Abstract
Aim: Characterize febrile neutropenia in the real-world and explore potentially modifiable risk factors. Patients & methods: Characteristics of patient presenting with febrile neutropenia after systemic cancer treatment were investigated, with a thorough evaluation of potential risk factors. Results: The rate of febrile neutropenia requiring hospitalization was comparable with clinical trials (mean absolute difference 2%, 95% CI: -1–4%; p = 0.29). The in-hospital mortality rate was 6%. Most cases resulted from low-risk regimens (50%) and 18.2% presented no apparent risk factors. 42.4% of patients presented modifiable factors potentially involved in the occurrence of febrile neutropenia. Conclusion: Febrile neutropenia rate in contemporary real-world evidence is comparable with clinical trials. Appropriate G-CSF administration and avoidance of potentially harmful drug-interactions represent potential areas for improvement.
Even with the advance of new cancer treatment strategies, chemotherapy remain an important part of the treatment of many cancer patients.
Febrile neutropenia remains one of the most common causes of cancer treatment morbidity and mortality.
Other factors may come into play in the occurrence of febrile neutropenia in the real-world setting, compared with the strictly controlled environment of clinical trials.
The febrile neutropenia rate in this real-world setting was comparable to rate in clinical trials.
Many patients presented a modifiable factor potentially involved in the occurrence of febrile neutropenia, such as an abnormal pre-treatment laboratory value, a potentially significant drug interaction between a comedication and chemotherapy, or not receiving G-CSF in accordance with the guidelines.
Despite a thorough review, we found no risk factor for 18 patients (18.2%) treated with low-risk regimen.
Despite a thorough evaluation of known risk factors, febrile neutropenia remains difficult to predict for individual patients and presents an opportunity for further study.
Appropriate G-CSF administration and avoidance of potentially harmful drug interactions could help reduce the burden of febrile neutropenia.
1. Background
Cytotoxic chemotherapy remains a standard of care for the majority of cancer patients [Citation1,Citation2], and can expose patients to significant risk of medullary toxicity and febrile neutropenia. Febrile neutropenia is defined by the occurrence of fever (temperature >38.3°C or >38.0°C on two consecutive readings) and a low absolute neutrophil count (<0.5 × 109/l). Febrile neutropenia is a major cause of morbidity, mortality and cost related to systemic cancer treatment [Citation3]. Even if some patients can be managed in the outpatient setting, most require hospitalization with an inpatient mortality of 4–10% [Citation3,Citation4]. Febrile neutropenia may also result in treatment delays, dose reductions or premature termination of chemotherapy, causing a significant reduction of dose intensity, that may negatively affect patient outcomes[Citation5,Citation6].
The risk of febrile neutropenia varies between chemotherapy regimens [Citation7]. Major clinical guidelines classify chemotherapy regimens based on rates of febrile neutropenia as high-risk (>20%), intermediate risk (10–20%) and low-risk (<10%) [Citation8,Citation9]. This estimation is based on data coming from clinical trials. However, the risk of febrile neutropenia in the real-world may differ from clinical trials, due to patient selection and variable follow-up procedures. Indeed, some data indicate higher rates of febrile neutropenia in the real-world setting [Citation10]. It is also possible that myelosuppression and other adverse events have been inadequately reported or underestimated in oncology trials [Citation11,Citation12]. A systematic review of neutropenic complications of chemotherapy reported in clinical trials in non-Hodgkin lymphoma found that only one in four trials reported the rate of febrile neutropenia [Citation13]. The estimation of febrile neutropenia risk for a particular regimen based on data from clinical trial only can be difficult, as the rate of febrile neutropenia can vary widely between trials of the same regimen, reflecting different patient populations and also maybe different supportive care protocols and practices between trials [Citation14].
Febrile neutropenia risk also depends on patient-related factors. Age, poor performance status and certain comorbidities are associated with a higher risk [Citation7,Citation15]. However, a significant number of patients without any apparent risk factors presents febrile neutropenia, suggesting that factors that are not routinely studied, such as drug interactions or pharmacogenomics, could contribute.
Other than a reduction in treatment intensity, few options exist to prevent febrile neutropenia. The incidence and mortality of febrile neutropenia can be reduced with prophylactic G-CSF, showing a reduction of 45% in infection-related mortality in a meta-analysis, with an absolute risk reduction from 2.8 to 1.5% [Citation16]. G-CSF is recommended by international guidelines for all patients treated with high-risk regimens, and for patients treated with intermediate risk regimens who present another risk factor [Citation8,Citation9]. G-CSF use in daily practice is frequently inadequate in regards of the guidelines, with underuse in some high-risk patients – carrying a risk of insufficient protection against febrile neutropenia – and overuse in low-risk patients [Citation17–20]. It is unclear whether extending G-CSF to a broader patient population including low-risk patients would be beneficial [Citation21]. Of note, most of the prospective trials evaluating G-CSF were conducted in patients receiving moderate or high risk regimens [Citation16].
