ABSTRACT
Oxytocin and vasopressin are neuropeptides implicated as modulators of human aggressive behavior. In animal models, administration of oxytocin generally attenuates aggressive behavior, but in humans the effects of oxytocin appear to be more nuanced. Vasopressin seems to have an opposing influence on aggression in animal studies, but much less research has been done in humans. We performed a cross-sectional study in which we measured oxytocin and vasopressin levels in forensic psychiatric male patients with a personality disorder (N = 38) and healthy male controls (N = 108). Elevated salivary oxytocin (B = −0.10, P = 0.02) and reduced urinary vasopressin (B = 0,19, P < 0.01) levels were found in patients compared to controls. Within the patient group urinary oxytocin levels were positively associated with psychopathy scores as measured with the PCL-R (B = 0.02, P = 0.02). These findings suggest that baseline levels in forensic psychiatric patients diagnosed with a primary personality disorder might be counterintuitive, as oxytocin levels are higher than expected and vasopressin levels are lower than those of healthy controls. More generally, the results imply a complex role of these neuropeptides on human behavior, in line with the social salience theory.
Introduction
Neuropeptides such as oxytocin and vasopressin are increasingly recognized as potent modulators of human behavior. Oxytocin is a neuropeptide that is associated with pro-social human behaviors such as trust (Kosfeld et al., Citation2005), affiliation (Feldman, Citation2012) and empathy (De Dreu & Kret, Citation2016). This raises the question of whether oxytocin might have an attenuating effect on aggressive behavior. In animal models, intracerebroventricularly administered oxytocin has a dose-dependent, decreasing effect on aggression (Calcagnoli et al., Citation2013), although more recent work shows that the effect of oxytocin on aggression depends on the social context and can be both pro-social and agonistic (Anpilov et al., Citation2020a). Mice with a total knockout of the oxytocin receptor gene show more aggressive behavior, while mice whose oxytocin receptor gene was not excised until approximately 21–28 days postnatally, had comparable levels of aggression to those of mice with a normal functioning oxytocin receptor gene (Dhakar et al., Citation2012). This evidence suggests a neurodevelopmental critical period (Silva-Santos et al., Citation2015) for the influence of oxytocin on brain function, including aggressive behavior. Vasopressin is chemically similar to oxytocin but its effect on aggressive behavior in animal models seems to be opposite. Vasopressin has an increasing effect on reactive maternal aggression in animal models (Bosch & Neumann, Citation2010).
In humans, the effect of oxytocin on behavior, particularly aggression, seems to be more complex than in animal models (Gedeon et al., Citation2019; J.a Bartz et al., Citation2011; Nave et al., Citation2015). Intranasal administration of oxytocin causes more readiness to interact with strangers (Cohen & Shamay-Tsoory, Citation2018), but social information seems to be crucial and there must be the expectation that the other participant will cooperate (Declerck et al., Citation2010). These effects seem especially to be orientated towards own group members: it enhances cooperation within a group and defensive behaviors such as protection of vulnerable group members (De Dreu et al., Citation2012). The direct effect of intranasal oxytocin on aggressive behavior in a laboratory task showed conflicting results (Alcorn, Green et al., Citation2015; Campbell & Hausmann, Citation2013; Ne’eman et al., Citation2016). It seems to be of great importance whether the environment is interpreted as ‘safe’ or ‘unsafe’. A ‘safe’ condition causes oxytocin to exert more pro-social behaviors, whereas in a more ‘unsafe’ environment oxytocin seems to cause more defensive aggressive behaviors (Olff et al., Citation2013). In line with this, the effects of oxytocin on behavior seem to differ depending on the presence of certain personality traits. Patients with a borderline personality disorder have lower oxytocin levels (Bertsch et al., Citation2013) and administration of oxytocin has divergent effects, with decreased trust and cooperation (J. Bartz et al., Citation2011) and decreased amygdala and insula reactivity, while this was increased in healthy controls (Lischke et al., Citation2017).
In forensic psychiatric patients, higher morning urinary oxytocin levels were found (Mitchell et al., Citation2013) and intranasal administration of oxytocin resulted in inconsistent effects on aggressive behavior in patients with an antisocial personality disorder (Alcorn, Rathnayaka et al., Citation2015). However, intranasal administration improved facial recognition in patients with antisocial personality disorder; while prior to the administration, they had deficits in recognizing fearful and happy faces (Timmermann et al., Citation2017). Moreover, different polymorphisms in the oxytocin receptor gene were found to be associated with aggressive and antisocial behavior and increased amygdala reactivity (Hovey et al., Citation2016; Waller et al., Citation2016).
The effect of vasopressin on aggression seems to depend on the condition of the subjects. If the production of vasopressin is disrupted in rats, it leads to a decrease of aggressive behaviors in lactating female rats and reproductively inexperienced male rats, whereas it has no effect in reproductively experienced males (Fodor et al., Citation2014) and even a reversed effect in female rats exposed to stressors in adolescence (Cordero et al., Citation2013). Social isolation in rats increases aggressive behaviors, presumably caused by an increase of the number of V1a vasopressin receptors in the anterior hypothalamus (Elliott Albers et al., Citation2006). Also, maternal separation causes increased inter-male aggression and upregulated mRNA expression for vasopressin in the hypothalamic periventricular and supraoptic nuclei (Veenema et al., Citation2006). Whereas levels of oxytocin in the human cerebrospinal fluid (CSF) are negatively associated with the life history of aggression (Lee et al., Citation2009), vasopressin levels in CSF are positively correlated with the life history of aggression in subjects diagnosed with a personality-disorder (Coccaro et al., Citation1998). However, administration of vasopressin in humans did not cause increased aggressive behavior (Brunnlieb et al., Citation2013). Genetic variants of the vasopressin receptor gene have been associated with impulsive aggressive behavior in patients with borderline personality disorder (Vogel et al., Citation2012).
Among those with a personality disorder, psychopathic traits are particularly interesting in the light of oxytocin and vasopressin. Psychopathy is defined by antisocial behaviour and emotional impairment. Only one-third of those who are diagnosed with antisocial personality disorder meet the criteria for psychopathy (Blair, Citation2003). People with psychopathy do not form authentic caring or love bonds with other people and their behavior is often based on obtaining what is in their own interest. On the other hand, they are particularly skillful in giving others the impression that they can be trusted. Basal urinary oxytocin levels were found to be higher in patients with psychopathic traits on the Psychopathy Check List – Revised (PCL-R, Hare, Citation2002), and to correlate with the factor 2 score on this list, that represents antisocial behavior, but notably not with factor 1, which represents callousness, unemotionality and superficial charm (Mitchell et al., Citation2013). Conversely, in children with conduct problems, low salivary levels of oxytocin were associated with callous and unemotional traits, which is often considered to be a precursor for psychopathic traits later in life (Levy et al., Citation2015). Lower levels of oxytocin in children with conduct problems might be explained by the higher methylation of different sites in the promotor region of the oxytocin receptor gene (Dadds et al., Citation2014).
