Abstract
Objective: This study evaluated the outcomes of the Oticon Medical Neuro Zti cochlear implant and the Neuro 2 sound processor.
Design: Neuro One users were upgraded to Neuro 2. Monosyllabic word identification was evaluated in adults with Neuro One after ≥5 months, with Neuro 2 at upgrade, and with Neuro 2 after 3 months. Self-reported listening ability, satisfaction, and usability were measured in adults and children.
Study sample: Participants were 44 adults and 26 children.
Results: Speech identification scores in quiet and noise were 58% and 45% with Neuro One and 67% and 55% with Neuro 2 after 3 months, respectively. Hearing impairment duration and number of active electrodes significantly predicted speech identification in noise with Neuro 2. Significantly higher questionnaire ratings were obtained for Neuro 2 than Neuro One regarding listening ability in complex listening situations, comfort and music, as well as nine aspects of satisfaction and usability.
Conclusion: This study demonstrates the clinical superiority of the Neuro 2 sound processor over Neuro One in terms of speech identification in quiet and in noise and reported patient benefit and satisfaction. Given the study design, sources of improvement may include factors unrelated to the sound processor itself.
Introduction
Upgrade of cochlear implant sound processors
Since the first cochlear implants, tremendous improvements have been made to the implant systems, to surgical techniques, and to rehabilitative care, leading to constantly improving outcomes for their users. Developments in electrode design, coding strategies, and sound processing features have led to increased benefits (Blamey et al. Citation2013; Di Lella et al. Citation2010; Lazard et al. Citation2010). As a result, patients can often benefit from an upgrade of their sound processor (Mauger et al. Citation2014; Mosnier et al. Citation2014; Plasmans et al. Citation2016).
Studies showing that cochlear implant users can benefit from newer sound processors typically use a paired research design where within-subject comparisons are made after a habituation period to the new sound processor (e.g. Mauger et al. Citation2014; Mosnier et al. Citation2014; Plasmans et al. Citation2016). The present study concerns the latest cochlear implant system of Oticon Medical (Smørum, Denmark), which the following section describes.
Oticon Medical neuro cochlear implant system
Oticon Medical has developed a cochlear implant system consisting of the Neuro Zti implant and the Neuro sound processor series (first generation: Neuro One; second generation: Neuro 2). The small and robust Neuro Zti implant has been available since 2015. A titanium flat base and a zirconia casing protect its receiver, which is fixed to the temporal bone with a dedicated fixation system using two titanium screws (Guevara et al. Citation2010). The Neuro Zti implant uses a stimulation mode that combines a common-ground and a monopolar pathway. It uses pseudo-monophasic electrical pulses with an anodic active phase and a capacitive discharge for charge balancing (Cogan Citation2008). The pulse amplitude is fixed, and the pulse duration is modulated to code loudness (Di Lella et al. Citation2010). The Neuro Zti implant is compatible with two electrode arrays, the Classic and the EVO, both with 20 channels.
Neuro one sound processor
The sound processor Neuro One became available in 2015. Compared to its predecessors, the Digisonic series (Bergeron and Hotton Citation2016), it offers Coordinated Adaptive Processing (CAP), which includes wide input dynamic range signal acquisition and constant automated environment detection from the Inium Sense platform that selects several signal processing strategies including Voice Track (noise reduction) and Voice Guard (output compression). Voice Track uses Wiener filter-based multiband single-channel noise reduction to significantly improve speech intelligibility in noise as well as sound quality (Guevara et al. Citation2016). Voice Guard applies back-end, post-spectral analysis compression to lead to a wide input dynamic range of up to 80 dB to maximise available information from the input signal. Voice Guard has been shown to improve identification of both soft and loud speech in 20 adult patients, compared to logarithmic wide-band compression (Lorenzi et al. Citation2004; Bozorg-Grayeli et al. Citation2016). Other features found to be useful for hearing aid users, namely Free Focus (automatic adaptive directionality) and Wind Noise (dual-microphone wind noise reduction algorithm), are borrowed from Oticon’s hearing aids. For more information regarding CAP, see Segovia-Martínez, Gnansia and Hoen (Citation2016).
Neuro 2 sound processor
Neuro 2 has been available since 2018: signal processing and coding strategies are unchanged from Neuro One to Neuro 2. Neuro 2 introduces improved design with smaller size and lighter weight as well as new powering options such as rechargeable batteries. Several studies have shown that visual appearance, wearing comfort on the ear, and robustness/reliability were key drivers for cochlear implant choices for both adults and parents of children (Chundu and Stephens Citation2013; Clamp et al. Citation2013; Geyer et al. Citation2006).
Whilst Neuro One is powered by two non-reusable 675 zinc-air batteries with life of approximately 3 days, in addition Neuro 2 can be powered by rechargeable lithium-ion batteries: the user can choose between a smaller battery (120 mAh) with life of approximately 1 day or a larger battery (200 mAh) with life of approximately 2 days. Neuro 2 incorporates additional ergonomic and mechanical design features, including more resistant microphones and antenna cables and a facility to check that the microphones are working, that are especially relevant for children.