The goal of this study was to estimate the rate and burden of febrile neutropenia in a real-life setting, and to determine, through in-depth analysis of a real-world cohort of patients hospitalized for febrile neutropenia, whether contributing factors could be identified, in order to identify potential avenues for improvement.
2. Methods
2.1. Patients
All patients who received systemic chemotherapy for solid tumors or lymphomas at our institution from the 1 January 2018 to the 31 December 2019 and were hospitalized up to 2 months after treatment administration were prospectively collected. All patients hospitalized for febrile neutropenia, defined as a temperature of >38.3°C or >38.0°C on two consecutive readings, and an absolute neutrophil count of <0.5 × 109/l, were included in this analysis. In order to capture the patient for which the occurrence of febrile neutropenia had an impact in terms of morbidity or mortality, only patients who were hospitalized due to febrile neutropenia were included in this analysis.
2.2. Data collection & analyses
Detailed information regarding patient's characteristics, cancer, systemic cancer treatments, concurrent medications and complications, according to the Common Terminology Criteria for Adverse Events (CTCAE, version 5.0) were collected. Presence of classical risk factors for febrile neutropenia, as defined by European Society of Medical Oncology (ESMO) and National Comprehensive Cancer Network (NCCN) guidelines, was recorded: age ≥ 65 years old, Eastern Cooperative Oncology Group Performance Status (ECOG PS) ≥ 2, significant comorbidities (cardiovascular disease, renal or liver dysfunction), or personal history of febrile neutropenia. Detailed information regarding pre-treatment laboratory values and values at complication were also collected, such as blood cell count, renal and liver function.
Each regimen was categorized into three categories: low-risk (<10%), intermediate-risk (10–20%) or high-risk (>20%), based on the ESMO and NCCN guidelines. If specific guidelines were missing, the classification was based on the proportion of febrile neutropenia in the largest available clinical trials of that regimen.
The number of cases related to each regimen were recorded and the regimens ranked based on this metric in decreasing order. The top regimens, which cumulatively were associated with more than 50% of cases were retained for further detailed analysis. Their respective rate of febrile neutropenia was compared with that of the most relevant clinical trials. Relevant studies for each regimen were searched on Medline. A study was deemed relevant if the treatment regimen corresponded in terms of dose and timing of administration, as well as tumor type, to the regimen used in our institution.
To determine if febrile neutropenia could have been hypothetically avoided in some patients, the presence of modifiable factors potentially contributing to febrile neutropenia were investigated. The prophylactic use of G-CSF was analyzed in relation to the intrinsic febrile neutropenia risk of each protocol and the presence of patient-specific risk factors. Potential drug interactions of chemotherapy with co-medications (treatment taken at the time of chemotherapy administration, in the outpatient setting) were reviewed by a pharmacist (SP), using different drug interaction analysis tools: Lexicomp drug interaction®, Theriaque®, Liverpool Cancer Drug Interactions®, EviQ®, Liverpool HIV Drug Interactions®, Micromedex Drug Interactions® and CYTP450 Drug Interactions®. Based on these tools, every drug interaction was classified as significant or insignificant with respect to its potential increase in the hematological toxicity of chemotherapy. Pre-treatment laboratory values and eventual dose adaptation were also examined by a medical oncologist (MB). For this evaluation, institutional guidelines were used. In cases where the institutional guidelines emit no recommendation, protocols of the pivotal clinical trials of the corresponding regimens were used, or prescriptions drug referential such as Lexicomp® and EviQ®. A potentially modifiable risk factor was defined as: the presence of a significant drug interaction between a comedication and the chemotherapy, deemed as a contraindicated association according to pharmacist’ expertise; an abnormal pre-treatment laboratory value that constitute a contraindication to the administration of chemotherapy as per-local guidelines; or the non-administration of G-CSF when recommended according to the ESMO-guidelines (high-risk regimens, or intermediate risk regimen in the presence of ≥ 1 additional risk factor). The presence of these modifiable risk factors was defined at the time of chemotherapy administration.
The study was approved by the local ethic committee (CCER 2018-02334) and was conducted in accordance with the declaration of Helsinki.
2.3. Statistical analysis
All statistical analyses were performed in the R environment for statistics (version 4.0). Meta-analysis was performed with the metafor package (version 3.0) [Citation22]. Statistical tests were two-sided and an alpha cut-off of 0.05 was applied.
3. Results
3.1. Patient characteristics
From the 1 January 2018 to 31 December 2019, 2018 patients received systemic cancer treatment at our institution, for a total of 13,822 distinct treatment administration events. During that same period, 1575 of these patients were hospitalized at least once within their treatment window, defined as the period extending up to 2 months after drug administration. 229 patients were hospitalized for complications attributed to systemic cancer treatment, and 1346 for other causes.