Overall, these data suggest that oxytocin and vasopressin function differently in patients with a personality disorder than in healthy subjects, and that their hormonal levels are associated with aggressive behavior. Although the hormonal effect on aggressive behavior is well described in animal models and to a lesser extent in healthy human subjects, relatively little is known about oxytocin and vasopressin in forensic psychiatric patients (Gedeon et al., Citation2019). We hypothesized that basal oxytocin and vasopressin levels might have an impact on aggressive behavior in patients with a personality disorder. Therefore, we performed a cross-sectional study in which we investigated the baseline levels of oxytocin and vasopressin in patients with a personality disorder in a forensic psychiatric hospital, and compared them with healthy subjects. We measured oxytocin and vasopressin in urine in the first void of the day, representing a pooled level overnight (Reyes et al., Citation2014) and a more temporally instantaneous oxytocin level in saliva which was taken in the afternoon, when oxytocin levels are most stable (Graugaard-Jensen et al., Citation2017).
Methods
This protocol was approved by the Medical Ethical Committee of the Erasmus University Medical Centre Rotterdam (MEC-2013-551) and performed according to the Declaration of Helsinki and the Good Clinical Practice guidelines. Al participants gave written informed consent.
Participants
Participants were male patients, admitted to the Forensic Psychiatric Centre ‘De Kijvelanden’ (Fivoor, Poortugaal, The Netherlands). Patients were admitted on court order, according to the Dutch Entrustment Act (TBS: Terbeschikkingstelling). This implies that there is a psychiatric disorder and/or personality disorder at the time of the offense and that there is a causal relation between the disorder and the offense. Because the participants were admitted involuntary, the study was conducted by an independent researcher, the psychiatrist that treated the patients was not informed about the participation and all data were handled strictly anonymously. All patients were diagnosed by a senior psychiatrist based on the DSM-IV-TR (Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision, Citation2000). Only patients with a primary diagnosis of personality disorder were included in the study. Recent drug use was unlikely as participants were regularly checked on the use of drugs by urine drug screenings (controlling for cannabis, amphetamine, alcohol, opioids or cocaine) within the facility. Most patients used psychoactive medication to prevent their disruptive behavior. Therefore, it was not possible to include only medication free patients. Medication use was documented carefully and participants were excluded when they used libido-inhibiting medication (androgen deprivation therapy such as Triptorelin and Cyproterone acetate), as they are known to influence the hormonal homeostasis. Participants were excluded when they had serious somatic comorbidity for which treatment in a somatic hospital was necessary. Healthy controls were recruited from the general population. All controls were screened by a medical doctor for having no mental or physical disorder; participants with a mental disorder, medication use, or (history of) drugs or alcohol abuse or dependence were not included.
Procedure
After the screening for inclusion- and exclusion criteria, participants that were eligible were given verbal and written information about the study, after which they provided written informed consent. To collect urine, they received a sterile container. On the day of collecting the saliva samples, they were asked to collect the first urinary void of the day. They were asked to store it in their fridge directly after collecting. The samples were stored at −80 C at the same day. Participants were asked to follow a normal daily routine on the day of the assessments, but to refrain from smoking, eating, drinking anything but water, and heavy physical activity one hour before the saliva collection. This was done to avoid bias in cortisol levels as a result of extreme activities or a deviance of the normal consumption. Collection of saliva took place between 2pm. and 3pm. Before collection of samples in the healthy controls, they were asked to fill in The Reactive Proactive Questionnaire (RPQ Raine et al., Citation2006). PCL-R data and diagnostic information of patients were available in their medical records.
Questionnaires
RPQ: healthy controls were asked to fill in the RPQ. The RPQ is a self-questionnaire to measure proactive and reactive aggression. The questionnaire exists of 23 items, of which 11 items measure reactive aggression and 12 items proactive aggression. Items can be answered on a three-point scale (never, sometimes or often). The Dutch version of the RPQ has been validated and showed the same two-factor structure (Cima et al., Citation2013).
PCL-R: the Psychopathy Checklist – revised (Hare, Citation2002) is the most widely used and accepted diagnostic instrument to measure the degree of psychopathy. It consists of a semi-structured interview and a review of the participants records. In the semi-structured interview, patients are asked to their school period, employment, intimate relationships, family, friends and criminal activity. In the records, data from the intake assessments and reports of institutional adjustment and transgressions are used. Each of the 20 items is scored on a three-point scale: 0: does not apply, 1: applies somewhat, 2: definitely applies. The resulting scores range from 0 to 40, the recommended diagnostic cut-off score for the diagnosis psychopathy is 30. However, in Europe the cut-off score of 26 is often used because this might represent better the same intensity of the disorder compared to the American population (Cooke, Michie, Hart & Clark, Citation2005). The PLC-R score is subdivided into two factors: Factor 1 is generally regarded as the core personality traits of psychopathy (i.e. selfish, callous and the remorseless use of other) and Factor 2 measures the antisocial and deviant behaviors (i.e. chronically unstable, socially deviant lifestyle) (Hare, Citation2002).
Urine samples
Urine samples were directly put on ice after collection and stored at −80°C as both vasopressin and oxytocin have a very short half-life when not cooled (Leng & Sabatier, Citation2016). Levels of oxytocin were determined using the Oxytocin EIA kit (Enzo Life Sciences Inc., USA), a competitive immunoassay for the quantitative determination of oxytocin. Levels of vasopressin were determined using the Arg-Vasopressin EIA kit (Enzo Life Sciences Inc., USA), a competitive immunoassay for the quantitative determination of vasopressin. Both the vasopressin and oxytocin kits could be used without pre-extraction for various materials besides plasma. The sensitivity of the methods was 3.39 and 11.7 pg/mL, respectively for vasopressin and oxytocin. The intra-assay CV was 10.2% at the level of 6.31 pg/mL for vasopressin, and 9.1% at the level of 21.4 pg/mL for oxytocin.
Urinary creatinine concentrations were measured using the Jaffé method. The basal urinary oxytocin and vasopressin levels were expressed as the oxytocin to creatinine ratio and the vasopressin to creatinine ratio, respectively.
Saliva samples
Testosterone and cortisol might interact with oxytocin. Oxytocin down regulates the cortisol release in the HPA axis and, therefore, dampens the stress reaction (Ring et al., Citation2006) especially in subjects with impaired emotion regulation abilities (Windle, Shanks, Lightman, & Ingram, Citation1997). Testosterone has opposite effects as compared to oxytocin: administration of testosterone leads to more aggressive behavior and dominance (Pope, Kouri, & Hudson, Citation2000). Testosterone can, therefore, be seen as an antagonist of oxytocin. To control for the influence of testosterone and cortisol on oxytocin, we also measured these hormones in saliva at the same moment that oxytocin was assessed. Saliva samples were collected using the Sarstedt cortisol salivette devices. The participants chewed on a synthetic swab during at least 2 minutes. Saliva was collected at 2pm. The samples were directly after collection put on ice, centrifuged at 2600 G for 15 minutes at 4°C. The supernatant was stored in Eppendorf tubes at −80°C until they were analyzed. The free cortisol levels in saliva were analyzed with a commercially available ELISA kit (Demeditec Diagnostics, Kiel, Germany). Limit of detection is 0.276 nmol/l. The inter- and intra-assay coefficients of variation are < 10% and <7%, respectively. The free testosterone levels in saliva were analyzed with a commercially available ELISA kit (DRG Diagnostics, Marburg, Germany). The limit of detection is 34.7 pmol/l. The inter- and intra-assay coefficient of variation is <10% and <15%, respectively. Determination of salivary oxytocin was performed using a 96-plate commercial oxytocin-ELISA kit (ENZO, NY, USA). Measurements were performed in duplicate. Samples underwent freeze drying to be concentrated by four, and dissolved in the assay buffer and treated according to the kit’s instructions.