Whilst professionals adjust the Neuro One with the DigiMap 4.0 fitting software, professionals adjust the Neuro 2 with a new fitting software, Genie Medical CI. The user interface of the new software was designed to facilitate adjustments of CI parameters.
Rationale for the study
This study upgraded habituated users of the Oticon Medical Neuro One sound processor to the Neuro 2 sound processor. These are the first published results pertaining to the Neuro 2 cochlear implant system. To quantify real-world clinical effectiveness of the Neuro 2, a clinical study with a large and heterogenous sample of participants composed of both adults and children cochlear implant users was conducted.
The study adopted a repeated-measures crossover within-subjects design. However, treatment order was not counterbalanced: all participants were Neuro One users at enrolment and were upgraded to Neuro 2. Word identification in quiet and in noise was measured before and after the sound processor upgrade from Neuro One to Neuro 2. Although there are no changes to the signal processing and coding strategies between Neuro One and Neuro 2, we expected that the standard clinical care that patients received during the study procedure and the mapping changes including activation of sound processing features described above would lead to improved performance, self-reported listening ability, and satisfaction and usability.
Both adults and children were included as it was of interest to assess user satisfaction and usability towards the Neuro 2 sound processor both in adults and in children. We hypothesised that the sound processor upgrade would lead to greater satisfaction and usability. Word identification was measured in adults (≥15 years old) only because development of speech and language is expected in children during the habituation period. This confounding factor cannot be controlled for and can lead to biases. Furthermore, it was of interest to recruit children of all ages and therefore some children would not have been able to complete a speech identification task.
Aim of the study
This study had two aims related to the upgrade of patients from the Neuro One to the Neuro 2 sound processors together with the provision of standard clinical care: (1) Document any changes in speech identification scores in adults, and (2) Document any changes in satisfaction towards self-reported listening ability, satisfaction, and usability in adults and children.
Materials and methods
Participants
All enrolled participants were: (1) recipients of one or two Oticon Medical cochlear implant Neuro Zti from one of the participating centres, (2) users of the Neuro One sound processor for ≥5 months at enrolment, (3) eligible for universal health coverage through France’s social security, and (4) able to understand both oral and written French. There were no age restrictions on participation.
Study design and procedures
Nine French cochlear implant centres were included, located in Bordeaux, Lille, Lyon, Nancy, Nantes, Paris (Pitié-Salpêtrière and Necker Hospital for Sick Children), and Nice (Nice University Hospital and Nice-Lenval).
Each centre invited their patients who met the inclusion criteria to take part in the study. Patients who agreed to participate attended three study visits over 3.5 months to replace their Neuro One sound processor with the Neuro 2 sound processor, to perform any required adjustments to the Neuro 2 sound processor, and to measure outcomes. Participants, or their parents in the case of children, provided written informed consent before enrolment. The study was conducted in accordance with the Declaration of Helsinki and the ethics committee Comité de Protection des Personnes Est-III approved its design and methods (IRB: 2017-A01188-45). The study was registered in the U.S. National Library of Medicine clinical trial database (NCT03288753). Participants kept their Neuro 2 sound processor after the end of the study and their travel expenses were reimbursed. Participants received no other financial incentive.
Each participant attended three study visits. The testing and/or questionnaire time for each study visit lasted approximately 45 minutes for adults and 15 minutes for children. Participants, or their parents in the case of children, completed the questionnaires.
Visit 1. For adult participants, speech identification was tested with the Neuro One sound processor. The participants completed the listening ability questionnaire as well as the satisfaction and usability questionnaire for the Neuro One sound processor.
Visit 2 (15 days after Visit 1). The patient’s sound processor was upgraded from Neuro One to Neuro 2. For adult participants, speech identification was tested with the Neuro 2 sound processor. The participants completed the listening ability questionnaire as well as the satisfaction and usability questionnaire for the Neuro 2 sound processor. The scores of the Visit 2 questionnaires are not reported as participants had not benefited from enough experience with their Neuro 2 sound processors to be able to provide reliable results.
Visit 3 (3 months after Visit 2). For adult participants, speech identification was tested with the Neuro 2 sound processor. The participants completed the listening ability questionnaire as well as the satisfaction and usability questionnaire for the Neuro 2 sound processor.
Cochlear implant mapping
Patients received standard clinical care. Adult patients were fitted with at least two programmes to cover their listening needs: one programme for quiet environments, which was used for the speech identification task in quiet, and one programme for noisy environments, which was used for the speech identification task in noise. (Adult patients who had difficulty handling the sound processor were fitted with a single programme.) The settings of the programmes varied from one participant to another, based on their needs and preferences, but the main difference for most patients was more noise reduction and more directionality in the noise programme: Voice Track can be set as off, low (20% suppression factor), medium (50% suppression factor), or high (70% suppression factor; Guevara et al. Citation2016). As this was a clinical effectiveness trial, participants were tested with their usual programmes. If required, some fine-tuning of the cochlear implant maps to address any reported patient needs could occur at planned or extra visits. Twelve participants required extra visits between Visit 2 and Visit 3.