Almost half of the treatment-related hospitalizations were due to febrile neutropenia (primary cause for hospital admission), with 109 hospitalizations involving 99 distinct patients (). Eight patients were hospitalized two-times for two distinct episodes of febrile neutropenia, and one patient was hospitalized three-times. Thirteen patients (13.1%) were 70 years or older, and almost one fourth (24/109) of the patients had a performance status of ECOG 2 or more at admission, with the remaining patients having a normal or almost normal performance status (ECOG 0-1). In addition, 36/99 patients (36%) presented at least one significant comorbidity. 40 patients (40.4%) did not have any classical risk factors for febrile neutropenia. Six patients died during their hospitalization for febrile neutropenia, for an in-hospital mortality rate of 6%.
Table 1. Characteristics of patient hospitalized for febrile neutropenia (patients baseline characteristics are reported per patients, while other characteristic such as ECOG or treatment intent, are reported per episode of febrile neutropenia, as they may evolve for patient presenting with multiple episodes of febrile neutropenia).
3.2. Treatment characteristics
A total of 52 distinct chemotherapeutic regimens were associated with neutropenic fever in this cohort. Eleven regimens accounted for more than 50% of the cases of febrile neutropenia requiring hospitalization, while the remaining cases were associated with 41 distinct regimens, each only related with one or two cases of febrile neutropenia ().
Table 2. Most common regimens associated with febrile neutropenia at our institution.
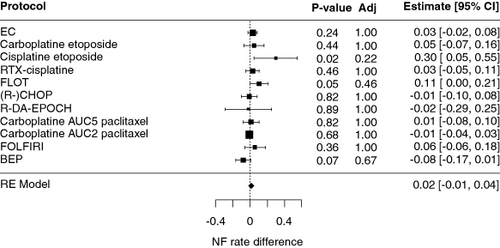
Overall, we found little difference for the neutropenia rate between the real-world and previously published trials () [Citation23–39]. As shown, the rate of febrile neutropenia seems to be higher than expected for the cisplatin-etoposide and FLOT regimens, but this finding did not reach statistical significance when adjusted for multiple comparisons. Supplementary Figure S1 shows the details of the comparison of the real-world data with relevant clinical trials, for each regimen.
Figure 1. Comparison of febrile neutropenia rates in the cohort with data from clinical trials.
AUC: Area under the curve; BEP: Bleomycin 30 mg D1 D8 D15, etoposide 100 mg/m2 D1-D5, cisplatin 20 mg/m2 D1-D5, pegfilgrastim 6 mg D6, q3W; Carboplatin etoposide: Carboplatin AUC 5 D1, etoposide 100 mg/m2 D1-D3 q3w; Ciplatin etoposide: Cisplatin 100 mg/m2 D1, etoposide 100 mg/m2 D1-D3 q3w; EC: Cyclophosphamide 600 mg/m2 D1, Epirubicin 90 mg/m2 D1 q3w; FLOT: 5-FU 2000 mg/m2 over 24 h, leucovorin 30 mg D1, Docetaxel 50 mg/m2 D1, oxaliplatin 85 mg/m2 D1 q2w; R-CHOP: Rituximab 375 mg/m2 D1, cyclophosphamide 750 mg/m2 D1, doxorubicin 50 mg/m2 D1, vincristine 1.4 mg/m2 D1, prednisone 40 mg/m2 D1-D5; R-DA-EPOCH: Rituximab 375 mg/m2 D1, dose-adjusted etoposide 50–124 mg/m2 (depending on dose level) D1-D5, prednisone 120 mg/m2 D1-D5, vincristin 0.4 mg/m2 D1-D4, cyclophosphamide 750–1866 mg/m2 (depending on dose level) D5, doxorubicin 10–25 mg/m2 (depending on dose level) D1-D4, filgrastim 0.5 mioU/kg starting from D6 until absolute neutrophile count >5000 cells/ul, q3w; RTX-cisplatin: Cisplatin 100 mg/m2 D1 q3w with concurrent radiotherapy.
3.3. G-CSF prescription
The administration of G-CSF was examined relative to the risk of each treatment regimen, in accordance with current guidelines of the ESMO. Interestingly, in most episode of febrile neutropenia (58/109), patients had received regimens considered at low-risk for febrile neutropenia, versus 38/109 (intermediate) and 13/99 (high-risk). Only 30 patients had received prophylactic G-CSF. Only one among 16 patients treated with a high-risk regimen did not receive G-CSF. For patients treated with intermediate risk regimens, careful review of associated risk factors (age ≥ 65 years old, ECOG ≥ 2, significant comorbidities (cardiovascular disease, renal or liver dysfunction), personal history of febrile neutropenia, low neutrophile count at the start of chemotherapy), revealed that in 27 episodes of febrile neutropenia, patients presented an additional risk factor for febrile neutropenia, among which eight received G-CSF. Thus, in 19/109 episodes of febrile neutropenia in the cohort, patients could have received G-CSF in accordance with the ESMO and NCCN guidelines but did not.
