Abstract
Aim: To describe real-world biomarker testing, treatment and survival in stage IA–IIIC non-small-cell lung cancer (NSCLC). Methods: Electronic records of USA-based patients in the CancerLinQ Discovery® database with stage IA–IIIC NSCLC (diagnosed between 2014 and 2018) were screened; a curated cohort of 14,452 records was identified for further analysis. Results: Of 3121 (21.6%) patients who had EGFR testing, 493 (15.8%) were EGFR-mutation positive. Of 974 patients who underwent surgical resection, 513 (52.7%) received adjuvant therapy. A quarter of patients with EGFR-mutation positive NSCLC received targeted adjuvant therapy. Conclusion: Approximately a fifth of patients underwent EGFR testing; biomarker testing is important to ensure optimal outcomes for patients with stage I–III NSCLC.
Plain language summary
A study investigating how many patients with early-stage non-small-cell lung cancer (NSCLC) had mutations in a protein called EGFR and which treatments they received in routine clinical practice: The treatment recommended by medical experts for stage IA–IIIA non-small-cell lung cancer (NSCLC) is surgical removal of the growth (tumor). Patients with stage II or III, and some with stage IB disease, are recommended to receive treatment with medications such as chemotherapy or oral cancer treatments after surgery (adjuvant treatment). In some lung cancers, there are mutations in a protein called EGFR. Osimertinib, a drug that blocks the activity of mutated EGFR on cancer cells, reducing their growth and spread, is recommended as an adjuvant treatment for patients with EGFR-mutated, stage IB–IIIA NSCLC. This study aimed to understand how many patients with stage I–III NSCLC have tumors with EGFR mutations, and which treatments patients received in everyday clinical practice, before new medicines such as osimertinib (that treat EGFR-mutated NSCLC) were recommended. We looked at anonymous data from 14,452 patients with stage I–III NSCLC treated at cancer clinics in the USA between 2014 and 2018. We found that 3121 (21.6%) patients had an EGFR mutation test and 493 (15.8%) had EGFR-mutation positive NSCLC. Of patients who had surgery to remove the tumor, 55% received adjuvant therapy (treatment after surgery). It is important to perform EGFR mutation testing in patients with stage IB–IIIA NSCLC so that patients with EGFR-mutation positive NSCLC can receive appropriate treatment.
Stage I–IIIA non-small-cell lung cancer (NSCLC) is typically treated surgically; adjuvant chemotherapy is recommended in patients with stage II/III, and select stage IB disease.
Osimertinib, an epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitor, is now approved for adjuvant treatment of resected EGFR-mutation positive stage IB–IIIA NSCLC. Immunotherapies have also been recently approved as neoadjuvant or adjuvant treatment in resectable NSCLC.
Given these recent advancements, we assessed real-world biomarker testing patterns, and treatment and survival of patients with stage I–III NSCLC using data from the CancerLinQ Discovery® database, a health information platform that collects real-world data from oncology clinics in the USA.
A cohort of 14,452 adults patients diagnosed with stage IA–IIIC NSCLC between January 2014 and December 2018 was identified.
Of this cohort, 21.6% had at least one EGFR mutation test between the index date (date of diagnosis) and end of follow-up, with around two-thirds of these tests performed in the first 60 days following NSCLC diagnosis.
Frequency of EGFR testing increased with increasing severity of NSCLC disease stage; female and Asian patients were more likely to receive an EGFR test.
Of those with an EGFR test, 15.8% had EGFR-mutation positive (EGFRm) NSCLC; EGFRm NSCLC was more common in females, non-smokers and Asian patients.
Surgical resection was the most common treatment for stage I and II NSCLC, while systemic therapy and/or radiotherapy without surgery was most common in stage III disease.
Among patients who had resection, almost half did not receive a neoadjuvant or adjuvant treatment.
Survival decreased with increasing disease stage; among patients with known EGFR status, median overall survival was longer in patients with EGFRm NSCLC than those with EGFRwt NSCLC.
1. Background
Lung cancer is the third most common cancer type in the USA, with non-small-cell lung cancer (NSCLC) accounting for approximately 80% of lung cancer diagnoses [Citation1]. Whereas the majority of patients with NSCLC present with advanced and/or metastatic disease (stage IV), approximately 40% are diagnosed in stage I–III in the USA [Citation2]. Survival rates vary considerably by stage of diagnosis. Five-year survival is 70–85% for stage IA and 60–75% for stage IB versus 30–45% for stage IIIA, while patients with stage IV NSCLC rarely achieve long-term survival [Citation3–5]. As a result, early diagnosis and treatment of NSCLC is critical to prolonging patient lives.
Stage I–IIIA NSCLC is typically treated surgically, with adjuvant chemotherapy recommended for patients with stage II or III disease, as well as some patients with high-risk stage IB disease (NCCN Clinical Practice Guidelines in Oncology [NCCN Guidelines®]) [Citation6]. However, postsurgical recurrence rates remain high [Citation7] and the addition of adjuvant chemotherapy has shown a modest survival benefit of ∼5% at 5 years [Citation8,Citation9]. Drugs that target oncogenic driver mutations have become standard of care for advanced NSCLC. Currently, these include kinase inhibitors that target NSCLC carrying sensitizing mutations of EGFR, as well as those targeting ALK rearrangements, ROS1 alterations, B-raf proto-oncogene mutations, ERBB2 mutations, KRAS proto-oncogene mutations, MET exon 14 skipping variants, RET rearrangements and NTRK gene fusions (NCCN Guidelines®) [Citation6]. However, not all of these are indicated or recommended for first-line treatment.