Statistical analyses
All statistical analyses were performed using SPSS software version 24.0 (IBM, Armonk, NY, USA). Descriptive statistics and correlation coefficients were calculated to summarize the basic features of the patient group and healthy controls. Oxytocin and vasopressin data became normally distributed after log-transformation. Multiple regression analyses were used to study group differences controlling for possible confounders. Generalized linear models for normal responses and identity link-function were used to study group differences while controlling for possible confounders. Model selection was based on Wald-tests with alpha set at the conventional 5% level. Associations with salivary oxytocin levels were controlled for salivary levels of cortisol, testosterone and the testosterone/cortisol ratio. Furthermore, regression models included age as a confounding variable as there is evidence that ageing alters the peripheral oxytocin levels (Elabd et al., Citation2015; Huffmeijer et al., Citation2012). Sensitivity analyses were planned to explore the impact of medication on oxytocin and vasopressin levels, but these were not followed up due to small subgroups.
Results
Eighty-four patients and 108 healthy controls were included in the study. Only patients with a primary diagnosis of personality disorder were included. Patients with a psychotic disorder were excluded. Four patients were excluded because they used libido inhibiting medication. One patient was excluded because of an insufficient amount of saliva. In total, 38 patients were available for further analyses. PCL-R data were missing or incomplete in 5 cases; further analyses with the PCL-R data are therefore performed on 33 patients. Descriptives for age, BMI and mean hormonal levels are summarized in . Diagnostic information, medication use and type of offense of the patients is summarized in . Overall, a positive correlation between age and salivary oxytocin levels was found (Pearson correlation coefficient: N = 146; r = 0.25, p < 0.01). Further analyses were corrected for age.
Table 1. Descriptives of the patients and healthy controls. Mean values with standard deviations are shown. RPQ = Reactive-Proactive Aggression Questionnaire. PCL-R = Psychopathy Check List, Revised version
Table 2. Diagnosis, medication use and type of offense (N, and % are indicated) within the group of forensic psychiatric patients
The regression model showed that patients had higher salivary oxytocin levels compared to healthy controls (B = −0.10, CI: −0.19; 0.02, P = 0.02, ). This result was not affected by salivary cortisol, testosterone and the testosterone-cortisol ratio (for details, see ). Urinary oxytocin levels did not differ significantly (B = −0.02, CI: −0.11; 0.07, P = 0.66, ) between groups.
Table 3. Linear regression model
Figure 1. Mean and standard errors of the raw data of salivary and urinary oxytocin and urinary vasopressin levels in healthy controls (Control) and personality disordered forensic psychiatric patients (Patient). Urinary levels are in pmol/mmol and salivary levels in pg/ml. Statistical analyses were performed on the log-transformed data.
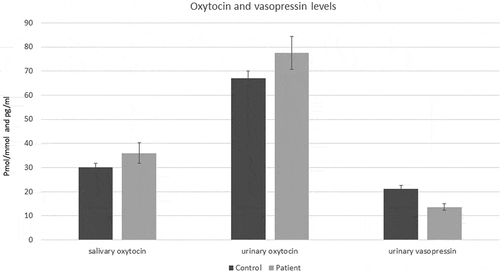
Urinary vasopressin levels were lower in patients compared to healthy controls (regression model: B = 0,19, CI: 0.06; 0.32, P < 0.01, ). Overall, urinary vasopressin level correlated positively with urinary oxytocin levels (N = 146; r = 0.17, P = 0.04).
Within the control group, the regression model showed a positive association between the RPQ total score and salivary oxytocin levels (B = 0.01, CI 0.00;0.02, P = 0.04). Both reactive aggression (B = 0.02, CI 0.00; 0.0.3, P = 0.01) and proactive aggression (B = 0.02, CI 0.01; 0.04, P = 0.01) were associated with salivary oxytocin levels. However, no association was found with urinary oxytocin levels (B = 0.00, CI-0.01; 0.01, P = 0.73) or with urinary vasopressin levels (B = 0.00, CI: −0.01;0.02, P = 0.87).
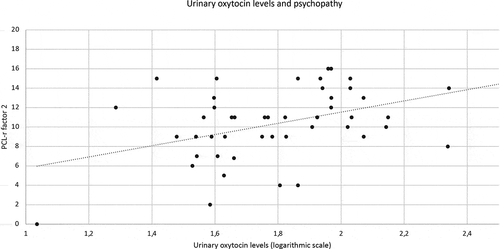
Within the patient group, psychopathy scores as measured with the PCL-R were positively associated with urinary oxytocin levels (regression model: B = 0.02, CI 0.00; 0.03, P = 0.02), especially with factor 2 (B = 0.04, CI 0.02; 0.06, P = <0.01; see ). There was no association between PCL-R data and salivary oxytocin levels (B = −0.00, CI −0.02; 0.01, P = 0.65) or with urinary vasopressin levels (B = 0.01, CI −0.01; 0.02, P = 0.24).
Discussion
In this study, we compared both urinary and salivary oxytocin levels and urinary vasopressin levels between forensic psychiatric patients with personality disorders and healthy controls. We found higher salivary oxytocin levels in forensic psychiatric patients diagnosed with a personality disorder compared with healthy controls. To the best of our knowledge, no previous studies of vasopressin levels have been conducted in a forensic psychiatric population. Within the patient group, a positive association between levels of psychopathy, mainly factor 2 as measured on the PCL-R, and urinary oxytocin levels was found. Within the control group, a positive association was observed between salivary oxytocin levels and aggressive behavior as measured with the RPQ.
During the last decade, the initial enthusiasm regarding oxytocin as a pro-social hormone with a promising therapeutic promise, was considerably tempered due to the emerging complexity of the association between oxytocin and social behavior (Zik & Roberts, Citation2015). In animal models, oxytocin seems to exert anti-aggressive effects (Calcagnoli et al., Citation2013, Citation2015), although depending on the social context (Anpilov et al., Citation2020a), but in healthy human subjects the findings are divergent (Alcorn, Green et al., Citation2015; Y.R. Berends, Tulen, Wierdsma, van Pelt, Feldman, et al., Citation2019a; Campbell & Hausmann, Citation2013; Ne’eman et al., Citation2016). In human beings with a psychiatric disorder, the effects of oxytocin can even be opposite to what one would expect. In borderline personality disorder, oxytocin can hinder trust and cooperation (J. Bartz et al., Citation2011). Among subjects currently abstinent following earlier heroin addiction, higher oxytocin levels have been observed which correlated positively with a lifetime burden of aggressive behavior (Gerra et al., Citation2017).
