Abstract
Evidence from the Phase III PACIFIC trial established durvalumab, a monoclonal antibody (mAb) targeting PD-L1, following concurrent chemoradiotherapy (cCRT) as a global standard of care for patients with unresectable, stage III non-small-cell lung cancer (NSCLC). There remains an unmet need to improve upon the outcomes achieved with the PACIFIC regimen. Combining durvalumab with other immunotherapies may improve outcomes further. Two such immunotherapies include oleclumab, an mAb targeting CD73, and monalizumab, an mAb targeting NKG2A. Both agents demonstrated antitumor activity in early-phase trials. PACIFIC-9 (NCT05221840) is an international, double-blind, randomized, placebo-controlled, Phase III trial comparing durvalumab plus either oleclumab or monalizumab with durvalumab plus placebo in patients with unresectable, stage III NSCLC and no disease progression following cCRT.
Clinical Trial Registration: NCT05221840 (ClinicalTrials.gov)
Plain language summary
Durvalumab is a treatment that helps the body's immune system to identify and attack cancer cells by binding to a protein called PD-L1. Studies show that durvalumab lowers the risk of cancer growing or spreading, and prolongs survival, when administered after chemotherapy and radiation therapy (‘chemoradiotherapy’) in patients with a type of lung cancer called stage III non-small-cell lung cancer (NSCLC) for whom surgery is not an option.
Two antibody treatments have been developed that may help a patient's immune system to identify and attack cancer cells. Oleclumab binds to a protein on cancer cells called CD73, which prevents the production of adenosine, a chemical that obstructs the immune system from attacking the cancer. Monalizumab binds to NKG2A, a protein on immune cells that inhibits their ability to destroy cancer cells. Early studies suggest that combining either of these treatments with durvalumab may be better than durvalumab alone for slowing the growth and spread of cancer in patients with NSCLC.
PACIFIC-9 is a study that aims to recruit approximately 999 patients with stage III NSCLC for whom surgery is not an option and who have completed chemoradiotherapy without the cancer growing or spreading. Patients will be randomly assigned in equal numbers to receive up to a year of treatment with durvalumab plus oleclumab, durvalumab plus monalizumab or durvalumab plus placebo. The primary measure of efficacy is the length of time that patients remain alive without the cancer growing or spreading for each combination versus durvalumab plus placebo.
The PACIFIC regimen
Durvalumab is a monoclonal antibody that binds to PD-L1 and overcomes PD-L1 mediated inhibition of T-cell activation, allowing T cells to recognize and kill tumor cells.
The Phase III placebo-controlled, PACIFIC trial (NCT02125461) demonstrated significant improvements in progression-free survival (PFS) and overall survival with durvalumab in patients with unresectable, stage III non-small-cell lung cancer (NSCLC) whose disease had not progressed following platinum-based, concurrent chemoradiotherapy (cCRT); the PACIFIC regimen is now a global standard of care in this population.
Oleclumab & monalizumab
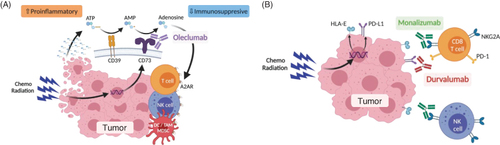
Extracellular adenosine can act as an immunosuppressant within the tumor microenvironment; oleclumab is a monoclonal antibody that binds to and blocks CD73, an enzyme on the surface of tumor cells that drives the production of extracellular adenosine.
NKG2A is a receptor on T cells and natural killer (NK) cells that can inhibit T cell and NK cell effector function when it binds to HLA-E; monalizumab is a monoclonal antibody that binds to NKG2A, preventing it from inhibiting NK and T cell function.
In the open-label, randomized, Phase II COAST trial (NCT03822351) the combination of durvalumab with oleclumab, and durvalumab with monalizumab, were both associated with encouraging clinical activity compared with durvalumab alone in patients with unresectable, stage III NSCLC and no progression after cCRT; the findings of COAST support further evaluation of these combinations in a Phase III trial.
PACIFIC-9
PACIFIC-9 (NCT05221840) is an international, double-blind, randomized, placebo-controlled, Phase III trial comparing the efficacy and safety of durvalumab plus oleclumab and durvalumab plus monalizumab versus durvalumab plus placebo in patients with unresectable, stage III NSCLC.
The trial is enrolling men and women aged 18 years or older with histologically or cytologically documented, unresectable, stage III NSCLC according to the TNM system as described in the International Association for the Study of Lung Cancer staging manual (8th edition), whose disease has not progressed following definitive, platinum-based cCRT.
The primary end point is PFS, defined as time from randomization until progression per Response Evaluation Criteria in Solid Tumors version 1.1 as assessed by blinded independent central review, or death due to any cause.
1. Background
Lung cancer accounts for significant morbidity and mortality worldwide, with an estimated 2.2 million new cases (11.4% of all new cancers) in 2020 [Citation1]. Moreover, lung cancer is a leading cause of cancer-related death worldwide, with approximately 2 million deaths annually [Citation2,Citation3]. Non-small-cell lung cancer (NSCLC) represents over 80% of all lung cancers, and 23–30% of patients with NSCLC present with unresectable, stage III disease [Citation4–6]. While some patients with stage IIIA NSCLC are suitable candidates for curative surgery and emerging perioperative systemic therapy, resection of the tumor is generally not recommended for patients with stage IIIB/IIIC disease [Citation7,Citation8]. The recommended standard treatment for those with unresectable, stage III NSCLC and good performance status includes definitive concurrent chemoradiotherapy (cCRT), involving the administration of radiotherapy with platinum-doublet chemotherapy [Citation7,Citation8]. For patients whose disease does not progress during cCRT, the recommended curative-intent treatment strategy is the administration of durvalumab as consolidation therapy for up to 12 months, based on data from the PACIFIC trial [Citation7–9].