Osimertinib is a third-generation, irreversible, central nervous system-active, oral EGFR-tyrosine kinase inhibitor (TKI), and is the preferred first-line treatment for EGFR-mutation positive (EGFRm) advanced NSCLC and the standard treatment for patients with acquired T790M following progression on other first-line EGFR-TKIs [Citation10–16]. More recently, the phase III ADAURA trial (NCT02511106) demonstrated that patients with surgically-resected stage IB–IIIA EGFRm NSCLC had significantly longer disease-free survival (DFS) and overall survival (OS) if receiving adjuvant osimertinib compared with placebo (DFS: hazard ratio [HR]: 0.27; 95% CI: 0.21–0.34; OS for stage IB–IIIA: HR: 0.49; 95.03% CI: 0.34–0.70) [Citation17–19]. Osimertinib was the first EGFR-TKI approved (in December 2020) for the adjuvant treatment of adult patients with resected stage IB–IIIA EGFRm NSCLC and is now widely approved for clinical use, including in the USA, Japan, China and the European Union [Citation20–23].
While genetic testing for actionable driver oncogenes is routine practice for patients with advanced NSCLC, there are limited data about the frequency of genetic testing in patients with stage I–III NSCLC or the frequency with which these patients are found to harbor EGFR mutations. In addition to the approval of osimertinib as an adjuvant treatment, immunotherapies have also recently been approved as neoadjuvant, adjuvant or perioperative treatment for patients with resectable NSCLC [Citation24–26]. The CancerLinQ Discovery® (CLQD) database is a health information platform that collects real-world data directly from patient electronic health records (EHRs) from oncology clinics in the USA. Here, records in the CLQD were retrospectively analyzed to summarize EGFR testing patterns before adjuvant osimertinib became the standard of care, as well as neoadjuvant and adjuvant treatment patterns and survival of patients with stage I–III NSCLC, to establish a benchmark against which future studies can measure improvement. Retrospective data can also contribute to our understanding of how clinical practice has changed, and the impact of new treatments. This study aimed to determine the proportion of EGFRm NSCLC by disease stage and age group, to determine OS by disease stage, age group and EGFR mutation status and to summarize treatment patterns in a cohort of patients with stage I–III NSCLC prior to approval of adjuvant osimertinib.
2. Methods
2.1. Data source, cohort selection & categorization
The CLQD process for collecting and curating patient EHRs has been previously described [Citation27]. At the time of this analysis, CLQD included clinical data aggregated from approximately 46 academic cancer centers and community clinics in the USA, representing more than one million patient records, including approximately 150,000 patients with NSCLC. The CLQD database was searched to identify patients who received an initial diagnosis of stage IA–IIIC NSCLC, and this cohort was refined to include patients who had received their initial diagnosis (the index date) between 1 January 2014 and 31 December 2018 to assess the treatment landscape prior to the approval of osimertinib as adjuvant treatment. The cohort was then further refined to include only patients who were at least 18 years of age. Patients with other co-existing primary cancer diagnoses at any point before index, except basal cell carcinoma, were excluded. EHRs of eligible patients were then examined for a documented EGFR genetic test (it was possible for patients to receive a test after progression to stage IV). If a patient had more than one EGFR mutation-positive test, the first positive test was used for this analysis. If a patient had EGFR mutation-positive and -negative test results, the first positive result was used.
Patients were assigned an EGFR mutation status according to the following definitions: EGFRm, reference to ‘EGFR positive’ or ‘EGFR genetic mutation’; EGFR wild-type (EGFRwt), reference to ‘negative’, ‘not detected’, ‘negative genetic finding’ or ‘wild-type’.
Presence of an EGFR test, with results available, was recorded within two timeframes: first, within 30 days before to any time after the index date and second, within 30 days before to 60 days following the index date. It was anticipated that an EGFR test recorded between 30 days before to 60 days following the index date would likely correspond to EGFR testing for stage I–III disease at the time of diagnosis, while tests recorded at any time after index may also include EGFR tests requested following recurrence. The primary objectives of the study were to determine the proportion of EGFRm NSCLC by disease stage and age group, assess OS by disease stage, age group and EGFR mutation status and summarize treatment patterns in the neoadjuvant and adjuvant settings. Other recorded outcomes included ALK, ROS1 and PD-L1 test results (tests performed within 30 days before to any time after the index date, rearrangements/expression status) overall and by NSCLC stage (I, II, III); as the CancerLinQ database reports information related to disease substages inconsistently (with some centers reporting information by substage [i.e. IIIA, IIIB and IIIC] and others reporting information by stage [i.e. III], we have reported results by stage [I, II and III]). The biomarkers were selected based on testing recommendations during the selection period. The proportion of EGFR mutations by patient age (<65 years, ≥65 years), sex (male, female) and ethnicity (white, black or African–American, Asian); OS by EGFR mutation status (EGFRm, EGFRwt); and treatment patterns (defined in Supplementary Table S1) overall and by NSCLC stage (I, II, III) and EGFR mutation status (EGFRm, EGFRwt) were also recorded. Data were collected for up to 12 months prior to diagnosis (pre-index period) for assessment of eligibility and for the follow-up period, which ran from the index date until death or the study cut-off date of 30 September 2020, allowing a potential minimum 21 months of post-index follow-up (Supplementary Figure S1A).
OS was defined as the time between the index date and death. Any patient who had not died before the cut-off date was right censored on their date of last clinical activity. Time to event analyses were performed using the Kaplan–Meier estimator. A Cox proportional hazard model with Efron approximation was used to calculate adjusted HRs of overall survival for patients with EGFRm NSCLC versus patients with EGFRwt NSCLC, after controlling for the effects of the following covariates: NSCLC stage at initial diagnosis; patient sex; age at initial diagnosis; ethnicity; smoking history; and treatment with surgery for the purpose of resection.
3. Results
3.1. Study cohort
The CLQD database included EHRs (with curated content) for 33,486 patients with stage IA–IIIC NSCLC, of whom 14,452 were adult patients who received the initial diagnosis between 2014 and 2018 (Supplementary Figure S1B). A total of 3121 of these patients (21.6%) had at least one EGFR genetic test administered from 30 days prior to index until the end of follow-up (up to 80.3 months of follow-up with a median of 24.4 months).