These findings suggest that the effect of oxytocin is moderated by other personal and environmental factors. This finding, among others, led to the formulation of the ‘Social salience hypothesis’ that states that oxytocin modulates the orienting responses to external cues, but is dependent on baseline individual differences such as gender, personality traits, and degree of psychopathology (Shamay-Tsoory & Abu-Akel, Citation2016).
The finding that oxytocin levels are most prominent associated with PCL-R factor 2, which represents antisocial lifestyle and associated with reactive anger, criminality and impulsive violence, is remarkable as oxytocin levels had been earlier associated with callous-unemotional traits, which are expressed in the PCL-R by factor 1 (Levy et al., Citation2015). In the control group, no distinction is found between pro-active and reactive patterns. These results support the idea that the effect of oxytocin depends on other factors, such as personality traits and contextual issues (here: placed in a maximum-secured hospital), as also suggested by the social salience hypothesis. Although our finding that patients with a personality disorder have higher oxytocin levels than healthy controls might be counterintuitive, this is in line with the findings of Mitchell et al. who found a positive correlation between urinary oxytocin levels and PCL-R factor 2 (Mitchell et al., Citation2013).
The current study was conducted in a forensic psychiatric hospital, in which patients have court-mandated compulsory treatment. It must be noted that this is a special ‘holding’ environment where intensive psychiatric treatment is given which might be experienced as stressful by the patients. Earlier research in this setting showed that these patients also have counterintuitively higher cortisol levels (Loomans et al., Citation2016), although in this population lower cortisol levels and hypo-reactivity to stress were to expect (Fairchild et al., Citation2018). Within this highly intensive therapeutic and closed environment it can be hypothesized that a higher level of salivary oxytocin is an adaptive physiological measure to bridge the permanent presence of therapists, sociotherapeutic staff and other patients with the former presence of low oxytocin in those persons before their incarceration. The relational urge stresses the production of oxytocin as a barrier against too much interactions and an auxiliary for attachment.
When the environment is perceived as ‘unsafe’, oxytocin tends to have an increasing effect on aggression, while it has a moderating influence on aggression when the environment is perceived as ‘safe’ (Olff et al., Citation2013). We suggest a protective and shielding function of this aggression to protect the integrity of the person and its next of kin (Berends et al., Citation2019b). The same phenomenon is confirmed by hostile attribution behavior. Aggressive individuals often exhibit a hostile attributional bias, which means that they have the tendency to interpret others’ behaviors as hostile and unsafe, even if their behavior does not have a hostile intent (Buck et al., Citation2012).
We found a positive association between the RPQ and salivary oxytocin levels in healthy subjects. In earlier research, an inverse correlation between baseline levels of oxytocin and the life time history of aggression in healthy subjects was observed, although these correlations are often mediated by other factors (Berends, Tulen, Wierdsma, Van Pelt, Kushner et al., Citation2019; Lee et al., Citation2009). It must be noted, however, that the healthy subjects in these studies were relatively young and highly educated. In this study, the healthy controls were matched with the forensic psychiatric patients for age and education level. Therefore, healthy controls were actively recruited among firefighters. It is known however, that firefighters tend to be more ‘sensation seeking’ than people not working within the rescue forces (Tschiesner, Citation2012). Notably, this personality trait is shared with people with an antisocial personality disorder (Mann et al., Citation2018), although firefighters can deploy this personality trait for social benefit while it leads to social damage in people with an antisocial personality disorder. Oxytocin seems to have an enhancing effect on defensive and protective aggressive behavior (Bosch et al., Citation2005), which might be beneficial effects in this group of firefighters.
Vasopressin is generally associated with more aggressive behavior both in animal and human models (Caldwell et al., Citation2008). However, manipulation of septal vasopressin activity does not alter the level of aggression in animal models (Beiderbeck et al., Citation2007) analogous to the lack of effect following intranasal administration in humans (Brunnlieb et al., Citation2013). It has been proposed that alterations of vasopressin could be the consequence, rather than the cause, of aggressive behavior (Veenema & Neumann, Citation2008). The patients in our study were all admitted in a forensic psychiatric hospital – a therapeutic, secured and predictable environment in which aggressive incidents are prevented to occur. This could be an explanation for the relatively low levels of vasopressin in our population. More idiographic research in which temporal effects can be studied (Van Ockenburg et al., Citation2015) is, therefore, warranted in this population.
Limitations and future directions
There is an ongoing debate regarding the extent to which peripheral oxytocin and vasopressin levels closely mirror central levels. The blood-brain-barrier is thought to be impermeable for neuropeptides such as vasopressin and oxytocin although recent findings indicate that there is a peripheral-to-central coupling by vagal afferent nerves (Iwasaki et al., Citation2019). Basal levels of plasma oxytocin and vasopressin therefore do not correlate with central levels. For urinary levels insufficient data are available to draw further conclusions (Rutigliano et al., Citation2016; Valstad et al., Citation2017). However, salivary oxytocin levels do correlate with central levels (Martin et al., Citation2018). In this study, we choose to identify oxytocin and vasopressin in morning urine and oxytocin in saliva in the afternoon. Plasma, saliva and cerebrospinal fluid oxytocin levels do not show significant diurnal fluctuations and the best correlation of peripheral with central oxytocin levels was found in the early morning (Kagerbauer et al., Citation2019). Besides that, we reasoned that the least behavioral effect on hormonal levels would be during the night. Because of these reasons, we collected urine in the morning as a pooled measure of these neuropeptides overnight.
However, there is also evidence that oxytocin levels decrease rapidly upon awakening and there might be an interaction with the cortisol awakening response (Van Dam et al., Citation2018). To minimize this effect in the salivary oxytocin sample, which represents a more momentary ‘state’ level, we choose to determine these levels in the afternoon, when more stable levels are reported (Graugaard-Jensen et al., Citation2017). These choices might be crucial in the divergent findings between urinary and salivary oxytocin levels.
Medication use might have affected oxytocin and vasopressin levels. Particularly for benzodiazepines (Yagi & Onaka, Citation1996) and antipsychotic medication (Kiss et al., Citation2010), there is evidence that these might affect oxytocin levels. However, for safety reasons and to avoid unnecessary stagnation of the treatment it was not possible to temporally interrupt this pharmacological treatment. In our sample, only 26% of the patients (N = 10) used anti-psychotic medication and only 13% of the patients (N = 5) used benzodiazepines. Further analyses with the equivalent dose of these medications were therefore underpowered.