Durvalumab is a high-affinity, human immunoglobulin (Ig) G1 monoclonal antibody that targets PD-L1, a protein on tumor cells that controls T-cell activation. By blocking the binding of PD-L1 with PD-1 and CD80 on T cells, durvalumab can overcome PD-L1-mediated inhibition of T-cell activation, allowing T cells to recognize and kill tumor cells [Citation10]. The efficacy and safety of durvalumab as consolidation therapy in patients with unresectable, stage III NSCLC and no disease progression after platinum-based cCRT was evaluated in the Phase III PACIFIC trial (NCT02125461), which demonstrated significant improvements in progression-free survival (PFS) (hazard ratio [HR]: 0.52; 95% confidence interval [CI]: 0.42–0.65; p < 0.001) and overall survival (OS) (HR: 0.68; 95% CI: 0.53–0.87; p = 0.0025) with durvalumab versus placebo [Citation9,Citation11–13]. Use of durvalumab was associated with a manageable safety profile (maximum-grade 3 or 4 adverse events of any cause increased by 4.4% in the durvalumab group compared with the placebo group) and no detrimental impact on patient-reported outcomes (PROs) compared with placebo [Citation9,Citation11,Citation14]. Long-term follow-up data from PACIFIC demonstrated that the benefits of durvalumab were sustained over time: the 5-year PFS rates were 33.1 vs 19.0% with durvalumab vs placebo, and the 5-year OS rates were 42.9 vs 33.4%, respectively [Citation13]. These outcomes established the PACIFIC regimen, comprising up to 12 months of consolidation therapy with durvalumab after platinum-based cCRT, as a global standard of care in this setting [Citation7,Citation8,Citation12,Citation15,Citation16].
2. Rationale for combining durvalumab with oleclumab or monalizumab
Despite the improvement in OS achieved with the PACIFIC regimen, many patients fail to respond, develop resistance or experience relapse [Citation13,Citation17]; thus, a significant unmet need exists to further improve outcomes for patients with unresectable, stage III NSCLC. Rational combination regimens targeting multiple pathophysiological pathways represent a promising avenue for further improvement in outcomes. Two targetable pathways, each with preclinical evidence for potential antitumor efficacy, are modulation of extracellular adenosine via inhibition of CD73 and blockade of the interaction between NKG2A with HLA-E [Citation18,Citation19].
Increases in extracellular adenosine can occur within the hypoxic tumor microenvironment and are known to exert immunosuppressive effects through a variety of mechanisms [Citation20,Citation21]. Therefore, modulating extracellular adenosine has gained interest as a possible immunotherapeutic approach for the treatment of solid tumors. CD73 is an ectonucleotidase found on the surfaces of immune cells and cancer cells, where it converts adenosine monophosphate to extracellular adenosine [Citation18,Citation20–22]. High expression of CD73 has been shown to increase extracellular adenosine production and suppress the local immune system in multiple cancers [Citation22,Citation23]. High expression of CD73 is associated with poor prognosis in several cancer types [Citation23–26]; in patients with NSCLC, overexpression of CD73 is associated with poor OS and recurrence-free survival compared with low CD73 expression [Citation26]. Moreover, the prospective biomarker study SUBMARINE (WJOG11518L; UMIN000035916), which analyzed tumor samples from patients who received the PACIFIC regimen, implicated CD73 as a possible mechanism of resistance to treatment [Citation17]. Consequently, CD73 has been proposed as a therapeutic target to reduce the immunosuppressive effects of the tumor microenvironment and overcome resistance to the PACIFIC regimen. Oleclumab is a human IgG1λ monoclonal antibody that binds to CD73 to decrease the creation of extracellular adenosine in the tumor microenvironment [Citation18,Citation27]. Combination of oleclumab with other immunotherapies, such as PD-1/PD-L1 inhibitors, has been shown to have antitumor activity in pre-clinical models [Citation18]. In a Phase I, first-in-human study of oleclumab in 192 patients with advanced solid tumors, including colorectal cancer, pancreatic ductal adenocarcinoma and EGFR-mutated NSCLC, oleclumab (alone or in combination with durvalumab) was associated with a manageable safety profile and exhibited isolated durable responses in some patients with NSCLC (partial responses observed in four patients [9.5%], with a median duration of response [DoR] of 14.8 months, and stable disease ≥8 weeks observed in another nine patients [21.4%]) [Citation28].
NKG2A is a receptor that is expressed as a heterodimer with CD94 on subsets of T cells and natural killer (NK) cells, where it recognizes the non-classical major histocompatibility complex 1 molecule, HLA-E, and inhibits T cell and NK cell effector function [Citation19]. Once bound to NKG2A, HLA-E triggers inhibitory signals that suppress cytokine secretion and direct cytotoxicity of T lymphocytes and NK cells [Citation29]. HLA-E is upregulated in some solid tumors, and high HLA-E can be associated with poor prognosis, though this is not consistent across cancer types [Citation30–37]. In an analysis of 197 patients with resected pulmonary adenocarcinoma, the expression of HLA-E by tumor cells was an independent negative prognostic factor for OS [Citation30]. In addition, tumoral expression of HLA-E has been shown to abolish the positive prognostic impact of CD8+ T cell infiltration in NSCLC and ovarian cancer [Citation30,Citation31]. Monalizumab is a first-in-class, humanized, IgG4 monoclonal antibody that targets NKG2A, preventing it from binding to HLA-E and thereby reducing inhibition of NK cells and CD8+ T cells, thus enhancing antitumor immunity [Citation19].
Pre-clinical studies indicate that expression of PD L1 [Citation38], CD73 [Citation39,Citation40] and the NKG2A ligands, HLA-E [Citation41] and its murine homolog Qa-1 [Citation42,Citation43], are increased in tumor cells in response to radiation (). Moreover, anti-PD-L1, anti-CD73 and a combination of anti-NKG2A and anti-PD-1 antibodies have demonstrated increased antitumor activity following radiotherapy in preclinical models [Citation38,Citation39,Citation42]. Consequently, simultaneous blockade of these immune checkpoints by combining anti-CD73 or anti-NKG2A monoclonal antibodies, such as oleclumab and monalizumab, with anti-PD-L1 antibodies, such as durvalumab, could represent a valuable approach for improving outcomes in patients with unresectable, stage III NSCLC who have undergone CRT.
