ABSTRACT
Background
Invasive fungal infections (IFI) are associated with significant morbidity and mortality. The objective of this work was to compare the costs per adult patient, associated with intravenous isavuconazole (ISAV) followed by oral ISAV versus the regimen of liposomal amphotericin B followed by posaconazole (L-AMB→POSA) in the treatment of IFI. The comparison was conducted from the perspective of the Spanish National Health System (SNS).
Methods
As indirect comparisons have demonstrated similar efficacy between the comparators, a cost-minimization approach was taken. Drug acquisition, administration, hospitalization, laboratory tests and adverse events costs were evaluated from SNS perspective. Deterministic and probabilistic sensitivity analyzes were performed.
Results
Total costs per-patient were €24,715.54 with ISAV versus €29,753.53 with L-AMB→POSA, resulting in cost-savings per patient treated with ISAV of €5,037.99 (−16.9%). Treatment costs of IFI remained lower for ISAV than for L-AMB→POSA across all sensitivity analyses (−7,968.89€ to −326.59€), being treatment duration the most influential parameter.
Conclusion
According to the present model, the treatment of IFIs with ISAV would generate savings for the SNS compared to L-AMB→POSA. These savings are attributed to the shorter duration of IV treatment, reduced use of healthcare resources and lower costs associated with managing adverse effects when ISAV was employed.
1. Introduction
Invasive fungal infections (IFIs) are opportunistic infections primarily affecting immunocompromised patients and critically ill patients, leading to high morbidity and mortality [Citation1,Citation2]. Invasive aspergillosis (IA) and mucormycosis are two IFIs that, despite their low incidence, are emerging as global concern [Citation3,Citation4]. The World Health Organization (WHO) has classified Aspergillus fumigatus and Mucorales order fungi that cause mucormycosis as critical and high-priority pathogens, respectively, based on criteria like antifungal resistance, mortality rates, and the availability of effective treatments [Citation5]. Isavuconazole (ISAV), granted orphan drug status, was the most recent antifungal approved in Europe in 2015 for treating IA and mucormycosis in adult patients for whom amphotericin B is not a suitable option [Citation6–8]. The efficacy and safety of ISAV were confirmed in two phase III clinical trials. The SECURE study in patients with IA concluded that the efficacy of ISAV was not inferior to voriconazole (VORI) [Citation9]. Furthermore, the VITAL study [Citation10], a case-control analysis in patients with mucormycosis, found that the efficacy of ISAV was similar to that of amphotericin B [Citation6–8]. Similarly, POSA new formulations (delayed-release tablets and intravenous) have shown to have similar efficacy than L-AMB in a matched-cohort study [Citation11]. Two meta-analyses assessed the efficacy of ISAV compared to other antifungals. A direct comparison meta-analysis concluded that ISAV’s efficacy was comparable to L-AMB and VORI in the treatment of IA [Citation12]. A network meta-analysis found that ISAV’s efficacy was not inferior to the control group (amphotericin B, VORI, and POSA) with significantly fewer drug-related adverse events and interruptions [Citation13].
Additionally, ISAV exhibits clinical features, like linear pharmacokinetics, a low profile of drug interactions, and a favorable safety profile (lacking nephrotoxicity) [Citation14], making it an attractive therapeutic option for critically ill, polypharmacy patients, or those with renal insufficiency. It can also serve as a second-line treatment after toxicity (hepatic, renal, and neurological) or failure of another azole [Citation15].
The 2017 guidelines from ESCMID-ECMM-ERS (European Societies of Clinical Microbiology and Infectious Diseases, Medical Mycology, and Respiratory) recommend ISAV and voriconazole as the first-line drugs for treating for pulmonary aspergillosis in neutropenic or allogenic hematopoietic stem cells transplantation (allo-HSCT) patients [Citation16]. In 2020, COVID-19-associated pulmonary aspergillosis (CAPA) was identified, and a document from ECMM/ISHAM (European Confederation of Medical Mycology and International Society for Human and Animal Mycology) also recommended ISAV or voriconazole as primary treatment [Citation17]. In both documents, amphotericin B is listed as an alternative treatment for pulmonary IA [Citation16,Citation17]. Regarding mucormycosis, the ECMM guideline recommends high-dose liposomal amphotericin B as the first-line treatment, with ISAV and POSA as alternative treatments [Citation18].