Our sample consist only of men and the results are, therefore, not necessarily applicable to women. We chose to only examine male patients because they constitute the vast majority of the forensic mental hospital population. Also oxytocin fluctuates throughout the menstrual cycle and the use of oral contraceptives influences these fluctuations (Salonia et al., Citation2005). Different hormones interact and cortisol and testosterone might affect oxytocin levels. In this study, we therefore controlled for cortisol and testosterone levels, but did not find an effect of saliva cortisol and testosterone on the differences in salivary oxytocin levels between the patients with a personality disorder and healthy controls.
The vasopressin data must be interpreted with caution as the release of vasopressin is also regulated by the osmoreceptor and baroreceptors in the hypothalamus and is released in response to extracellular hyperosmolality. Besides that, the use of alcohol, but also of vasopressin itself and different types of medication (such as those affecting Angiotensin II), can affect the levels of vasopressin in urine. The use of vasopressin or any antihypertensive agents was therefore an exclusion criterion for the patient group; the healthy controls did not use any medication at all. However, in the control group the use of alcohol in the period before sampling was not objectively disproved. Our results are partly in line with those of earlier research (Mitchell et al., Citation2013), but larger sample sizes are needed to draw definite conclusions. Further replication of our results and clarification of the effects of oxytocin and vasopressin by manipulation of oxytocin and vasopressin levels in a forensic psychiatric population is, therefore, warranted. Manipulation with intranasal oxytocin is now broadly applied and has led to many new insights in the social effects of this neuropeptide, while the effects of intranasal vasopressin are largely undiscovered.
The group of patients in our study consisted only of patients with a personality disorder. However, within the group of patients with a personality disorder high variability still exists. Our group of patients consisted mainly of patients with a cluster B personality disorder (N = 35), with the largest group having an antisocial personality disorder (N = 17). A relatively large group had the diagnosis of a personality disorder not otherwise specified with antisocial and/or narcissistic traits (N = 18). The large variability in levels of neuropeptides in this group of patients is most likely caused by the diagnostic heterogeneity that is characteristic for forensic psychiatry. There are multiple neurobiological pathways that influence aggressive behavior, in which there is a complicated interplay between neurobiological, developmental and environmental factors (A. Raine, Citation2013)
Conclusions
Oxytocin and vasopressin are important modulators of social behavior and might influence the emergence of violence. However, this study shows that baseline levels in forensic psychiatric patients diagnosed with a primary personality disorder can be other than expected, as oxytocin levels are higher and vasopressin levels are lower than those of healthy controls. These results implicate a complex role of these neuropeptides in human beings, in line with the social salience theory. There is an increasing amount of evidence that the functioning of these neuropeptides is different in patients with a personality disorder.
Acknowledgments
This research was funded by Fivoor, the Erasmus MC and the Koningsheide foundation (P2013/485). The authors thank M. Bennaars, A.F. Juriaans and T.L. van Dijl for their contribution to the data collection.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Alcorn, J. L., Green, C. E., Schmitz, J., & Lane, S. D. (2015). Effects of oxytocin on aggressive responding in healthy adult men. Behavioural Pharmacology, 26(8), 798–804. https://doi.org/https://doi.org/10.1097/FBP.0000000000000173
- Alcorn, J. L., Rathnayaka, N., Swann, A. C., Moeller, F. G., & Lane, S. D. (2015). Effects of Intranasal oxytocin on aggressive responding in antisocial personality disorder. The Psychological Record, 65(4), 691–703. https://doi.org/https://doi.org/10.1007/s40732-015-0139-y
- Anpilov, S., Shemesh, Y., Eren, N., Harony-Nicolas, H., Benjamin, A., Dine, J., Oliveira, V. E. M., Forkosh, O., Karamihalev, S., Hüttl, R.-E., Feldman, N., Berger, R., Dagan, A., Chen, G., Neumann, I. D., Wagner, S., Yizhar, O., & Chen, A. (2020a). Wireless optogenetic stimulation of oxytocin neurons in a semi-natural setup dynamically elevates both pro-social and agonistic behaviors. Neuron, 107(4), 644–655.e7. https://doi.org/https://doi.org/10.1016/j.neuron.2020.05.028
- Bartz, J., Simeon, D., Hamilton, H., Kim, S., Crystal, S., Braun, A., Vicens, V., & Hollander, E. (2011). Oxytocin can hinder trust and cooperation in borderline personality disorder. Social Cognitive and Affective Neuroscience, 6(5), 556–563. https://doi.org/https://doi.org/10.1093/scan/nsq085
- Bartz, J. A., Zaki, J., Bolger, N., & Ochsner, K. N. (2011). Social effects of oxytocin in humans: Context and person matter. Trends in Cognitive Sciences, 15(7), 301–309. https://doi.org/https://doi.org/10.1016/j.tics.2011.05.002
- Beiderbeck, D. I., Neumann, I. D., & Veenema, A. H. (2007). Differences in intermale aggression are accompanied by opposite vasopressin release patterns within the septum in rats bred for low and high anxiety. European Journal of Neuroscience, 26(12), 3597–3605. https://doi.org/https://doi.org/10.1111/j.1460-9568.2007.05974.x
- Berends, Y. R., Tulen, J. H. M., Wierdsma, A. I., Van Pelt, J., Feldman, R., Zagoory-Sharon, O., de Rijke, Y. B., Kushner, S. A., & van Marle, H. J. C. (2019a). Intranasal administration of oxytocin decreases task-related aggressive responses in healthy young males. Psychoneuroendocrinology, 106(March), 147–154. https://doi.org/https://doi.org/10.1016/j.psyneuen.2019.03.027
- Berends, Y. R., Tulen, J. H. M., Wierdsma, A. I., Van Pelt, J., Kushner, S. A., & van Marle, H. J. C. (2019b). Oxytocin, vasopressin and trust: Associations with aggressive behavior in healthy young males. Physiology & Behavior, 204(May), 180–185. https://doi.org/https://doi.org/10.1016/j.physbeh.2019.02.027
- Bertsch, K., Schmidinger, I., Neumann, I. D., & Herpertz, S. C. (2013). Reduced plasma oxytocin levels in female patients with borderline personality disorder. Hormones and Behavior, 63(3), 424–429. https://doi.org/https://doi.org/10.1016/j.yhbeh.2012.11.013
- Blair, R. J. R. (2003). Neurobiological basis of psychopathy. The British Journal of Psychiatry, 182(1), 5–7. https://doi.org/https://doi.org/10.1192/bjp.182.1.5
- Bosch, O. J., Meddle, S. L., Beiderbeck, D. I., Douglas, A. J., & Neumann, I. D. (2005). Brain oxytocin correlates with maternal aggression: Link to anxiety. Journal of Neuroscience, 25(29), 6807–6815. https://doi.org/https://doi.org/10.1523/JNEUROSCI.1342-05.2005