3.4. Drug interactions
Fourteen patients were taking 10 or more comedications at the time of the occurrence of febrile neutropenia. The average number of comedications per patients in the cohort was 5.5. Overall, in 18/99 patients (18%), we found drug interactions with 30 comedications which could increase the hematological toxicity of chemotherapy. Furthermore, two patients presented the same drug interaction in two distinct episodes of febrile neutropenia, meaning that drug interactions may have contributed to 20 out of 109 episodes of febrile neutropenia. Five patients (5%) had more than one comedication with potential significant interaction with chemotherapy. For 11 comedications, the combination with chemotherapy should have been avoided, because of a clear contraindication (). Among these, we can mention the combination of the antiretroviral ritonavir and darunavir, with docetaxel, or the association of proton pumps inhibitors or Trimethoprim/sulfamethoxazole with methotrexate.
Table 3. Among patients hospitalized for febrile neutropenia, potential drug interaction between chemotherapy and comedications: associations that are usually contra-indicated.
3.5. Pre-treatment laboratory values & dose adjustment
Pre-treatment laboratory values, patient weight and body surface and prescribed dose of chemotherapy were analyzed. Significant abnormalities in pre-treatment laboratory values were defined as laboratory values that would necessitate a dose adjustment or withholding a dose in the protocols of the pivotal clinical trials of the corresponding regimens. Overall, in 90 out of the 109 episodes of febrile neutropenia, patients had received treatment at full dose. For 10/109 episodes of febrile neutropenia (9.25%), patients presented at least one significant abnormality in pre-treatment laboratory value (). Six out of these ten cases did not undergo dose reduction despite the abnormal laboratory value. Six patients presented abnormal blood cell count, for which the dose would have been reduced or postponed according to the protocol of the pivotal clinical trial: for example, a patient with an absolute neutrophil count of 0.6 G/l, who received gemcitabine 1000 mg/m2 and carboplatin AUC5. In five patients, the reason was abnormal liver or renal function, as for example a patient who received a TIP regimen (cisplatin 25 mg/m2 [day 1–day 3], ifosfamide 1200 mg/m2 [day 1–day 3] and paclitaxel 175 mg/m2 [day 1]), with an altered renal function: GFR (MDRD formula) of 49 ml/min. Most of these patients presenting with abnormal laboratory values were treated with a palliative intent, and the majority did not have a dose reduction in their chemotherapy regimen, as illustrated in .
Table 4. Patients hospitalized for febrile neutropenia who presented significant abnormal pre-treatment laboratory values, and the regimen they received, with dose-adjustment.
Overall, in 42/109 episodes of febrile neutropenia, patients presented at least one modifiable risk factor. Six patients had more than one modifiable risk factor (e.g., a significant abnormal laboratory values and a significant drug interaction).
4. Discussion
In this prospective real-world study of all consecutive patients hospitalized for systemic cancer treatment complication in a single tertiary academic center, febrile neutropenia was the most common cause of hospitalization, occurring in 4.9% (95% CI: 4.0–5.9%) of the patients during that period and associated with an in-hospital mortality rate of 6%. Interestingly, only 11 regimens accounted for the majority of episodes of febrile neutropenia in our cohort, suggesting that efforts for the prevention of febrile neutropenia could be focused on a small number of regimens. Surprisingly, most of the patients hospitalized in our cohort were treated with low-risk regimens. Overall, the rate of febrile neutropenia requiring hospitalization in our cohort was generally comparable to the rate of febrile neutropenia in prospective clinical trials for most of these treatment regimens and no statistically significant differences were found after adjustment for multiple comparisons.
Few studies have compared real-world febrile neutropenia rate with randomized trials. A systematic review and meta-analysis by Truong et al. compared febrile neutropenia rate in 65 observational real-world studies with 110 randomized trials involving 29 treatment regimens in breast cancer and found a higher risk in the real world (11.7 vs 7.6%), even after adjustment for age, regimen and treatment intent [Citation10]. The inpatient mortality rate was 6% in our cohort, in range with previously published data [Citation23]. Some data suggest that mortality rate from febrile neutropenia could be lower in high-volume centers, which is the case in our institution, treating more than 50 episodes of febrile neutropenia each year.
Overall, 42 patients presented at least one modifiable risk factor. In ten episodes of febrile neutropenia, patients presented abnormal pre-treatment laboratory values that may have contributed to the occurrence of febrile neutropenia. Interestingly, in six of these cases, no dose reduction was performed. It is possible that the risk-benefit ratio was considered favorable for treatment despite the presence of laboratory abnormalities, for example in the setting of very aggressive disease or when delivered with curative intent. In some cases, it might have been appropriate to reduce treatment intensity, especially in the palliative setting, representing an area of potential improvement.
