Abstract
Objective: To compare speech perception between children with a different age at cochlear implantation. Design: We evaluated speech perception by comparing consonant–vowel–consonant (auditory) (CVC(A)) scores at five-year follow-up of children implanted between 1997 and 2010. The proportion of children from each age-at-implantation group reaching the 95%CI of CVC(A) ceiling scores (>95%) was calculated to identify speech perception differences masked by ceiling effects. Study sample: 54 children implanted between 8 and 36 months. Results: Although ceiling effects occurred, a CVC(A) score difference between age-at-implantation groups was confirmed (H (4) = 30.36; p < 0.001). Outperformance of early (<18 months) compared to later implanted children was demonstrated (p <0.001). A larger proportion of children implanted before 13 months compared to children implanted between 13 and 18 months reached ceiling scores. Logistic regression confirmed that age at implantation predicted whether a child reached a ceiling score. Conclusions: Ceiling effects can mask thorough delineation of speech perception. However, this study showed long-term speech perception outperformance of early implanted children (<18 months) either including or not accounting for ceiling effects during analysis. Development of long-term assessment tools not affected by ceiling effects is essential to maintain adequate assessment of young implanted infants.
Introduction
Cochlear implants (CIs) are beneficial in providing hearing, speech and language rehabilitation to children presenting with profound hearing loss (Vaca et al, Citation2015). The timing of cochlear implantation is essential for prompt rehabilitation in developing age appropriate hearing, and in turn, other skills (Levine et al, Citation2016). Early cochlear implantation has shown promising outcomes. The current literature indicates that infants who receive a CI before 12 months of age outperform children receiving CIs after this age on a variety of language outcome measures (Houston et al, Citation2001; Miyamoto et al, Citation2005; Dettman et al, Citation2007, Vlastarakos et al, Citation2010; Colletti et al, Citation2011; Houston et al, Citation2012; Ching et al, Citation2013a,Citationb; Holman et al, Citation2013; Leigh et al, Citation2013; Cuda et al, Citation2014; Dettman et al, Citation2016; Levine et al, Citation2016). However, in order to reach consensus within the medical community, both Vlastarakos et al. (Citation2010) and Dettman et al. (Citation2016) highlight the need to define the optimal age for paediatric cochlear implantation. Several factors may influence why this optimal age for cochlear implantation is not explicitly defined. The absence of objective paediatric speech and language outcome measures without the occurrence of ceiling effects is known to be one of these underlying factors (Vlastarakos et al, Citation2010).
Following paediatric CI surgery, regular postoperative follow-up is necessary to closely monitor speech and language development. Since there is no clear guideline for universal postoperative assessment of children using CIs, a wide variety of speech and language outcome measures are currently used (Uhler & Gifford, Citation2014). Some of these tests could provide data that are hindered by ceiling effects, as they are not able to adequately monitor the rapid speech and language improvement following paediatric CI surgery (Helms et al, Citation2004). Ceiling effects occur when the majority of tested patients reach the maximum or near maximum test score (Helms et al, Citation2004). Helms et al. (Citation2004) identified that speech perception ceiling effects occurred already 1 month following CI device activation in 51% of their adult CI population. Similarly, Massa & Ruckenstein (Citation2014) reported postoperative plateau scores between six months and three years following implantation in adult CI users. Since ceiling effects limit measuring the maximum performance of CI users, current postoperative tests may not accurately reflect the speech and language performance of CI patients. Therefore, application of these scores can potentially bias the interpretation of the postoperative test results (Helms et al, Citation2004). To prevent this bias from affecting speech and language outcome scores and to accurately depict the competence of the CI user, the difficulty of testing should increase when a CI user reaches a ceiling score on a less complex test (Spahr et al, Citation2014).
The occurrence of ceiling effects could be more pronounced in the paediatric CI population compared to adult CI users due to the use of categorical outcome measures and advances in CI technologies. First, current categorical outcome measures used in children have a limited number of test categories. For example, in speech intelligibility rating (SIR) and categories of auditory performance (CAP) scores, paediatric CI users can rapidly achieve the highest category (Cox & McDaniel, Citation1989; Archbold et al, Citation1995). For this reason, two additional category levels have been added in the CAP assessment (CAP-8 and CAP-9; CAP-II score) (NEAP© [Nottingham Early Assessment Package]; The Ear Foundation, Nottingham, UK, 2009). Colletti et al. (Citation2012) showed that younger patients (implanted between two and six months) significantly (p < 0.001) outperformed later implanted children on CAP-II scores at four-year follow-up. Results indicated that CAP-II scores greatly aided in identifying important differences between children implanted before two years of age (Colletti et al, Citation2012). Secondly, several authors demonstrated that young adult patients (mean age of 44.1 years [19.2–67.3] and between 26 and 39 years, respectively) who used newer CI technologies achieved plateau scores sooner (Ruffin et al, Citation2007; Krueger et al, Citation2008). This effect could be even more pronounced in children using newer technologies.