Measures
Speech identification in quiet and in noise
Speech identification was tested in a cochlear implant-only condition in adult participants, i.e., participants with hearing aids removed those for the speech identification test. An open-set monosyllabic word identification task was used. The French Lafon cochlear implant word lists (Lafon Citation1972) were presented in a sound-proof test booth at 65 dB SPL from a front loudspeaker located one metre away from the participant. Each word contains three phonemes. The recording contains 20 lists of 17 words per list and examples of words include: fil (thread), mur (wall), and vol (flight). Two conditions were tested: in quiet and in noise. For the noise condition, in addition to the monosyllabic words, a speech-shaped noise was presented at 55 dB SPL from the same front loudspeaker (signal-to-noise ratio SNR of +10 dB).
At each of the three visits, in each of the two conditions (quiet and noise), patients were presented with two lists of monosyllabic words, for a total of 34 words per condition. Each patient was presented with a total of 12 lists (three visits x two conditions x two lists per condition per visit): lists were picked at random and, to reduce learning effects, no patient was presented with the same list twice. Word order within each list was however not counterbalanced. The percentage of correctly identified phonemes for each test condition was scored.
Listening ability questionnaire
A 16-item questionnaire was custom made to measure self-reported listening ability in different real-life listening situations (see Supplementary Appendix A for English translation from the authors; please contact the authors for more information). Example items include “In a quiet environment, I can have a conversation with someone I know, for example a family member or friend” and “On the telephone I can understand someone I do not know”. Seven response options were provided: Strongly disagree, Disagree, Slightly disagree, Neither agree nor disagree, Slightly agree, Agree, and Strongly agree. Response options were coded from 1 to 7, with higher scores representing better listening ability.
Satisfaction and usability questionnaire
A 30-item questionnaire was custom made to measure satisfaction and usability with the Neuro 2 sound processor. The questionnaire was developed as no validated measure of sound processor satisfaction and usability was readily available. The items were designed to tap into four topics: (1) size and appearance, (2) battery options, (3) handling and usability, and (4) benefits reported by the user and by family and friends. Examples of items include “I am satisfied with the size and appearance of my sound processor” and “It is easy for me to change the battery of my sound processor”. We focus the reporting on 15 items for which seven response options were provided: Strongly disagree, Disagree, Slightly disagree, neither agree nor disagree, Slightly agree, Agree, and Strongly agree. Response options were coded from 1 to 7, with higher scores representing higher satisfaction and usability.
Data analysis
A two-way repeated measures analysis of variance (ANOVA) investigated the effect of condition and study visit on speech identification scores in quiet and in noise. Post hoc Tukey’s tests were performed. Prior to running the ANOVA, assumptions were confirmed with Kolmogorov–Smirnov’s test showing normal distribution of the dependant variables, probability plots (residuals and expected values, showing normal distribution of the residuals), and Levene's test showing homogeneity of variances (homoscedasticity).
A multivariate analysis, that is, a linear regression model, was conducted to identify the significant predictors of speech identification scores in noise with the Neuro 2 after three months. Six potential predictors were identified: age, duration of hearing impairment, duration of CI experience, duration of Neuro experience, number of active electrodes, and CI configuration (CI unilateral with or without contralateral HA and bilateral CI). A full model approach was used: all six potential predictors were entered at once in the linear regression model.
A factor analysis with varimax rotation was conducted on the listening ability questionnaire scores. Three factors had eigenvalues greater than 1. The factor scores were normally distributed (Shapiro-Wilk test not significant), and therefore, t-tests were conducted to compare factor scores with the Neuro One and with the Neuro 2 after 3 months, combined for adults and children.
Wilcoxon signed-rank tests were used to compare scores on the satisfaction and usability questionnaire with the Neuro One and after 3 months with the Neuro 2, combined for adults and children. A non-parametric test was used as the scores were not normally distributed.
Missing data were not imputed. Factor scores on the listening ability questionnaire were averaged across the data available for each participant. Analyses were run with the statistical package Statistica 13.
Results
presents the 70 participants in terms of their demographics, whether hearing impairment (in the implanted ear in case of unilateral implantation) occurred before or after language acquisition and its duration, as well as a summary of their cochlear implant history. A total of 44 adults and 26 children were recruited. The sample reflects the characteristics of the population of cochlear implant users in France. Hearing impairment occurred post-lingually for most adults, but pre-lingually for most children. Most adults were implanted in one ear only, while most children were implanted in both ears. Of the 41 participants implanted unilaterally, 28 used a contralateral hearing aid. Participants had on average used their Neuro One sound processor(s) for 9–10 months. Implants had between 7 and 20 active electrodes. For participants implanted bilaterally, if the number of active electrodes was different in both implants, an average was calculated.
Table 1. Description of the 70 participants.