Figure 1. Schematic mechanisms of action for oleclumab and monalizumab. The mechanisms of action of (A) oleclumab and (B) monalizumab are shown. Figure adapted from Martinez-Marti, et al. ‘COAST: an open-label, Phase II, multidrug platform study of durvalumab alone or in combination with novel agents in patients with locally advanced, unresectable, stage III NSCLC’. Oral presentation at the European Society for Medical Oncology (ESMO) Congress 2021. Figures originally created with BioRender.com.
AMP: Adenosine monophosphate; ATP: Adenosine triphosphate; DC: Dendritic cell; NK: Natural killer; TAM: Tumor-associated macrophage.
3. Clinical activity observed with durvalumab combined with oleclumab or monalizumab in the randomized Phase II COAST trial
Encouraging clinical activity has been observed with durvalumab plus oleclumab and durvalumab plus monalizumab, compared with durvalumab plus placebo, in the open-label, Phase II COAST trial (NCT03822351), which randomized 189 patients with unresectable, stage III NSCLC and no progression following definitive, platinum-based cCRT [Citation44]. Objective response rate (ORR) was greater with durvalumab plus oleclumab (30.0%) and durvalumab plus monalizumab (35.5%) vs durvalumab alone (17.9%) [Citation44]. Moreover, PFS was prolonged with durvalumab plus oleclumab (HR: 0.44; 95% CI: 0.26–0.75) and durvalumab plus monalizumab (HR: 0.42; 95% CI: 0.24–0.72) versus durvalumab alone, with 12-month PFS rates of 62.6, 72.7 and 33.9%, respectively [Citation44]. In exploratory subgroup analyses, PFS benefit was observed with both combinations across a range of clinically important subgroups, including those on the basis of histology, type of prior platinum-based chemotherapy, and Eastern Cooperative Oncology Group performance status (ECOG PS), although patient numbers in these subgroups were small [Citation44]. PFS benefit was also consistently demonstrated with both combinations versus durvalumab alone irrespective of tumor CD73, NKG2A or HLA-E expression [Citation44]. Furthermore, evidence of a PFS benefit was observed in patients with unknown PD-L1 status and PD-L1 tumor cell (TC) expression ≥1%; analysis of patients with PD-L1 TC <1% was limited due to small sample size [Citation44]. The safety profile of each combination was manageable and similar to the safety profile of durvalumab alone; no new significant safety signals were observed with either combination [Citation44]. Preliminary evidence of antitumor activity with monalizumab and oleclumab in combination with durvalumab for patients with resectable, early-stage NSCLC has also been observed in the neoadjuvant setting in the ongoing Phase II NeoCOAST trial (NCT03794544), and additional investigation of monalizumab and oleclumab in combination with both durvalumab and chemotherapy in a similar setting is ongoing in the Phase II NeoCOAST-2 trial (NCT05061550) [Citation45,Citation46].
The available preclinical and early clinical data strongly support further investigation of oleclumab and monalizumab in combination with durvalumab in a global Phase III trial in the unresectable, stage III NSCLC setting. Here, we describe the design of the Phase III PACIFIC-9 trial (NCT05221840), which is evaluating the efficacy and safety of durvalumab in combination with either oleclumab or monalizumab, compared with durvalumab plus placebo, in patients with unresectable, stage III NSCLC whose disease has not progressed following definitive, platinum-based cCRT.
4. Methods
4.1. Study design
PACIFIC-9 is an international, double-blind, randomized, placebo-controlled, Phase III trial (). The study aims to enroll approximately 999 patients from across 20 countries (Australia, Brazil, Canada, China, Colombia, France, Germany, Italy, Japan, Peru, Poland, Portugal, the Republic of Korea, Spain, Taiwan, Thailand, Turkey, the UK, USA and Vietnam).
Figure 2. Design of the PACIFIC 9 trial.
BICR: Blinded independent central review; cCRT: Concurrent chemoradiotherapy; IV: Intravenous; NSCLC: Non-small-cell lung cancer; PFS: Progression-free survival; Q2W: Once every 2 weeks; Q4W: Once every 4 weeks; RECIST v1.1: Response Evaluation Criteria in Solid Tumors version 1.1; WHO PS: World Health Organization performance status.
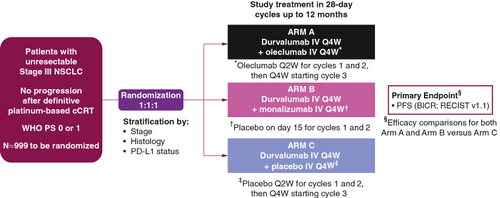
Patients will be randomized in a 1:1:1 ratio to receive one of the following three regimens, stratified by disease stage prior to cCRT, tumor histology, and PD-L1 status: (1) intravenous (IV) infusion of durvalumab on Day 1 of each 28 day cycle, plus IV infusions of oleclumab given on Day 1 and Day 15 of cycles 1 and 2 and then on Day 1 of each subsequent 28-day cycle for up to 12 months; (2) IV infusions of durvalumab and monalizumab, both given on Day 1 of each 28-day cycle for up to 12 months (patients will also receive an IV infusion of placebo on Day 15 of cycles 1 and 2); or (3) IV infusion of durvalumab on Day 1 of each 28-day cycle plus IV infusion of placebo on Days 1 and 15 of cycles 1 and 2 and then on Day 1 of each subsequent 28-day cycle for up to 12 months. Treatment will continue until end of planned treatment, clinical progression, confirmed Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST v1.1)-defined radiological progression, unacceptable toxicity, withdrawal of consent or another criterion for discontinuation is met. As results from the PACIFIC study suggest that patients who start durvalumab <14 days after cCRT may achieve better outcomes than those who start treatment ≥14 days post cCRT [Citation9,Citation11], investigators will be encouraged to initiate study treatment as soon as possible after completion of cCRT.
