Abstract
Objective: The objective of this study is to evaluate its safety and effectiveness of the bone conduction implant (BCI) having an implanted transducer and to review similar bone conduction devices.
Design: This is a consecutive prospective case series study where the patients were evaluated after 1, 3, 6 and 12 months. Outcome measures were focussed on intraoperative and postoperative safety, the effectiveness of the device in terms of audiological performance and patient’s experience.
Study sample: Sixteen patients with average age of 40.2 (range 18–74) years have been included. Thirteen patients were operated in Gothenburg and three in Stockholm.
Results: It was found that the procedure for installing the BCI is safe and the transmission condition was stable over the follow-up time. No serious adverse events or severe adverse device effects occurred. The hearing sensitivity, speech in noise and the self-assessment as compared with the unaided condition improved significantly with the BCI. These patients also performed similar or better than with a conventional bone conduction reference device on a softband.
Conclusions: In summary, it was found that the BCI can provide a safe and effective hearing rehabilitation alternative for patients with mild-to-moderate conductive or mixed hearing impairments.
Introduction
There is a long history of treatment options for patients who suffer from a conduction hearing loss due to middle ear infection or syndromic malformations of the outer or middle ear. Not until the percutaneous bone anchored hearing aid (BAHA) was introduced in 1977, these groups of patients could be offered an alternative that had the potential to successfully treat their hearing loss (Håkansson et al. Citation1985; Tjellström and Håkansson Citation1995). We estimate from company sales information, that an accumulated number of up to 300,000 patients may have been treated (June 2019), where this estimated figure, although not officially confirmed, also includes a significant number of single sided deaf patients and passive/active transcutaneous bone-anchored implants. This trend has also catalysed new developments in other bone conduction applications such as for hearing and vertigo diagnostics (Håkansson Citation2003; Fredén Jansson et al. Citation2015a; Håkansson et al. Citation2018) and in consumer applications (Everyday Hearing Citation2018).
Although the hearing rehabilitation effect using the BAHA is significant and well documented, there are still some inherent drawbacks with the percutaneous solution. These drawbacks include a lifelong commitment of everyday care and potential adverse events related to skin infections, skin overgrowth and implant loss. Moreover, some patients find the percutaneous solution aesthetically unattractive. Secondary drawbacks are related to feedback when using head wear, and the stigma of having an implant sticking out through the skin.
Since the BAHA was introduced, several other bone conduction solutions have been developed in order to improve the situation for these patients (Reinfeldt et al. Citation2015a). The presently known types of bone conduction devices can be divided into two main categories according to their transmission and attachment to the skull bone. As illustrated in , the transducer is either attached over the intact skin (Over skin drive) or directly to the skull bone (Direct bone drive). To the former principle belong the conventional bone conduction devices (pressure attached using Headband, Eyeglasses or Baha Sound arc) which are completely non-invasive but suffer from soft tissue dampening of sound transmission at higher frequencies and that the static force needed for efficient sound transmission might cause some wearing discomfort. To solve the pressure issues MedEl (Innsbruck, Austria) launched the ADHEAR device where the transducer is glue attached without pressure using an acrylic plate. This solution seems to work reasonably well for patients with good cochlear function (Westerkull Citation2018). To the direct bone, drive principle belongs the percutaneous BAHA snapped to a skin penetrating abutment attached to an osseointegrated bone-anchored titanium implant. Two commercially available product systems belong to this group, the Ponto® from Oticon Medical, Askim, Sweden, and the Baha® from Cochlear Bone anchored solutions, Mölnlycke, Sweden.
Figure 1. Diagram showing present modalities of bone conduction devices that can be either directly attached to the skull bone (Direct bone drive) or applied over the intact skin (Over skin drive).
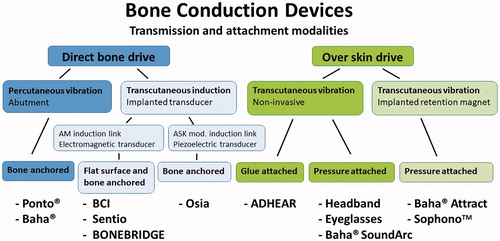
In recent years, other intact skin solutions have been developed, such as the Baha® Attract and the Sophono™ where a retention magnet-s are implanted but where the transducer is pressure attached to the skin over the implanted magnet. These devices, here classified as transcutaneous “Over skin drive” devices, have been reported to have an acceptable sound amplification in patients having mild sensorineural hearing loss (den Besten et al. Citation2018; Nelissen et al. Citation2016). These devices do not have the issues related to the skin penetrating implant in the BAHA but have to some degree the same challenges with pressure over the skin and skin dampening as previously described for conventional bone conduction devices.
In the late 1990s, the idea of a completely implanted transducer was presented. One suggestion was to implant the transducer and drive it electrically via a percutaneous electric connection (Håkansson Citation2005), not included in . This solution would have the potential to offer a very powerful system with a reasonable size of the external device but with the drawback of still requiring a percutaneous system. Interestingly, this idea never reached the patient phase but came into clinical use in a completely different area namely in the mind controlled bone anchored prosthetic arm project as developed by Ortiz-Catalan et al. (Citation2012).
The bone conduction implant
The development of the new project called the bone conduction implant (BCI), where the skin is kept intact using an induction transmission system started around 1997 at Chalmers University of Technology (Gothenburg, Sweden) and later further developed in close collaboration with Sahlgrenska Academy (Gothenburg, Sweden). The aim was to combine the advantages of direct bone conduction stimulation with the benefit of keeping the skin intact.
A number of pre-clinical studies of the BCI were performed under various conditions using a skull simulator, on a dry human skull, on cadaver heads, in an animal model (sheep) and in patients (threshold testing, sound probe measurements and laser Doppler vibrometry). Results from these preclinical studies have been presented in numerous scientific journals, conferences and have been defended in several PhD theses (Stenfelt Citation1999; Reinfeldt Citation2009; Eeg Olofsson Citation2012; Taghavi Citation2014; Fredén Jansson Citation2017; Rigato Citation2019). A very crucial aspect is the mechanical robustness of the implanted unit, which should be safe, reliable and last for at least 10 years, preferably lifelong. The reliability of the BCI was tested long-term in a study by Fredén Jansson et al. (Citation2019) who also propose guidelines for quality assurance testing in similar implantable devices.