The majority of the 3121 patients tested were ≥65 years of age (62.4%), over half were female (55.6%) and most were white (72.9%); these proportions were similar across NSCLC stage I, II and III subgroups. Most patients had tumors with a non-squamous histology (85.6%; Supplementary Table S2).
3.2. EGFR testing patterns
In total, 65.5% of EGFR tests were administered in the 60 days following initial NSCLC diagnosis and thus were considered to examine the EGFR mutational status at the time of diagnosis of stage I–III NSCLC, while the other 34.5% were administered after the 60-day period and thus may include EGFR tests performed after recurrence (Supplementary Figure S1B). The frequency of EGFR testing was found to increase with increasing NSCLC disease stage (17.6, 22.4 and 25.2% of patients for stage I, II and III NSCLC, respectively, received an EGFR test at any time; Supplementary Figure S1B). In terms of patient characteristics, median age at diagnosis was similar among patients who received an EGFR test versus those who did not; however, older patients were less likely to receive an EGFR test. Female and Asian patients were more likely to receive an EGFR test compared with male and non-Asian patients (Supplementary Table S3).
3.3. EGFR mutation status
Of the 3121 patients with a documented EGFR test at any time during follow-up, 2466 (79.0%) did not harbor an EGFRm (EGFRwt), 493 (15.8%) had EGFRm and 162 (5.2%) had an unsuccessful/indeterminate result. Patients with stage I disease had a higher proportion of EGFRm than those with stage II or III disease (19.9, 13.2 and 13.9%, respectively; A). Overall and by stage, the proportions of patients with EGFR tests reporting EGFRwt or EGFRm were almost identical for the subset of tests executed within 60 days following initial diagnosis, suggesting that tumor EGFR mutational status remained constant between initial diagnosis and later progressive disease (A & Supplementary Table S4).
Figure 1. The proportion of EGFRm NSCLC by disease stage, patient age and sex. (A) Bars show the percentage of electronic health records from patients with stage I–III NSCLC where genetic testing returned a result of EGFRwt or EGFRm, or the result was not recorded/unsuccessful. ‘Any time' refers to results of tests conducted at diagnosis or during follow-up. ‘Within 60 days’ refers to the subset of tests conducted within 60 days of the index date. (B) Bars show the percentage of EHRs that reported EGFRwt or EGFRm stage I, II or III NSCLC for patients 65 years and over versus under 65 years. (C) Bars show the percentage of EHRs that reported EGFRwt or EGFRm stage I, II or III NSCLC from male versus female patients. Values under the bars show the total number of patient EHRs.
EGFRm: EGFR-mutation positive; EGFRwt: EGFR wild-type; EHR: Electronic health record; NSCLC: Non-small-cell lung cancer.
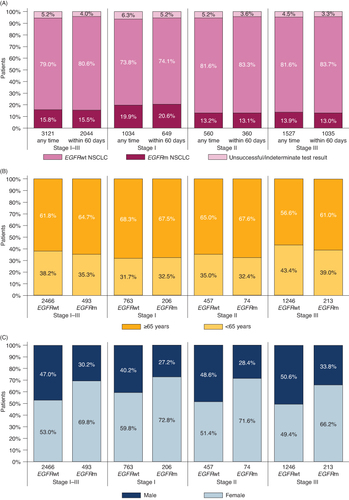
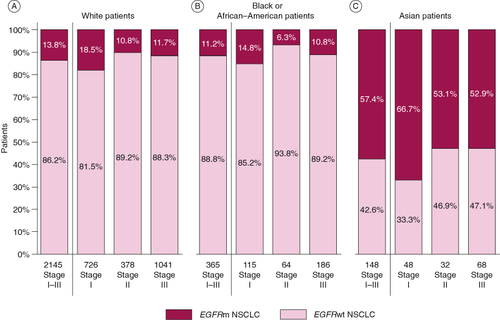
The distribution of EGFRm across age groups (≥65 years and <65 years) was similar to that of EGFRwt across all stages combined and for individual stages (B). In contrast, although the proportion of males and females was even among patients with EGFRwt NSCLC (47.0 and 53.0%, respectively), females accounted for the majority of patients with EGFRm (69.8% across all stages and 72.8, 71.6 and 66.2% across stages I, II and III; C). The proportion of patients with EGFRm NSCLC among White and Black/African–American patient populations was generally similar to that seen in the overall population (18.5, 10.8, 11.7 and 14.8, 6.3, 10.8% respectively, for stages I, II and III; A & B), however the proportion of Asian patients with EGFRm was much higher (66.7, 53.1 and 52.9% for stages I, II and III; C).
Figure 2. The frequency of EGFRm NSCLC by patient ethnicity. (A–C) Bars show the percentage of electronic health records from (A) White, (B) Black or African–American or (C) Asian patients that reported stage I, II or III NSCLC that was EGFRwt or EGFRm. Values under the bars show the total number of patient EHRs.
EGFRm: EGFR-mutation positive; EGFRwt: EGFR wild-type; EHR: Electronic health record; NSCLC: Non-small-cell lung cancer.
Fewer patients with EGFRm had a history of smoking than those with EGFRwt NSCLC (41.4 and 81.8%, respectively) and a larger proportion of EGFRm tumors than EGFRwt were non-squamous (96.3 and 83.9%, respectively).
3.4. Testing for additional clinically relevant biomarkers
Among all patients with stage I–III NSCLC who had a documented EGFR test (n = 3121), the majority (∼80%; stage I: 77.5%, stage II: 84.8%, stage III: 82.6%) also had tests for ALK alterations, while around half had tests for ROS1 alterations and approximately a third had tests for PD-L1 expression level at any time during follow-up (). Across all stages, over 90% of ALK and ROS1 tests were negative, while ∼30% of PD-L1 tests indicated positive PD-L1 expression. Very few tumors which had ALK or ROS1 alterations also had EGFRm (0.3 and 0.6% of tumors, respectively). Less than 5% of stage I–III NSCLC tumors that expressed PD-L1 were also EGFRm ().