The primary end point is PFS, defined as time from randomization until progression per RECIST v1.1 [Citation47] as assessed by blinded independent central review (BICR), or death from any cause. The secondary end points are: OS; PFS by investigator assessment; ORR and DoR (RECIST v1.1; BICR); time from randomization to second progression (‘PFS2’; earliest of the progression events following initial progression, subsequent to first subsequent therapy or death); time to distant metastasis or death; time to first subsequent therapy or death; immunohistochemistry analysis of tumoral PD-L1 expression relative to efficacy outcomes (OS, PFS and ORR); time to first confirmed deterioration of pulmonary symptoms (cough, dyspnea and chest pain as single items from the Non-Small Cell Lung Cancer Symptom Assessment Questionnaire [NSCLC-SAQ]); pharmacokinetics and immunogenicity of durvalumab, oleclumab and monalizumab; and safety and tolerability.
Safety and tolerability in terms of reported adverse events, which will be coded using the latest version of the Medical Dictionary for Regulatory Activities and graded using National Cancer Institute Common Toxicity Criteria for Adverse Events (CTCAE) version 5.0, will be assessed continuously from screening until 90 days after the last dose of study intervention. Adverse events will be treated according to toxicity management guidelines for each study drug. Other safety data will be assessed in terms of physical examination, clinical chemistry, hematology, vital signs and electrocardiograms. Several PRO questionnaires will be administered during the trial, including (but not limited to) the NSCLC Symptom Assessment Questionnaire and the European Organization for Research and Treatment of Cancer (EORTC) quality-of-life questionnaire (EORTC QLQ-C30).
4.2. Eligibility criteria
Key inclusion and exclusion criteria are listed in .
Table 1. Key eligibility criteria for the PACIFIC-9 trial.
4.2.1. Patient & disease characteristics
Men and women aged 18 years or above are eligible for the study if they have World Health Organization performance status 0 or 1, histologically or cytologically documented NSCLC and have been treated with cCRT for unresectable, stage III NSCLC without disease progression on this treatment. Patients must be staged according to the Tumor, Node, Metastasis (TNM) system as described in the International Association for the Study of Lung Cancer staging manual (8th edition) [Citation48]. Except for overt cT4 disease, biopsy is strongly preferred to prove nodal status N2 or N3 via endobronchial ultrasound, mediastinoscopy or thoracoscopy at initial diagnosis and staging. Brain imaging with magnetic resonance imaging (preferred) or high-quality computed tomography (CT) scan with IV contrast is strongly encouraged during initial staging. Positron emission tomography (PET)-CT scan from at least the base of skull to mid-thigh is required within 3 months prior to the first dose of cCRT to avoid enrolling patients who have upstaged during initial diagnosis.
4.2.2. Biomarker testing
As we enter a new era for studies of patients with unresectable, stage III NSCLC, in which novel immunotherapy combinations that build upon the backbone of PD-L1/PD-1 inhibition are being explored, future treatment selection will need to be informed by a biomarker-driven approach. In recognition of this, the provision of a tumor tissue sample for biomarker testing is a mandatory requirement of PACIFIC-9. The sample must be obtained before cCRT; irradiated samples are not acceptable. Central confirmation of tumor PD-L1 status must occur before randomization. Confirmation of EGFR and ALK status by central confirmation or locally-approved assay must also be available before randomization; patients with EGFR mutations or ALK translocations are excluded from the study considering their potential to benefit from targeted therapies and emerging evidence from external studies that suggests these patients have limited response to immunotherapy [Citation49–51]. Following completion of PD-L1 testing, and testing of EGFR and ALK if required, any residual tissue collected at screening may be used for the assessment of exploratory biomarkers.
4.2.3. Prior concurrent chemoradiotherapy
PACIFIC-9 is aligned with international guidelines for chemotherapy and radiation [Citation7,Citation8]. Patient selection should involve early input from multidisciplinary teams to identify patients who are most suited to benefit from cCRT. Randomization to study treatment occurs after receipt of cCRT. Participants must not have progressed following at least two cycles of definitive, platinum-based, cCRT. Consolidation chemotherapy after radiotherapy (RT) is not permitted, but up to two cycles of induction chemotherapy before cCRT is allowed to account for regional variability in clinical practice. Chemotherapy must be cisplatin- or carboplatin-based and contain one of the following agents (per local standard-of-care regimens): etoposide, vinblastine, vinorelbine, a taxane (docetaxel/paclitaxel) or pemetrexed; use of gemcitabine is not permitted. Although RT is delivered before study randomization, PACIFIC-9 requires all randomized subjects to have completed a full course of definitive cCRT that includes a total RT prescription dose of 60 Gy ±10% (i.e., 54–66 Gy; with individualized prescribed doses agreed between the attending physician and patient) via intensity-modulated radiation therapy or 3D conformal radiation therapy [Citation52]. The type of RT administered was not selected as a stratification factor, as no differential prognostic impact is expected [Citation53]; however, RT guidance consistent with global standards is provided to all participating institutions for their reference in the protocol and accessory documents. Additionally, robust data will be collected to allow potential analysis of the impact of type of RT on outcomes.
4.3. Statistical methods
The two primary objectives of this trial are to assess the efficacy of durvalumab plus oleclumab vs durvalumab plus placebo and durvalumab plus monalizumab vs durvalumab plus placebo in terms of PFS (BICR; RECIST v1.1). The key secondary objective is to assess efficacy in terms of OS. The study will be considered positive (a success) if the primary analysis of PFS for either treatment comparison is statistically significant. To control the type I error at 5% (2-sided) between the primary (PFS) and key secondary (OS) end points, a multiple testing procedure (MTP) with gatekeeping strategy will be used for each treatment comparison.
PFS and OS will be analyzed using a log rank test stratified by disease stage, tumor histology and PD-L1 status. HRs and 95% CIs will be estimated from a stratified Cox proportional hazards model. The intention-to-treat population, comprising all patients randomized in the trial, will be used for all efficacy measures. Safety data will be summarized descriptively based on all randomized patients who receive any amount of study treatment.