Vlastarakos et al. (Citation2010) suggest that using assessment tools with possible ceiling effects limits accurate identification of implant success for early implanted paediatric patients. To assess whether the influence of age at implantation on long-term speech perception was not masked by ceiling effects, we compared two speech perception analyses: a data assessment between paediatric CI cohorts grouped according to age at implantation and groups divided by the proportion of children reaching a ceiling consonant–vowel–consonant (auditory) (CVC(A)) score. By comparing aforementioned analyses, we attempted to clarify whether ceiling effects masked performance differences initiated by a different age at implantation. Furthermore, the ceiling effect analysis allowed us to assess speech perception performance differences between the youngest age-at-implantation groups (implanted before 13 months and between 13 and 18 months): an analysis that could not have been performed by comparing raw scores only since all children performed at the highest (ceiling) CVC(A) range.
Methods
Study design – participants
We conducted a retrospective review of children implanted before 36 months of age between 1997 and 2010 at our institution (UMC Utrecht). All included patients presented with prelingual hearing loss; defined as hearing loss that occurred before the acquisition of spoken language skills (before two years of age) (Vincenti et al, Citation2014). All CI candidates used preoperative hearing aids for a minimum of six weeks. CI indication was established following standardised multidisciplinary assessment. Five surgeons performed cochlear implantation through the suprameatal (SMA) or mastoidectomy with posterior tympanotomy approach (MPTA) (Clark et al, Citation1979; House, Citation1976; Kronenberg et al, Citation2001; Bruijnzeel et al, Citation2016). Patients receiving unilateral or bilateral implants were included ().
Table 1. Baseline characteristics of included patients arranged by age-at-implantation group.
Two authors conducted a retrospective review of institutional digitalised outpatient reports. Outcome measures included baseline demographic and hearing characteristics, surgical details and postoperative speech perception scores. In line with previous studies, children with a significant cognitive delay were excluded from our analysis (Dettman et al, Citation2016). Reporting was conducted according to STROBE guidelines (von Elm et al, Citation2008). Local ethical committee provided approval for this study (protocol number: METC 14-486/C).
Variables
Speech and language therapists from a certified CI revalidation team performed speech perception assessment by administering CVC(A) word lists during postoperative follow-up (Bosman, Citation1989; Bosman & Smoorenburg, Citation1995). This stimulus-repetition task is based on presenting recorded, open-set meaningful monosyllabic words (consonant–vowel–consonants [CVCs]) in a quiet room at a level of 65 dB SPL without providing visual cues. The participant should correctly repeat aforementioned meaningful CVC(A) words. Bosman (Citation1989) and Bosman & Smoorenburg (Citation1995) developed 60 different lists of CVC(A) words of which 15 word lists can be applied in the paediatric population. Each of these 15 CVC(A) lists contains 11 words, which contain a total of 33 phonemes. During the speech perception assessment in this study, the speech and language therapist performed an assessment using two randomly selected, different, CVC(A) word lists. The speech and language therapist calculated the percentage of correctly repeated CVC(A) words from the total of 22 presented words (containing a total of 66 phonemes). Example of CVC(A) phonemes are: lip, bus, pop, men, net and big. The speech perception of included children was evaluated for five years after their initial CI surgery. In our institution, paediatric CI users are evaluated every three months during the first year following implantation and on an annual basis thereafter. During each postoperative evaluation, different CVC(A)-word lists are selected. The five-year postoperative data were selected in this study to reduce relative maturation effects (Suh et al, Citation2009).
To account for ceiling effects, Helms et al. (Citation2004) used the 95% confidence interval around maximum speech perception scores to show CI performance differences between patients. In line with this approach, we allocated CVC(A) scores above 95% correct phonemes as ceiling scores. Secondly, to evaluate whether particular age groups demonstrated significant differences in speech perception, patients were divided into five different age-at-implantation groups based on six-months intervals (). This approach was in line with various other studies evaluating age-at-implantation effects in paediatric CI populations (Robbins et al., Citation2004; May-Mederake, Citation2012; Leigh et al, Citation2013; Dettman et al, Citation2016). We evaluated the number of children per age-at-implantation group attaining the ceiling score (>95% correct CVC(A) phonemes). Then, we compared the number of children between age-at-implantation groups reaching this CVC(A) ceiling score. We defined a between group performance difference as a significant difference between the 95% confidence interval of the number of ceiling scoring children between age-at-implantation groups.
