severe cases treated in a tertiary care hospital setting. Additionally, a higher proportion of microbiologically confirmed infections were observed in the severe group (28.9%) compared to the moderate group (12.7%). This may be due to the dysregulated immune system in severe COVID-19 patients making them prone to secondary bacterial infection (Citation35).
Bacteremia and respiratory tract were the most common sites of infection, consistent with studies that observed positive blood cultures followed by respiratory cultures to be most reported (Citation36,Citation37). Alarmingly, around 35.0% of A. baumannii isolates were multi-drug resistant (MDR). Our findings align with studies (Citation15,Citation17,Citation38) that emphasize the high prevalence of MDR and extended-drug resistant (XDR) Acinetobacter in India and their association with higher mortality. A higher proportion of resistant organisms were observed among those initiated on empirical therapy. This may be due to the potential of early initiation of empirical therapy contributing to infection with drug-resistant strains, though a causal relationship cannot be established.
Our study revealed that despite the lack of evidence supporting the use of broad-spectrum antibiotics in COVID-19 patients, many hospitalized patients were still prescribed these antibiotics. Ceftriaxone and piperacillin-tazobactam were the most prescribed empirical antibiotics in our study, consistent with prescribing patterns observed in other studies conducted in India (Citation15,Citation16). Among all prescribed antibiotics (inclusive of empirical and non-empirical therapy), the most prescribed belonged to the ‘Watch’ group. There was also significant utilization of antibiotics from the ‘Not recommended’ group. Additionally, ‘Reserve’ group antibiotics, such as polymyxin B, were used, particularly in those with severe COVID-19. Similarly, high antibiotic usage from the ‘Watch’ and ‘Reserve’ groups have been reported in Vietnamese and Indian studies (Citation15,Citation39). This emphasizes the importance of monitoring antibiotic consumption and strict implementation of antimicrobial stewardship programs to ensure the appropriate and rational use of antibiotics.
It is worth noting that empirical antibiotic therapy was discontinued in one-third of the patients within 5 days of initiation, which may indicate good antimicrobial stewardship practices at our center. However, rational prescribing still has a long way to go as evidenced by a study that reported only 37.9% of the study cohort being prescribed empirical antibiotics due to proper implementation of antimicrobial stewardship programs (Citation40).
The median duration of hospitalization did not differ between patients initiated on empirical therapy and those who were not. This suggests that anti-bacterial therapy may not have an impact on the hospital stay and that judicious use of empirical therapy can lead to fewer hospitalization days (Citation4). However, differences in outcome parameters were observed between the moderate and severe groups, which may be attributed to the nature of the disease and the pattern of prescribing more antibiotics in severe cases of COVID-19. Higher mortality rates were associated with those initiated on empirical therapy but that may be due to more severe cases being prescribed antibiotics and a causal relation cannot be established in this study.
The study has few limitations, primarily being a single-center study. Although efforts were made to address various factors influencing antibiotic decision-making, there may be some variables that we could not account for due to the retrospective nature of the study. A prospective study is warranted to better understand those factors. Another limitation is that most of the patients in our study received antibiotics empirically, thus making it difficult to distinguish characteristics between the two groups. Additionally, antibiotics prescribed at referral centers were not available.
5. Conclusion
In conclusion, the study evaluated the predictors and patterns of antibiotic usage and the associated bacterial infection in hospitalized COVID patients during the second wave of the pandemic. The findings underscore the importance of practicing culture-directed therapy and implementing stringent antimicrobial stewardship programs. Rational prescribing practices, including prioritizing medications from the ‘Access’ category of the AWaRe classification, are crucial to prevent the development of antibiotic-resistant bacteria and ensure effective antibiotic use in the future. Therefore, it is important to implement stringent antimicrobial stewardship programs, establish protocols for empirical antibiotic treatment, and adhere to them to prevent future challenges associated with resistant bacteria.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Ethics statement
Ethical approval was obtained from the Institutional Ethics Committee (IEC) of Kasturba Medical College and Kasturba Hospital (KMC&KH), Manipal (Reference No: IEC 693/2021, dated 8 January 2022). The study was registered in the Clinical Trial Registry of India (CTRI), Reg No: CTRI/2022/01/039784.
Author contribution statement
Lipin Lukose, Gursimran Kaur, Kanav Khera, Viswam K Subeesh, Ronald L Castelino, and Sonal Sekhar Miraj conceptualized the study. Lipin Lukose and Gursimran Kaur collected the data. Gail Ann Abraham, Mohammed Asif M, Sonal Sekhar Miraj, and Shubhada Karanth cured the data. Lipin Lukose, Gursimran Kaur, and Viswam K Subeesh analyzed the data. Lipin Lukose, Gursimran Kaur, and Sonal Sekhar Miraj wrote the manuscript. Viswam K Subeesh, Kanav Khera, Ronald L Castelino, Shubhada Karanth, Chandrashekar Udyavara Kudru, Muralidhar Varma, and Sonal Sekhar Miraj critically evaluated the manuscript. All the authors participated in the manuscript review and approved the final draft of the revised manuscript. All authors agreed on the submission of the manuscript to the journal. All authors had access to the study data and took responsibility for the integrity of the data and the accuracy of the data analysis.