Table 1. ALK, ROS1 and PD-L1 testing in patients with stage I–III NSCLC with a documented EGFR test at any time during follow-up.
3.5. Overall survival
Among all patients with a documented EGFR test (n=3121), OS decreased with increasing disease stage. Median follow-up for all stages combined was 24.4 months. Estimated 2-year survival probabilities for disease diagnosed at stage I, II and III were 81.9 (95% CI: 79.4–84.4%), 72.9 (69.1–77.0%) and 57.1% (54.4–59.8%), respectively. Estimated 5-year survival probabilities for disease diagnosed at stage I, II and III were 53.3 (95% CI: 49.0–57.9), 49.7 (44.5–55.6%) and 29.1% (25.8–32.7%), respectively, and median survival times were (months, 95% CI) 72.0 (56.4–not estimable [NE]), 55.5 (45.9–NE) and 29.5 (27.4–33.6), respectively.
Among patients with known EGFR mutation status (n = 2959), median follow-up for all stages combined was 22.2 months for patients without EGFRm (n=2466) and 32.7 months for those with EGFRm NSCLC (n = 493; ). Median OS in months for the EGFRm subgroup was as follows: all stages, not reached (NR) (95% CI: 55.2–NE); stage I, NR (NE–NE); stage II, NR (47.1–NE); stage III, 48.2 (41.8–NE) and for the EGFRwt subgroup was: all stages, 38.5 (35.3–41.5); stage I, 55.9 (49.9–NE); stage II, 49.4 (41.7–NE); stage III, 26.8 (24.5–29; ). Both 2- and 5-year OS were numerically longer for the EGFRm subgroup compared with the EGFRwt subgroup, for all stages combined and each stage individually ( & A & B). The Cox model for OS demonstrated significantly lower HRs in patients with EGFRm NSCLC compared with those with EGFRwt NSCLC (all patients, n = 3121, 0.63 [95% CI: 0.52–0.75]; C). EGFRm patients also had reduced hazards of death for all stages (stage I: n = 1034 [HR: 0.59, 95% CI: 0.41–0.84]; stage II: n = 560 [HR: 0.68, 95% CI: 0.43–1.09]; stage III: n = 1527 [HR: 0.63, 95% CI: 0.49–0.80]; C). However, for the stage II group, the CI of the HR crossed 1; this is likely due to the smaller number of patients in the stage II subgroup.
Figure 3. Overall survival as determined from EHRs of patients with stage I–III NSCLC and known EGFR status. (A & B) Kaplan–Meier plots showing the overall survival calculated from EHRs from patients with stage I, II or III NSCLC that was (A) EGFRwt or (B) EGFRm. Colored bands represent 95% CI. (C) HR and confidence bounds stratified by stage and adjusted for gender, age at initial diagnosis, ethnicity, smoking history and whether the patient record reported surgery for the purpose of resection (within 16 weeks of initial diagnosis). The Cox proportional model for all patients has stage included as a covariate in addition to all other covariates. A HR below 1 indicates lower risk of mortality (longer overall survival) for patients with EGFRm compared with EGFRwt NSCLC.
EGFRm: EGFR-mutation positive; EGFRwt: EGFR wild-type; EHR: Electronic health record; HR: Hazard ratio; NSCLC: Non-small-cell lung cancer.
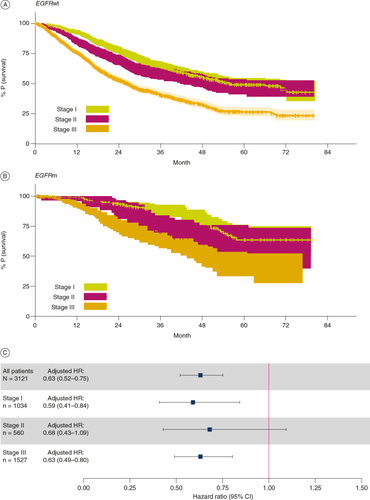
Figure 4. Percentage of EHRs with known EGFR status reporting a surgery to resect stage I–III NSCLC, alone or with neoadjuvant and/or adjuvant therapy, by disease stage (A) and by disease stage and mutational status (B). Bars show the percentage of patients reporting a surgery to resect stage I–III NSCLC, alone or with neoadjuvant and/or adjuvant therapy. EHRs are classified by disease stage (A) and by disease stage and EGFR mutational status (B). Values under the bars show the total number of patients.
EGFRm: EGFR-mutation positive; EGFRwt: EGFR wild-type; EHR: Electronic health record; NSCLC: Non-small-cell lung cancer.
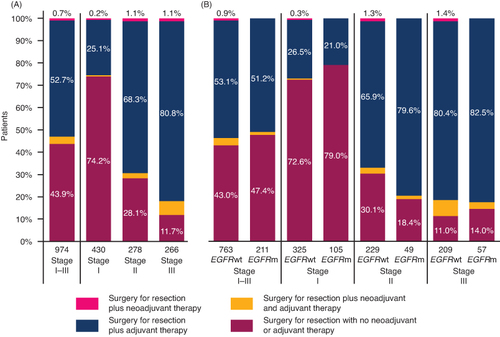
Table 2. Overall survival calculated from EHRs from patients with stage I–III NSCLC and known EGFR status by disease stage and EGFR status.
3.6. Treatment patterns
Among patients with known EGFR mutation status (n = 2959), surgical resection (defined as surgery within 16 weeks of the initial NSCLC diagnosis) with or without neoadjuvant and/or adjuvant therapy was the most commonly reported treatment for NSCLC diagnosed at stage I and II (430/969 [44.4%] and 278/531 [52.4%] of patients, respectively) but was less commonly reported for stage III disease (266/1459 [18.2%]). The reverse was true for the frequency of systemic therapy and/or radiotherapy without surgery (reported in 264/969 [27.2%], 145/531 [27.3%] and 979/1459 [67.1%] of patient EHRs for NSCLC diagnosed at stage I, II and III, respectively). A small proportion of patient EHRs did not report anti-cancer treatment, including surgery, at any time after the original diagnosis ().