Statistical analysis
Statistical analysis was performed using SPSS (IBM Corp. Released 2013. IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY). During analysis of baseline characteristics () and correlations between variables (), we deemed statistical tests significant at a value of p = 0.05. We applied Bonferroni-correction (p = 0.005) when multiple between age-at-implantation group comparisons were performed (). Shapiro–Wilk test was used to confirm non-normal data distribution. To examine whether age at implantation affected speech perception we used implantation age both as a continuous (CVC(A) raw score comparisons between age-at-implantation groups) and a categorised variable (CVC(A) ceiling score comparisons between age-at-implantation groups).
Table 2. Mann–Whitney U-tests between age-at-implantation groups on raw CVC(A) scores.
Table 3. Report of Spearman's rho correlation among variables.
Baseline characteristics
Univariate relations between variables and confounders were studied using Spearman correlation (e.g. age at implantation and bilateral implantation). Relations between dichotomous variables were studied using Fisher exact test (e.g. prematurity and bilateral implantation). The Pierson Chi-square was elected for comparisons between multiple unpaired groups on discrete outcomes (e.g. age-at-implantation groups and aetiology of hearing loss).
CVC(A) scores
CVC(A) raw score comparisons between age-at-implantation groups
As CVC(A)-scores were not normally distributed, we elected two statistical tests to study between group differences. First, the Kruskal–Wallis test was used to determine whether significant differences in speech perception scores (CVC(A) values) existed between all age-at-implantation groups. Secondly, Mann–Whitney U-tests were used to perform comparison between sets of two specific age-at-implantation groups.
CVC(A) ceiling score comparisons between age-at-implantation groups
Fisher exact tests were used to perform age-at-implantation group comparisons to assess whether a greater proportion of children from a specific age-at-implantation group reached a ceiling CVC(A) score.
Logistic regression
Since we subdivided our data into binary outcome measures (reaching or not reaching a CVC(A) ceiling score), we used logistic regression to study which variables influenced the probability to reach the ceiling CVC(A) score (>95% correct CVC(A) phonemes). This analysis included variables that are reported in the literature to affect CI speech perception performance: gender, level of hearing loss (measured by auditory brainstem response [ABR]), hearing loss aetiology, comorbidities, prematurity, CI device type, surgical implantation technique and unilateral or bilateral implantation.
Results
Between 1997 and 2014, 122 children were implanted before the age of 36 months at the UMC Utrecht, the Netherlands. Ninety patients completed five-year CVC(A) score follow-up and were selected for this study. We included 54 out of the 90 selected children. Thirty-six patients were excluded due to: CI-induced facial nerve excitation (n = 1), Dutch not as primary language (n = 9), post-lingual hearing loss (n = 2) and incomplete speech perception follow-up scores (n = 24). Incomplete speech perception follow-up occurred due to: migration (n = 5), immigration (n = 5) or the inability to fulfil CVC(A) assessment due to cognitive delay (n = 14).
Statistical analysis – baseline characteristics
shows baseline characteristics of the included children. Children were grouped according to age at implantation. Median age at implantation of the 54 included patients was 22.92 months [8.52–34.08 months]. Median hearing loss at indication was 100.0 deciBel (dB) [90–110 dB]. The youngest age-at-implantation group showed significantly less hearing loss compared to older implanted children (p = 0.005). The MED-EL CI device was only used in the youngest two age-at-implantation groups (p = 0.012). The number of unilateral and bilateral implanted patients per age-at-implantation groups significantly differed at five-year follow-up (p = 0.018). Nine patients presented with comorbidities not intervening with speech perception testing (Usher/Beckwith–Wiedemann syndrome [n = 4], diabetes mellitus [DM] type I [n = 1], motoric developmental disorders [n = 2], asthma [n = 1] and antibody synthesis defect [n = 1]) (data not presented in ).