Supplemental Material
Download MS Word (21 KB)Supplemental Material
Download MS Word (250.6 KB)Acknowledgments
The authors gratefully acknowledge the help and facilities provided by Manipal College of Pharmaceutical Sciences, Kasturba Hospital, Manipal, Manipal Center for Infectious Diseases and Manipal Academy of Higher Education, Manipal, India.
Declaration of Interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Data availability statement
The dataset used or analyzed during the current study available from the corresponding author on reasonable request.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/14787210.2024.2303019
Additional information
Funding
References
- Lenharo M WHO declares end to COVID-19’s emergency phase. Nature News Article [Internet]. 2023 May 05 [cited 2023 May 25]. Available from: https://www.nature.com/articles/d41586-023-01559-z
- Mygov.in [Internet] India: Government of India; [cited 2023 Jun 19]. Available from: https://www.mygov.in/covid-19/
- Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet (London, England). 2020;395(10229):1054–1062. doi: 10.1016/s0140-6736(20)30566-3
- Pettit NN, Nguyen CT, Lew AK, et al. Reducing the use of empiric antibiotic therapy in COVID-19 on hospital admission. BMC Infect Dis. 2021;21(1):516. doi: 10.1186/s12879-021-06219-z
- Garcia-Vidal C, Sanjuan G, Moreno-García E, et al. Incidence of co-infections and superinfections in hospitalized patients with COVID-19: a retrospective cohort study. Clin Microbiol Infect. 2021;27(1):83–88. doi: 10.1016/j.cmi.2020.07.041
- Alhazzani W, Møller MH, Arabi YM, et al. Surviving sepsis campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Intensive care Med. 2020;46(5):854–887. doi: 10.1007/s00134-020-06022-5
- Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. Bethesda (MD): National Institutes of Health (US); 2021 [cited 2023 May 30]. Available from: https://files.covid19treatmentguidelines.nih.gov/guidelines/covid19treatmentguidelines.pdf
- WHO. Clinical Management Of COVID-19: Living Guideline. Geneva (CH): World Health Organisation; 2023 Jan 13 [cited 2023 May 30]. Available from: https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2023.1
- Langford BJ, So M, Raybardhan S, et al. Bacterial co-infection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis. Clin Microbiol Infect. 2020;26(12):1622–1629. doi: 10.1016/j.cmi.2020.07.016
- Thapa B, Pathak SB, Jha N, et al. Antibiotics use in hospitalised COVID-19 patients in a tertiary care centre: a descriptive cross-sectional study. JNMA J Nepal Med Assoc. 2022;60(251):625–630. doi: 10.31729/jnma.7394
- Spigaglia P. COVID-19 and clostridioides difficile infection (CDI): possible implications for elderly patients. Anaerobe. 2020;64:102233. doi: 10.1016/j.anaerobe.2020.102233
- Llor C, Ouchi D, Giner-Soriano M, et al. Correlation between previous antibiotic exposure and COVID-19 severity. A population-based cohort study. Antibiotics. 2021;10(11):1364. doi: 10.3390/antibiotics10111364
- Rawson TM, Moore LSP, Castro-Sanchez E, et al. COVID-19 and the potential long-term impact on antimicrobial resistance. J Antimicrob Chemother. 2020;75(7):1681–1684. doi: 10.1093/jac/dkaa194
- Huttner BD, Catho G, Pano-Pardo JR, et al. COVID-19: don’t neglect antimicrobial stewardship principles! Clinical microbiology and infection! Clin Microbiol Infect. 2020;26(7):808–810.
- Vijay S, Bansal N, Rao BK, et al. Secondary infections in hospitalized COVID-19 patients: Indian experience. Infect Drug Resist. 2021;14:1893–1903. doi: 10.2147/idr.S299774
- Chindhalore CA, SVG GND, Gajbhiye SV, et al. Prescription pattern for antimicrobials and the potential predictors for antibiotics among patients with COVID-19: a retrospective observational study. J Clin Diagnostic Res. 2022;16(9):FC15–FC19.