4.4. Ethical considerations
The PACIFIC-9 trial is being conducted in accordance with the ethical principles derived from the Declaration of Helsinki and conducted in adherence with the protocol as well as International Conference on Harmonization Good Clinical Practice guidelines and applicable laws and country-specific regulations. The protocol, revisions, and other relevant documents are to be reviewed and approved by Institutional Review Boards/Independent Ethics Committees at each participating site. Written informed consent will be obtained from patients before trial-related procedures are performed. It will be possible to obtain data underlying the findings described in the final report for this study in accordance with AstraZeneca's data sharing policy (www.astrazenecaclinicaltrials.com/our-transparency-commitments/).
5. Conclusion
The treatment landscape for patients with non-metastatic NSCLC is evolving rapidly. In the last few years, novel immunotherapy-based regimens have received approvals in the neoadjuvant and adjuvant treatment settings for patients with resectable NSCLC based on the robust clinical benefits demonstrated in pivotal, Phase III trials [Citation54–58]. Nevertheless, the PACIFIC regimen remains the global standard of care for patients with unresectable, stage III NSCLC [Citation7,Citation8], and is associated with robust and sustained PFS and OS benefit in both clinical trial [Citation13] and real-world settings [Citation59,Citation60]. Although the use of durvalumab after cCRT has improved clinical outcomes for this population, further improvements may be achieved through the use of novel immunotherapy combinations that build upon the backbone of PD-1/PD-L1 inhibition. Novel immunotherapies such as oleclumab or monalizumab may synergize with the immunomodulatory effects of durvalumab and augment clinical efficacy. PACIFIC-9 is the first randomized Phase III trial to investigate the efficacy and safety of durvalumab in combination with these novel agents.
Clinical data have demonstrated an acceptable safety profile for oleclumab and monalizumab in combination with durvalumab, and the potential for clinical benefit with these combinations is supported by recent findings of the open-label, randomized, Phase II COAST trial [Citation44]. When administered after cCRT in patients with unresectable, stage III NSCLC, the combination of either of these agents with durvalumab was associated with encouraging improvements in PFS and ORR versus durvalumab alone. Both combinations were also well tolerated, with a safety profile comparable to durvalumab alone. Thus, the benefit/risk assessment favors the conduct of a large, blinded, Phase III trial such as PACIFIC-9 to confirm these findings. Trial enrollment began in February 2022; this trial is ongoing.
Author contributions
All authors made substantial contributions to the design and/or conduct of the study, and to drafting or reviewing the article for intellectual content. All authors approved the final version of the article and agree to be accountable for all aspects of the work.
Financial disclosure
The PACIFIC-9 trial (NCT05221840) is sponsored by AstraZeneca.
Writing disclosure
Medical writing support, under the direction of the authors, was provided by G Allcock, A Korpal and E Exner of Ashfield MedComms (New York, NY), an Inizio Company, and was funded by AstraZeneca.
Ethical conduct of research
The study protocol was approved by the appropriate ethics committee or institutional review board at each participating site. The study will be conducted in accordance with the principles outlined in the Declaration of Helsinki and the International Conference on Harmonisation Guidelines for Good Clinical Practice. Written informed consent will be obtained from all trial participants prior to enrollment.
Competing interests disclosure
F Barlesi reports Institutional financial interest from AbbVie, ACEA, Amgen, AstraZeneca, Bayer, BMS, Boehringer Ingelheim, Eisai, Eli Lilly Oncology, F. Hoffmann-La Roche Ltd, Genentech, Ipsen, Ignyta, Innate Pharma, Loxo, Novartis, Medimmune, Merck, MSD, Pierre Fabre, Pfizer, Sanofi-Aventis, and Takeda; and non-financial interests as principal investigator for AstraZeneca, BMS, Innate Pharma, Merck, Pierre Fabre, and F. Hoffmann-La Roche Ltd, sponsored trials (or ISR). BC Cho reports research funding from MOGAM Institute, LG Chem, Oscotec, Interpark Bio Convergence Corp, GI Innovation, GI Cell, Abion, AbbVie, AstraZeneca, Bayer, Blueprint Medicines, Boehringer Ingelheim, Champions Onoclogy, CJ Bioscience, CJ Blossom Park, Cyrus, Dizal Pharma, Genexine, Janssen, Lilly, MSD, Novartis, Nuvalent, Oncternal, Ono, Regeneron, Dong-A ST, BridgeBio Therapeutics, Yuhan, ImmuneOncia, Illumina, Kanaph Therapeutics, Therapex, J INTS BIO, Hanmi, CHA Bundang Medical Center, and Vertical Bio AG; royalties from Champions Oncology, Crown Bioscience, Imagen, and PearlRiver Bio GmbH; consulting fees from Abion, BeiGene, Novartis, AstraZeneca, Boehringer Ingelheim, Roche, BMS, CJ, CureLogen, Cyrus Therapeutics, Ono, Onegene Biotechnology, Yuhan, Pfizer, Eli Lilly, GI Cell, Guardant, HK inno.N, Imnewrun Biosciences