CVC(A) scores
CVC(A) raw score comparisons between age-at-implant groups
The median CVC(A) score at five-year follow-up was 92.00% [57–100%]. Shapiro–Wilk testing showed that data were not normally distributed: most children scored the highest possible CVC(A) scores (CVC(A) ceiling scores). Although ceiling scores occurred, a Kruskal–Willis test confirmed a significant CVC(A) score difference between age-at-implantation groups (H (4) = 30.36; p < 0.001) (). Mann–Whitney U-tests between age-at-implantation groups showed that the youngest age-at-implantation group (implanted before 13 months) outperformed children implanted after 18 months (). No statistical speech performance difference was found between age-at-implantation groups 1 (<13 months) and 2 (13–18 months) (data not shown in ).
More recently implanted children (implanted after 2006) significantly outperformed children implanted before 2006 (data not presented). Within the CI patient cohort that was implanted after 2006, age at cochlear implantation still significantly (p < 0.001) influenced the level of the CVC(A) score.
Since the number of unilateral and bilateral implanted patients per age-at-implantation group significantly differed at five-year follow-up, children using an unilateral CI were separately analysed. Following Bonferroni correction, all aforementioned CVC(A) raw score comparisons remained significant, except for the comparison between age group 1 and 3 (using a Mann–Whitney U-test). Although exclusion of bilaterally implanted children showed similar results of between group comparisons, within age-at-implantation groups 2 and 4 significant CVC(A) score differences were identified in favour of bilaterally implanted children (U = 28.00, z = 2.42, p = 0.017, r = 0.73; U = 28.00, z = 2.23, p = 0.028, r = 0.62).
CVC(A) ceiling score comparisons between age-at-implantation groups
Between-group analysis using Fisher exact test demonstrated performance differences between groups 1 and 4 (p < 0.001), groups 1 and 5 (p < 0.001) and groups 2 and 4 (p < 0.001) following Bonferroni correction ().
Figure 1. Proportion of children from each age-at-implantation group reaching the CVC(A) ceiling score at five-year follow-up. Error bars represent the 95% confidence interval. Between-groups analysis using Fisher exact test confirmed performance differences between group 1 and 4 (p < 0.001), group 1 and 5 (p < 0.001) and group 2 and 4 (p < 0.001) following Bonferroni correction.
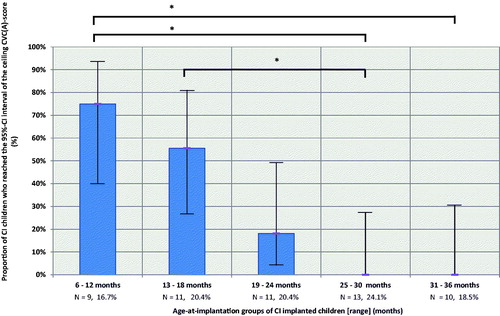
Logistic regression
Binominal logistic regression showed that a larger proportion of young implanted children (<18 months) reached CVC(A) ceiling levels (χ2(1) = 11.77; p < 0.05) compared to older (>18 months) implanted children. shows correlations among variables that are reported in the literature to affect CI speech perception performance. Results marked in bold show a strong correlation (p < 0.01). We included the variables that significantly correlated to age at CI surgery into our logistic regression: hearing loss level at CI indication, comorbidities, the CI device type and unilateral or bilateral implantation. This logistic regression analysis showed that age at implantation was the only significant predictor for reaching a five-year CVC(A) ceiling score (). Logistic regression did not confirm bilateral CI use advantage in reaching a CVC(A) ceiling score (> 95% CVC) at five-year follow-up ().
Table 4. Report of results of binominal logistic regression analysis.
Discussion
In this study, we evaluated paediatric age-at-implantation effects on postoperative speech perception. Analyses on raw CVC(A) scores showed that the two youngest age-at-implantation groups (implanted between 6 and 18 months) outperformed older implanted children (implanted between 18 and 36 months) at five-year follow-up. When accounting for ceiling effects a larger proportion of young implanted children (<18 months) reached CVC(A) ceiling levels compared to older (>18 months) implanted children. Since we demonstrated benefits of early implantation in both separate analyses (raw CVC(A) score and CVC(A) ceiling score comparisons), we can derive that ceiling effects can be successfully measured and its effect can be weighed on speech perception outcomes. However, current speech perception evaluation tools such as the CVC(A) score have their limitations thus, prevented further in-depth (statistical) comparison between the two youngest implanted groups. By comparing the proportion of children reaching the 95%CI CVC(A) ceiling score between age-at-implantation groups, we were able to show that a relatively larger proportion of earlier implanted children (<13 months) reached CVC(A) ceiling scores compared to those implanted between 13 and 18 months. This comparison not being statistically significant could be due to either ceiling effects, the limited number of children per group or a diminishing age-at-implantation effect during long-term follow-up (Boons et al, Citation2012).