The final solution of the BCI system comprises an externally worn audio processor and a bridging bone conductor (BBC) unit, implanted under the skin and soft tissues, see details in . A commercial version of the BCI system is developed by Oticon Medical under the name Sentio and is currently in a regulatory process for CE marking.
Figure 2. The BCI system (left) consists of an external audio processor which includes a digital sound processor (DSP) that drives a power amplifier (PA) and an amplitude modulation (AM) induction link connected to the transducer secured in a recess of the mastoid portion of the temporal bone. The audio processor is held in place by magnets (N and S poles) over the implanted unit called the Bridging Bone Conductor. Sizes of the Bridging Bone Conductor are shown in the middle and the real appearance, including the audio processor to the right.
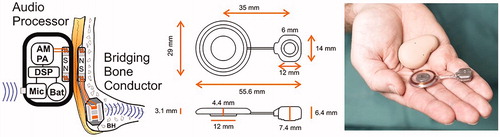
Similar direct bone drive systems, with transcutaneous induction transmission through the intact skin and implanted transducer, are the Bonebridge (BB) from MedEl (Innsbruck, Austria) and the Osia system from Cochlear Bone Anchored Solutions (Gothenburg, Sweden). The BB has been internationally commercialised (CE approval 2012, FDA approval 2018) and scientific reports are available from several research groups showing effective rehabilitation and safe results in follow-up of such device, see e.g. Sprinzl and Wolf-Magele (Citation2016). The Osia system, which is in a clinical study phase for obtaining a CE mark, uses a piezoelectric transducer instead of an electromagnetic transducer as is used in BCI, Sentio and BB. As we are aware, it also uses a different induction link based on amplitude shift keying (ASK) which requires active electronic circuits implanted for the demodulation similar to in cochlear implants whereas the BCI, Sentio and BB use amplitude demodulation that require only a few passive components in the implanted unit.
Objectives
The main objective of this clinical study is to show that the BCI system is safe for the patients and that the performance of the implantable BBC unit does not deteriorate over time. The secondary objective is to investigate the performance of the full BCI system in patients compared with the unaided condition as well as compared to a conventional bone conduction reference device.
Material and methods
This study is a prospective, non-randomised, multi-centre, 12 months clinical investigation of the BCI device on 16 patients (13 patients in Gothenburg and 3 in Stockholm). In the preoperative phase the patients were screened, enrolled and fitted with a reference device. After 1 month of use in the home environment, audiometric baseline measurements were made on the reference device. In the next phase, preparation for surgery was made, including preoperative cone-beam computed tomography of the implant site in patients with known defects in the mastoid region, either congenital or from previous surgery. Following surgery, a postoperative follow-up was made 7–10 d after implantation. As a last phase, the sound processor was fitted after 4–6 weeks postoperatively, and four follow-up visits after 1, 3, 6 and 12 months after fitting were carried out.
Audiometric measurements (sound field unaided vs. BCI aided) were performed at all follow-up visits from fitting and onwards to allow for comparison to the measurements made on the reference device prior to implantation. At all follow-up visits, skin status and adverse events were noted. Two different questionnaires regarding the patient’s hearing situation and perceived benefit were completed at three points during the study: first regarding the use of the reference device preoperatively, and then regarding the BCI device after 6 and 12 months of use.
The trial was approved by the Swedish Medical Products Agency (461:2012/513308) and the Regional ethical review board in Gothenburg (445-12). The study was performed in accordance with the ISO standard 14155 (ISO 14155 Citation2011) and the CitationDeclaration of Helsinki. Additional follow-up of these patients will be reported after 3 and 5 years.
Inclusion criteria
Patients fulfilling the following inclusion criteria were invited to the clinical study using the BCI: unilateral or bilateral conductive hearing loss with air-bone gap of at least 20 dB (average of 0.5, 1, 2 and 4 kHz); normal or near-normal sensorineural hearing with a PTA bone conduction (BC) of 30 dB HL or better; either rejected or being unable to use conventional air conduction (AC) hearing aids; and agreeing to be accessible for multiple follow-up visits according to the protocol and be motivated to be one of the first patients using the BCI.
Patients
Demographic and audiometric data of the 16 operated patients are presented in . Average age at implantation was 40.2 (range 18–74) years, 44% were men and 56% women and the implant side was 62.5% left and 37.5% right side. Half of the patients were unilateral and half bilateral.
Table 1. Demographic data and hearing thresholds of included patients.
Surgical procedure
The surgical procedure has been presented by Eeg-Olofsson et al. (Citation2014) and Reinfeldt et al. (Citation2015b) and will only briefly be repeated here. Patients were operated under general anaesthesia. A straight line indicating the level of the audio processor was marked on the patient so that the microphone openings were positioned just above the superior level of the pinna; see red dashed line .
Figure 3. Positioning of the Bridging Bone Conductor (BBC) behind the ear (a) and the transducer in a prepared recess secured by a titanium wire. (b) The transducer casing in patient 1 was secured by a flexible titanium plate with screws at the ends, whereas in patient 2–16 it was secured by a thin titanium wire.
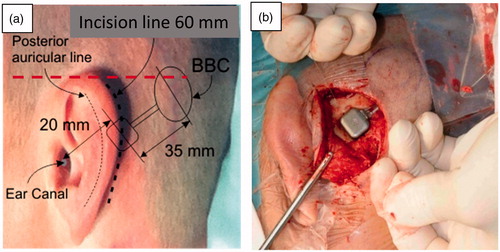
A postauricular incision was made down to the bone and an anterior flap was raised so that the posterior border of the ear-canal opening was exposed. A posterior flap was also separated from the bone to allow for the coil and retention magnet of the BBC to be inserted. Then, a recess 3–5 mm deep for the transducer casing (allowing the transducer to protrude 2–3 mm over the bone surface) was drilled 20 mm from the ear canal opening to the transducer centre. A small channel for the neck of the BBC was thereafter drilled posteriorly. After the drilling procedure, the BBC was inserted under the posterior flap and the transducer was secured in the drilled recess in one of two ways. For the first patient, an approximately 40 mm long flexible titanium plate was placed above the transducer casing and attached with two 3 mm titanium screws with pre-drilling on both sides of the transducer. For the remaining patients (pat no 2–16), small holes were drilled 1–2 mm from the edge, one on each side of the recess, and through these holes a titanium wire was inserted (. The wire was stretched and tightened over the transducer casing, which has a compliant layer of silicone on top, and finally the ends were twisted three turns, and then cut and placed in the bone bed on the side of the transducer. The wire solution was chosen as it allows for more flexibility regarding the depth of the recess where the transducer is placed. Before closing the incision, the implant functionality was verified by stimulating the implant electrically and measuring the resulting nasal sound pressure (NSP) according to Reinfeldt et al. (Citation2019) and/or an acoustic emission method based on an audio processor programmed to generate a sequence of tones. During both measurements, the test devices used was sealed in a sterile plastic tube.