Inc., Janssen, Takeda, MSD, Janssen, Medpacto, Blueprint Medicines, RandBio, and Hanmi; honoraria from ASCO, AstraZeneca, Guardant, Roche, ESMO, IASLC, Korean Cancer Association, Korean Society of Medical Onoclogy, Korean Society of Thyroid-Head and Neck Surgery, Korean Cancer Study Group, Novartis, MSD, The Chinese Thoracic Oncology Society, and Pfizer; scientific advisory board participation for KANAPH Therapeutic Inc, BridgeBio Therapeutics, Cyrus Therapeutics, Guardant Health, Oscotec Inc, J INTS Bio, Therapex Co. Ltd, Gilead, and Amgen; membership on the board of directors for J INTS BIO; stock ownership in TheraCanVac Inc., Gencurix Inc., BridgeBio Therapeutics, KANAPH Therapeutic Inc., Cyrus therapeutics, Interpark Bio Convergence Corp., J INTS BIO; employment by the Yonsei University Health System; and is the founder of DAAN Biotherapeutics. SB Goldberg reports research funding from AstraZeneca, Boehringer Ingelheim, and Mirati; and consulting/advisory board membership for AstraZeneca, Boehringer Ingelheim, BMS, Genentech, Amgen, Blueprint Medicine, Sanofi Genzyme, Daiichi-Sankyo, Takeda, Janssen, Summit Therapeutics, Merck, and Regeneron. K Yoh reports grants or contracts from AbbVie, Amgen, AstraZeneca, Boehringer Ingelheim, Chugai, Daiichi-Sankyo, Lilly, MSD, Pfizer, Taiho, and Takeda; consulting fees from Boehringer Ingelheim; and payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from AbbVie, Amgen, AstraZeneca, Boehringer Ingelheim, BMS, Chugai, Daiichi-Sankyo, Janssen, Kyowa Kirin, Lilly, Merck Serono, Novartis, Ono, Otsuka, Taiho, and Takeda. AC Zimmer Gelatti reports grants or contracts from Roche, Amgen, AstraZeneca, Boehringer Ingelheim, BMS, Daiichi-Sankyo, Lilly, MSD, Pfizer, Taiho, and Takeda; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Roche, Amgen, AstraZeneca, Boehringer Ingelheim, BMS, MSD, Daiichi-Sankyo, Janssen, Pfizer, Lilly, Novartis, and Takeda; travel support from Daiichi-Sankyo, Amgen, and Janssen; advisory board or data safety monitoring board participation for AstraZeneca, MSD, BeiGene, Pfizer, and Amgen. H Mann is an employee of and owns stock in AstraZeneca. A Gopinathan is an employee of and owns stock in AstraZeneca. ZF Bielecka is an employee of and owns stock in AstraZeneca. M Newton is an employee of and owns stock in AstraZeneca. C Aggarwal reports research funding from AstraZeneca, Genentech, Incyte, Macrogenics, Medimmune, MSD, and Lilly; and consulting or advisory fees from Genentech, Lilly, Celgene Merck, AstraZeneca, Blueprint Genetics, Shionogi, Daiichi-Sankyo/AstraZeneca, Regeneron/Sanofi, Eisai, BeiGene, Turning Point Therapeutics, Pfizer, Janssen, and Boehringer Ingelheim. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Data availability statement
This is a clinical trial protocol manuscript, and no data are being reported. On completion of the trial, data will be available in accordance with AstraZeneca's data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure. Data for studies directly listed on Vivli can be requested through Vivli at www.vivli.org. Data for studies not listed on Vivli could be requested through Vivli at https://vivli.org/members/enquiries-about-studies-not-listed-on-the-vivli-platform/. AstraZeneca Vivli member page is also available outlining further details: https://vivli.org/ourmember/astrazeneca/.
Additional information
Funding
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
- Siegel RL, Miller KD, Wagle NS, et al. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17–48. doi:10.3322/caac.21763
- Global Burden of Disease Cancer C Kocarnik JM, Compton K, et al. Cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life years for 29 cancer groups from 2010 to 2019: a systematic analysis for the Global Burden of Disease Study 2019. JAMA Oncol. 2022;8(3):420–444. doi:10.1001/jamaoncol.2021.6987
- Casal-Mourino A, Ruano-Ravina A, Lorenzo-Gonzalez M, et al. Epidemiology of stage III lung cancer: frequency, diagnostic characteristics, and survival. Transl Lung Cancer Res. 2021;10(1):506–518. doi:10.21037/tlcr.2020.03.40
- Provencio M, Carcereny E, Rodriguez-Abreu D, et al. Lung cancer in Spain: information from the Thoracic Tumors Registry (TTR study). Transl Lung Cancer Res. 2019;8(4):461–475. doi:10.21037/tlcr.2019.08.05
- Walters S, Maringe C, Coleman MP, et al. Lung cancer survival and stage at diagnosis in Australia, Canada, Denmark, Norway, Sweden and the UK: a population-based study, 2004–2007. Thorax. 2013;68(6):551–564. doi:10.1136/thoraxjnl-2012-202297
- Daly ME, Singh N, Ismaila N, et al. Management of stage III non-small-cell lung cancer: ASCO Guideline. J Clin Oncol. 2022;40(12):1356–1384. doi:10.1200/JCO.21.02528
- Remon J, Soria JC, Peters S, et al. Early and locally advanced non-small-cell lung cancer: an update of the ESMO Clinical Practice Guidelines focusing on diagnosis, staging, systemic and local therapy. Ann Oncol. 2021;32(12):1637–1642. doi:10.1016/j.annonc.2021.08.1994
- Antonia SJ, Villegas A, Daniel D, et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med. 2018;379(24):2342–2350. doi:10.1056/NEJMoa1809697