- Bosch, O. J., & Neumann, I. D. (2010). Vasopressin released within the central amygdala promotes maternal aggression. European Journal of Neuroscience, 31(5), 883–891. https://doi.org/https://doi.org/10.1111/j.1460-9568.2010.07115.x
- Brunnlieb, C., Münte, T. F., Krämer, U., Tempelmann, C., & Heldmann, M. (2013). Vasopressin modulates neural responses during human reactive aggression. Social Neuroscience, 8(2), 148–164. https://doi.org/https://doi.org/10.1080/17470919.2013.763654
- Buck, N. M. L., Cima, M., Lancel, M., & Van Marle, H. J. C. (2012). On the explanation of the hostile attributional bias: Traumatic experiences, schemas, and migrant status. Journal of Aggression, Maltreatment & Trauma, 21(2), 223–236. https://doi.org/https://doi.org/10.1080/10926771.2012.639051
- Calcagnoli, F., De Boer, S. F., Althaus, M., den Boer, J. A., & Koolhaas, J. M. (2013). Antiaggressive activity of central oxytocin in male rats. Psychopharmacology, 229(4), 639–651. https://doi.org/https://doi.org/10.1007/s00213-013-3124-7
- Calcagnoli, F., Stubbendorff, C., Meyer, N., De Boer, S. F., Althaus, M., & Koolhaas, J. M. (2015). Oxytocin microinjected into the central amygdaloid nuclei exerts anti-aggressive effects in male rats. Neuropharmacology, 90(March), 74–81. https://doi.org/https://doi.org/10.1016/j.neuropharm.2014.11.012
- Caldwell, H. K., Lee, H. J., Macbeth, A. H., & Young, W. S. (2008). Vasopressin: Behavioral roles of an “original” neuropeptide. Progress in Neurobiology, 84(1), 1–24. https://doi.org/https://doi.org/10.1016/j.pneurobio.2007.10.007
- Campbell, A., & Hausmann, M. (2013). Effects of oxytocin on women ’ s aggression depend on state anxiety. Aggressive Behavior, 39(April), 316–322. https://doi.org/https://doi.org/10.1002/ab.21478
- Cima, M., Raine, A., Meesters, C., & Popma, A. (2013). Validation of the Dutch reactive proactive questionnaire (RPQ): Differential correlates of reactive and proactive aggression from childhood to adulthood. Aggressive Behavior, 39(2), 99–113. https://doi.org/https://doi.org/10.1002/ab.21458
- Coccaro, E. F., Kavoussi, R. J., Hauger, R. L., Cooper, T. B., & Ferris, C. F. (1998). Cerebrospinal Fluid Vasopressin Levels. Archives General Psychiatry, 55(8), 708–714. https://doi.org/https://doi.org/10.1001/archpsyc.55.8.708
- Cohen, D., & Shamay-Tsoory, S. G. (2018). Oxytocin regulates social approach. Social Neuroscience, 13(6), 680–687. https://doi.org/https://doi.org/10.1080/17470919.2017.1418428
- Cooke, D. J., Michie, C., Hart, S. D., & Clark, D., (2005). Assessing psychopathy in the UK: concerns about cross-cultural generalisability. British Journal of Psychiatry 186, 335–341. https://doi.org/https://doi.org/10.1192/bjp.186.4.335
- Cordero, M. I., Ansermet, F., & Sandi, C. (2013). Long-term program- ming of enhanced aggression by peripuberty stress in female rats. Psychoneuroendocrinology, 38(11), 2758–2769. https://doi.org/https://doi.org/10.1016/j.psyneuen.2013.07.005
- Dadds, M. R., Moul, C., Cauchi, A., Dobson-Stone, C., Hawes, D. J., Brennan, J., & Ebstein, R. E. (2014). Methylation of the oxytocin receptor gene and oxytocin blood levels in the development of psychopathy. Development and Psychopathology, 26(1), 33–40. https://doi.org/https://doi.org/10.1017/S0954579413000497
- De Dreu, C. K. W., & Kret, M. E. (2016). Oxytocin conditions intergroup relations through upregulated in-group empathy, cooperation, conformity, and defense. Biological Psychiatry, 79(3), 165–173. https://doi.org/https://doi.org/10.1016/j.biopsych.2015.03.020
- De Dreu, C. K. W., Shalvi, S., Greer, L. L., Van Kleef, G. A., Handgraaf, M. J. J., & Denson, T. (2012). Oxytocin motivates non-cooperation in intergroup conflict to protect vulnerable in-group members. PloS One, 7(11), e46751. https://doi.org/https://doi.org/10.1371/journal.pone.0046751
- Declerck, C. H., Boone, C., & Kiyonari, T. (2010). Oxytocin and cooperation under conditions of uncertainty: The modulating role of incentives and social information. Hormones and Behavior, 57(3), 368–374. https://doi.org/https://doi.org/10.1016/j.yhbeh.2010.01.006
- Dhakar, M. B., Rich, M. E., Reno, E. L., Lee, H.-J., & Caldwell, H. K. (2012). Heightened aggressive behavior in mice with lifelong versus postweaning knockout of the oxytocin receptor. Hormones and Behavior, 62(1), 86–92. https://doi.org/https://doi.org/10.1016/j.yhbeh.2012.05.007
- Diagnostic and Statistical Manual of Mental Disorders. (2000). Text Revision (DSM-IV-TR) (Vol. 1). Fourth. American Psychiatric Association.
- Elabd, C., Cousin, W., Upadhyayula, P., Chen, R. Y., Chooljian, M. S., Li, J., Kung, S., Jiang, K. P., & Conboy, I. M. (2015). Oxytocin is an age-specific circulating hormone that is necessary for muscle maintenance and regeneration. Nature Communications, 5(4082), 1–26. https://doi.org/https://doi.org/10.1038/ncomms5082
- Elliott Albers, H., Dean, A., Karom, M. C., Smith, D., & Huhman, K. L. (2006). Role of V1a vasopressin receptors in the control of aggression in Syrian hamsters. Brain Research, 1073–1074(February), 425–430. https://doi.org/https://doi.org/10.1016/j.brainres.2005.12.081
- Fairchild, G., Baker, E., & Eaton, S. (2018). Hypothalamic-Pituitary-Adrenal axis function in children and adults with severe antisocial behavior and the impact of early adversity. Current Psychiatry Reports, 20(10), 84. https://doi.org/https://doi.org/10.1007/s11920-018-0952-5
- Feldman, R. (2012). Oxytocin and social affiliation in humans. Hormones and Behavior, 61(3), 380–391. https://doi.org/https://doi.org/10.1016/j.yhbeh.2012.01.008
- Fodor, A., Barsvari, B., Aliczki, M., Balogh, Z., Zelena, D., Goldberg, S. R., & Haller, J. (2014). The effects of vasopressin deficiency on aggression and impulsiveness in male and female rats. Psychoneuroendocrinology, 47(September), 141–150. https://doi.org/https://doi.org/10.1016/j.psyneuen.2014.05.010
- Gedeon, T., Parry, J., & Völlm, B. (2019). The role of oxytocin in antisocial personality disorders: A systematic review of the literature. Frontiers in Psychiatry, 10(February), 76. https://doi.org/https://doi.org/10.3389/fpsyt.2019.00076
- Gerra, L. M., Gerra, G., Mercolini, L., Manfredini, M., Somaini, L., Pieri, C. M., Antonioni, M., Protti, M., Ossola, P., & Marchesi, C. (2017). Increased oxytocin levels among abstinent heroin addicts: Association with aggressiveness, psychiatric symptoms and perceived childhood neglect. Progress in Neuro-psychopharmacology & Biological Psychiatry, 75(April), 70–76. https://doi.org/https://doi.org/10.1016/j.pnpbp.2017.01.005
- Graugaard-Jensen, C., Hvistendahl, G. M., Frøkiær, J., Bie, P., & Djurhuus, J. C. (2017). Oral contraceptives and renal water handling: A diurnal study in young women. Physiological Reports, 5(23), 1–9. https://doi.org/https://doi.org/10.14814/phy2.13547
- Hare, R. D. (2003). The Hare Psychopathy Checklist-Revised, 2nd edition. Toronto, ON: Multi-Health Systems.