However, in decision-making, especially within antibiotic stewardship teams (Programs for the Optimization of Antibiotic Use), the choice between treatment alternatives should be based on efficiency criteria to ensure the use of cost-effective treatments [Citation8,Citation19]. Since no differences in efficacy between ISAV and L-AMB have been demonstrated, according to available meta-analyses [Citation12,Citation13] and the VITAL clinical trial [Citation10], a cost-effectiveness analysis may not be warranted; instead, a cost-minimization analysis may be appropriate [Citation20].
A United Kingdom (UK) study compared the economic impact of treating IFIs and concluded that intravenous ISAV followed by oral ISAV has the potential to reduce costs per patient compared to intravenous L-AMB followed by oral POSA (L-AMB/POSA) [Citation20]. However, there are no economic studies comparing ISAV to L-AMB in Spain.
The aim of this study was to compare the costs per adult patient associated with intravenous ISAV followed by oral ISAV versus L-AMB/POSA in the treatment of IFIs from the perspective of the Spanish National Health System (SNS).
2. Methods
2.1. Economic model
Assuming no discernible differences in efficacy between ISAV and L-AMB/POSA in the treatment of IFIs, as supported by available meta-analyses [Citation12,Citation13] and the VITAL clinical trial [Citation10], a cost-minimization analysis was conducted using a methodology similar to a previously published UK model [Citation21]. The analysis was conducted from the perspective of the SNS and assessed the costs associated with each patient’s complete IFI treatment. The following direct healthcare costs were taken into consideration: (i) pharmacological treatment; (ii) medication administration; (iii) laboratory tests; (iv) patient hospitalization; and (v) adverse effects of pharmacological treatment [Citation21]. The time horizon extended to the completion of the full IFI treatment, either due to infection resolution or patient death. provides an overview of resource utilization and average unit costs of these resources. All costs were adjusted for inflation to €2023.
Table 1. Unit costs (€ 2023) and resource use considered in the model, validated by the panel of clinical experts.
2.2. Drug acquisition costs
Acquisition costs encompassed ISAV, L-AMB, and POSA. These costs were estimated from the laboratory’s reported sale prices (PVL) [Citation22] (). The treatment regimens are summarized in . ISAV dosing was assumed as per its technical data sheets [Citation6,Citation7]: an initial loading dose of 3 units of 200 mg intravenous (IV) or oral, followed by a daily dose of 200 mg IV or 2 daily capsules of 100 mg. According to the consensus of an expert panel, it was estimated that 85% of patients started treatment with IV ISAV, and 15% with oral ISAV. The duration of IV treatment was assumed to be 8.1 days based on the SECURE study [Citation9,Citation21,Citation23] ().
Figure 1. Pharmacological treatment regimes.
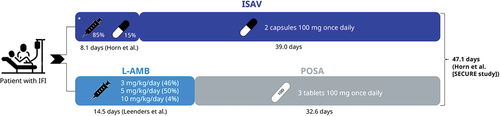
For IV treatment with L-AMB, it was assumed that 46% received 3 mg/kg, 50% received 5 mg/kg, and 4% received 10 mg/kg, following the opinion of the expert panel, aligning with the dose range used in the L-AMB study [Citation10]. The median body weight was presumed to be 68.6 kg, as per the SECURE study [Citation9]. The assumed duration of L-AMB treatment was 14.5 days, based on the study by Leenders, et al. [Citation24]. According to the technical data sheet for POSA, patients were administered 3 daily tablets of 100 mg (300 mg/day) orally, with a loading dose of 600 mg on day 1 [Citation21,Citation25]. The total treatment duration was assumed to be the same in both treatment arms, estimated based on the total mean duration with ISAV in the SECURE study, which was 47.1 days [Citation9] ().
We decided not to include the oral suspension and intravenous infusion formulations of POSA in the model for different reasons. POSA oral suspension was not considered because it is currently marginally used in adults due to bad absorption and pharmacokinetic problems [Citation26]. Regarding IV formulation, we did not include it because our goal was to focus on the use of POSA as oral step-down from L-AMB. Besides this use, POSA in Spain is rarely used as a first line antifungal treatment for IA in patients that need IV therapy. To conclude, POSA IV is mostly used as IFI prophylaxis in hematological patients, which would require a completely different methodology to analyze costs.