Before starting the surgery, the skin depth was measured with a needle at the planned position for the centre of the coil, and the average thickness was 5.9 (range 5–8) mm. The surgical time was measured from the first cut until surgery finished and only suturing remained, and the average surgery duration was found to be 61 (range 47–81) min. This time also included measurement of implant performance and abundant photo documentation.
Fitting of the audio processor
The BCI
The fitting of the external audio processor for the BCI took place 4–6 weeks after surgery. Fitting was generally done with linear amplification using computer-based software ARK base (ON Semiconductor, Phoenix, AZ). No automatic features were activated but modest compression for high level sounds was used in some patients, decided in the interaction between the patient and the operator. A more detailed description of the digital signal processor used can be found in Taghavi et al. (Citation2015). All patients were provided with up to four programmes with different frequency characteristics to be tested in various listening situations. Most patients preferred to use the first programme most of the time, typically adjusted for their normal listening situations. In some cases the patients used a second programme with a high-frequency cut in order to get a softer sound in some noisy environments. In the audiometric tests, the first programme was used all the time.
Individual frequency responses after fitting and adjustments were measured as output force level (OFL dB re 1 µN) at 60 and 90 dB sound pressure level re 20 μ Pascal (SPL) are shown in . Measurements were taken when the audio processor was attached over a BBC using 5 mm artificial skin and where the BCI transducer was attached to a Skull simulator with a snap adaptor (Håkansson and Carlsson Citation1989). From the BCI frequency response in the OFL domain in , it can be seen that the transducer has a main resonance frequency at approximately 900 Hz but also by design, a high-frequency resonance at approximately 5.5 kHz. The frequency responses at 60 dB SPL were the ones used by the patients at the 12-month follow-up measurements. Using these frequency responses, it is also possible to illustrate the audibility of ordinary speech. This is done by plotting the free sound field thresholds transferred to the OFL domain together with the maximum force output (MFO) and the long term average speech spectrum (LTASS using a female voice) from Byrne et al. (Citation1994), see . It is assumed that the sound field thresholds represent the device performance also at higher sound levels as the device has a linear amplification for low and medium sound levels.
Figure 4. (a) Frequency responses of individual audio processors at 60 dB SPL input, average response at 60 and 90 dB SPL input, and maximum dynamic range of the system determined by MFO minus noise floor (vertical lines at 0.5, 1, 2 and 4 kHz) and (b) illustrates the audibility of average ordinary speech corresponding to a 70 dB overall long term average speech spectrum (LTASS) including peak and valley levels giving a 30 dB speech dynamic range (transformed from SPL to force level) as well as the clinical dynamic range dB for these patients determined by MFO minus thresholds.
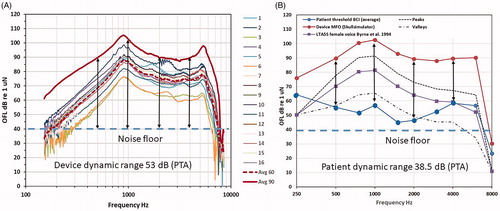
The LTASS values correspond to an overall 70 dB speech level that is extended to cover louder sounds (peaks up to +18 dB) and softer sounds (valleys down to −12 dB) forming a desired audibility speech area. This corresponds to approximately 30 dB of dynamic range of speech (except at 250 and 8 kHz where it is deliberately set to zero) which is slightly frequency dependent and determined by the 30th and 99th percentiles of speech power levels from Holube et al. (Citation2010). Sound field threshold data as well as LTASS values from Byrne et al. (Citation1994) were transformed by the average OFL 60 to dB re 1 μN. Although the diagram in could be analysed patient by patient, for simplicity, only the average device response and the average free sound field thresholds are shown. It can be seen from that the audibility is quite good in the important frequency range from 500 to 4 kHz where speech sounds are well above the noise floor and below MFO (the noise floor is in the range of 30–40 dB given by the randomly fluctuating curve at lowest frequencies in . This graph indicates that the patients are well amplified at low and medium frequencies but most likely a bit under-amplified at high frequencies. In another study, we have also shown that the gain margin to oscillation is sufficient and will not be a problem (Taghavi et al. Citation2012), so in our case the lower high-frequency gain setting may reflect the patients’ preference to comfortable and less sharp sound rather than audibility. Finally, maximum possible dynamic range (MFO minus noise floor) is 53 dB taken from vertical lines) and the clinical dynamic range in these particular patients (MFO minus thresholds) was on the average 38.5 dB (PTA taken from vertical lines) are most likely sufficient in these patients.
Reference device
Before the BCI surgery, the patients were fitted with a reference device, a BAHA on a softband (Ponto Pro Power (PPP) from Oticon Medical, Askim, Sweden) using Oticon software Genie Medical, based on in-situ thresholds and patients' own wishes, including skin compensation (up to 10 dB extra gain at high frequencies), and with all automatic functions disabled. After one month of use of the reference device, the full test battery as for the BCI was performed.
Transmission properties over time
The transmission properties of the implant were objectively tested by the NSP, first at surgery and then at fitting and at 1, 3, 6 and 12-month follow-up for patients 1–13 (Gothenburg patients). The NSP values measured were analysed at 0.5, 1, 2 and 4 kHz in post processing. The consecutive values over time at each frequency were modelled by a linear regression line where a positive slope indicates an increase in transmission and a negative slope indicates a decrease. If stable transmission conditions are maintained over time, the slope is expected to be approximately zero.