Cancer patients frequently receive multiple medications, including anticancer drugs, supportive care drugs and medications for comorbid conditions, increasing the probability of having significant drug interactions with chemotherapy [Citation40,Citation41]. After expert review, we found potentially significant drug interactions with chemotherapy in 18% of patients, a proportion that was higher than what we expected. It is however impossible to draw conclusions on the association between these potential drug interactions and the occurrence of neutropenia in these patients. There is little evidence on the prevalence of drug interactions, which has been previously estimated to concern between 27 and 58% of patients, of which 9–34% were considered of major importance, but not necessarily involving chemotherapy [Citation42,Citation43]. The clinical impact of drug interaction in the real-life setting is also poorly studied. A retrospective study estimated that 4% of deaths could be related to severe drug interactions in hospitalized cancer patients [Citation44,Citation45]. Another study reported that 2% of more than 450 unplanned hospital admission among cancer patients were due to drug interaction [Citation28]. The frequency of such event are probably underestimated as drug interaction frequently go unrecognized.
Finally, in a significant proportion of situation (17.4%), patients could have received G-CSF according to the ESMO guidelines. These results can inform future strategies to decrease febrile neutropenia rates, for example with decision aids or computer alerts. It should be noted that we cannot infer how many of the 13,822 treatment cycles delivered in our institution during the study period were coupled with appropriate G-CSF administration. International guidelines generally do not recommend G-CSF use for low-risk these regimens based on cost–effectiveness studies, but the majority of febrile neutropenia episodes were associated with a low-risk regimen in our study [Citation46]. Some real-world evidence suggests that G-CSF use in lower risk situation could also have clinical benefit [Citation21] and could be considered, especially as prices of G-CSF decreased with the advent of biosimilars. However, given the absence of an overall survival benefit with G-CSF and the risk of side effects, albeit rare (such as myelodysplastic syndrome or ARDS), a recommendation for low-risk patients should only be made if supported by prospective studies. It could be also argued that some regimens carrying a low risk of febrile neutropenia in the rigorous setting of a clinical trial could be more toxic in the real world, but this is not supported by our data.
Even after a very thorough investigation of all potential risk factors and after excluding high-risk regimens, 18 patients had no clear identifiable risk factor for febrile neutropenia, demonstrating the difficulty of predicting toxicity at the individual level. This could be due to individual pharmacogenetic differences or unknown exposures, such as concomitant viral illnesses or even factors that are less well studied, such as diet or the microbiome.
To alleviate the burden of febrile neutropenia in clinical practice, additional approaches are also of paramount importance such as providing prompt intravenous antibiotics to patients, or the recognition of mild cases that do not require hospitalization and can be managed in the outpatient setting.
There are some important differences between clinical trial data and contemporary real-world data. Clinical trials have strict inclusion and exclusion criteria, that do not naturally translate to routine care. Patients with febrile neutropenia in our cohort frequently had comorbid conditions, and ECOG performance status of 2 or more was frequent. On the other hand, data from clinical trials are generally older. For example, nine of the 17 of the pivotal trials we used as reference have been published before 2010. Supportive care, chemotherapy delivery protocols and management guidelines for sepsis may have improved in the meantime. In that sense, it is entirely possible that these factors compensate for a population that would have otherwise been more vulnerable to febrile neutropenia and its consequences.
A potential limitation of our study is that we reported here only the cases of febrile neutropenia requiring hospitalization. Even if a majority of febrile neutropenia cases usually require hospitalization [Citation47], a small number of patients with febrile neutropenia may not have been admitted. Another limitation is that we draw data only from our institution, but which employs multiple practicing oncologists and serves a metropolitan area of over 1 million inhabitants of various ethnic ancestries. Given variation in local practices, it is possible that some of the regimens that we used are applied differently in other institutions. Finally, some of our observations may not translate to small practices, especially in settings that are scarce in resources.
5. Conclusion
Despite the advent of new systemic cancer therapies, febrile neutropenia associated with cytotoxic chemotherapy remains a significant burden for cancer patients and the healthcare system. Treating physicians should primarily focus on strategies that have demonstrated a survival benefit, especially the prompt management of cases of febrile neutropenia. Aside from the management of declared cases, strategies aiming at decreasing the risk of febrile neutropenia requires careful identification of intrinsic and extrinsic risk factors, and adequate preventive measures such as dose modulation and G-CSF administration. Based on our observation, particular attention should be paid to a small number of regimens. Whether G-CSF administration should be generalized to include some low-risk regimens is an interesting question that may be worth investigating in a future trial. Finally, almost one fifth of patients presented febrile neutropenia without any apparent risk factors. Further investigations, such as pharmacogenomics studies or studies of various exposures, are warranted in this population.