2.3. Administration and monitoring
The model included the cost of IV infusion preparation, as well as patient monitoring through appropriate laboratory tests [Citation21,Citation27]. The time required for IV infusion preparation, as estimated by the expert panel and the UK economic study, was 10 and 30 minutes for ISAV and L-AMB, respectively. Based on the IV administration duration (8.1 days for ISAV and 14.5 days for L-AMB) and dosing guidelines for both drugs [Citation6,Citation7,Citation24], IV administration for ISAV was estimated to take 121 minutes ((10 minutes * 6 loading infusions) + (10 minutes * 6.1 maintenance infusions)), and for L-AMB, it was estimated to take 435 minutes (14.5 days * 1 daily infusion *30 minutes). The cost of 1 minute of IV infusion preparation by nursing was estimated at €1.07, based on a previously published Spanish study [Citation28]. The frequency of laboratory tests (urinalysis, serum creatinine, blood magnesium, liver function) was obtained from the UK study and validated by Spanish experts. The unit costs of the tests were obtained from a previously published Spanish study [Citation27] ().
2.4. Hospitalizations
The cost of hospitalization was calculated by multiplying the duration of hospitalization by the respective daily cost [Citation21]. The same duration of hospitalization was applied to both treatments (ISAV and L-AMB/POSA), specifically, the duration observed in the SECURE study for ISAV since there was no available data for L-AMB/POSA [Citation21] for a conservative approach. Therefore, this variable’s value remained the same for both treatments (19.7 days). It was estimated that 15.6% of hospitalization days took place in emergency rooms [Citation29], with the remaining days on the general ward (16.6 ward days, 3.1 emergency room days). The unit costs of general ward or emergency room admission were calculated as averages of the public healthcare prices of the autonomous communities [Citation30–33].
2.5. Adverse effects
The frequencies of adverse effects associated with the compared treatments were sourced from medical literature [Citation25,Citation34,Citation35]. The unit costs for adverse effects were drawn from a Spanish study [Citation27] and regional public healthcare prices.
2.6. Presentation of results
Three analyses were performed:
A deterministic base case analysis employing mean values for all variables.
A deterministic univariate sensitivity analysis, which considered minimum and maximum values or standard deviations when mean values were available. Variables under examination included those used to calculate the cost of treatment: medication acquisition costs, the percentage of patients receiving IV ISAV as initial treatment, total treatment duration, IV treatment duration, average body weight, and L-AMB dosing. No others variables were considered due to their low impact on the result.
A probabilistic analysis using second-order Monte Carlo simulations [Citation36] to assess uncertainty and variability applying a range of ±20% in the study costs and calculating 95% confidence intervals (CI) for mean results [Citation36]. In this analysis, one thousand simulations were performed using gamma distributions for costs. The alpha and beta parameters of the gamma distributions, representing the slope and intersection with the curve axis, respectively, were obtained from the means and standard deviations of each variable using the method of moments [Citation37]. In the 1,000 simulations (akin to a hypothetical cohort of 1,000 patients), random cost values from the model were taken according to the alpha and beta statistics of the gamma distributions. This methodology has been described previously [Citation37–39].
3. Results
3.1. Deterministic base case
and present the results of the deterministic analysis. Regarding pharmacological costs, ISAV demonstrated substantial savings of €4,029.25 (€8,195.50 - €12,224.75) compared to L-AMB/POSA. This was attributed to the shorter IV treatment duration with ISAV (8.1 days) compared to L-AMB/POSA (14.5 days), amounting to significant 33.0% reduction in costs.
Figure 2. Cost analysis results. Deterministic base case.
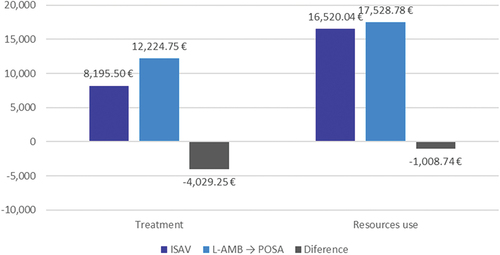
Table 2. Cost analysis results. Deterministic base case.