Audiometric test battery
The following tests were performed with the reference device after 1 month of use and with the BCI at each follow-up visit:
Sound field warble tone thresholds (0.25, 0.5, 0.75, 1, 1.5, 2, 3, 4, 6 and 8 kHz), sound field speech recognition threshold (SRT) in quiet with Swedish spondees, sound field speech recognition score (SRS) in noise with speech at 4 dB higher level than the noise was measured at 63 dB SPL for both aided and unaided condition (Swedish phonemically balanced words and pre-recorded noise), sound field signal-to-noise ratio (SNR) threshold that yields 50% speech intelligibility were determined (Swedish Hagerman sentences, Hagerman Citation1982, Citation1993), where the speech level was kept constant at 63 dB SPL, while the noise level was adjusted in order to achieve the 50% intelligibility.
All measurements were controlled by an AC40 (Interacoustics A/S, Middlefart, Denmark) audiometer in a sound-insulated room of 16 m3. Two speakers placed at a distance of 1 m in front of the patient at the level of the head one on top of the other were used, one for all speech and warble tones and one for the noise. Prior to the clinical study, all equipment was calibrated according to standard procedures ISO 8253-1 (Citation2010) and ISO 8253-3 (Citation2012) where applicable.
Blocking of the non-test ear was applied during all measurements for patients with better AC hearing at the non-implanted ear than at the implanted ear. The purpose of blocking was to remove the AC sound in the contralateral ear, which was done effectively by inserting a foam ear-plug (E-A-R Classic Soft) as deep as possible to minimise the occlusion effect (Stenfelt and Reinfeldt Citation2007), and also covering that ear with an aural earmuff (Peltor™ 3 M™ Svenska AB, Sollentuna, Sweden). The non-implanted ear was thus blocked but not masked since masking would reduce the sensitivity of that ear to bone-conducted sound that normally is also heard from an implant on the other side of the skull.
To minimise order effects, the measurement order was randomised between the follow-up visits for each patient.
Questionnaires
In addition to the audiometric testing two different patients related outcome measures, the Swedish Abbreviated Profile of Hearing Aid Benefit (APHAB) and Glasgow Benefit Inventory (GBI) questionnaires, were completed 12 months after fitting of the BCI audio processor. In APHAB, four subscales are covered: ease of communication (EC), listening against background noise (BN), listening under reverberant conditions (RV) and aversiveness of sound (AV) (Cox and Alexander Citation1995). A difference of at least 22 points is needed for individual subscales in EC, BN and RV to be judged as a real difference between unaided and aided condition (Cox and Alexander Citation1995). The GBI is measuring the patient benefit in the general, the social support and the physical health (Robinson, Gatehouse, and Browning Citation1996). The scores are given on a scale of −100 to +100, where positive scores imply benefit in general, and in health status from the surgical intervention implanting the BCI.
Test conditions
The following conditions were tested and reported in this study: BCI at 12 months after fitting, PPP on softband as reference (ref) – device after 1 month of usage before the BCI surgery, and unaided as average of all visits (fitting of the ref and BCI device, 1, 3, 6 and 12-month follow-up). The unaided average was used in order to reduce intra subject variability that sometimes is large due to conditions of the middle ear in this type of patients.
Hypotheses
Six hypotheses are formulated as follows. The BCI: (1) involves a safe surgical intervention; (2) can be used with low risk for the patients over time; (3) has stable transmission conditions over time; (4) improves the sensitivity to sound and the intelligibility of speech at normal conversation levels relative to the unaided condition which is the primary effect hypothesis; (5) has similar or better sensitivity to sound and intelligibility of speech at normal conversation levels relative to a conventional bone conduction reference device which is the secondary effect hypothesis; and (6) has similar or better scores in the questionnaires comparing with both the unaided situation and the reference device in support for both the primary and secondary effect hypothesis.
Sample size calculation and statistical methods
In this clinical study, a sample size of 16 patients is regarded to be sufficient to statistically evaluate the effect hypotheses. This assumption is based on a previous study by Håkansson et al. (Citation1990) on BAHA patients (n = 49), where all patients had a positive improvement of the sound field tone thresholds over the unaided condition. Mean values, standard deviations and Wilcoxon signed rank test were used in the statistical analysis in this report. “The last-observation carried forward” principle was used in the case of missing data.
Results
Safety outcomes
All implants were tested for NSP transmission and the implanted device was found to be functioning as expected before the wound was closed. No intra-operative complications were encountered in any of the patients and no serious adverse events were reported at surgery or before discharge. During the follow-up, from time of surgery until the 12-month follow-up was completed, no adverse events classified as serious occurred in any of the 16 patients. The magnet retention force was measured at each follow-up visit showing a tendency to increase over time. The magnet was, therefore, changed to a weaker one in six of the patients with rationale that the force should not exceed 0.8–1 N or when the patient expressed a complaint that could be related to high pressure. The average force at 12-month follow up was 0.73 N (range 0.37–0.96 N).
In four cases, device-related adverse events were reported during the follow-up time: Patient 1: Experienced persistent pain at site of implantation after surgery. This pain gradually disappeared during the first 2 months, and no pain was reported at end-of-study; Patient 9: Experienced numbness and pain at the site of implantation after surgery. Numbness at the site of implantation has decreased but some numbness remains at end-of-study. The pain was managed by change to a weaker magnet in the processor, and no pain was reported at end-of-study; Patient 11: Reported pain at the site of implantation at one follow-up visit. This pain was managed by change to a weaker magnet in the audio processor. No pain was reported at the end-of-study; Patient 16: Mild wound issue at the site of implantation after surgery. The complication was resolved by antibiotic treatment.
Device deficiency
One device deficiency, classified as not serious, was reported in Patient 5. The device deficiency occurred 8 months after surgery and there was no additional medical complication in the patient, neither before, nor during or after the re-implantation of the BBC. After explantation of the BBC, it was confirmed that the retention magnet had become loose inside the titanium housing due to a defective gluing of the magnet inside the hermetic sealed titanium casing. This loose magnet created a “clicking” sound when attaching and removing the audio processor from its position on the head. The loose magnet did not create any disturbance to the patient when the audio processor was in place, and under normal use.