Minor recruitment deviations from the protocol
Recruitment resulted in three minor deviations from the protocol. First, the protocol proposed to recruit 90 participants (45 adults and 45 children), while it was only possible to recruit 70 participants (44 adults and 26 children). Second, one teenager aged 16 was inadvertently included in the group of children, although the protocol stated that participants ≥15 years old should be included in the group of adults: the consequence is that this participant did not complete the word identification task. Third, one child aged 2 with 3 months of experience with the Neuro One was included in the study, although the protocol stated that participants should have ≥5 months of Neuro experience. The research team chose to keep this participant’s data in the analyses as the children in this study did not complete word identification task and therefore habituation to the Neuro One sound processor was not as critical for children as for adults.
Speech identification in quiet and in noise
reports open-set monosyllabic word identification performance in quiet and in noise (+10 dB SNR) for the adult participants. As expected, performance in terms of % of correctly identified phonemes was better in quiet (mean of 58.3% SD = 22.7% and 67.4% SD = 19.3% for Neuro One and Neuro 2 after 3 months, respectively) than in noise (mean of 44.5% SD = 23.1% and 54.8% SD = 20.8% for Neuro One and Neuro 2 after 3 months, respectively).
Figure 1. Box-plot of speech identification (monosyllabic words with phoneme scoring) scores for the Neuro One and the Neuro 2 sound processors in quiet and in noise in adults. The middle line represents the median, the + sign, the mean, the lower and upper boundaries of the boxes, the 25th and 75th percentiles, the whiskers, the 5th and 95th percentiles, and the dots, the outliers. As some data were missing, the number of data points is reported.
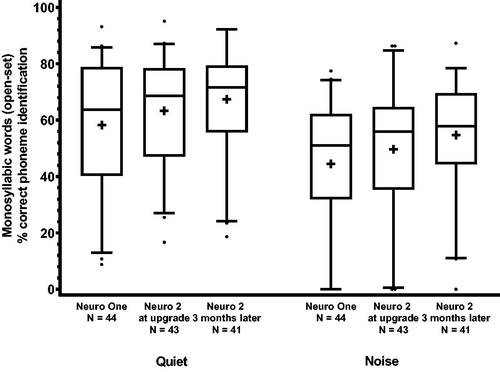
A repeated-measures ANOVA showed that both the factors condition [F(1,38) = 116.0, p < 0.001] and study visit [F(2,76) = 18.0; p < 0.001] were significant, while the interaction condition*study visit was not significant [F(2,76) = 0.49; p = 0.62]. Post hoc Tukey tests revealed that speech identification in quiet was significantly higher at Visit 2 (Neuro 2; p = 0.01) and at Visit 3 (Neuro 2 3 months later; p < 0.001) than at Visit 1 (Neuro One). The improvement in scores in quiet from Visit 2 to Visit 3 did not reach statistical significance. Furthermore, speech identification in noise was significantly higher at Visit 2 (Neuro 2; p = 0.008) and at Visit 3 (Neuro 2, 3 months later; p < 0.001) than at Visit 1 (Neuro One), and significantly higher at Visit 3 (Neuro 2, 3 months later; p = 0.003) than at Visit 2 (Neuro 2).
The partial eta-squared was calculated to describe effect size: for condition partial η2 = .75 and for study visit, η2 = .32. An effect leading to η2 > .14 is considered large (Cohen Citation1988).
Factors associated with speech identification in noise with neuro 2
The following six factors were considered as potential predictors of speech identification in noise with the Neuro 2 after 3 months: age, duration of hearing impairment, duration of CI experience, duration of Neuro experience, number of active electrodes, and CI configuration (CI unilateral with or without contralateral HA and bilateral CI). These six potential predictors were entered into a linear regression model which included 40 participants, as speech identification in noise scores were available for 41 of the 44 adults and duration of hearing impairment was missing for one of these participants.
The model was significant with F (6, 33) = 2.91: number of active electrodes (b = 0.43, p = 0.006) and hearing impairment duration (b = −0.31, p = 0.045) remained significant for an adjusted R2 of .23. In other words, speech identification in noise in this population of adult Neuro 2 cochlear implant users increases with number of active electrodes and decreases with hearing impairment duration. However, the model accounts for only approximately 23% of the variance observed in speech identification scores in noise.
Listening ability
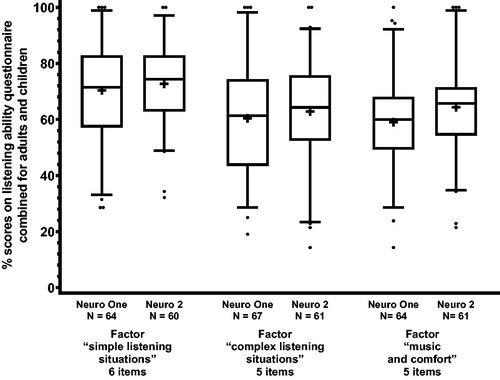
We ran a factor analysis with varimax rotation on the listening ability questionnaire. Three factors had eigenvalues greater than 1: we named these factors simple listening situations (5 items), complex listening situations (6 items), and music and comfort (5 items). For each participant, we computed an average percentage score for each factor with Neuro One and also with Neuro 2.