In terms of other healthcare costs, ISAV also led to savings in IV administration (€354.17), laboratory tests (€144.00), and the management of adverse effects (€510.56), with a total savings of €1,008.74 (€16,520.04 - €17,528.78). The reduction in these costs represented a saving of 5.8% of total costs ( , ). The reduction in IV administration costs were due to the shorter duration of ISAV IV. Additionally, ISAV exhibited fewer laboratory tests and incurred lower costs for managing adverse effects compared to L-AMB/POSA. Notably, hospitalization costs remain unchanged since both treatments (ISAV and L-AMB/POSA) assumed the same hospitalization duration.
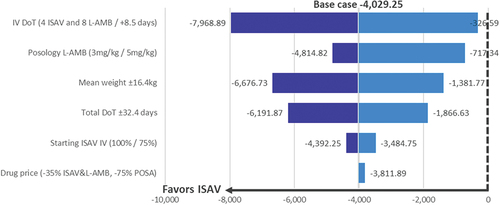
Figure 3. Cost analysis results. Univariate sensitivity analysis: tornado diagram. Difference ISAV vs L-AMB → POSA (MAX/MIN) (€).
The total cost per patient treated with ISAV amounted to €24,715.54, whereas with L-AMB/POSA, it reached €29,753.53. Consequently, the savings per patient treated with ISAV totaled €5,037.99, making a substantial 16.9% reduction in costs (, ).
3.2. Univariate sensitivity analysis
Across all conducted analyses, consistent cost savings were observed in the treatment with ISAV when compared to L-AMB/POSA. These savings ranged from a minimum of €326.59 to a maximum of €7,968.89 with ISAV, depending on the modified parameter. The parameter with the most significant impact on the outcome was the IV administration time, while the least influential parameter was the drug acquisition price ( , ).
Table 3. Cost analysis results. Univariate sensitivity analysis (€ 2023).
3.3. Probabilistic analysis
Following 1,000 second-order Monte Carlo simulations, the average total savings per patient treated with ISAV compared to L-AMB/POSA amounted to €5,029, with a 95% CI ranging from -€4,088 to -€6,107 (, ). Importantly, the probability of achieving savings with ISAV was 100%.
Figure 4. Cost analysis results. Probabilistic analysis.
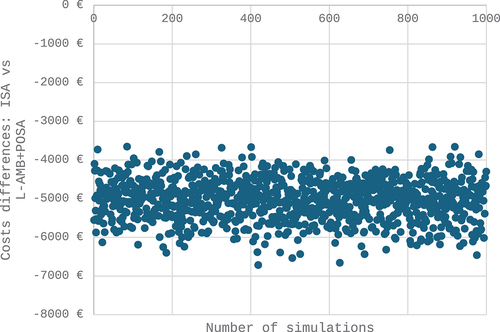
Table 4. Cost analysis results (€ 2023). Probabilistic analysis.
4. Discussion
The present study adapted a previous economic model from the United Kingdom [Citation21,Citation40]. According to the probabilistic analysis in our study, the savings per patient are €5,029 (95% CI: -€4,088 to -€6,107), and the probability of achieving savings is 100%. ISAV has the potential to yield substantial costs reductions in the management of IFIs when compared to L-AMB/POSA. This cost reduction would allow treating an additional 17 patients for every 100 treatments. This saving would not only be due to the shorter treatment duration of ISAV IV, which providing a cost savings of a significant 33.0%, but also to the lower number of laboratory tests or the lower frequency of adverse events ( and ), representing a 5.8% of savings, overall due to ISAV has no impact on nephrotoxicity and low impact on blood disorders comparing with L-AMB/POSA. The findings in this Spanish study are consistent with those obtained in the UK, both concerning mucormycosis [Citation40] and for overall IFIs [Citation21]. Furthermore, the high bioavailability of ISAV’s oral form allows for the interchangeability of both formulations and a seamless transition to oral treatment, maintaining the same safety profile [Citation7]. The once-daily dosing with ISAV, coupled with its improved safety profile relative to POSA, which requires three tablets per day, may contribute to enhanced treatment adherence [Citation41].
One question that could be raised concerns the need to carry out economic analyzes in different European countries. The differences between the health systems of different countries are multiple: different health organization, different clinical practice, different unit costs of health resources, differences in access to health services, co-payments, differences in budget constraints, etc. An economic analysis carried out in Spain is not valid for the UK, in the same way that an economic analysis carried out in the UK is not valid in Spain, France, Germany or US. The economic analyzes carried out in one country must be replicated in other countries for the reasons indicated. Consequently, economic analyzes at the national level are required both by the Spanish Health Authorities and by health professionals in Spain.