- Khurana S, Singh P, Sharad N, et al. Profile of co-infections & secondary infections in COVID-19 patients at a dedicated COVID-19 facility of a tertiary care Indian hospital: implication on antimicrobial resistance. Indian J Med Microbiol. 2021;39(2):147–153. doi: 10.1016/j.ijmmb.2020.10.014
- Clinical guidelines for management of COVID-19 patients [Internet]. India: AIIMS/ICMR-COVID-19 National Task Force/Joint Monitoring Group; [cited 2023 May 30]. Available from: https://www.mohfw.gov.in/pdf/ClinicalGuidanceforManagementofAdultCOVID19Patientsupdatedason05thjan2023.pdf
- WHO: Anatomic Therapeutic Chemical (ATC) Classification. Geneva (CH): World Health Organization; [cited 2023 May 30]. Available from: https://www.who.int/tools/atc-ddd-toolkit/atc-classification
- WHO: 2021 AWaRe classification. Geneva (CH): World Health Organization; [cited 2023 May 30]. Available from: https://www.who.int/publications/i/item/2021-aware-classification
- Gyselinck I, Janssens W, Verhamme P, et al. Rationale for azithromycin in COVID-19: an overview of existing evidence. BMJ Open Respir Res. 2021;8(1):e000806. doi: 10.1136/bmjresp-2020-000806
- Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48(3):452–8. doi: 10.1038/bmt.2012.244
- Nandi A, Pecetta S, Bloom DE. Global antibiotic use during the COVID-19 pandemic: analysis of pharmaceutical sales data from 71 countries, 2020-2022. EClinicalMedicine. 2023;57:101848. doi: 10.1016/j.eclinm.2023.101848
- Rizvi SG, Ahammad SZ. COVID-19 and antimicrobial resistance: A cross-study. Sci Total Environ. 2022;807(Pt 2):150873. doi: 10.1016/j.scitotenv.2021.150873
- Vaughn VM, Gandhi TN, Petty LA, et al. Empiric antibacterial therapy and community-onset bacterial coinfection in patients hospitalized with coronavirus disease 2019 (COVID-19): a multi-hospital cohort study. Clin Infect Dis. 2021;72(10):e533–e541. doi: 10.1093/cid/ciaa1239
- Garg S, Kim L, Whitaker M, et al. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019 - COVID-NET, 14 states, March 1-30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(15):458–464.
- Schuetz P, Albrich W, Mueller B. Procalcitonin for diagnosis of infection and guide to antibiotic decisions: past, present and future. BMC Med. 2011;9(1):107. doi: 10.1186/1741-7015-9-107
- May M, Chang M, Dietz D, et al. Limited utility of procalcitonin in identifying community-associated bacterial infections in patients presenting with coronavirus disease 2019. Antimicrob Agents Chemother. 2021;65(4). doi: 10.1128/aac.02167-20
- van Berkel M, Kox M, Frenzel T, et al. Biomarkers for antimicrobial stewardship: a reappraisal in COVID-19 times? Crit Care. 2020;24(1):600.
- Ahava MJ, Kortela E, Forsblom E, et al. Low incidence of severe bacterial infections in hospitalised patients with COVID-19: a population-based registry study. Infect Dis. 2023;55(2):132–141.
- Townsend L, Hughes G, Kerr C, et al. Bacterial pneumonia coinfection and antimicrobial therapy duration in SARS-CoV-2 (COVID-19) infection. JAC Antimicrob Resist. 2020;2(3):dlaa071. doi: 10.1093/jacamr/dlaa071
- Rawson TM, Moore LSP, Zhu N, et al. Bacterial and Fungal Coinfection in individuals with coronavirus: a rapid review to support COVID-19 antimicrobial prescribing. Clin Infect Dis. 2020;71(9):2459–2468. doi: 10.1093/cid/ciaa530
- Lansbury L, Lim B, Baskaran V, et al. Co-infections in people with COVID-19: a systematic review and meta-analysis. J Infect. 2020;81(2):266–275. doi: 10.1016/j.jinf.2020.05.046
- Baghdadi JD, Coffey KC, Adediran T, et al. Antibiotic use and bacterial infection among inpatients in the first wave of COVID-19: a retrospective cohort study of 64,691 patients. Antimicrob Agents Chemother. 2021;65(11):e0134121. doi: 10.1128/aac.01341-21
- Nag VL, Kaur N. Superinfections in COVID-19 patients: role of antimicrobials. Dubai Med J. 2021;4(2):81–90. doi: 10.1159/000515067
- Moreno-Torres V, de Mendoza C, de la Fuente S, et al. Bacterial infections in patients hospitalized with COVID-19. Intern Emerg Med. 2022;17(2):431–438. doi: 10.1007/s11739-021-02824-7
- Shah MM, Patel K, Milucky J, et al. Bacterial and viral infections among adults hospitalized with COVID-19, COVID-NET, 14 states, March 2020-April 2022. Influenza Other Respir Viruses. 2023;17(3):e13107.
- Gandra S, Tseng KK, Arora A, et al. The mortality burden of multidrug-resistant pathogens in India: a retrospective, observational study. Clin Infect Dis. 2019;69(4):563–570. doi: 10.1093/cid/ciy955
- Dat VQ, Dat TT, Hieu VQ, et al. Antibiotic use for empirical therapy in the critical care units in primary and secondary hospitals in Vietnam: a multicenter cross-sectional study. Lancet Reg Health West Pac. 2022;18:100306. doi: 10.1016/j.lanwpc.2021.100306
- Stevens RW, Jensen K, Kooda K, et al. A retrospective antibiotic prescribing assessment and examination of potential antibiotic stewardship targets in patients with COVID-19. JAC Antimicrob Resist. 2021;3(4):dlab170. doi: 10.1093/jacamr/dlab170