- Stewart R, Morrow M, Hammond SA, et al. Identification and characterization of MEDI4736, an antagonistic anti-PD-L1 monoclonal antibody. Cancer Immunol Res. 2015;3(9):1052–1062. doi:10.1158/2326-6066.CIR-14-0191
- Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017;377(20):1919–1929. doi:10.1056/NEJMoa1709937
- European Medicines Agency. Durvalumab (imfinzi). Summary of product characteristics. 2023 [ cited 2023 Oct 26]. Available from: www.ema.europa.eu/en/documents/product-information/imfinzi-epar-product-information_en.pdf
- Spigel DR, Faivre-Finn C, Gray JE, et al. Five-year survival outcomes from the PACIFIC trial: durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. J Clin Oncol. 2022;40(12):1301–1311. doi:10.1200/JCO.21.01308
- Hui R, Ozguroglu M, Villegas A, et al. Patient-reported outcomes with durvalumab after chemoradiotherapy in stage III, unresectable non-small-cell lung cancer (PACIFIC): a randomised, controlled, phase 3 study. Lancet Oncol. 2019;20(12):1670–1680. doi:10.1016/S1470-2045(19)30519-4
- Food and Drug Administration. IMFINZI (durvalumab) label. 2023 [ cited 2023 Aug 8]. Available from: www.accessdata.fda.gov/drugsatfda_docs/label/2022/761069s035lbl.pdf
- Park K, Vansteenkiste J, Lee KH, et al. Pan-Asian adapted ESMO Clinical Practice Guidelines for the management of patients with locally-advanced unresectable non-small-cell lung cancer: a KSMO-ESMO initiative endorsed by CSCO, ISMPO, JSMO, MOS, SSO and TOS. Ann Oncol. 2020;31(2):191–201. doi:10.1016/j.annonc.2019.10.026
- Haratani K, Nakamura A, Mamesaya N, et al. Tumor microenvironment landscape of NSCLC reveals resistance mechanisms for programmed death-ligand 1 blockade after chemoradiotherapy: a multicenter prospective biomarker study (WJOG11518L:SUBMARINE). J Thorac Oncol. 2023;18(10):1334–1350. doi:10.1016/j.jtho.2023.06.012
- Hay CM, Sult E, Huang Q, et al. Targeting CD73 in the tumor microenvironment with MEDI9447. Oncoimmunology. 2016;5(8):e1208875. doi:10.1080/2162402X.2016.1208875
- Andre P, Denis C, Soulas C, et al. Anti-NKG2A mAb Is a checkpoint inhibitor that promotes anti-tumor immunity by unleashing both T and NK cells. Cell. 2018;175(7):1731–1743.e13. doi:10.1016/j.cell.2018.10.014
- Young A, Mittal D, Stagg J, et al. Targeting cancer-derived adenosine: new therapeutic approaches. Cancer Discov. 2014;4(8):879–888. doi:10.1158/2159-8290.CD-14-0341
- Ohta A. A metabolic immune checkpoint: adenosine in tumor microenvironment. Front Immunol. 2016;7:109. doi:10.3389/fimmu.2016.00109
- Vijayan D, Young A, Teng MWL, et al. Targeting immunosuppressive adenosine in cancer. Nat Rev Cancer. 2017;17(12):709–724. doi:10.1038/nrc.2017.86
- Turcotte M, Spring K, Pommey S, et al. CD73 is associated with poor prognosis in high-grade serous ovarian cancer. Cancer Res. 2015;75(21):4494–4503. doi:10.1158/0008-5472.CAN-14-3569
- Harvey JB, Phan LH, Villarreal OE, et al. CD73's potential as an immunotherapy target in gastrointestinal cancers. Front Immunol. 2020;11:508. doi:10.3389/fimmu.2020.00508
- Buisseret L, Pommey S, Allard B, et al. Clinical significance of CD73 in triple-negative breast cancer: multiplex analysis of a Phase III clinical trial. Ann Oncol. 2018;29(4):1056–1062. doi:10.1093/annonc/mdx730
- Inoue Y, Yoshimura K, Kurabe N, et al. Prognostic impact of CD73 and A2A adenosine receptor expression in non-small-cell lung cancer. Oncotarget. 2017;8(5):8738–8751. doi:10.18632/oncotarget.14434
- Geoghegan JC, Diedrich G, Lu X, et al. Inhibition of CD73 AMP hydrolysis by a therapeutic antibody with a dual, non-competitive mechanism of action. MAbs. 2016;8(3):454–467. doi:10.1080/19420862.2016.1143182
- Bendell J, LoRusso P, Overman M, et al. First-in-human study of oleclumab, a potent, selective anti-CD73 monoclonal antibody, alone or in combination with durvalumab in patients with advanced solid tumors. Cancer Immunol Immunother. 2023;72(7):2443–2458. doi:10.1007/s00262-023-03430-6
- van Hall T, Andre P, Horowitz A, et al. Monalizumab: inhibiting the novel immune checkpoint NKG2A. J Immunother Cancer. 2019;7(1):263. doi:10.1186/s40425-019-0761-3
- Talebian Yazdi M, van Riet S, van Schadewijk A, et al. The positive prognostic effect of stromal CD8+ tumor-infiltrating T cells is restrained by the expression of HLA-E in non-small cell lung carcinoma. Oncotarget. 2016;7(3):3477–3488. doi:10.18632/oncotarget.6506
- Gooden M, Lampen M, Jordanova ES, et al. HLA-E expression by gynecological cancers restrains tumor-infiltrating CD8(+) T lymphocytes. Proc Natl Acad Sci USA. 2011;108(26):10656–10661. doi:10.1073/pnas.1100354108
- de Kruijf EM, Sajet A, van Nes JG, et al. HLA-E and HLA-G expression in classical HLA class I-negative tumors is of prognostic value for clinical outcome of early breast cancer patients. J Immunol. 2010;185(12):7452–7459. doi:10.4049/jimmunol.1002629
- Platonova S, Cherfils-Vicini J, Damotte D, et al. Profound coordinated alterations of intratumoral NK cell phenotype and function in lung carcinoma. Cancer Res. 2011;71(16):5412–5422. doi:10.1158/0008-5472.CAN-10-4179
- Levy EM, Bianchini M, Von Euw EM, et al. Human leukocyte antigen-E protein is overexpressed in primary human colorectal cancer. Int J Oncol. 2008;32(3):633–641.