Moreover, we have introduced new variables in our study that differentiate it from the UK studies: first, we introduced the 10 mg/kg posology for L-AMB, since there is a number of cases where this posology is utilized for mucormycosis, as recommended by the experts panel and clinical guidelines; second, we have included as hospitalization costs the differential between the Emergency Room and General Ward costs; third, we have included cost of adverse events, since we considered that adverse events could have an important impact on final treatment costs; forth, we included a probabilistic analysis with a Monte Carlo simulation to provide more robustness to the study; and finally, all resource use data costs come from Spanish data.
As for potential limitations of the study, firstly, it should be noted that a theoretical model has been employed, representing a simplified simulation of reality. Secondly, as there are no clinical studies directly comparing ISAV and L-AMB/POSA, several estimations and assumptions were necessary. Two specific weakness of the study were that the IV infusion duration taking data from different trials and as in the study by Bagshaw et al. [Citation21], the same total duration of treatment and total duration of hospitalization was considered for both arms (ISAV and L-AMB+POSA), assuming the mean total duration of ISAV in the SECURE study as a conservative approach, however it could be different taking into account the safety profile.
It is of interest to take into account that in a post hoc analysis from trials SECURE and VITAL in older patients > 65 is not favorable for ISAV in terms of safety and efficacy [Citation42]. However, the difference in safety was driven by a lower proportion of patients under 65 with adverse events in the ISA arm (61.9%, ≥65 vs 49%, <65), in contrast to the voriconazole arm, that showed a similar proportion of adverse events for both age cohorts (58.1%, ≥65 vs 57.4%, <65). Consistently, despite lower mycological and radiological response observed in patients ≥ 65 in the ISA arm, overall clinical response was similar in both treatment arms. In addition, in the present study we have taken overall results from VITAL and SECURE studies, accounting for both ≥ 65 and < 65 cohorts, therefore we do not expect that age may have an impact in our reported results. Additionally, due to a lack of reliable data, the study did not account for costs associated with patients discharged from the hospital who either enter specialized care centers or receive hospitalization at home.
Among the strengths of the study, it is worth emphasizing that the deterministic sensitivity analysis in all variables assessing in the model and Monte Carlo simulations confirmed the robustness of the base case findings. The model assumptions were also clinically validated by a panel of multidisciplinary Spanish experts with extensive experience in treating fungal infections. Also, the consistency of the results with a previously published British study adds credibility to the findings.
5. Conclusions
According to the current model, the treatment of IFIs with ISAV would led to cost savings for the SNS when compared to L-AMB/POSA. These savings are attributed to the shorter duration of IV treatment, reduced use of healthcare resources, and decreased costs for managing adverse effects with ISAV. Furthermore, ISAV maintains consistent efficacy and tolerability throughout the entire treatment period without switching to another therapeutic class. This analysis provides valuable insights for healthcare professionals, enabling informed therapeutic decisions based on efficiency data within the antimicrobial stewardship teams (Programs for Optimization of Antimicrobials) of Spanish hospitals.
Declaration of interest
Carlota Moya-Alarcón and M. Gálvez-Santisteban are employees of Pfizer SLU, C Rubio-Terrés is an employee of HEALTH VALUE, JR Azanza has participated in sessions, meetings and consulting of the following companies: Andromaco, Angelini, Astellas, Astra Zeneca, Baxter, Boehringer, Bial, Biogen, Bioberica, Cassen, Cinfa, Eisai, Esteve, Ferrer, Geiser, GSK, Gilead, Idifarma, Leo, Menarini, MSD, Merck, Norgine, Novartis, Nycomed, Oger. Pfizer, Recordati, Rovi, Roche, Sanofi, Seagen, Shionogi, UCB and Zambon, J Barberan has received honoraria for speaking engagements and scientific advice from Pfizer, Wyeth, Merck, Angelini, Menarini, Meiji Pharma Spain, Shionogi and Gilead, R Ferrer Consulting fees: Inotrem, Pfizer, Cytosorbent. Payment honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events: Shionogi, MSD, Gilead, Menarini, Thermofisher. Stock or stock options: Grifols, M Kwon has received speaker/honoraria from Pfizer, Jazz, Gilead. Consultancy for Incyte, Janssen, A Moreno served as a temporary scientific advisor and received payments for giving presentations from Pfizer.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contribution
M. Gálvez-Santisteban and C. Moya-Alarcón and C Rubio-Terrés made the conceptualization of the economic model. JR Azanza, J Barberan, R Ferrer, M Kwon and A Moreno validated data included in the economic model. C Rubio-Terrés wrote the first draft. All authors interpreted the data, reviewed, edited and agreed on the final version.