Author contributions
M Borgeaud and P Tsantoulis designed the study protocol, M Borgeaud collected all the data. S Perano reviewed drugs interactions and prescription of G-CSF. P Tsantoulis performed the statistical analysis. M Borgeaud, P Tsantoulis and A Addeo wrote the manuscript.
Financial disclosure
The authors have no financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Writing disclosure
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
This study was approved by the local ethic committee (Commission Cantonale d'Ethique de la Recherche sur l’être humain, Genève, CCER 2018-02334), and have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Supplementary Figure S1
Download PNG Image (219.6 KB)Supplemental material
Supplemental data for this article can be accessed at https://doi.org/10.1080/14796694.2024.2349510
Competing interests disclosure
The authors have no competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Data availability statement
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
References
- Maldonado EB, Parsons S, Chen EY, Haslam A, Prasad V. Estimation of US patients with cancer who may respond to cytotoxic chemotherapy. Future Sci OA. 2020;6(8):FSO600. doi:10.2144/fsoa-2020-0024
- Haslam A, Gill J, Prasad V. Estimation of the percentage of US patients with cancer who are eligible for immune checkpoint inhibitor drugs. JAMA Netw Open. 2020;3(3):e200423. doi:10.1001/jamanetworkopen.2020.0423
- Kuderer NM, Dale DC, Crawford J, Cosler LE, Lyman GH. Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer. 2006;106(10):2258–2266. doi:10.1002/cncr.21847
- Shah C, Du X, Bishnoi R, Bian J. Risk of mortality in adult cancer febrile neutropenia patients with a machine learning approach. JCO. 2018;36(Suppl. 15):e13562–e13562. doi:10.1200/JCO.2018.36.15_suppl.e13562
- Chirivella I, Bermejo B, Insa A, et al. Optimal delivery of anthracycline-based chemotherapy in the adjuvant setting improves outcome of breast cancer patients. Breast Cancer Res Treat. 2009;114(3):479–484. doi:10.1007/s10549-008-0018-1
- Bosly A, Bron D, Van Hoof A, et al. Achievement of optimal average relative dose intensity and correlation with survival in diffuse large B-cell lymphoma patients treated with CHOP. Ann Hematol. 2008;87(4):277–283. doi:10.1007/s00277-007-0399-y
- Lyman GH, Abella E, Pettengell R. Risk factors for febrile neutropenia among patients with cancer receiving chemotherapy: a systematic review. Crit Rev Oncol Hematol. 2014;90(3):190–199. doi:10.1016/j.critrevonc.2013.12.006
- Klastersky J, de Naurois J, Rolston K, et al. Management of febrile neutropaenia: ESMO Clinical Practice Guidelines. Ann Oncol. 2016;27(Suppl. 5):v111–v118. doi:10.1093/annonc/mdw325
- Becker PS, Griffiths EA, Alwan LM, et al. NCCN guidelines insights: hematopoietic growth factors, Version 1.2020. J Natl Compr Canc Netw. 2020;18(1):12–22. doi:10.6004/jnccn.2020.0002
- Truong J, Lee EK, Trudeau ME, Chan KKW. Interpreting febrile neutropenia rates from randomized, controlled trials for consideration of primary prophylaxis in the real world: a systematic review and meta-analysis. Ann Oncol. 2016;27(4):608–618. doi:10.1093/annonc/mdv619
- Dale DC, McCarter GC, Crawford J, Lyman GH. Myelotoxicity and dose intensity of chemotherapy: reporting practices from randomized clinical trials. J Natl Compr Canc Netw. 2003;1(3):440–454. doi:10.6004/jnccn.2003.0038
- Péron J, Maillet D, Gan HK, Chen EX, You B. Adherence to CONSORT adverse event reporting guidelines in randomized clinical trials evaluating systemic cancer therapy: a systematic review. J Clin Oncol. 2013;31(31):3957–3963. doi:10.1200/JCO.2013.49.3981
- Lyman GH, Poniewierski MS, Culakova E. Risk of chemotherapy-induced neutropenic complications when treating patients with non-Hodgkin lymphoma. Expert Opin Drug Saf. 2016;15(4):483–492. doi:10.1517/14740338.2016.1146675
- Crawford J, Dale DC, Lyman GH. Chemotherapy-induced neutropenia: risks, consequences, and new directions for its management. Cancer 2004;100(2):228–237. doi:10.1002/cncr.11882