Transmission properties over time
It was found that the overall NSP change over time is around zero, indicating that sound transmission is stable over time. It was concluded in this study that the transmission to bone has not changed over the 1-year follow-up, neither on a group nor on an individual level. A detailed report of the NSP results is presented in Reinfeldt et al. (Citation2019).
Effectiveness of the device
Hearing sensitivity – warble tones and SRT
The average warble tone thresholds were used to test the primary effect hypothesis, namely that the BCI improves the sensitivity to sound relative the unaided condition. The difference between PTA threshold for the BCI at 12-month follow-up and unaided was statistically significant at 5% confidence level according to the Wilcoxon signed-rank test, with an average of 28.0 ± 6.4 dB (±1 standard deviation). shows the mean improvement at each tested frequency for the BCI and for the reference device, compared to the unaided situation as baseline. The BCI provided these patients with an average improvement at test frequencies by 6–36 dB (5–30 dB for the reference device). Wilcoxon signed-rank test showed that the improvements for the BCI were statistically significant over the unaided condition for all frequencies except at 8 kHz, which confirms the primary effect hypothesis. When comparing the improvements of the devices, for the secondary effect hypothesis, with the null hypothesis that the BCI and the reference device have the same improvements, it could be rejected at 750 Hz and at 1500–6000 Hz in favour for the BCI and at 250 Hz in favour for the reference device. The PTA for the reference device is 23.7 ± 7.2 dB over the unaided condition.
Figure 5. (a) Warble tone threshold improvements for BCI and reference device compared to the unaided situation (baseline at 0 dB), with mean and standard deviations. Stars are included at frequencies where the BCI or the Ref device has statistically higher improvement than the other device (α < 0.05). (b) Speech Recognition Threshold (SRT) improvements for BCI and reference device (Ref) over the unaided condition for all patients. The rightmost column shows mean improvement and standard deviation.
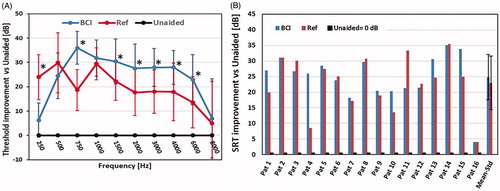
The improvement in SRT using the BCI and the reference device over the unaided condition is presented in . The BCI was better than the unaided condition in all patients with an average improvement of 24.9 ± 7.3 and 23.0 ± 8.6 dB for the reference device. This average improvement over the unaided condition was statistically significant for both devices (α < 0.05), but there was no significant advantage for the BCI over the reference device.
Speech-in-noise tests
shows the improvement in SRS of monosyllabic words in noise over the unaided condition. Improvement in SRS with the BCI is statistically significant (α < 0.05) with 45 ± 16% over the unaided condition. Also, the improvement of the reference device (43 ± 19%) is statistically significant over the unaided condition. Comparing the BCI and the reference device, the average SRS improvement was slightly higher using the BCI compared to using the reference device but the difference between the devices was not statistically significant.
Figure 6. (a) Speech Recognition Score (SRS) improvements for BCI and reference (Ref) device over the unaided condition for all patients. The rightmost column shows mean improvement and standard deviation. (b) Signal-to-noise ratio threshold (SNR-threshold) for 50% intelligibility for the BCI and the reference (Ref) device for all patients. The rightmost column shows mean improvement and standard deviation.
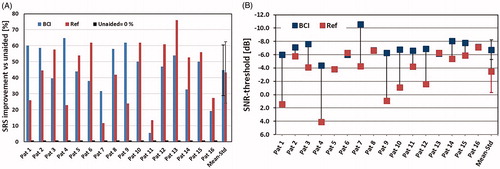
In , the SNR-thresholds using the BCI and the reference device are shown. Increasing negative SNR-thresholds mean that the patient reaches the same speech intelligibility despite a higher noise level. The difference between the BCI and reference device was statistically significantly better (lower score) with the BCI (Wilcoxon signed rank test).
As can be seen in , the BCI shows better or similar results compared to the reference device for all patients. On average the BCI gave a SNR-threshold of −6.8 ± 1.5 dB, while the reference device gave an average SNR-threshold of −3.5 ± 3.2 dB. A plausible explanation for the better SNR-threshold of the BCI could be that the high-frequency content in the speech signal, which is important for speech understanding, is more attenuated when vibrations pass through the skin using the reference device.
Questionnaires
The APHAB and GBI questionnaires were completed by the patients for the reference device before the surgery, and for the BCI after 6 and 12 months. Questionnaire results for the reference device preoperatively and the 12-month results for the BCI are reported for all sixteen patients in . For the subscales EC, BN and AV, the average score among all patients experienced an improvement above the 22-point level, which indicates that there is a clinically significant improvement over the unaided condition. All improvements, seen in , for the first three categories, EC, BN and RV, are statistically significant. No significant differences between the two devices were found.
Figure 7. (a) APHAB improvements in the four categories (ease of communication (EC), listening against background noise (BN), listening under reverberant conditions (RV), and aversiveness of sound (AV)) for the BCI and for the reference device (Ref). (b) GBI results in the categories total score, general subscale score, social support score and physical health score. Results for both the BCI and the reference device (Ref) are presented. Results show the mean improvement and standard deviation.
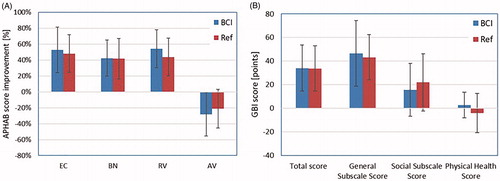
The GBI result showed statistically significant improvements in the average total score for both devices (α < 0.05), see . For the subscale analyses, the general subscale scores were significantly improved over the unaided condition for both devices. The social subscale score and the physical health score were not significantly changed for either of the devices, and furthermore, no statistically significant differences were found between the devices. On an individual level, an improvement in the GBI evaluation was experienced by all patients for the total score. When analysing sub-scales on an individual level, all patients reported an improvement for the general subscale score, and a majority of the patients for the social support score.
Protocol deviations and analysis population
Patient 3 was not participating in the 12-month follow-up visit and the results presented was thus taken from the 6 months visit. Additionally, the 12 months SNR-threshold (−0.9 dB), recorded for patient 6, was considered erroneous and was replaced by the 6months value (−6 dB), adhering to the last observation carried forward method of handling missing data. As a sensitivity analysis, the aided results were analysed with and without these corrections, with no effect on the outcome from the Wilcoxon signed-rank test.