Data availability statement
Data available on reasonable request and in line with permission approval with the study sponsor’s confidentiality policy.
Additional information
Funding
References
- Enoch DA, Yang H, Aliyu SH, et al. The changing epidemiology of invasive fungal infections. Methods Mol Biol. 2017;1508:17–65.
- Fernández B, Mendoza NA, Ramos JT. Infección fúngica invasiva (IFI). Protoc Diagn Ter Pediatr. 2023;2:411–420.
- Kriengkauykiat J, Ito JI, Dadwal S. Epidemiology and treatment approaches in management of invasive fungal infections. Clin Epidemiol. 2011;3:175–191. doi: 10.2147/CLEP.S12502
- Sung AH, Martin S, Phan B, et al. Patient characteristics and risk factors in invasive mold infections: comparison from a systematic review and database analysis. Clinicoecon Outcomes Res. 2021;13:593–602. doi: 10.2147/CEOR.S308744
- WHO fungal priority pathogens list to guide research, development and public health action. Geneva: World Health Organization; Licence: CC BY-NC-SA 3.0 IGO. 2022. [cited 2023 Aug 4]. Available from: https://www.who.int/publications/i/item/9789240060241
- Cresemba 200 mg polvo para concentrado para solución para perfusión. Ficha técnica. [cited 2023 Aug 4]. Available from: https://www.ema.europa.eu/en/documents/product-information/cresemba-epar-product-information_es.pdf
- Cresemba, cápsulas duras de 100 mg. Ficha técnica. [cited 2023 Aug 4]. Available from: https://cima.aemps.es/cima/dochtml/ft/1151036002/FT_1151036002.html
- Informe de Posicionamiento Terapéutico de isavuconazol (Cresemba®) en el tratamiento de la aspergilosis invasora y la mucormicosis. IPT, 55/2016. Versión 1. Agencia Española de Medicamentos y Productos Sanitarios, 21 de noviembre de 2016. [cited 2023 Aug 4]. Available from: https://www.aemps.gob.es/medicamentosUsoHumano/informesPublicos/docs/IPT-isavuconazol-Cresemba-aspergilosis-mucormicosis.pdf
- Maertens JA, Raad II, Marr KA, et al. Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by aspergillus and other filamentous fungi (SECURE): a phase 3, randomised-controlled, non-inferiority trial. Lancet. 2016;387(10020):760–769. doi: 10.1016/S0140-6736(15)01159-9
- Marty FM, Ostrosky-Zeichner L, Cornely OA, et al. VITAL and FungiScope mucormycosis investigators. Isavuconazole treatment for mucormycosis: a single-arm open-label trial and case-control analysis. Lancet Infect Dis. 2016;16(7):828–837. doi: 10.1016/S1473-3099(16)00071-2
- FungiScope® ECMM/ISHAM Working Group, Salmanton-García J, Seidel D, et al. Matched-paired analysis of patients treated for invasive mucormycosis: standard treatment versus posaconazole new formulations (MoveOn). J Antimicrob Chemother. 2019;74(11):3315–3327.