- Chao C, Page JH, Yang S-J, Rodriguez R, Huynh J, Chia VM. History of chronic comorbidity and risk of chemotherapy-induced febrile neutropenia in cancer patients not receiving G-CSF prophylaxis. Ann Oncol. 2014;25(9):1821–1829. doi:10.1093/annonc/mdu203
- Kuderer NM, Dale DC, Crawford J, Lyman GH. Impact of primary prophylaxis with granulocyte colony-stimulating factor on febrile neutropenia and mortality in adult cancer patients receiving chemotherapy: a systematic review. J Clin Oncol. 2007;25(21):3158–3167. doi:10.1200/JCO.2006.08.8823
- Baig H, Somlo B, Eisen M, Stryker S, Bensink M, Morrow PK. Appropriateness of granulocyte colony-stimulating factor use in patients receiving chemotherapy by febrile neutropenia risk level. J Oncol Pharm Pract. 2019;25(7):1576–1585. doi:10.1177/1078155218799859
- Wang C-Y, Heldermon CD, Vouri SM, et al. Trends in use of granulocyte colony-stimulating factor following introduction of biosimilars among adults with cancer and commercial or Medicare insurance From 2014 to 2019. JAMA Netw Open. 2021;4(11):e2133474. doi:10.1001/jamanetworkopen.2021.33474
- Weycker D, Li X, Tzivelekis S, et al. Burden of chemotherapy-induced febrile neutropenia hospitalizations in US Clinical Practice, by use and patterns of prophylaxis with colony-stimulating factor. Support Care Cancer 2017;25(2):439–447. doi:10.1007/s00520-016-3421-x
- Zullo AR, Lou U, Cabral SE, Huynh J, Berard-Collins CM. Overuse and underuse of pegfilgrastim for primary prophylaxis of febrile neutropenia. J Oncol Pharm Pract. 2019;25(6):1357–1365. doi:10.1177/1078155218792698
- Aapro M, Ludwig H, Bokemeyer C, et al. Predictive modeling of the outcomes of chemotherapy-induced (febrile) neutropenia prophylaxis with biosimilar filgrastim (MONITOR-GCSF study). Ann Oncol. 2016;27(11):2039–2045. doi:10.1093/annonc/mdw309
- Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Soft. 2010;36(3):1–48. doi:10.18637/jss.v036.i03
- Del Mastro L, De Placido S, Bruzzi P, et al. Fluorouracil and dose-dense chemotherapy in adjuvant treatment of patients with early-stage breast cancer: an open-label, 2 × 2 factorial, randomised Phase III trial. Lancet 2015;385(9980):1863–1872. doi:10.1016/S0140-6736(14)62048-1
- Blohmer J-U, Schmid P, Hilfrich J, et al. Epirubicin and cyclophosphamide versus epirubicin and docetaxel as first-line therapy for women with metastatic breast cancer: final results of a randomised Phase III trial. Ann Oncol. 2010;21(7):1430–1435. doi:10.1093/annonc/mdp585
- Socinski MA, Smit EF, Lorigan P, et al. Phase III study of pemetrexed plus carboplatin compared with etoposide plus carboplatin in chemotherapy-naive patients with extensive-stage small-cell lung cancer. J Clin Oncol. 2009;27(28):4787–4792. doi:10.1200/JCO.2009.23.1548
- Quoix E, Breton JL, Daniel C, et al. Etoposide phosphate with carboplatin in the treatment of elderly patients with small-cell lung cancer: a Phase II study. Ann. Oncol. 2001;12(7):957–962. doi:10.1023/A:1011171722175
- Hügli A, Moro D, Mermillod B, et al. Phase II trial of up-front accelerated thoracic radiotherapy combined with chemotherapy and optional up-front prophylactic cranial irradiation in limited small-cell lung cancer. Groupe d'Oncologie Thoracique des Régions Alpines. J Clin Oncol. 2000;18(8):1662–1667. doi:10.1200/JCO.2000.18.8.1662
- Zatloukal P, Cardenal F, Szczesna A, et al. A multicenter international randomized Phase III study comparing cisplatin in combination with irinotecan or etoposide in previously untreated small-cell lung cancer patients with extensive disease. Ann Oncol. 2010;21(9):1810–1816. doi:10.1093/annonc/mdq036
- Cardenal F, López-Cabrerizo MP, Antón A, et al. Randomized Phase III study of gemcitabine-cisplatin versus etoposide-cisplatin in the treatment of locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol. 1999;17(1):12–18. doi:10.1200/JCO.1999.17.1.12
- Szturz P, Wouters K, Kiyota N, et al. Weekly low-dose versus three-weekly high-dose cisplatin for concurrent chemoradiation in locoregionally advanced non-nasopharyngeal head and neck cancer: a systematic review and meta-analysis of aggregate data. Oncologist. 2017;22(9):1056–1066. doi:10.1634/theoncologist.2017-0015