Discussion
To date (June 2019), 14 patients are regular daily users of the BCI whereas one patient is a temporary user and one patient is a non-user. The non-user (patient 5) was just at the border of being included (PTA BC = 30 dB at inclusion) and has since then developed slightly poorer residual sensorineural hearing. The temporary user is the youngest one (patient 3, with perfect hearing on the non-implanted side) and he has moved around in the world during last 3–4 years and do quite well with his normal hearing in the contra lateral ear. Living on a different continent was also the reason that this patient missed the 12-month follow-up.
Safety
The procedure for installing the BCI device was found to be safe and relatively uncomplicated. During the 12-month follow-up time no “serious adverse events” or “severe adverse device effects” have occurred in any of the 16 patients. Only a few adverse events, mainly related to the choice of magnet strength, were reported. This is indeed a very good result if compared to the prevailing BAHA treatment, where some of the patients have more frequent problems related to the skin-penetration over time (Dimitriadis et al. Citation2016). Main explanations for the positive wearing and safety results with the BCI might be that the skin is kept intact and that the retention force for the audio processor is relatively low. From a surgery perspective, the engagement of the BBC in the mastoid bone is shallow and it is placed far from delicate structures of the mastoid bone and the inner ear.
The BBC has been found to very robust in a laboratory study by Fredén Jansson et al. (Citation2019) who subjected an implant to relevant impact testing procedures originally developed for cochlear implants. One complete BCI system was in the same study subjected to accelerated use (24 h/d with 78.6 dBA mixed speech/music radio sound) in a long-term endurance/fatigue test. This device has to date survived a time corresponding to more than 26 years of normal use without malfunction or deteriorated performance. This experiment is currently ongoing and will continue until the implant fails.
In a pilot study by Fredén Jansson et al. (Citation2015b), the magnetic resonance imaging (MRI) compatibility of the BBC was tested at 1.5 Tesla. The study indicated that the effects on the implants’ output force, distortion and retention force were minor, but the image was distorted near the implant to a maximum distance of 10 cm, which is similar to other active transcutaneous bone conduction devices. In that study, it was concluded that the current BCI design may pass an approval to be MR conditional up to 1.5 Tesla.
Effectiveness
It should be noted that the performance of the Ponto Pro Power is underestimated when measuring the 750 Hz warble tone threshold, because of that device is tailor made for the percutaneous application where a notch filter is used in that frequency region in order to damp the transducer’s resonance frequency which is not possible to change. The promising results in this study may indicate that some patients with bone thresholds below 30 dB HL could benefit from the present model of the BCI but most likely not the whole cohort of patients who are currently using or are presumptive candidates for a power BAHA. However, the present BCI design is just the first generation of direct bone drive transcutaneous induction devices and future power models may be expected to offer additional maximum output and other prescription rules allowing for a more sophisticated use of compression. Theoretically, 6 dB increased maximum force level output may be achieved by using a full bridge instead of the present half bridge power stage as used today (Taghavi et al. Citation2015).
Implant verification and transmission properties over time
When the first patients were operated, NSP measurements were vital to verify the implant functionality before the wound was closed, and two spare devices were kept ready in case of anomalies detected. This tool was also valuable during follow-up, when the stability of the transmission condition through the implanted unit (BBC) to the skull bone could be followed and confirmed over time. A simplified alternative to the NSP measurement was also used in this study where a battery-operated audio processor was verifying the implant functionality by sound emission from the surrounding skull bone. This audio processor was programmed to produce a series of tones that can be heard via the implant in the operating room.
Another potential tool for implant verification is the surface microphone developed by Hodgetts et al. (Citation2018). The surface microphone solution may offer an easy and effective method to verify the fitting and audibility of the audio processor.
Comparison to other devices
The main difference between the BCI and the BB system is the depth of the required recess in the temporal bone needed for the transducer installation. The BCI requires a shallower recess of 2–4 mm compared to the 8–10 mm needed to install the BB implant (Reinfeldt et al. Citation2015c). In a review by Reinfeldt et al. Citation2015a, it was found that the audiometric and questionnaire results in this clinical study are very similar for the BCI and the BB system.
When compared to the two commercially available passive transcutaneous devices, the Sophono device (Medtronic, Jacksonville, FL, USA) and the Baha Attract (Cochlear Bone Anchored Solutions, Mölnlycke, Sweden), the BCI showed comparable threshold improvement but superior speech recognition in noise results. Furthermore, complications related to the stronger retention force in the passive systems (need also to carry the transducer and suffer from less sensitivity if this force is too low) can be reduced with active transcutaneous devices.
Present results are also compared with the BAHA results reported for the 122 first BAHA users in Gothenburg (Tjellström and Håkansson Citation1995) and there is a surprising resemblance in comparable measures comparing the unaided condition: warble tone thresholds (then 29.4 dB, now 28.0 dB); SRT (then 26.5 dB, now 24.9 dB); SRS (then 41.6%; now 45%). The resemblance is also remarkable regarding the SNR threshold as in the study by Carlsson et al. (Citation1986), comparing the 21 first BAHA patients, it was found that the difference was 3.3 dB (improvement with the BAHA) whereas in this study the improvement in SNR threshold between the BCI and the reference device was also 3.3 dB. In a study by Rigato et al. (Citation2016) comparing BCI patients with matched BAHA patients the audiometric similarities between the two treatment options was also confirmed.
Conclusion
In this study, it was shown that the procedure for implanting the BCI is safe and that the transmission condition is stable over the follow-up time. The hearing ability of the BCI patients was significantly improved compared to the unaided condition (primary effect hypothesis) as measured by hearing thresholds, speech reception thresholds and speech-in-noise performance. Improvement was shown also in the patients’ quality of life as assessed by the GBI questionnaire, and self-assessed reduction of disability as measured by the APHAB questionnaire. Furthermore, the hearing rehabilitation provided by the BCI was found to be comparable, and in some measurements superior, to the use of a power BC device on a softband (secondary effect hypothesis).
The BCI was found to be a good option for the indicated patients and is assumed beneficial for those with skin problems or those who refuses a percutaneous system. Future studies are planned to continue to follow these patients in a 3- and 5-year follow-up.