Earlier age at implantation was related to a higher score on long-term speech perception. However, current studies report different time points for cochlear implantation to reach optimal speech perception benefit. Houston & Miyamoto (2010) assessed speech perception using two closed-set word recognition tests (grammatical analysis of elicited language-pre-sentence level [GAEL-P] and the paediatric speech intelligibility test [PSI]) and an open-set word recognition test (lexical neighbourhood test [LNT]). Authors showed that speech perception outcomes of children implanted before 13 months were largely similar to those implanted between 16 and 23 months (Houston & Miyamoto, Citation2010). In line with this finding, Dettman et al. (Citation2016) reported that their three youngest age-at-implantation groups (all implanted before 24 months) outperformed older implanted children on the following speech perception outcome measures: open-set monosyllabic word (OSW) recognition (including CVC words) and open-set sentence recognition (using Bench-Kowal-Bamford [BKB] sentences). Dettman et al. (Citation2016) suggested that children develop speech perception skills if they have access to CIs before and after 12 months and emphasised that children should receive CIs before 24 months of age. Similar to their results, we were unable to show statistical long-term performance differences between children implanted before 13 months and those implanted between 13 and 18 months. Our ceiling effect analysis showed that a relatively larger proportion of children implanted before 13 months reached a CVC(A) ceiling score. Although Dettman et al. (Citation2016)’s study retrospectively assessed children implanted in a similar time frame as our cohort; they used different outcome measures evaluated over a shorter follow-up that limits additional comparison between Dettman et al. (Citation2016)’s and ours.
Colletti et al. (Citation2012) concluded that earlier implantation (<6 months) is essential for adequate speech and language development. These authors based their conclusions on a combination of speech perception, receptive language development, receptive vocabulary and speech production scores. However, CI indication and year of surgery data were not provided. The latter could have affected outcomes of the youngest implanted patients similar to our study in which patients implanted after 2006 outperformed patients implanted before 2005. This finding can be explained by age at implantation being significantly correlated to the year of CI surgery in our study. Since the year at CI surgery correlated with the age at cochlear implantation, we further investigated this correlation. To assess whether a positive age-at-implantation effect could still be shown in the most recent (>2005) implanted children, we performed a sub-analysis including these patients and only age at implantation remained to significantly (p < 0.001) affect the level of the CVC(A) score.
Boons et al. (Citation2012) showed that the first CI fitting effect disappeared after three-year follow-up. This finding justifies electing the five-year follow-up moment in the current study to prevent measuring first fitting or maturation effects due to different duration of CI use. Regression models did not show a reduced age-at-implantation effect in time, however, some have warned age-at-implantation effects decreasing during long-term follow-up (Boons et al., Citation2012). Our findings confirmed age-at-implantation effects in a young paediatric population using data measured at five-year follow-up. However, a relative decrease in age-at-implantation effects during long-term follow-up (Boons et al, Citation2012) could explain why we were not able to show a significant difference between the proportion of children reaching a ceiling CVC(A) score comparing the youngest two age-at-implantation groups.
Niparko et al. (Citation2010) showed that bilateral implantation did not result in significant improvement of verbal language development, while Boons et al. (Citation2012) demonstrated improved language test scores in bilaterally implanted children. In the current study, bilateral implantation significantly correlated with age at implantation and year of CI surgery. More recently implanted children were implanted at a younger age and more frequently bilateral due to application of newer guidelines and reimbursement rates; e.g. bilateral cochlear implantation is only reimbursed in the Netherlands since September 2013 which could explain why 85.7% of our included bilateral patients were implanted after 2006. Therefore, age at implantation, the year of CI surgery and receiving bilateral implants could affect the CVC(A) ceiling score at five-year follow-up. However, logistic regression confirmed that only age at implantation significantly influenced reaching CVC(A) ceiling scores at five-year follow-up.
Although logistic regression and between group analysis did not show a bilateral implant effect in reaching a CVC(A) ceiling score, within group analysis showed a relative benefit of being bilaterally implanted in age groups 2 (13–18 months) and 4 (25–30 months). shows that this effect could not be explained by a difference in age at bilateral implantation. Most likely this effect was only retrieved within aforementioned groups because unilateral and bilateral patients were equally distributed while in other age groups numbers of bilateral children were too small to detect a relative benefit.