- Hovey, D., Lindstedt, M., Zettergren, A., Jonsson, L., Johansson, A., Melke, J., Kerekes, N., Anckasäter, H., Lichtenstein, P., Lundström, S., & Westberg, L. (2016). Antisocial behavior and polymorphisms in the oxytocin receptor gene: Findings in two independent samples. Molecular Psychiatry, 21(7), 983–988. https://doi.org/https://doi.org/10.1038/mp.2015.144
- Huffmeijer, R., Van Ijzendoorn, M. H., & Bakermans-Kranenburg, M. J. (2012). Ageing and oxytocin: A call for extending human oxytocin research to ageing populations - A mini-review. Gerontology, 59(1), 32–39. https://doi.org/https://doi.org/10.1159/000341333
- Iwasaki, Y., Kumari, P., Wang, L., Hidema, S., Nishimori, K., & Yada, T. (2019). Relay of peripheral oxytocin to central oxytocin neurons via vagal afferents for regulating feeding. Biochemical and Biophysical Research Communications, 519(3), 553–558. https://doi.org/https://doi.org/10.1016/j.bbrc.2019.09.039
- Kagerbauer, S. M., Debus, J. M., Martin, J., Gempt, J., Jungwirth, B., Hapfelmeier, A., & Podtschaske, A. H. (2019). Absence of a diurnal rhythm of oxytocin and arginine-vasopressin in human cerebrospinal fluid, blood and saliva. Neuropeptides, 78(October), 101977. https://doi.org/https://doi.org/10.1016/j.npep.2019.101977
- Kiss, A., Bundzikova, J., Pirnik, Z., & Mikkelsen, J. D. (2010). Different antipsychotics elicit different effects on magnocellular oxytocinergic and vasopressinergic neurons as revealed by Fos immunohistochemistry. Journal of Neuroscience Research, 88(3), 677–685. https://doi.org/https://doi.org/10.1002/jnr.22226
- Kosfeld, M., Heinrichs, M., Zak, P. J., Fischbacher, U., & Fehr, E. (2005). Oxytocin increases trust in humans. Nature, 435(7042), 673–676. https://doi.org/https://doi.org/10.1038/nature03701
- Lee, R., Ferris, C., Van de Kar, L. D., & Coccaro, E. F. (2009). Cerebrospinal fluid oxytocin, life history of aggression, and personality disorder. Psychoneuroendocrinology, 34(10), 1567–1573. https://doi.org/https://doi.org/10.1016/j.psyneuen.2009.06.002
- Leng, G., & Sabatier, N. (2016). Measuring oxytocin and vasopressin: Bioassays, immunoassays and random numbers. Journal of Neuroendocrinology, 28(10), 1-13. https://doi.org/https://doi.org/10.1111/jne.12413
- Levy, T., Bloch, Y., Bar-Maisels, M., Gat-Yablonski, G., Djalovski, A., Borodkin, K., & Apter, A. (2015). Salivary oxytocin in adolescents with conduct problems and callous-unemotional traits. European Child & Adolescent Psychiatry, 24(12), 1543–1551. https://doi.org/https://doi.org/10.1007/s00787-015-0765-6
- Lischke, A., Herpertz, S. C., Berger, C., Domes, G., & Gamer, M. (2017). Divergent effects of oxytocin on (para-)limbic reactivity to emotional and neutral scenes in females with and without borderline personality disorder. Social Cognitive and Affective Neuroscience, 12(11), 1783–1792. https://doi.org/https://doi.org/10.1093/scan/nsx107
- Loomans, M. M., Tulen, J. H. M., de Rijke, Y. B., & van Marle, H. J. C. (2016). A hormonal approach to anti-social behaviour. Criminal Behaviour and Mental Health, 26(5), 380–394. https://doi.org/https://doi.org/10.1002/cbm.1968
- Mann, F. D., . L., Paul, S., Tackett, J. L., Tucker-Drob, E. M., & Harden, K. P. (2018). Personality risk for antisocial behavior: Testing the intersections between callous–unemotional traits, sensation seeking, and impulse control in adolescence. Development and Psychopathology, 30(1), 267–282. https://doi.org/https://doi.org/10.1017/S095457941700061X
- Martin, J., Kagerbauer, S. M., Gempt, J., Podtschaske, A., Hapfelmeier, A., & Schneider, G. (2018). Oxytocin levels in saliva correlate better than plasma levels with concentrations in the cerebrospinal fluid of patients in neurocritical care. Journal of Neuroendocrinology, 30(5), 1–9. https://doi.org/https://doi.org/10.1111/jne.12596
- Mitchell, I. J., Smid, W., Troelstra, J., Wever, E., Ziegler, T. E., & Beech, A. R. (2013). Psychopathic characteristics are related to high basal urinary oxytocin levels in male forensic patients. Journal of Forensic Psychiatry & Psychology, 24(3), 309–318. https://doi.org/https://doi.org/10.1080/14789949.2013.773455
- Nave, G., Camerer, C., & McCullough, M. (2015). Does oxytocin increase trust in humans? A critical review of research. Perspectives on Psychological Science, 10(6), 772–789. https://doi.org/https://doi.org/10.1177/1745691615600138
- Ne’eman, R., Perach-Barzilay, N., Fischer-Shofty, M., Atias, A., & Shamay-Tsoory, S. G. (2016). Intranasal administration of oxytocin increases human aggressive behavior. Hormones and Behavior, 80(April), 125–131. https://doi.org/https://doi.org/10.1016/j.yhbeh.2016.01.015
- Olff, M., Frijling, J. L., Kubzansky, L. D., Bradley, B., Ellenbogen, M. A., Cardoso, C., Bartz, J. A., Yee, J. R., & van Zuiden, M. (2013). The role of oxytocin in social bonding, stress regulation and mental health: An update on the moderating effects of context and interindividual differences. Psychoneuroendocrinology, 38(9), 1883–1894. https://doi.org/https://doi.org/10.1016/j.psyneuen.2013.06.019
- Pope, H. G., Kouri, E. M., & Hudson, J. I. (2000). Effects of supraphysiologic doses of testosterone on mood and aggression in normal men. Archives General Psychiatry, 57(2), 133–140.
- Raine, A. (2013). The anatomy of violence: The biological roots of crime. Pantheon books.