Table 3. The frequency that EHRs from patients with stage I–III NSCLC and known EGFR status reported each type of treatment.
Neoadjuvant therapy was defined as systemic treatment and/or radiotherapy started between initial diagnosis and surgery, while adjuvant therapy was defined as systemic treatment and/or radiotherapy started no later than 26 weeks after surgery (Supplementary Table S1). Surgical resection, either alone or combined with neoadjuvant and/or adjuvant therapy tended to be more common in patients with EGFRm versus EGFRwt NSCLC (42.8 vs 30.9%); 51.0 and 42.6%, respectively, for stage I, 66.2 and 50.1% at stage II, and 26.8 and 16.8% at stage III. In contrast, the proportion of patients receiving systemic therapy and/or radiotherapy (without surgery) was higher in patients with EGFRwt NSCLC than in those with EGFRm ().
Among the 974 patients with known EGFR status who underwent resection, almost half (43.9%) did not report either a neoadjuvant or adjuvant treatment (A), with neoadjuvant treatment use being particularly low (less than 1.0% of patients). Adjuvant treatment was reported more frequently in patients whose NSCLC was diagnosed at stage III (80.8%) compared with those diagnosed at stage II (68.3%) or stage I (25.1%; A). In general, the frequency of adjuvant treatment use was similar in patients with EGFRm versus EGFRwt disease, although in patients whose NSCLC was diagnosed at stage II, it was used more frequently in patients with EGFRm than in those with EGFRwt (79.6 and 65.9%, respectively; B).
Among patients with known EGFR status who underwent resection and received adjuvant treatment, chemotherapy, either alone or in combination with radiotherapy, was the most common adjuvant treatment overall and across the three disease stage subgroups (). This was the case for both EGFRwt and EGFRm disease, although chemotherapy was more commonly used in EGFRwt than EGFRm NSCLC (57.2 and 40.5%, respectively, across all stages combined; ).
Table 4. The frequency that EHRs from patients with stage I–III NSCLC and known EGFR status reported each type of adjuvant therapy.
4. Discussion
The CLQD database is a large real-world data asset that includes EHR data for patients from all 50 US states. Here, CLQD data were used to examine the landscape of molecular testing and treatments received by patients diagnosed with stage I–III NSCLC between 2014 and 2018, as well as patient survival. This was a period when the EGFR-TKI, osimertinib, was established as standard of care for EGFRm stage IV NSCLC, but before practice changing results reported from the ADAURA study showed a DFS benefit for adjuvant osimertinib in patients with surgically-resected EGFRm stage IB–IIIA NSCLC [Citation18]. Guidelines during the above time period recommended surgical resection combined with adjuvant chemotherapy for patients with stage II–IIIA (and high-risk stage IB) NSCLC. Neoadjuvant therapy was recommended in certain circumstances when disease was present in lymph nodes, to downgrade the cancer prior to surgery [Citation28].
This dataset showed that only a few patients diagnosed with stage I–III NSCLC between 2014 and 2018 were treated with neoadjuvant therapy, which is in line with clinical guidelines. More surprising was that a substantial number of patients with resected stage II and III disease did not receive adjuvant therapy. The EHRs also suggest that a surprisingly large number of patients with stage I and II NSCLC did not undergo surgical resection. Additionally, compared with patients with EGFRm NSCLC, a higher proportion of those with EGFRwt NSCLC received systemic therapy and/or radiotherapy (without surgery). This may be because the CLQD database is curated from predominately community hospital records, which may not have full access to patient surgery notes. This means that patients who are recorded as not having undergone surgery may either have missing records in the CancerLinQ database, or may not have undergone surgical resection. The differences related to EGFR mutation status may be due to EGFR mutations being more common in non-smokers, who are more likely to be surgical candidates.
The analysis uncovered that around one in five patients had molecular EGFR testing of NSCLC tumors diagnosed at stage I–III in this database. The relatively low frequency is expected given that, during this period, there were no approved targeted therapies in the adjuvant setting. The rate of EGFR testing appeared higher for patients with stage III versus stage I/II NSCLC, potentially because stage III NSCLC would have been more likely to recur and require EGFR testing to determine the appropriateness of EGFR-TKI therapy. The overall proportion that EGFR tests detected EGFRm versus EGFRwt NSCLC in this US patient cohort was in line with previously reported data [Citation29]. Previously reported associations between more frequent EGFRm status (than EGFRwt) for NSCLC diagnosed at stage I, in patients of Asian ethnicity, with a non-smoking history and of female sex were also all present in the EHR dataset curated by CLQD [Citation30–35].
Data from EHRs were also used to calculate the OS of patients diagnosed with each stage of NSCLC and to examine the relationship between OS and EGFR status. Since CLQD records inconsistently reported disease substage, the analysis was restricted to considering diagnosis by stages I, II or III, which limited direct comparisons with other reports. It is also important to note that the CLQD includes patients from academic and community centers, and the availability of treatments may differ between centers, which may impact survival. Additionally, given the early stage of disease and the relatively short duration of follow-up, median OS was not always reached. A retrospective analysis of patients with resected non-squamous NSCLC in patients from a center in Italy showed that median OS was not reached (with median follow-up of 36 months) [Citation36]. Two-year survival probabilities were 100% for stage I, 90% for stage II and 64% for stage III. In comparison, 2-year survival probabilities in CLQD were slightly lower (82% for stage I, 73% for stage II and 57% for stage III). Two-year survival probabilities in CLQD were similar to those reported from a Portuguese clinical center for stage I disease (82% in CLQD vs 80% in Portugal) but were higher in CLQD for stage II disease (73% for stage II in CLQD vs 50% in Portugal) [Citation37].