Limitations
Most CI studies assess a combination of several auditory, speech perception and production and language outcomes (Colletti et al, Citation2012; Dettman et al, Citation2016). Therefore, evaluation of a single speech perception test score might not allow recommendations regarding an optimal age at paediatric cochlear implantation. In our centre, CVC(A) scores are part of a larger postoperative test battery, including for example CAP and SIR scores (Cox & McDaniel, Citation1989; Archbold et al, Citation1995). However, outcome measures on other language domains were performed on fewer children and therefore lacked statistical power to be included in the current study.
Secondly, the fact that data was collected retrospectively led to the inability to correct for several variables in our regression analysis that could affect speech perception. Szagun & Stumper (Citation2012) marked that the child’s home language environment significantly contributed to the child’s opportunities to derive benefit from early cochlear implantation. Besides the aforementioned variable, we were also not able to account for: age at hearing aid fitting and family factors (maternal education and relative socio-economic advantage).
Thirdly, our youngest cohorts had significantly less hearing loss at CI indication (90 dB [90–100]) than older age-at-implantation groups. Since these younger children had relatively better thresholds, they probably had greater stimulation of their auditory system before implantation compared to children presenting with worse thresholds (older implanted children). However, children from both groups showed no auditory benefit from an obligatory preoperative six-week hearing aid trial. Aforementioned auditory benefit was defined by observing reactions to sound, parental assessment (e.g. LittlEARS [Tsiakpini et al, Citation2004]) and measuring aided thresholds. Furthermore, recent studies comparing long-term outcomes of children using conventional hearing aids or wearing CIs showed that 90 dB is already a hearing loss margin at which a CI is more beneficial regarding speech and language development compared to using hearing aids (Lovett et al, Citation2015).
Future studies
Since young implanted children (<19 months) all scored CVC(A) ceiling scores, current follow-up methods did not enable us to assess long-term speech perception outcomes between the youngest two age-at-implantation groups. We only showed relative differences between proportions of children reaching a ceiling CVC(A) value, whereas actual significant differences between groups could exist. These could be elucidated by newly developed tests that are not affected by ceiling effects. In addition, a wide variation of CI follow-up tests is currently used to evaluate performance, which makes comparison of outcomes between CI studies difficult (Uhler & Gifford, Citation2014). Both findings indicate that there is a major need for development of additional and universal assessment methods to monitor paediatric age-at-implantation impact on speech and language performance without ceiling effects masking performance differences (Gifford et al, Citation2008). Colletti et al. (Citation2012) showed that application of the CAP-II score could be essential to identify differences between children implanted before 24 months of age. However, since no normed scores are available, the CAP-II could be most useful in combination with other outcomes measures (test-battery setting). An example of a language test that could be used independently and could be less prone to be affected by ceiling effects is the digits-in-noise (DIN) test, a newly developed Dutch speech recognition test (Smits et al, Citation2013).
Furthermore, there is a need for large, prospective, multi-centre clinical studies that consistently assess long-term outcome measures in a standardised prospective protocolled manner to elucidate the benefits of early cochlear implantation (<12 months). These outcomes could eventually shift current FDA guidelines to a younger permitted implantation age than 12 months (Dettman et al, Citation2016). An example of such a prospective, long-term follow-up study is the longitudinal outcomes of children with hearing impairment study (LOCHI) study (Ching et al, Citation2013a,Citationb). Findings from this study regarding children using CIs showed that earlier age at activation of the first CI was associated with better language scores (Ching, Citation2015). Furthermore, results showed clear evidence that earlier age at intervention was associated with better outcomes at five years of age (Ching, Citation2015).
Conclusions
Ceiling effects can mask thorough delineation of speech performance following paediatric cochlear implantation. However, we did show improved speech perception of the youngest age-at-implantation cohorts compared to older implanted children (after 18 months) at five-year follow-up. In addition, a larger, however not significantly different, proportion of earlier implanted children (before 13 months) reached speech perception ceiling scores (>95% CVC(A)) compared to the proportion of children who was implanted between 13 and 18 months. Age at implantation was the only significant predictor in reaching a CVC(A) ceiling score at five-year follow-up. To assess speech perception differences in future studies initiated by different age at implantation, it is essential to develop and use alternative assessment methods without ceiling effects masking postoperative performance.
Declaration of interest
The authors report no declarations of interest.
Acknowledgements
We thank A. Bezdjian, MSc, for reviewing the language and grammar of the manuscript.
In addition, we thank the CI revalidation team of the UMC Utrecht for data collection.