Disclosure statement
The corresponding author (BH) holds patents related to this project. BH, SR and MEO have been consultants to Oticon Medical in various stages. The other authors report no declaration of interest.
Additional information
Funding
References
- Byrne, D., H. Dillon, K. Tran, S. Arlinger, K. Wilbraham, R. Cox, B. Hagerman, R. Hetu, J. Kei, and C. Lui. 1994. “An International Comparison of Long-Term Average Speech Spectra.” The Journal of the Acoustical Society of America 96 (4): 2108–2120. doi:10.1121/1.410152.
- Carlsson, P., B. Håkansson, U. Rosenhall, and A. Tjellström. 1986. “A Speech-to-Noise Ratio Test with the Bone-Anchored Hearing Aid: A Comparative Study.” Otolaryngology-Head and Neck Surgery: Official Journal of American Academy of Otolaryngology-Head and Neck Surgery 94 (4): 421–426. doi:10.1177/019459988609400402.
- Cox, R. M., and G. C. Alexander. 1995. “The Abbreviated Profile of Hearing Aid Benefit.” Ear and Hearing 16 (2): 176–186.
- Declaration of Helsinki, Ethical Principles for Medical Research Involving Human Subjects, World Medical Association.
- den Besten, C. A., P. Monksfield, A. Bosman, P. H. Skarzynski, K. Green, C. Runge, S. Wigren, et al. 2018. “Audiological and Clinical Outcomes of a Transcutaneous Bone Conduction Hearing Implant: Six-Month Results from a Multicentre Study.” Clinical Otolaryngology 44: 144–157. doi:10.1111/coa.13248.
- Dimitriadis, P., M. Farr, A. Allam, and J. Ray. 2016. “Three Year Experience with the Cochlear BAHA Attract Implant: A Systematic Review of the Literature.” BMC Ear, Nose, and Throat Disorders 16: 12. doi:10.1186/s12901-016-0033-5.
- Eeg Olofsson, M. 2012. “Transmission of Bone-conducted Sound in the Human Skull Based on Vibration and Perceptual Measures.” PhD thesis, Institute of Clinical Sciences at Sahlgrenska Academy University of Gothenburg.
- Eeg-Olofsson, M., B. Håkansson, S. Reinfeldt, H. Taghavi, H. Lund, KJ. Fredén Jansson, E. Håkansson, and J. Stalfors, 2014. “The Bone Conduction Implant – First Implantation, Surgical and Audiologic Aspects.” Otology and Neurotology 35 (4): 679–685. doi:10.1097/MAO.0000000000000203.
- Everyday Hearing. 2018. Best Bone Conduction Headphones of A Complete Guide. https://www.everydayhearing.com/hearing-technology/articles/bone-conduction-headphones/
- Fredén Jansson, K. J. 2017. “The Balanced Electromagnetic Separation Transducer for Bone Conduction Audiometry and Hearing Rehabilitation.” PhD thesis, Chalmers University of Technology.
- Fredén Jansson, K. J., B. Håkansson, L. Johannsen, and T. Tengstrand. 2015a. “Electro-Acoustic Performance of the New Bone Vibrator Radioear B81: A Comparison with the Conventional Radioear B71.” International Journal of Audiology 54 (5): 334–340. doi:10.3109/14992027.2014.980521.
- Fredén Jansson, K. J., B. Håkansson, C. Rigato, M. Eeg-Olofsson, and S. Reinfeldt. 2019. Robustness and Lifetime of the Bone Conduction Implant – A Pilot Study. Medical Devices: Evidence and Research 12: 89–100. doi:10.2147/MDER.S192860.
- Fredén Jansson, K. J., C. Rigato, B. Håkansson, S. Reinfeldt, and M. Eeg-Olofsson. 2015b. “2015. Magnetic Resonance Imaging Investigation of the Bone Conduction Implant – a Pilot Study at 1.5 Tesla.” Medical Devices: Evidence and Research 8: 413–423. doi:10.2147/MDER.S90704.
- Hagerman, B. 1982. “Sentences for Testing Speech Intelligibility in noise.” Scandinavian Audiology 11 (2): 79–87. doi: 10.3109/01050398209076203
- Hagerman, B. 1993. “Efficiency of Speech Audiometry and Other Tests.” British Journal of Audiology 27 (6): 423–425. doi: 10.3109/03005369309076719
- Håkansson, B. 2003. “The Balanced Electromagnetic Separation Transducer a New Bone Conduction Transducer.” The Journal of the Acoustical Society of America 113 (2): 818–825. doi:10.1121/1.1536633.
- Håkansson, B., and P. Carlsson. 1989. “Skull Simulator for Direct Bone Conduction Hearing Devices.” Scandinavian Audiology 18 (2): 91–98. doi:10.3109/01050398909070728.
- Håkansson, B., K. J. Fredén Jansson, T. Tengstrand, L. Johannsen, M. Eeg-Olofsson, C. Rigato, E. Dahlström, and S. Reinfeldt. 2018. “VEMP Using a New Low Frequency Bone Conduction Transducer.” Medical Devices: Evidence and Research 11 (11): 301–312. doi:10.2147/MDER.S171369.
- Håkansson, B., G. Lidén, A. Tjellström, A. Ringdahl, M. Jacobsson, P. Carlsson, and B. E. Erlandson. 1990. “Ten Years of Experience with the Swedish Bone-Anchored Hearing System.”Annals of Otology, Rhinology and Laryngology 99 (10): 1–16. doi:10.1177/0003489490099S1001.
- Håkansson, B., A. Tjellström, U. Rosenhall, and P. Carlsson. 1985. “The Bone-Anchored Hearing Aid: Principle Design and a Psychoacoustical Evaluation.” Acta Oto-Laryngologica 100 (3–4): 229–239. doi:10.3109/00016488509104785.
- Hodgetts, W., D. Scott, P. Maas, and L. Westover. 2018. “Development of a Novel Bone Conduction Verification Tool Using a Surface Microphone: Validation With Percutaneous Bone Conduction Users.” Ear Hear 39 (6): 1157–1164. doi:10.1097/AUD.0000000000000572.