Average listening ability was for the simple listening situations factor 70.4% for Neuro One and 72.7% for Neuro 2, for the complex listening situations factor 60.4% for Neuro One and 62.9% for Neuro 2, and for the music and comfort factor 59.0% for Neuro One and 64.4% for Neuro 2. portrays these results. We ran paired t-tests for each factor. Scores with Neuro 2 proved to be significantly higher than scores with Neuro One for the factors complex listening situations (t = 3.14, p = 0.003) and music and comfort (t = 3.25, p = 0.002), but not for the factor simple listening situations (t = 1.53; p = 0.131).
Figure 2. Box-plot of self-reported listening ability scores for the Neuro One and the Neuro 2 sound processors for the three factors, combined for adults and children. The middle line represents the median, the + sign, the mean, the lower and upper boundaries of the boxes, the 25th and 75th percentiles, the whiskers, the 5th and 95th percentiles, and the dots, the outliers. As some data were missing, the number of data points is reported.
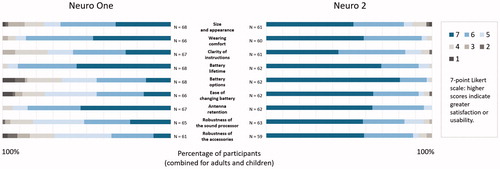
Satisfaction and usability
Nine of the 15 items showed significantly greater satisfaction and usability with the Neuro 2 compared to the Neuro One. These items target size and appearance (Z = 5.48, p < 0.001), wearing comfort (Z = 4.36, p < 0.001), clarity of instructions (Z = 2.49, p = 0.01), battery lifetime (Z = 4.86, p < 0.001), battery options (Z = 5.76, p < 0.001), ease of changing battery (Z = 2.02, p = 0.04), antenna retention (Z = 2.95, p = 0.003), robustness of the sound processor (Z = 2.52, p = 0.01), and robustness of the accessories (Z = 3.30, p < 0.001). presents results for these nine questionnaire items for all participants. For the other six items, ratings did not differ significantly between the Neuro One and the Neuro 2: these covered ease of changing volume, ease of changing programme, ease of replacing cable, overall ease of use, benefit reported by others, and overall satisfaction (all ps > 0.05).
Discussion
Summary of results
This is the first report of outcomes with the Oticon Medical Neuro 2 sound processor. This multicentric clinical evaluation measured outcomes in 44 adults and 26 children at three study visits: with the Neuro One sound processor after ≥5 months of usage, with the Neuro 2 sound processor at upgrade, and with the Neuro 2 sound processor after 3 months of use. In adults, speech identification in quiet shows scores of 58% with Neuro One and 67% with Neuro 2 after 3 months. In noise, scores were 45% with Neuro One and 55% after 3 months with Neuro 2. Self-reported listening ability was significantly improved in complex listening situations as well as for music and comfort. In adults and children, Neuro 2 led to high levels of satisfaction and usability, with the smaller size and weight, introduction of rechargeable batteries, usability, and robustness all appreciated.
Differences observed in speech identification results
Speech identification results were significantly better with Neuro 2 than with Neuro One and the effect size was large. As the available signal processing and coding strategies are the same in both sound processors, this could appear surprising. However, at least three reasons could explain this difference in scores.
First, as mentioned above, participants received standard clinical care during the study and were tested with their usual programmes. Although this was not measured systematically for all participants, there was a tendency for more signal processing to be applied in the Neuro 2 programmes than in the Neuro One programmes, that is, the CAP signal processing features were more likely to have been engaged in the Genie Medical CI fitting software. This is because patients reported during the study visits 2 and 3 needs that more signal processing, for example, automatic adaptive directionality and noise reduction, could address and because the new fitting software, Genie Medical CI, eases professionals’ adjustments of parameters. The professionals involved in the study reported adjusting the maps of most participants during the study. It is possible that refining of the maps led to significantly improved outcomes.
Second, participants benefited from 3.5 months of extra CI experience from Visit 1 to Visit 3. Most participants had less than one year of Neuro One experience at enrolment, yet a previous study showed that CI users improved their speech identification ability over the year following implantation (Lazard et al. Citation2010). The Neuro One outcomes at Visit 1 might have been measured too early and therefore not a real representation of a plateau in performance or habituation. Performance could also have improved due to increased experience with the test procedures (i.e. procedural learning).
Third, hearing aid device users are motivated to perform well with new technology: they do better in speech tests when they believe they are trialling new hearing aids (Dawes et al. Citation2011; Citation2013). This supports the idea that speech identification tests are a behavioural measure sensitive to motivation as the Framework for Understand of Effortful Listening underlines (Pichora-Fuller et al. Citation2016). As the participants in this study were not blinded to the sound processor with which they completed the speech identification task, they might have been motivated to perform better with the latest sound processor.