Funding
Wilko Grolman received unrestricted grants from Cochlear, MED-EL and Advanced Bionics under formal legal hospital contract. None of the other authors receives any funding.
References
- Archbold, S., Lutman, M.E. & Marshall, D.H. 1995. Categories of auditory performance. Ann Otol Rhinol Laryngol Suppl, 166, 312–314.
- Boons, T., Brokx, J.P., Dhooge, I., Frijns, J.H., Peeraer, L., et al. 2012. Predictors of spoken language development following pediatric cochlear implantation. Ear Hear, 33, 617–639.
- Bosman, A.J. 1989. Speech perception by the hearing impaired [doctoral thesis]. Utrecht, the Netherlands: University of Utrecht.
- Bosman, A.J., & Smoorenburg, G.F. 1995. Intelligibility of Dutch CVC syllables and sentences for listeners with normal hearing and with three types of hearing impairment. Audiology, 34, 260–284.
- Bruijnzeel, H., Draaisma, K., van Grootel, R., Stegeman, I., Topsakal, V., et al. 2016. Systematic review on surgical outcomes and hearing preservation for cochlear implantation in children and adults. Otolaryngol Head Neck Surg, 154, 586–596.
- Ching, T.Y., Dillon, H., Marnane, V., et al. 2013a. Outcomes of early- and late-identified children at 3 years of age: findings from a prospective population-based study. Ear Hear, 34, 535–552.
- Ching, T.Y., Leigh, G., & Dillon, H. 2013b. Introduction to the longitudinal outcomes of children with hearing impairment (LOCHI) study: Background, design, sample characteristics. Int J Audiol, 52, S4–S9.
- Ching, T.Y. 2015. Is early intervention effective in improving spoken language outcomes of children with congenital hearing loss? Am J Audiol, 24, 345–348.
- Clark, G.M., Pyman, B.C. & Bailey, Q.R. 1979. The surgery for multiple-electrode cochlear implantations. J Laryngol Otol, 93, 215–223.
- Colletti, L., Mandala, M., Zoccante, L., et al. 2011. Infants versus older children fitted with cochlear implants: Performance over 10 years. Int J Pediatr Otorhinolaryngol, 75, 504–509.
- Colletti, L., Mandala, M. & Colletti, V. 2012. Cochlear implants in children younger than 6 months. Otolaryngol Head Neck Surg, 147, 139–146.
- Cox, R.M. & Mcdaniel, D.M. 1989. Development of the speech intelligibility rating (SIR) test for hearing aid comparisons. J Speech Hear Res, 32, 347–352.
- Cuda, D., Murri, A., Guerzoni, L., et al. 2014. Pre-school children have better spoken language when early implanted. Int J Otorhinolaryngol, 78, 1327–1331.
- Dettman, S., Pinder, D., Briggs, R., et al. 2007. Communication development in children who receive the cochlear implant younger than 12 months: Risks versus benefits. Ear Hear, 28, 11S–18S.
- Dettman, S.J., Dowell, R.C., Choo, D., et al. 2016. Long-term communication outcomes for children receiving cochlear implants younger than 12 months: a multicenter study. Otol Neurotol, 37, e82–e95.
- Gifford, R.H., Shallop, J.K. & Peterson, A.M. 2008. Speech recognition materials and ceiling effects: considerations for cochlear implant programs. Audiol Neurootol, 13, 193–205.
- Helms, J., Weichbold, V., Baumann, U., Von Specht, H., Schon, F., et al. 2004. Analysis of ceiling effects occurring with speech recognition tests in adult cochlear-implanted patients. ORL J Otorhinolaryngol Relat Spec, 66, 130–135.
- Holman, M.A., Carlson, M.L., Driscoll, C.L., et al. 2013. Cochlear implantation in children aged 12 months and younger. Otol Neurotol, 34, 251–258.
- House, W.F. 1976. Cochlear implants. Ann Otol Rhinol Laryngol, 85, 1–93.
- Houston, D., Ying, E., Pisoni, D., et al. 2001. Development of pre-word learning skills in infants with cochlear implants. Volta Rev, 103, 303–326.
- Houston, D. & Miyamoto, R. 2010. Effects of early auditory experience on word learning and speech perception in deaf children with cochlear implants. Otol Neurotol, 31, 1248–1253.
- Houston, D., Stewart, J., Moberly, A., et al. 2012. Word learning in deaf children with cochlear implants: Effects of early auditory experience. Dev Sci, 15, 448–461.