- Holube, I., S. Fredelake, M. Vlaming, and B. Kollmeier. 2010. “Development and Analysis of an International Speech Test Signal (ISTS).” International Journal of Audiology 49 (12): 891–903. doi:10.3109/14992027.2010.506889.
- ISO 14155. 2011. Clinical Investigation of Medical Devices for Human Subjects – Good Clinical Practice International Organization for Standardization. Geneva, Switzerland: ISO.
- ISO 389-3. 2016. Acoustics – Reference Zero for the Calibration of Audiometric Equipment – Part 3: Reference Equivalent Threshold Vibratory Force Levels for Pure Tones and Bone Vibrators. Geneva, Switzerland: ISO.
- ISO 8253-1. 2010. Acoustics – Audiometric Test Methods – Part 1: Pure-tone Air and Bone Conduction Audiometry. Geneva, Switzerland: ISO.
- ISO 8253-3. 2012. Acoustics – Audiometric Test Methods – Part 3: Speech Audiometry. Geneva, Switzerland: ISO.
- Nelissen, R.C., M. J. H. Agterberg, M. K. S. Hol, and A. F. M. Snik. 2016. “Three-Year Experience with the Sophono in Children with Congenital Conductive Unilateral Hearing Loss: Tolerability, Audiometry, and Sound Localization Compared to a Bone-Anchored Hearing Aid.” European Archives of Oto-Rhino-Laryngology 273 (10): 3149–3156. doi:10.1007/s00405-016-3908-6.
- Ortiz-Catalan, M., R. Brånemark, B. Håkansson, and J. Delbeke. 2012. “On the Viability of Implantable Electrodes for the Natural Control of Artificial Limbs: Review and Discussion.” Biomedical Engineering Online 11 (1): 33. doi:10.1186/1475-925X-11-33.
- Reinfeldt, S. 2009. “Bone Conduction Hearing in Human Communication - Sensitivity, Transmission, and Applications.” PhD thesis, Chalmers University of Technology.
- Reinfeldt, S., B. Håkansson, H. Taghavi, and M. Eeg-Olofsson. 2015a. “New Developments in Bone Conduction Hearing Implants: A Review.” Medical Devices: Evidence and Research 8: 79–93. doi:10.2147/MDER.S39691.
- Reinfeldt, S., B. Håkansson, H. Taghavi, K. J. Fredén Jansson, and M. Eeg-Olofsson. 2015b. “The Bone Conduction Implant – Clinical Results of the First Six Patients.” International Journal of Audiology 54 (6): 408–416. doi:10.3109/14992027.2014.996826.
- Reinfeldt, S., P. Östli, B. Håkansson, H. Taghavi, M. Eeg-Olofsson, and J. Stalfors. 2015c. “Study of the Feasible Size of a Bone Conduction Implant Transducer in the Temporal Bone.” Otology and Neurotology 36 (4): 631–637. doi:10.1097/MAO.0000000000000682.
- Reinfeldt, S., C. Rigato, B. Håkansson, KJ. Fredén Jansson, and M. Eeg-Olofsson. 2019. “Nasal Sound Pressure as Objective Verification of Implant in Active Transcutaneous Bone Conduction Devices, Submitted to Medical Devices.” Medical Devices: Evidence and Research 12: 193–202. doi:10.2147/MDER.S197919.
- Rigato, C. 2019. “On Direct Drive Bone Conduction Devices - Hearing Rehabilitation and Objective Assessment of Stimulation Methods.” PhD thesis, Chalmers University of Technology.
- Rigato, C., S. Reinfeldt, B. Håkansson, K. J. Jansson, M. K. Hol, and M. Eeg-Olofsson. 2016. “Audiometric Comparison between the First Patients with the Transcutaneous Bone Conduction Implant and Matched Percutaneous Bone Anchored Hearing Device Users.” Otology and Neurotology 37 (9): 1381–1387. doi:10.1097/MAO.0000000000001183.
- Robinson, K., S. Gatehouse, and GG. Browning. 1996. “Measuring Patient Benefit from 672 Otorhinolaryngological Surgery and Therapy.” Annals of Otology, Rhinology and Laryngology 105 (6): 415–422. doi:10.1177/000348949610500601.
- Sprinzl, G. M., and A. Wolf-Magele. 2016. “The Bonebridge Bone Conduction Hearing Implant: Indication Criteria, Surgery and a Systematic Review of the Literature.” Clinical Otolaryngology 41 (2): 131–143. doi:10.1111/coa.12484.
- Stenfelt, S. 1999. “Hearing by Bone Conduction: Physical and Physiological Aspects.” PhD thesis, Chalmers University of Technology.
- Stenfelt, S., and S. Reinfeldt. 2007. “A Model of the Occlusion Effect with Bone-Conducted Stimulation.” International Journal of Audiology 46 (10): 595–608. doi:10.1080/14992020701545880.
- Taghavi, H. 2014. “The Bone Conduction Implant (BCI) - Pre clinical Studies, Technical Design and a Clinical Evaluation.” PhD thesis, Chalmers University of Technology.
- Taghavi, H., B. Håkansson, S. Reinfeldt, M. Eeg-Olofsson, and S. Akhshijan. 2012. “Feedback Analysis in Percutaneous Bone-Conduction Device and Bone-Conduction Implant on a Dry Cranium.” Otology & Neurotology: Official Publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology 33 (3): 413–420. doi:10.1097/MAO.0b013e3182487fc8.
- Taghavi, H., B. Håkansson, S. Reinfeldt, M. Eeg-Olofsson, K. J. F. Jansson, E. Håkansson, and B. Nasri. 2015. “Technical Design of a New Bone Conduction Implant (BCI) System.” International Journal of Audiology 54 (10): 736–744. doi:10.3109/14992027.2015.1051665.
- Tjellström, A., and B. Håkansson. 1995. “The Bone-anchored Hearing Aid. Design Principles, Indications, and Long-term Clinical Results.” Otolaryngologic Clinics of North America 28 (1): 53–72.
- Håkansson 2005. Percutaneous bone anchored transferring device. US patent 6840919 (SE 9704752).
- Westerkull, P. 2018. “An Adhesive Bone Conduction System, Adhear, a New Treatment Option for Conductive Hearing Losses.” Journal of Hearing Science 8 (2): 35–43. doi:10.17430/1003045.