When considering EGFR status, the data from CLQD records showed longer OS when EHRs reported EGFRm NSCLC versus EGFRwt NSCLC. A cohort study using data from patients with NSCLC registered in the Danish Lung Cancer Registry also showed that EGFRm NSCLC was associated with longer median OS compared with patients with EGFRwt status [Citation38]. The association of longer OS among patients with EGFRm NSCLC was also shown in the retrospective analysis of patients with resected non-squamous NSCLC from an Italian center [Citation36]. The reasons for the differences in OS are complex, and may be due to demographic differences between subgroups in this study; a higher proportion of patients in the EGFRm subgroup than the EGFRwt subgroup had stage I disease. Additionally, compared with the EGFRwt subgroup, a lower proportion of patients in the EGFRm subgroup had stage III disease. It is well documented that patients with earlier stage disease have longer OS [Citation3–5]. Similarly, in this study, a higher proportion of patients with EGFRm than EGFRwt NSCLC were female; previous studies have demonstrated that female patients have superior survival outcomes to male patients [Citation39–41]. The differences in OS may also be due to variations in subsequent treatment patterns for advanced disease, and specifically the use of targeted therapies for EGFRm NSCLC; this was not captured within the current study, but the reported real-world use of first- and second-generation EGFR-TKIs varied between approximately 50 and 70% during the period of the current study [Citation42,Citation43]. Future studies should examine the subsequent therapies received by patients whose stage I–III NSCLC recurred after initial treatment and the impact on survival outcomes.
There are several limitations to consider for this analysis. First, the CLQD dataset is a convenience sample of records at medical oncology clinics in the USA and is not a systematically collected dataset. While CLQD provides access to de-identified data representing more than one million patient records (and ~150,000 patients with NSCLC) at the time of this analysis, the data may not be representative of all patients diagnosed and treated in the USA during this period. For example, while CLQD includes data from patients across the USA, the largest proportion of patients come from the South and Midwest regions. Given the nature of retrospective assessments of real-world data, there is also a potential for missing data, leading to a high rate of censoring. Indeed, previous analysis suggests that 5-year OS estimates in the CLQD database were higher than those reported by SEER for several tumor types, possibly due to incomplete capture of mortality data in patient EHRs [Citation44]. It should also be noted that for comparison of outcomes between EGFRm and EGFRwt NSCLC, patient numbers were much larger for the EGFRwt group, and as discussed, treatment for advanced disease may have differed according to EGFR mutation status. Additionally, information on molecular testing is typically curated from unstructured data in EHRs and only some EHRs had sufficient unstructured content to qualify for CLQD curation. It is therefore possible that not all EGFR tests and results were detected. This might explain why there was a lower testing rate in this study (21.6%) than reported by another retrospective analysis of patients treated at a USA clinic, where testing was reported in 40% of patients with stage IB–IIIA NSCLC [Citation29]. It may also reflect differences in testing rates between academic and community centers but data were not available on the type of center in which patients were treated. We were also unable to determine how many patients received their EGFR test results prior to starting treatment. Additionally, the CLQD curation process had insufficient data to differentiate between common EGFR mutations (Ex19del and L858R) and uncommon mutations. The latter make up about 10–20% of EGFR mutations and have variable sensitivity to EGFR-TKIs compared with the common mutations [Citation26,Citation45,Citation46]. Finally, as previously discussed, the lower than expected rates of surgery may be due to missing records in the CLQD; direct comparisons with other studies are limited by the need to group results by overall stage, due to inconsistencies in the CLQD.
5. Conclusion
The analysis of CLQD data uncovered that around one in five patients received an EGFR test during this period, with the highest frequency for stage III disease versus stage I/II NSCLC. The analysis also uncovered that many EHRs reporting surgeries for disease resection did not also report an adjuvant therapy, even though adjuvant chemotherapy was indicated during this period. The approval of targeted adjuvant therapy, as well as (neo)adjuvant immunotherapy, for resectable stage IB–IIIA NSCLC, will likely impact the testing and treatment patterns we observed. Indeed, it will be important to ensure that genetic testing is performed in this patient population to ensure patients receive the most appropriate treatment thus improving patient outcomes.
Author contributions
Contributions to the study conception or design: M Muthusamy, M Berktas, J Li, A Taylor, N Pennell. Contribution to the acquisition of data for the work: P Sun. Contribution to the analysis and interpretation of data for the work: M Berktas, J Li, D Thomas, P Sun, A Taylor, N Pennell. Drafting the work: M Berktas, D Thomas. Revising the work critically for important intellectual content: M Muthusamy, M Berktas, J Li, P Sun, A Taylor, N Pennell. Final approval of the version to be published: all authors. Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: all authors.
Financial disclosure
This analysis was funded by AstraZeneca. M. Muthusamy reports no conflict of interest. M Berktas received a consulting fee from AstraZeneca. J Li reports employment at AstraZeneca and owns stocks/shares in AstraZeneca. D Thomas reports employment at AstraZeneca and owns stocks/shares in AstraZeneca. P Sun reports employment at AstraZeneca and owns stocks/shares in AstraZeneca. A Taylor reports employment at Gilead Science and previous employment at AstraZeneca and owns stocks/shares in Gilead Science and previously owned stocks/shares in AstraZeneca. N Pennell reports participation in advisory councils for Anheart, Bayer, Eli Lilly, Genentech, Janssen, Merck, Novartis, Pfizer, Sanofi Genzyme, Summit Therapeutics, and Takeda. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Writing disclosure
The authors would like to acknowledge R Goodchild, of Oxford Pharmagenesis, and C Allinson, of Ashfield MedComms, an Inizio Company, for medical writing support that was funded by AstraZeneca in accordance with Good Publication Practice (GPP) guidelines (www.ismpp.org/gpp-2022).
Ethical conduct of research
This observational study was performed in accordance with the ethical principles of the Declaration of Helsinki, the International Conference on Harmonisation (ICH) Good Clinical Practice, Good Pharmacoepidemiology Practice and the applicable legislation on non-interventional studies and/or observational studies.