- Kronenberg, J., Migirov, L. & Dagan, T. 2001. Suprameatal approach: new surgical approach for cochlear implantation. J Laryngol Otol, 115, 283–285. in cochlear implant surgery: our experience with 80 patients. ORL J Otorhinolaryngol Relat Spec, 64, 403-5.
- Krueger, B., Joseph, G., Rost, U., Strauss-Schier, A., Lenarz, T., et al. 2008. Performance groups in adult cochlear implant users: speech perception results from 1984 until today. Otol Neurotol, 29, 509–512.
- Leigh, J., Dettman, S., Dowell, R., et al. 2013. Communication development in children who receive a cochlear implant by 12 months of age. Otol Neurotol, 34, 443–450.
- Levine, D., Strother-Garcia, K., Michnick golinkoff, R. & Hirsh-Pasek, K. 2016. Language development in the first year of life: what deaf children might be missing before cochlear implantation. Otol Neurotol, 37, e56–e62.
- Lovett, R.E.S., Vickers, D.A. & Summerfield, A.Q. 2015. Bilateral cochlear implantation for hearing-impaired children: criterion of candidacy derived from an observational study. Ear Hear, 36, 14–23.
- Massa, S.T. & Ruckenstein, M.J. 2014. Comparing the performance plateau in adult cochlear implant patients using HINT and AzBio. Otol Neurotol, 35, 598–604.
- May-Mederake, B. 2012. Early intervention and assessment of speech and language development in young children with cochlear implants. Int J Pediatr Otorhinolaryngol, 76, 939–946.
- Miyamoto, R., Houston, D. & Bergeson, T. 2005. Cochlear implantation in deaf infants. Laryngoscope, 115, 1376–1380.
- Niparko, J.K., Tobey, E.A., Thal, D.J., Eisenberg, L.S., Wang, N.Y., et al. 2010. Spoken language development in children following cochlear implantation. JAMA, 303, 1498–1506.
- Robbins, A., Koch, D.B., Osberger, M.J., et al. 2004. Effect of age at cochlear implantation on auditory skill development in infants and toddlers. Arch Otolaryngol Head Neck Surg, 130, 570–574.
- Ruffin, C.V., Tyler, R.S., Witt, S.A., Dunn, C.C., Gantz, B.J., et al. 2007. Long-term performance of Clarion 1.0 cochlear implant users. Laryngoscope, 117, 1183–1190.
- Smits, C., Goverts, T.S. & Festen, J.M. 2013. The digits-in-noise test: Assessing auditory speech recognition abilities in noise. J Acoust Soc Am, 133, 1693–1706.
- Spahr, A.J., Dorman, M.F., Litvak, L.M., Cook, S., Loiselle, L.M., et al. 2014. Development and validation of the pediatric AzBio sentence lists. Ear Hear, 35, 418–422.
- Suh, M.W., Cho, E.K., Kim, B.J., Chang, S.O., Kim, C.S., et al. 2009. Long term outcomes of early cochlear implantation in Korea. Clin Exp Otorhinolaryngol, 2, 120–125.
- Szagun, G. & Stumper, B. 2012. Age or experience? The influence of age at implantation and social and linguistic environment on language development in children with cochlear implants. J Speech Lang Hear Res, 55, 1640–1654.
- Tsiakpini, L., Weichbold, V., Kuehn-Inacker, H., Coninx, F., D'haese, P., et al. 2004. LittlEARS Auditory Questionnaire. Inssbruck; MED-EL.
- Uhler, K. & Gifford, R.H. 2014. Current trends in pediatric cochlear implant candidate selection and postoperative follow-up. Am J Audiol, 23, 309–325.
- Vaca, M., Gutierrez, A., Polo, R., Alonso, A. & Alvarez, F. 2015. Long-term results of the transattical approach: an alternative technique for cochlear implantation. Eur Arch Otorhinolaryngol, 272, 35–41.
- Vincenti, V., Bacciu, A., Guida, M., Marra, F., Bertoldi, B., et al. 2014. Pediatric cochlear implantation: an update. Ital J Pediatr, 40, 72.
- Vlastarakos, P.V., Proikas, K., Papacharalampous, G., Exadaktylou, I., Mochloulis, G., et al. 2010. Cochlear implantation under the first year of age–the outcomes. A critical systematic review and meta-analysis. Int J Pediatr Otorhinolaryngol, 74, 119–126.
- Von Elm, E., Altman, D.G., Egger, M., Pocock, S.J., Gotzsche, P.C., et al. 2008. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol, 61, 344–349.