Comparisons with other studies
Another recent multicentric evaluation, this time of the latest Cochlear Nucleus CI532 cochlear implant system, recruited patients from several countries and their French patients were tested after 6 months of CI experience with the same Lafon monosyllabic words as in the present study and with the same scoring at the phoneme level (Hey et al. Citation2019). It should be noted that the words were presented at 65 dB SPL in the present study, while they were presented at 60 dB SPL in the Hey study. While the samples and the test conditions were slightly different, and therefore, direct comparisons should not be attempted, the 40 patients of the Hey et al study scored on average 55–60% in quiet, which is comparable to the results of the present study. Earlier studies have scored Lafon words at the word level therefore leading to lower scores that are not directly comparable to the present study (e.g. Esquia Medina et al. Citation2013; Mosnier et al. Citation2017).
Importance of number of active electrodes and deafness duration
When investigating factors related to speech identification in noise in the sample of 44 adults, number of active electrodes was positively correlated and deafness duration was negatively correlated with the outcome. This follows the results of a retrospective study of over 2000 patients showing the importance of these factors towards outcomes (Blamey et al. Citation2013; Lazard et al. Citation2012). Although the maximal number of channels that cochlear implant users can derive benefits from is open to debate, some recent work suggests that this could be at least 20 active electrodes (Croghan, Duran and Smith Citation2017).
Self-reported listening ability, satisfaction, and usability
Adults and children reported greater listening ability for two of the three factors identified in the factor analysis, that is, complex listening situations as well as music and comfort.
Furthermore, adults and children reported higher satisfaction and usability with several aspects of the Neuro 2, compared to the Neuro One: size and appearance, wearing comfort, clarity of instructions, battery lifetime, options (rechargeable and single-use), and changing ease, antenna retention, and robustness of the sound processor and accessories. This shows how both adult users and parents of paediatric users highly value the appearance, wearing comfort, and robustness of cochlear implant sound processors (Chundu and Stephens Citation2013; Clamp et al. Citation2013; Geyer et al. Citation2006).
Strengths and limitations
This study benefited from a multicentric design where participants were recruited from nine cochlear implant centres. In total, 44 adults and 26 children participated in three study visits spread over 3.5 months.
However, the clinical effectiveness approach means that a heterogenous sample of participants was recruited. Standard clinical practice was followed and maps were adjusted during the study, and therefore participants did not complete the speech identification tasks with the same signal processing settings. Furthermore, some participants received fine-tuning during the study whilst others did not, based on their expressed needs. Also, it could have been interesting to counterbalance treatment order (Neuro One versus Neuro 2) or to repeat another round of measures with Neuro One to isolate the effects of signal processing versus habituation; however, this was beyond the scope of this observational upgrade study. Furthermore, it would have been interesting to test patients not only in a cochlear implant only configuration, but also in a best-aided configuration, i.e., with a contralateral hearing aid for the 28 patients (40% of the sample) who wore one. This could have led to a different pattern of results. Nonetheless, this study summarises realistic outcomes in a mixed caseload of cochlear implant users with a recent sound processor.
It would also have been relevant to use a validated measure of listening ability, such as the 60-item Nijmegen Cochlear Implantation Questionnaire (NCIQ; Hinderink, Krabbe and Van Den Broek Citation2000), which measures quality of life, or more specifically physical, psychological and social functioning. This would have allowed comparisons between results of the present study with those of other studies. However, it would have resulted in participants having to complete a total of almost 100 items, which is why we created a shorter 16-item custom questionnaire to measure listening ability. Interestingly, two of the factors that emerged from our factor analysis, Simple listening situations and Complex listening situations, are equivalent to two of six factors of the NCIQ. In our sample, music and comfort loaded into a separate factor, while it is part of the advanced sound perception factor in the NCIQ. The new Cochlear Implant Quality of Life instruments (CIQOL; McRackan et al., Cochlear Implant Quality of Life Development Consortium Citation2019), which were carefully developed, show strong psychometric properties, and are available in a version with 35 items and a version with 10 items, are very relevant measures for future similar studies.
Future research should also include adaptive tasks of sentence comprehension in noise such as the Vocale Rapide dans le Bruit (VRB), a French version of the QuickSpeech-In-Noise (SIN) Test (Leclercq, Renard and Vincent Citation2018). While the speech test reported here, the Lafon monosyllabic words, was performed at a fixed SNR in a speech-shaped noise, the VRB test is adaptive and therefore performed at different SNRs in a four-talker babble noise, making its results particularly informative of functioning in different listening situations.
Conclusion
The latest sound processor of Oticon Medical, Neuro 2, enables speech identification in quiet and in noise for adults and leads to high levels of patient satisfaction both for adults and children.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Supplemental Material
Download MS Word (31.9 KB)Acknowledgements
The authors thank all participating cochlear implant centres, patients, and families.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Bergeron, F., and M. Hotton. 2016. “Perception in Noise with the Digisonic SP Cochlear Implant: Clinical Trial of Saphyr Processor's Upgraded Signal Processing.” European Annals of Otorhinolaryngology, Head and Neck Diseases 16: S1879–S7296. doi:10.1016/j.anorl.2016.04.019.