Supplementary Materials
Download Zip (430.7 KB)Supplemental material
Supplemental data for this article can be accessed at https://doi.org/10.1080/14796694.2024.2347826
Competing interests disclosure
The authors have no competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, stock ownership or options and expert testimony.
Data availability statement
This study was based on data from the CancerLinQ Discovery® database. Researchers accessed data with all required permissions and based on a specific study protocol. Other parties may apply for their own data access, using the standard application/approval process. Further information is available from the corresponding author upon request.
Additional information
Funding
References
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71:7–33. doi:10.3322/caac.21654
- SEER [Internet]. Bethesda (MD) USA: National Cancer Institute; Cancer Stat Facts: Lung and Bronchus Cancer; 2022 [ February 2024]. Available from: https://seer.cancer.gov/statfacts/html/lungb.html
- Peters S, Weder W, Dafni U, et al. Lungscape: resected non-small-cell lung cancer outcome by clinical and pathological parameters. J Thorac Oncol. 2014;9:1675–1684. doi:10.1097/jto.0000000000000320
- Aokage K, Miyoshi T, Ishii G, et al. Clinical and pathological staging validation in the eighth edition of the TNM classification for lung cancer: correlation between solid size on thin-section computed tomography and invasive size in pathological findings in the new T classification. J Thorac Oncol. 2017;12:1403–1412. doi:10.1016/j.jtho.2017.06.003
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11:39–51. doi:10.1016/j.jtho.2015.09.009
- National Comprehensive Cancer Network®. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Non-Small-Cell Lung Cancer Version 3.2023.© National Comprehensive Cancer Network, Inc. 2023. All rights reserved. To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way. [ accessed 2023 May].
- Lou F, Huang J, Sima CS, et al. Patterns of recurrence and second primary lung cancer in early-stage lung cancer survivors followed with routine computed tomography surveillance. J Thorac Cardiovasc Surg. 2013;145:75–81; discussion 81–72. doi:10.1016/j.jtcvs.2012.09.030
- Arriagada R, Auperin A, Burdett S, et al. Adjuvant chemotherapy, with or without postoperative radiotherapy, in operable non-small-cell lung cancer: two meta-analyses of individual patient data. Lancet. 2010;375:1267–1277. doi:10.1016/s0140-6736(10)60059-1
- Pignon JP, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE collaborative group. J Clin Oncol. 2008;26:3552–3559. doi:10.1200/jco.2007.13.9030
- Cross DA, Ashton SE, Ghiorghiu S, et al. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov. 2014;4:1046–1061. doi:10.1158/2159-8290.CD-14-0337
- Mok TS, Wu YL, Ahn MJ, et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med. 2017;376:629–640. doi:10.1056/NEJMoa1612674
- Hendriks LE, Kerr KM, Menis J, et al. Oncogene-addicted metastatic non-small-cell lung cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023;34:339–357. doi:10.1016/j.annonc.2022.12.009
- Ramalingam SS, Vansteenkiste J, Planchard D, et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med. 2020;382:41–50. doi:10.1056/NEJMoa1913662
- Reungwetwattana T, Nakagawa K, Cho BC, et al. CNS response to osimertinib versus standard epidermal growth factor receptor tyrosine kinase inhibitors in patients with untreated EGFR-mutated advanced non-small-cell lung cancer. J Clin Oncol. 2018;36:3290–3297. doi:10.1200/JCO.2018.78.3118
- Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378:113–125. doi:10.1056/NEJMoa1713137
- Wu YL, Ahn MJ, Garassino MC, et al. CNS efficacy of osimertinib in patients with T790M-positive advanced non-small-cell lung cancer: data from a randomized phase III trial (AURA3). J Clin Oncol. 2018;36:2702–2709. doi:10.1200/JCO.2018.77.9363
- Herbst RS, Wu YL, John T, et al. Adjuvant osimertinib for resected EGFR-mutated stage IB-IIIA non-small-cell lung cancer: updated results from the phase III randomized ADAURA trial. J Clin Oncol. 2023;41:1830–1840. doi:10.1200/JCO.22.02186
- Wu YL, Tsuboi M, He J, et al. Osimertinib in resected EGFR-mutated non-small-cell lung cancer. N Engl J Med. 2020;383:1711–1723. doi:10.1056/NEJMoa2027071
- Tsuboi M, Herbst RS, John T, et al. Overall survival with osimertinib in resected EGFR-mutated NSCLC. N Engl J Med. 2023;389:137–147. doi:10.1056/NEJMoa2304594
- AstraZeneca [Internet]. AstraZeneca; Tagrisso approved in China for the adjuvant treatment of patients with early-stage EGFR-mutated lung cancer. 2021 [ February 2024]. Available from: www.astrazeneca.com/media-centre/press-releases/2021/tagrisso-approved-in-china-in-early-lung-cancer.html#
- AstraZeneca [Internet]. AstraZeneca; Tagrisso approved in Japan for the adjuvant treatment of patients with early-stage EGFR-mutated lung cancer. 2022 [ February 2024]. Available from: www.astrazeneca.com/media-centre/press-releases/2022/tagrisso-approved-in-japan-for-early-lung-cancer.html
- European Medicines Agency [Internet]. European Medicines Agency; Osimertinib summary of product characteristics. 2022 [ June 2022]. Available from: www.ema.europa.eu/en/documents/product-information/tagrisso-epar-product-information_en.pdf
- Food and Drug Administration [Internet]. Food and Drug Administration; Osimertinib prescribing information. 2020 [ June 2022]. Available from: www.accessdata.fda.gov/drugsatfda_docs/label/2022/208065s025lbl.pdf