- Herbrecht R, Kuessner D, Pooley N, et al. Systematic review and network meta-analysis of clinical outcomes associated with isavuconazole versus relevant comparators for patients with invasive aspergillosis. Curr Med Res Opin. 2018;34(12):2187–2195. doi: 10.1080/03007995.2018.1502659
- Kato H, Hagihara M, Asai N, et al. A systematic review and meta-analysis of efficacy and safety of isavuconazole for the treatment and prophylaxis of invasive fungal infections. Mycoses. 2023;66(9):815–824. doi: 10.1111/myc.13622
- Natesan SK, Chandrasekar PH. Isavuconazole for the treatment of invasive aspergillosis and mucormycosis: current evidence, safety, efficacy, and clinical recommendations. Infect Drug Resist. 2016;9:291–300. doi: 10.2147/IDR.S102207
- Fortún J. Diagnostic and therapeutic approach to fungal pneumonia in the critically ill patient. Rev Esp Quimioter. 2022;35(Suppl 1):97–103. doi: 10.37201/req/s01.21.2022
- Ullmann AJ, Aguado JM, Arikan-Akdagli S, et al. Diagnosis and management of aspergillus diseases: executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin Microbiol Infect. 2018;24(Suppl 1):e1–e38. doi: 10.1016/j.cmi.2018.01.002
- European Confederation of Medical Mycology; International Society for Human Animal Mycology; Asia Fungal Working Group; INFOCUS LATAM/ISHAM Working Group; ISHAM Pan Africa Mycology Working Group; European Society for Clinical Microbiology; Infectious Diseases Fungal Infection Study Group; ESCMID Study Group for Infections in Critically Ill Patients; Interregional Association of Clinical Microbiology and Antimicrobial Chemotherapy; Medical Mycology Society of Nigeria; Medical Mycology Society of China Medicine Education Association; Infectious Diseases Working Party of the German Society for Haematology and Medical Oncology; Association of Medical Microbiology; Infectious Disease Canada, Koehler P, Bassetti M, et al. Defining and managing COVID-19-associated pulmonary aspergillosis: the 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. Lancet Infect Dis. 2021;21(6):e149–e162.
- Mucormycosis ECMM MSG Global Guideline Writing Group, Cornely OA, Alastruey-Izquierdo A, et al. Global guideline for the diagnosis and management of mucormycosis: an initiative of the European confederation of medical mycology in cooperation with the mycoses study group education and research consortium. Lancet Infect Dis. 2019;19(12):e405–e421.
- Grupo de Estudio de la Infección Hospitalaria-Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica; Sociedad Española de Farmacia Hospitalaria; Sociedad Española de Medicina Preventiva, Salud Pública e Higiene, Rodríguez-Baño J, Paño-Pardo JR, et al. Programas de optimización de uso de antimicrobianos (PROA) en hospitales españoles: documento de consenso GEIH-SEIMC, SEFH y SEMPSPH [Programs for optimizing the use of antibiotics (PROA) in Spanish hospitals: GEIH-SEIMC, SEFH and SEMPSPH consensus document]. Enferm Infec Microbiol Clin. 2012;30(1):22.e1–22.e23.
- Prieto L, Sacristán JA, Antoñanzas F, et al. Análisis coste-efectividad en la evaluación económica de intervenciones sanitarias. Medicina Clínica. 2004;122(13):505–510. doi: 10.1016/S0025-7753(04)74288-8
- Bagshaw E, Enoch DA, Blackney M, et al. Economic impact of treating invasive mold disease with isavuconazole compared with liposomal amphotericin B in the UK. Future Microbiol. 2018;13(11):1283–1293. doi: 10.2217/fmb-2018-0119
- BotPlus. [cited 2023 Aug 6]. Available from: https://botplusweb.farmaceuticos.com/
- Horn D, Goff D, Khandelwal N, et al. Hospital resource use of patients receiving isavuconazole vs voriconazole for invasive mold infections in the phase III SECURE trial. J Med Econ. 2016;19(7):728–734. doi: 10.3111/13696998.2016.1164175
- Leenders AC, Daenen S, Jansen RL, et al. Liposomal amphotericin B compared with amphotericin B deoxycholate in the treatment of documented and suspected neutropenia-associated invasive fungal infections. Br J Haematol. 1998;103(1):205–212. doi: 10.1046/j.1365-2141.1998.00944.x
- Noxafil. Assessment report. Procedure No. EMEA/H/C/000610/II/0062. EMA/590500/2021. European Medicines Agency. 2021 Sep 16 [cited 2023 Aug 6]. Available from: https://www.ema.europa.eu/en/documents/variation-report/noxafil-h-c-610-ii-0062-epar-assessment-report-variation_en.pdf
- Soysal A. Prevention of invasive fungal infections in immunocompromised patients: the role of delayed-release posaconazole. Infect Drug Resist. 2015;8:321–331. doi: 10.2147/IDR.S65592
- Azanza JR, Grau S, Vázquez L, et al. The cost-effectiveness of isavuconazole compared to voriconazole, the standard of care in the treatment of patients with invasive mould diseases, prior to differential pathogen diagnosis in Spain. Mycoses. 2021;64(1):66–77. doi: 10.1111/myc.13189
- Nolla JM, Martín E, Llamas P, et al. An estimate of the cost of administering intravenous biological agents in Spanish day hospitals. Ther Clin Risk Manag. 2017;13:325–334. doi: 10.2147/TCRM.S112062
- Batista MV, Ussetti MP, Jiang Y, et al. Comparing the real-world use of Isavuconazole to other anti-fungal therapy for invasive fungal infections in patients with and without underlying disparities: a multi-center retrospective study. J Fungi. 2023;9(2):166. doi: 10.3390/jof9020166
- Instituto catalán de la salud. Resolución SLT/3911/2022 de 14 de diciembre. Diari Oficial de la Generalitat de Catalunya, Nº 8816, 20 de diciembre de 2022. [cited 2023 Aug 6]. Available from: https://noticias.juridicas.com/base_datos/CCAA/744047-r-slt-3911-2022-de-14-dic-ca-cataluna-revision-de-precios-publicos-correspondientes.html
- Presidencia de la Generalitat Valenciana. Ley 20/2017, de 28 de diciembre, de tasas. Boletín Oficial del Estado, Nº 38, 12 de febrero 2018. [cited 2023 Aug 6]. Available from: https://www.boe.es/boe/dias/2018/02/12/pdfs/BOE-A-2018-1870.pdf.