- Raine, A., Dodge, K., Loeber, R., Gatzke-kopp, L., Lynam, D., Stouthamer-loeber, M., Liu, J., & Liu, J. (2006). The reactive-proactive aggression questionaire: Diferential correlates of reactive and proactive aggression in adolescent boys. Aggressive Behavior, 32(2), 159–171. https://doi.org/https://doi.org/10.1002/ab.20115.The
- Reyes, T. L., Galinsky, A. M., Hoffmann, J. N., You, H. M., Ziegler, T. E., & McClintock, M. K. (2014). Social peptides: Measuring Urinary oxytocin and vasopressin in a home field study of older adults at risk for dehydration. The Journals of Gerontology. Series B, Psychological Sciences and Social Sciences, 69(Suppl 2), S229–S237. https://doi.org/https://doi.org/10.1093/geronb/gbu104
- Ring, R. H., Malberg, J. E., Potestio, L., Ping, J., Boikess, S., Luo, B., … Rosenzweig-Lipson, S. (2006). Anxiolytic-like activity of oxytocin in male mice: behavioral and autonomic evidence, therapeutic implications. Psychopharmacology, 185(2), 218–225. https://doi.org/https://doi.org/10.1007/s00213-005-0293–z
- Rutigliano, G., Rocchetti, M., Paloyelis, Y., Gilleen, J., Sardella, A., Cappucciati, M., Palombini, E., Dell’Osso, L., Caverzasi, E., Politi, P., McGuire, P., & Fusar-Poli, P. (2016). Peripheral oxytocin and vasopressin: Biomarkers of psychiatric disorders? A comprehensive systematic review and preliminary meta-analysis. Psychiatry Research, 241(July), 207–220. https://doi.org/https://doi.org/10.1016/j.psychres.2016.04.117
- Salonia, A., Nappi, R. E., Pontillo, M., Daverio, R., Smeraldi, A., Briganti, A., Fabbri, F., Zanni, G., Rigatti, P., & Montorsi, F. (2005). Menstrual cycle-related changes in plasma oxytocin are relevant to normal sexual function in healthy women. Hormones and Behavior, 47(2), 164–169. https://doi.org/https://doi.org/10.1016/j.yhbeh.2004.10.002
- Shamay-Tsoory, S. G., & Abu-Akel, A. (2016). The social salience hypothesis of oxytocin. Biological Psychiatry, 79(3), 194–202. https://doi.org/https://doi.org/10.1016/j.biopsych.2015.07.020
- Silva-Santos, S., van Woerden, G. M., Bruinsma, C. F., Mientjes, E., Jolfaei, M. A., Distel, B., Kushner, S. A., & Elgersma, Y. (2015). Ube3a reinstatement identifies distinct developmental windows in a murine Angelman syndrome model. Journal of Clinical Investigation, 125(5), 2069–2076. https://doi.org/https://doi.org/10.1172/JCI80554
- Timmermann, M., Jeung, H., Schmitt, R., Boll, S., Freitag, C. M., Bertsch, K., & Herpertz, S. C. (2017). Oxytocin improves facial emotion recognition in young adults with antisocial personality disorder. Psychoneuroendocrinology, 85(April), 158–164. https://doi.org/https://doi.org/10.1016/j.psyneuen.2017.07.483
- Tschiesner, R. (2012). Sensation Seeking, Traumaerleben und Bewältigungsstrategien: Eine empirische Untersuchung an Einsatzkräften. Neuropsychiatrie, 26(1), 28–34. https://doi.org/https://doi.org/10.1007/s40211-012-0002-1
- Valstad, M., Alvares, G. A., Egknud, M., Matziorinis, A. M., Andreassen, O. A., Westlye, L. T., & Quintana, D. S. (2017). The correlation between central and peripheral oxytocin concentrations: A systematic review and meta-analysis. Neuroscience and Biobehavioral Reviews, 78(February), 117–124. https://doi.org/https://doi.org/10.1016/j.neubiorev.2017.04.017
- Van Dam, J. M., Garrett, A. J., Schneider, L. A., Buisman-Pijlman, F. T. A., Short, M. A., Hodyl, N. A., Edwards, H. K., Goldsworthy, M. R., & Pitcher, J. B. (2018). Variability of the cortisol awakening response and morning salivary oxytocin in late adolescence. Journal of Neuroendocrinology, 30(11), 1–9. https://doi.org/https://doi.org/10.1111/jne.12645
- Van Ockenburg, S. L., Booij, S. H., Riese, H., Rosmalen, J. G. M., & Janssens, K. A. M. (2015). How to assess stress biomarkers for idiographic research? Psychoneuroendocrinology, 62(December), 189–199. https://doi.org/https://doi.org/10.1016/j.psyneuen.2015.08.002
- Veenema, A. H., Blume, A., Niederle, D., Buwalda, B., & Neumann, I. D. (2006). Effects of early life stress on adult male aggression and hypothalamic vasopressin and serotonin. The European Journal of Neuroscience, 24(6), 1711–1720. https://doi.org/https://doi.org/10.1111/j.1460-9568.2006.05045.x
- Veenema, A. H., & Neumann, I. D. (2008). Central vasopressin and oxytocin release: Regulation of complex social behaviours. In Advances in vasopressin and oxytocin; from genes to behaviour to disease (Vol. 170, 8, pp. 261–276). Elsevier. https://doi.org/https://doi.org/10.1016/S0079-6123(08)00422-6
- Vogel, F., Wagner, S., Başkaya, Ö., Leuenberger, B., Mobascher, A., Dahmen, N., Lieb, K., & Tadić, A. (2012). Variable number of tandem repeat polymorphisms of the arginine vasopressin receptor 1A gene and impulsive aggression in patients with borderline personality disorder. Psychiatric Genetics, 22(2), 105–106. https://doi.org/https://doi.org/10.1097/YPG.0b013e32834accad
- Waller, R., Corral-Frías, N. S., Vannucci, B., Bogdan, R., Knodt, A. R., Hariri, A. R., & Hyde, L. W. (2016). An oxytocin receptor polymorphism predicts amygdala reactivity and antisocial behavior in men. Social Cognitive and Affective Neuroscience, 11(8), 1218–1226. https://doi.org/https://doi.org/10.1093/scan/nsw042
- Windle, R. J., Shanks, N., Lightman, S. L., & Ingram, C. D. (1997). Central oxytocin administration reduces stress-induced corticosterone release and anxiety behavior in rats. Endocrinology, 138(7), 2829–2834. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9202224
- Yagi, K., & Onaka, T. (1996). A benzodiazepine, chlordiazepoxide, blocks vasopressin and oxytocin release after footshocks but not osmotic stimulus in the rat. Neuroscience Letters, 203(1), 49–52. https://doi.org/https://doi.org/10.1016/0304-3940(95)12262-1
- Zik, J. B., & Roberts, D. L. (2015). The many faces of oxytocin: Implications for psychiatry. Psychiatry Research, 226(1), 31–37. https://doi.org/https://doi.org/10.1016/j.psychres.2014.11.048