- Food and Drug Administration [Internet]. Food and Drug Administration; FDA approves neoadjuvant nivolumab and platinum-doublet chemotherapy for early-stage non-small cell lung cancer. 2022 [ January 2023]. Available from: www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-neoadjuvant-nivolumab-and-platinum-doublet-chemotherapy-early-stage-non-small-cell-lung
- Food and Drug Administration [Internet]. Food and Drug Administration; FDA approves atezolizumab as adjuvant treatment for non-small cell lung cancer. 2023 [ January 2023]. Available from: www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-atezolizumab-adjuvant-treatment-non-small-cell-lung-cancer
- Russo A, Franchina T, Ricciardi G, et al. Heterogeneous responses to epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) in patients with uncommon EGFR mutations: new insights and future perspectives in this complex clinical scenario. Int J Mol Sci. 2019;20:1431. doi:10.3390/ijms20061431
- Potter D, Brothers R, Kolacevski A, et al. Development of CancerLinQ, a health information learning platform from multiple electronic health record systems to support improved quality of care. JCO Clin Cancer Inform. 2020;4:929–937. doi:10.1200/cci.20.00064
- Kris MG, Gaspar LE, Chaft JE, et al. Adjuvant systemic therapy and adjuvant radiation therapy for stage I to IIIA completely resected non-small-cell lung cancers: American Society of Clinical Oncology/Cancer Care Ontario clinical practice guideline update. J Clin Oncol. 2017;35:2960–2974. doi:10.1200/jco.2017.72.4401
- Rodriguez E, Dawar R, Basher F, et al. Prevalence of EGFR mutation testing in early-stage lung cancer: implications of the ADAURA trial for clinical practice. J Clin Oncol. 2021;39:e20507–e20507. doi:10.1200/JCO.2021.39.15_suppl.e20507
- Boch C, Kollmeier J, Roth A, et al. The frequency of EGFR and KRAS mutations in non-small cell lung cancer (NSCLC): routine screening data for central Europe from a cohort study. BMJ Open. 2013;3:e002560. doi:10.1136/bmjopen-2013-002560
- Midha A, Dearden S, McCormack R. EGFR mutation incidence in non-small-cell lung cancer of adenocarcinoma histology: a systematic review and global map by ethnicity (mutMapII). Am J Cancer Res. 2015;5:2892–2911.
- Pi C, Xu CR, Zhang MF, et al. EGFR mutations in early-stage and advanced-stage lung adenocarcinoma: analysis based on large-scale data from China. Thorac Cancer. 2018;9:814–819. doi:10.1111/1759-7714.12651
- Schmid S, Garcia M, Hueniken K, et al. FP01.03 Prevalence, treatment patterns and long-term clinical outcomes of patients with EGFR positive resected stage IB-IIIA NSCLC. J Thorac Oncol. 2021;16:S945. doi:10.1016/j.jtho.2021.08.206
- Shi Y, Au JS, Thongprasert S, et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol. 2014;9:154–162. doi:10.1097/jto.0000000000000033
- Zhang YL, Yuan JQ, Wang KF, et al. The prevalence of EGFR mutation in patients with non-small-cell lung cancer: a systematic review and meta-analysis. Oncotarget. 2016;7:78985–78993. doi:10.18632/oncotarget.12587
- Pasello G, Lorenzi M, Pretelli G, et al. Diagnostic-therapeutic pathway and outcomes of early stage NSCLC: a focus on EGFR testing in the real-world. Front Oncol. 2022;12:909064. doi:10.3389/fonc.2022.909064
- Soares M, Antunes L, Redondo P, et al. Treatment and outcomes for early non-small-cell lung cancer: a retrospective analysis of a Portuguese hospital database. Lung Cancer Manag. 2021;10:LMT46. doi:10.2217/lmt-2020-0028
- Ehrenstein V, Eriksen K, Taylor A, et al. Characteristics and overall survival of patients with early-stage non-small cell lung cancer: a cohort study in Denmark. Cancer Med. 2023;12:30–37. doi:10.1002/cam4.4946
- May L, Shows K, Nana-Sinkam P, et al. Sex differences in lung cancer. Cancers. 2023;15:3111. doi:10.3390/cancers15123111
- Yu XQ, Yap ML, Cheng ES, et al. Evaluating prognostic factors for sex differences in lung cancer survival: findings from a large Australian cohort. J Thorac Oncol. 2022;17:688–699. doi:10.1016/j.jtho.2022.01.016
- Jeon DS, Kim JW, Kim SG, et al. Sex differences in the characteristics and survival of patients with non-small-cell lung cancer: a retrospective analytical study based on real-world clinical data of the Korean population. Thorac Cancer. 2022;13:2584–2591. doi:10.1111/1759-7714.14594
- Samol J, Demedts I, Erman M, et al. Real-world use of tyrosine kinase inhibitors (TKI) in epidermal growth factor receptor mutated (EGFRm) advanced non-small cell lung cancer (NSCLC) in nine countries. J Thorac Oncol. 2023;18:S55–S56. doi:10.1016/S1556-0864(23)00280-0
- Nieva J, Reckamp KL, Potter D, et al. Retrospective analysis of real-world management of EGFR-mutated advanced NSCLC, after first-line EGFR-TKI treatment: US treatment patterns, attrition, and survival data. Drugs Real World Outcomes. 2022;9:333–345. doi:10.1007/s40801-022-00302-w
- Potter DM, Riffon MF, Manning B, et al. Summary of the 12 most common cancers in the CancerLinQ Discovery (CLQD) database. JCO Clin Cancer Inform. 2021;5:658–667. doi:10.1200/cci.21.00011
- John T, Taylor A, Wang H, et al. Uncommon EGFR mutations in non-small-cell lung cancer: a systematic literature review of prevalence and clinical outcomes. Cancer Epidemiol. 2022;76:102080. doi:10.1016/j.canep.2021.102080
- Passaro A, Mok T, Peters S, et al. Recent advances on the role of EGFR tyrosine kinase inhibitors in the management of NSCLC with uncommon, non exon 20 insertions, EGFR mutations. J Thorac Oncol. 2021;16:764–773. doi:10.1016/j.jtho.2020.12.002