- Consejería de Sanidad y Servicios Sociales. Resolución de 16 de enero de 2023. Diario Oficial de Extremadura, Nº 14, 20 de enero de 2023. [cited 2023 Aug 6]. Available from: https://doe.juntaex.es/pdfs/doe/2023/140o/23060215.pdf
- Xunta de Galicia.Consellería de Facenda, Axencia Tributaria de Galicia. Normativa vigente. Tasas y precios de la Comunidad Autónoma 2020. Edición actualizada en mayo de 2020. [cited 2023 Aug 6]. Available from: https://www.conselleriadefacenda.gal/documents/16561/24448624/Libro-Tasas-2020-es.pdf/24d78ef5-bb5f-4d53-945c-76c443ed7d8b
- EMA. Assessment Report: Cresemba. In. Vol EMA/596950/2015: London: European Medicines Agency; 2015 [cited 2023 Nov 21]. Available from: https://www.ema.europa.eu/en/documents/assessment-report/cresemba-epar-public-assessment-report_en.pdf
- Wade RL, Chaudhari P, Natoli JL, et al. Nephrotoxicity and other adverse events among inpatients receiving liposomal amphotericin B or amphotericin B lipid complex. Diagn Microbiol Infect Dis. 2013;76(3):361–367. doi: 10.1016/j.diagmicrobio.2013.04.001
- Stan Ulam ER. John von Neumann, and the Monte Carlo method. Los Alamos Science. 1987;15:131–137.
- Peral C, Cordido F, Gimeno-Ballester V, et al. Cost-effectiveness analysis of second-line pharmacological treatment of acromegaly in Spain. Expert Rev Pharmacoecon Outcomes Res. 2020;20(1):105–114. doi: 10.1080/14737167.2019.1610396
- Arlandis Guzmán S, Jiménez Cidre MA, Rubio-Rodríguez D, et al. [Analysis of costs and consequences related to the persistence of Mirabegron and antimuscarinic treatments and their impact on quality of life in patients with overactive bladder in Spain: results of a probabilistic model.]. Arch Esp Urol. 2020;73(6):509–522.
- Formiga F, García-Pavía P, Martín Sánchez FJ, et al. Health and economic impact of the correct diagnosis of transthyretin cardiac amyloidosis in Spain. Expert Rev Pharmacoecon Outcomes Res. 2021;21(5):1127–1133. doi: 10.1080/14737167.2021.1933948
- Bagshaw E, Kuessner D, Posthumus J, et al. The cost of treating mucormycosis with isavuconazole compared with standard therapy in the UK. Future Microbiol. 2017;12(6):515–525. doi: 10.2217/fmb-2016-0231
- Gast A, Mathes T. Medication adherence influencing factors—an (updated) overview of systematic reviews. Syst Rev. 2019;8(1):112. doi: 10.1186/s13643-019-1014-8
- Hamed K, Engelhardt M, Kovanda LL, et al. Post-hoc analysis of the safety and efficacy of isavuconazole in older patients with invasive fungal disease from the VITAL and SECURE studies. Sci Rep. 2023;13(1):6730. doi: 10.1038/s41598-023-31788-1