- Blamey, P., F. Artieres, D. Baskent, F. Bergeron, A. Beynon, E. Burke, N. Dillier, et al. 2013. “Factors Affecting Auditory Performance of Postlinguistically Deaf Adults Using Cochlear Implants: An Update with 2251 Patients.” Audiology and Neurotology 18 (1): 36–47. doi:10.1159/000343189.
- Bozorg-Grayeli A., N. Guevara, J-P. Bebear, M. Ardoint, S. Saaï, M. Hoen, D. Gnansia, P. Romanet, and J-P. Lavieille. 2016. “Clinical Evaluation of the xDP Output Compression Strategy for Cochlear Implants.” European Archives of Oto-Rhino-Laryngology 273 (9): 2363–2371. doi:10.1007/s00405-015-3796-1.
- Chundu, S., and N. Stephens. 2013. “Patients' Involvement in Choosing a Cochlear Implant.” Cochlear Implants International 14 (3): 165–168. doi:10.1179/1754762812Y.0000000010.
- Clamp, P.J., T. Rotchell, J. Maddocks, and P.J. Robinson. 2013. “What Factors Influence Patient and Parent Choice of Cochlear Implant Model for Children?” Cochlear Implants International 14 (3): 130–134. doi:10.1179/1754762812Y.0000000007.
- Cogan, SF. 2008. “Neural Stimulation and Recording Electrodes.” Annual Review of Biomedical Engineering 10 (1): 275–309. doi:10.1146/annurev.bioeng.10.061807.160518.
- Cohen, J. 1988. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Erlbaum.
- Croghan, NB., SI. Duran, and ZM. Smith. 2017. “Re-Examining the Relationship between Number of Cochlear Implant Channels and Maximal Speech Intelligibility.” The Journal of the Acoustical Society of America 142 (6): EL537–EL543. doi:10.1121/1.5016044.
- Dawes, P., R. Hopkins, and K. J. Munro. 2013. “Placebo Effects in Hearing-Aid Trials Are Reliable.” International Journal of Audiology 52 (7): 472–477. doi:10.3109/14992027.2013.783718.
- Dawes, P., S. Powell, and K. J. Munro. 2011. “The Placebo Effect and the Influence of Participant Expectation on Hearing Aid Trials.” Ear and Hearing 32 (6): 767–774. doi:10.1097/AUD.0b013e3182251a0e
- Di Lella, F., A. Bacciu, E. Pasanisi, V. Vincenti, M. Guida, and S. Bacciu. 2010. “Main Peak Interleaved Sampling (MPIS) Strategy: Effect of Stimulation Rate Variations on Speech Perception in Adult Cochlear Implant Recipients Using the Digisonic SP Cochlear Implant.” Acta Oto-Laryngologica 130 (1): 102–107. doi:10.3109/00016480902896113.
- Esquia Medina, G.N., S. Borel, Y. Nguyen, E. Ambert-Dahan, E. Ferrary, O. Sterkers, and A.B. Grayeli. 2013. “Is Electrode-Modiolus Distance a Prognostic Factor for Hearing Performances after Cochlear Implant Surgery?.” Audiology and Neurotology 18 (6): 406–413. doi:10.1159/000354115.
- Geyer, M., F.K. Seymour, L. Stott, C. Lynch, M. Beukes, W. Aleksy, and J.M. Graham. 2006. “How we Do It: Patient Participation in Cochlear Implant Selection.” Clinical Otolaryngology: Official Journal of Ent-UK; Official Journal of Netherlands Society for Oto-Rhino-Laryngology & Cervico-Facial Surgery 31 (1): 56–61. doi:10.1111/j.1749-4486.2006.01127.x
- Guevara, N., A. Bozorg-Grayeli, J-P. Bebear, M. Ardoint, S. Saaï, D. Gnansia, M. Hoen, P. Romanet, and J-P. Lavieille. 2016. “The Voice Track Multiband Single-Channel Modified Wiener-Filter Noise Reduction System for Cochlear Implants: Patients' Outcomes and Subjective Appraisal.” International Journal of Audiology 55 (8): 431–438. doi:10.3109/14992027.2016.1172267.
- Guevara, N., O. Sterkers, J.P. Bébéar, R. Meller, J. Magnan, I. Mosnier, I. Amstutz, et al. 2010. “Multicenter Evaluation of the Digisonic SP Cochlear Implant Fixation System with Titanium Screws in 156 Patients. Annals of Otology.” Annals of Otology, Rhinology & Laryngology 119: 501–505. doi:10.1177/000348941011900801.