- Wu Z, Liang J, Wang Z, et al. HLA-E expression in diffuse glioma: relationship with clinicopathological features and patient survival. BMC Neurol. 2020;20(1):59. doi:10.1186/s12883-020-01640-4
- Baysal H, Siozopoulou V, Zaryouh H, et al. The prognostic impact of the immune signature in head and neck squamous cell carcinoma. Front Immunol. 2022;13:1001161. doi:10.3389/fimmu.2022.1001161
- Spaans VM, Peters AA, Fleuren GJ, et al. HLA-E expression in cervical adenocarcinomas: association with improved long-term survival. J Transl Med. 2012;10:184. doi:10.1186/1479-5876-10-184
- Gong X, Li X, Jiang T, et al. Combined radiotherapy and anti-PD-L1 antibody synergistically enhances antitumor effect in non-small cell lung cancer. J Thorac Oncol. 2017;12(7):1085–1097. doi:10.1016/j.jtho.2017.04.014
- Tsukui H, Horie H, Koinuma K, et al. CD73 blockade enhances the local and abscopal effects of radiotherapy in a murine rectal cancer model. BMC Cancer. 2020;20(1):411. doi:10.1186/s12885-020-06893-3
- Wennerberg E, Spada S, Rudqvist NP, et al. CD73 blockade promotes dendritic cell infiltration of irradiated tumors and tumor rejection. Cancer Immunol Res. 2020;8(4):465–478. doi:10.1158/2326-6066.CIR-19-0449
- Hrbac T, Kopkova A, Siegl F, et al. HLA-E and HLA-F are overexpressed in glioblastoma and HLA-E increased after exposure to ionizing radiation. Cancer Genomics Proteomics. 2022;19(2):151–162. doi:10.21873/cgp.20311
- Battaglia NG, Caldon JJ, Okoshi EN, et al. NKG2A inhibits the anti-tumor CD8 T cell immune response elicited by radiotherapy. J Immunol. 2020;204(Suppl. 1):241.24. doi:10.4049/jimmunol.204.Supp.241.24
- Battaglia NG, Murphy JD, Uccello TP, et al. Combination of NKG2A and PD-1 blockade improves radiotherapy response in radioresistant tumors. J Immunol. 2022;209(3):629–640. doi:10.4049/jimmunol.2100044
- Herbst RS, Majem M, Barlesi F, et al. COAST: an open-label, Phase II, multidrug platform study of durvalumab alone or in combination with oleclumab or monalizumab in patients with unresectable, stage III non-small-cell lung cancer. J Clin Oncol. 2022;40(29):3383–3393. doi:10.1200/JCO.22.00227
- Cascone T, Kar G, Spicer JD, et al. Neoadjuvant durvalumab alone or combined with novel immuno-oncology agents in resectable lung cancer: the Phase II NeoCOAST platform trial. Cancer Discovery. 2023;13(11):2394–2411. doi:10.1158/2159-8290.Cd-23-0436
- Guisier F, Bennouna J, Spira AI, et al. NeoCOAST-2: a phase 2 study of neoadjuvant durvalumab plus novel immunotherapies (IO) and chemotherapy (CT) or MEDI5752 (volrustomig) plus CT, followed by surgery and adjuvant durvalumab plus novel IO or volrustomig alone in patients with resectable non-small-cell lung cancer (NSCLC). J Clin Oncol. 2023;41(Suppl. 16):TPS8604. doi:10.1200/JCO.2023.41.16_suppl.TPS8604
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. doi:10.1016/j.ejca.2008.10.026
- Detterbeck FC, Boffa DJ, Kim AW, et al. The Eighth Edition lung cancer stage classification. Chest. 2017;151(1):193–203. doi:10.1016/j.chest.2016.10.010
- Mazieres J, Drilon A, Lusque A, et al. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: results from the IMMUNOTARGET registry. Ann Oncol. 2019;30(8):1321–1328. doi:10.1093/annonc/mdz167
- Huang Q, Zhang H, Hai J, et al. Impact of PD-L1 expression, driver mutations and clinical characteristics on survival after anti-PD-1/PD-L1 immunotherapy versus chemotherapy in non-small-cell lung cancer: a meta-analysis of randomized trials. Oncoimmunology. 2018;7(12):e1396403. doi:10.1080/2162402X.2017.1396403
- Lee CK, Man J, Lord S, et al. Clinical and molecular characteristics associated with survival among patients treated with checkpoint inhibitors for advanced non-small cell lung carcinoma: a systematic review and meta-analysis. JAMA Oncol. 2018;4(2):210–216. doi:10.1001/jamaoncol.2017.4427
- Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015;16(2):187–199. doi:10.1016/S1470-2045(14)71207-0
- Chun SG, Hu C, Choy H, et al. Impact of intensity-modulated radiation therapy technique for locally advanced non-small-cell lung cancer: a secondary analysis of the nrg oncology RTOG 0617 randomized clinical trial. J Clin Oncol. 2017;35(1):56–62. doi:10.1200/JCO.2016.69.1378
- O'Brien M, Paz-Ares L, Marreaud S, et al. Pembrolizumab versus placebo as adjuvant therapy for completely resected stage IB-IIIA non-small-cell lung cancer (PEARLS/KEYNOTE-091): an interim analysis of a randomised, triple-blind, phase 3 trial. Lancet Oncol. 2022;23(10):1274–1286. doi:10.1016/S1470-2045(22)00518-6
- Felip E, Altorki N, Zhou C, et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet. 2021;398(10308):1344–1357. doi:10.1016/S0140-6736(21)02098-5
- Forde PM, Spicer J, Lu S, et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N Engl J Med. 2022;386(21):1973–1985. doi:10.1056/NEJMoa2202170
- Food and Drug Administration. KEYTRUDA (pembrolizumab) label. 2023 [ cited 2023 Oct 16]. Available from: www.accessdata.fda.gov/drugsatfda_docs/label/2023/125514s138lbl.pdf
- Food and Drug Administration. OPDIVO (nivolumab) label. 2023 [ cited 2023 Oct 16]. Available from: www.accessdata.fda.gov/drugsatfda_docs/label/2023/125554Orig1s121lbl.pdf
- Girard N, Bar J, Garrido P, et al. Treatment characteristics and real-world progression-free survival in patients with unresectable stage III NSCLC who received durvalumab after chemoradiotherapy: findings from the PACIFIC-R study. J Thorac Oncol. 2023;18(2):181–193. doi:10.1016/j.jtho.2022.10.003
- Girard N, Christoph DCC, Garassino MC, et al. Real-world overall survival (OS) with durvalumab (D) after chemoradiotherapy (CRT) in patients (pts) with unresectable stage III non-small cell lung cancer (NSCLC): interim analysis from the PACIFIC-R study. IOTECH. 2022;16:100163. doi:10.1016/j.iotech.2022.100163