- Noronha V, Joshi A, Patil VM, et al. Once-a-week versus once-every-3-weeks cisplatin chemoradiation for locally advanced head and neck cancer: a Phase III Randomized noninferiority trial. J Clin Oncol. 2018;36(11):1064–1072. doi:10.1200/JCO.2017.74.9457
- Al-Batran S-E, Homann N, Pauligk C, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, Phase II/3 trial. Lancet. 2019;393(10184):1948–1957. doi:10.1016/S0140-6736(18)32557-1
- Bartlett NL, Wilson WH, Jung S-H, et al. Dose-adjusted EPOCH-R compared with R-CHOP as frontline therapy for diffuse large B-cell lymphoma: clinical outcomes of the Phase III intergroup trial alliance/CALGB 50303. J Clin Oncol. 2019;37(21):1790–1799. doi:10.1200/JCO.18.01994
- Pignata S, Scambia G, Ferrandina G, et al. Carboplatin plus paclitaxel versus carboplatin plus pegylated liposomal doxorubicin as first-line treatment for patients with ovarian cancer: the MITO-2 randomized Phase III trial. J Clin Oncol. 2011;29(27):3628–3635. doi:10.1200/JCO.2010.33.8566
- Katsumata N, Yasuda M, Takahashi F, et al. Dose-dense paclitaxel once a week in combination with carboplatin every 3 weeks for advanced ovarian cancer: a Phase III, open-label, randomised controlled trial. Lancet. 2009;374(9698):1331–1338. doi:10.1016/S0140-6736(09)61157-0
- Clamp AR, James EC, McNeish IA, et al. Weekly dose-dense chemotherapy in first-line epithelial ovarian, fallopian tube, or primary peritoneal carcinoma treatment (ICON8): primary progression free survival analysis results from a GCIG Phase III randomised controlled trial. Lancet. 2019;394(10214):2084–2095. doi:10.1016/S0140-6736(19)32259-7
- Tournigand C, André T, Achille E, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004;22(2):229–237. doi:10.1200/JCO.2004.05.113
- de Wit R, Roberts JT, Wilkinson PM, et al. Equivalence of three or four cycles of bleomycin, etoposide, and cisplatin chemotherapy and of a 3- or 5-day schedule in good-prognosis germ cell cancer: a randomized study of the European Organization for Research and Treatment of Cancer Genitourinary Tract Cancer Cooperative Group and the Medical Research Council. J Clin Oncol. 2001;19(6):1629–1640. doi:10.1200/JCO.2001.19.6.1629
- Culine S, Kramar A, Théodore C, et al. Randomized trial comparing bleomycin/etoposide/cisplatin with alternating cisplatin/cyclophosphamide/doxorubicin and vinblastine/bleomycin regimens of chemotherapy for patients with intermediate- and poor-risk metastatic nonseminomatous germ cell tumors: Genito-Urinary Group of the French Federation of Cancer Centers Trial T93MP. J Clin Oncol. 2008;26(3):421–427. doi:10.1200/JCO.2007.13.8461
- Urban D, Urban GE, Margalit O, et al. Mortality among neutropenic cancer patients within the United States: the association with hospital volume. JCO Oncol Pract. 2021;17(4):e582–e592. doi:10.1200/OP.20.00115
- Scripture CD, Figg WD. Drug interactions in cancer therapy. Nat Rev Cancer. 2006;6(7):546–558. doi:10.1038/nrc1887
- van Leeuwen RWF, Swart EL, Boven E, Boom FA, Schuitenmaker MG, Hugtenburg JG. Potential drug interactions in cancer therapy: a prevalence study using an advanced screening method. Ann Oncol. 2011;22(10):2334–2341. doi:10.1093/annonc/mdq761
- Riechelmann RP, Tannock IF, Wang L, Saad ED, Taback NA, Krzyzanowska MK. Potential drug interactions and duplicate prescriptions among cancer patients. J Natl Cancer Inst. 2007;99(8):592–600. doi:10.1093/jnci/djk130
- Buajordet I, Ebbesen J, Erikssen J, Brørs O, Hilberg T. Fatal adverse drug events: the paradox of drug treatment. J Intern Med. 2001;250(4):327–341. doi:10.1046/j.1365-2796.2001.00892.x
- Miranda V, Fede A, Nobuo M, et al. Adverse drug reactions and drug interactions as causes of hospital admission in oncology. J Pain Symptom Manage. 2011;42(3):342–353. doi:10.1016/j.jpainsymman.2010.11.014
- Lyman GH, Kuderer NM. The economics of the colony-stimulating factors in the prevention and treatment of febrile neutropenia. Crit Rev Oncol Hematol. 2004;50(2):129–146. doi:10.1016/j.critrevonc.2004.01.001
- Weycker D, Li X, Edelsberg J, et al. Risk and consequences of chemotherapy-induced febrile neutropenia in patients with metastatic solid tumors. J Oncol Pract. 2015;11(1):47–54. doi:10.1200/JOP.2014.001492