- Hey, M., T. Wesarg, A. Mewes, S. Helbig, J. Hornung, T. Lenarz, R. Briggs, et al. 2019. “Objective, Audiological and Quality of Life Measures with the CI532 Slim Modiolar Electrode.” Cochlear Implants International 20 (2): 80–90. doi:10.1080/14670100.2018.1544684.
- Hinderink, J. B., P.F. Krabbe, and P. Van Den Broek. 2000. “Development and Application of a Health-Related Quality-of-Life Instrument for Adults with Cochlear Implants: The Nijmegen Cochlear Implant Questionnaire.” Otolaryngology–Head and Neck Surgery 123 (6): 756–765. doi:10.1067/mhn.2000.108203.
- Lafon, J. C. 1972. “Phonetic Test, Phonation, Audition.” Journal Français D'oto-Rhino-Laryngologie, Audiophonologie et Chirurgie Maxillo-Faciale 21: 223–229.
- Lazard, D. S., C. Vincent, F. Venail, P. Van de Heyning, E. Truy, O. Sterkers, P. H. Skarzynski, et al. 2012. “Pre-, per-and Postoperative Factors Affecting Performance of Postlinguistically Deaf Adults Using Cochlear Implants: A New Conceptual Model over Time.” PLoS One 7 (11): e48739. doi:10.1371/journal.pone.0048739.
- Lazard, D.S., P. Bordure, G. Lina-Granade, J. Magnan, R. Meller, B. Meyer, E. Radafy, et al. 2010. “Speech Perception Performance for 100 Post-Lingually Deaf Adults Fitted with Neurelec Cochlear Implants: Comparison between Digisonic Convex and Digisonic SP Devices after a 1-Year Follow-up.” Acta Oto-Laryngologica 130 (11): 1267–1273. doi:10.3109/00016481003769972.
- Leclercq, F., C. Renard, and C. Vincent. 2018. “Speech Audiometry in Noise: Development of the French-Language VRB (Vocale Rapide Dans le Bruit) Test.” European Annals of Oto-Rhino-Laryngology, Head and Neck Diseases 135 (5): 315–319. doi:10.1016/j.anorl.2018.07.002.
- Lorenzi, C., J. Sibellas, C. Füllgrabe, S. Gallégo, C. Fugain, and B. Meyer. 2004. “Effects of Amplitude Compression on First-and Second-Order Modulation Detection Thresholds in Cochlear Implant Listeners.” International Journal of Audiology 43 (5): 264–270. doi:10.1080/14992020400050035.
- Mauger, S. J., C. D. Warren, M. R. Knight, M. Goorevich, and E. Nel. 2014. “Clinical Evaluation of the Nucleus® 6 Cochlear Implant System: Performance Improvements with SmartSound iQ.” International Journal of Audiology 53 (8): 564–576. doi:10.3109/14992027.2014.895431.
- McRackan, T. R., B. N. Hand, C. A. Velozo, and J. R. Dubno, and Cochlear Implant Quality of Life Development Consortium 2019. “Development of the Cochlear Implant Quality of Life Item Bank.” Ear and Hearing 40 (4): 1016–1024. doi:10.1097/AUD.0000000000000684.
- Mosnier, I., M. Marx, F. Venail, N. Loundon, S. Roux-Vaillard, and O. Sterkers. 2014. “Benefits from Upgrade to the CP810™ Sound Processor for Nucleus® 24 Cochlear Implant Recipients.” European Archives of Oto-Rhino-Laryngology 271 (1): 49–57. doi:10.1007/s00405-013-2381-8.
- Mosnier, I., N. Mathias, J. Flament, D. Amar, A. Liagre-Callies, S. Borel, E. Ambert-Dahan, O. Sterkers, and D. Bernardeschi. 2017. “Benefit of the UltraZoom Beamforming Technology in Noise in Cochlear Implant Users.” European Archives of Oto-Rhino-Laryngology 274 (9): 3335–3342. doi:10.1007/s00405-017-4651-3.
- Pichora-Fuller, M. K., S. E. Kramer, M. A. Eckert, B. Edwards, B. W.Y. Hornsby, L. E. Humes, U. Lemke, et al. 2016. “Hearing Impairment and Cognitive Energy: The Framework for Understanding Effortful Listening (FUEL).” Ear and Hearing 37: 5S–27S. doi:10.1097/AUD.0000000000000312.
- Plasmans, A., E. Rushbrooke, M. Moran, C. Spence, L. Theuwis, A. Zarowski, E. Offeciers, et al. 2016. “A Multicentre Clinical Evaluation of Paediatric Cochlear Implant Users Upgrading to the Nucleus® 6 System.” International Journal of Pediatric Otorhinolaryngology 83: 193–199. doi:10.1016/j.ijporl.2016.02.004.
- Segovia-Martínez, M.,. D. Gnansia, and M. Hoen. 2016. “Coordinated Adaptive Processing in the Neuro Cochlear Implant System. Oticon Medical White Paper.” Available at https://protect-us.mimecast.com/s/a-LxCkRwomfn6nxjqTQ8Fzq?domain=oticonmedical.com