Abstract
Objective: To investigate the variance in reported prevalence rates of permanent neonatal hearing impairment (HI) worldwide.
Design: A systematic review and meta-analysis was performed on reported prevalence rates of sensorineural and permanent conductive or mixed HI worse than 40 dB in neonates, detected as a result of a screening programme or audiometric study.
Study sample: For meta-analysis, 35 articles were selected, 25 from high-income countries and 10 from middle-income countries according to the world bank classification system.
Results: The prevalence rate of permanent uni- and bilateral HI worse than 40 dB in neonates varied from 1 to 6 per 1000, the overall prevalence was 2.21 per 1000 [1.71, 2.8]. In NICU populations the prevalence rate was higher with a larger fraction of bilateral cases. Although not significant, prevalence rates were slightly higher in Asia compared to Europe and the number of infants lost to follow-up appeared higher in countries with lower gross national income.
Conclusion: Substantial variations exist in prevalence rates of neonatal permanent HI across countries and regions. There is a strong need for more data from low-income countries to identify demographic factors that account for this variability in reported prevalence rates. Reporting these data in a uniform way is advocated.
Introduction
Hearing impairment (HI) is a rather common sensory deficit in children in their first years of life, but it is not easily noticed. Deficiency in auditory input at a young age may cause a delay in speech, language and general development and poor academic performance (Yoshinaga-Itano and Apuzzo Citation1998a, Citation1998b). Early detection of congenital HI and appropriate intervention is crucial to minimise its impact (Joint Committee on Infant Hearing, American Academy of Pediatrics Citation2007; Downs and Yoshinaga-Itano Citation1999).
Prevalence rates of HI at various ages and from all over the world are indispensable to better understand the pathophysiology of HI and to organise health care provisions such as detection, intervention and prevention.
Permanent hearing impairment (PHI) in childhood is caused by both genetic factors and environmental factors. These factors are likely to vary across the world (Grundfast Citation2002; Smith and Taggart Citation2004). Neonatal PHI of genetic origin is in many cases autosomal recessive (Grundfast Citation2002; Smith and Taggart Citation2004; Denoyelle et al. Citation1997; Zelante et al. Citation1997). PHI can also be acquired in utero or during early childhood as a complication of infectious disease, a consequence of ototoxic medication, or in connection with premature birth. This explains the higher prevalence of PHI in infants treated at the neonatal intensive care unit (NICU) (Grundfast Citation2002). Variations exist in the worldwide availability and quality of health care provisions such as birth clinics, NICU’s, medication and vaccination programmes. The aforementioned factors all influence the prevalence of neonatal PHI to some extent an may cause variations across the world.
The World Health Organization (WHO) (Citation2018) reported that prevalence rates of HI are higher in low-income countries (Mathers, Smith, and Concha Citation2003; Stevens et al. Citation2013). However, these concerns both conductive and sensorineural HI in children at school age, when otitis media plays an important role (Mathers, Smith, and Concha Citation2003; Stevens et al. Citation2013; Smith Citation2008). The Global Research on Developmental Disabilities Collaborators (Citation2018) estimated the number of children with HI to be the highest in South Asia and the lowest in North America. The highest number of children with or at risk for developmental disabilities were found in low- and middle-income countries such as India, China and Nigeria.
During the past 20 years universal neonatal hearing screening (NHS) programmes, targeted at detecting PHI, have been introduced in many high- and middle-income countries (Joint Committee on Infant Hearing, American Academy of Pediatrics Citation2007). In some countries, NHS has been implemented at a local level or for a limited period of time. As a result, prevalence rates of PHI in neonates from a variety of countries are increasingly becoming available. This new knowledge may help to find explanations for variations in prevalence rates.
Most NHS programmes aim to detect PHI >40 dB HL, and use a two- or three-step screening protocol (Sloot et al. Citation2015; Vos et al. Citation2016; Kanji, Khoza-Shangase, and Moroe Citation2018). Two types of objective screening tests are most commonly used: otoacoustic emission tests (OAE) and automated auditory brainstem response tests (aABR). An OAE measures the reaction (emissions) of the outer hair cells in the cochlea to an auditory stimulus, presented in the infant’s ear canal. An aABR measures the electrical response of the auditory brainstem to an auditory stimulus set at 35 or 40 dB nHL. Both tests detect HI worse than 40 dB HL, and have high sensitivity and specificity (Wolff et al. Citation2010). In contrast to OAE, aABR screening is able to also detect cases of auditory neuropathy. An infant who fails the first step of the screening programme is invited to return for the next step of screening. The specificity of a screening programme increases with the number of steps used.
Infants are referred for full diagnostic assessment when they have failed the final step of the screening programme. Auditory brainstem response (ABR) and auditory steady-state responses (ASSR), which measure the electrical response in the central auditory pathway, are used to objectively estimate the hearing threshold. Quite often more extensive examinations are needed to obtain a final diagnostic result that includes type of HI and the threshold.
The aim of this study is to investigate the prevalence of PHI in neonates by means of a systematic literature review and analyse the results using a Bayesian meta-analysis. In more detail, we aim to:
Summarise the literature that reports on the prevalence of neonatal PHI of 40 dB or worse;
Combine the findings in a meta-analysis to obtain an accurate estimation of the overall prevalence rate of neonatal PHI;
Analyse the degree of variation in reported prevalence rates of neonatal PHI and identify explanatory factors.
Materials and methods
Literature search
Current scientific literature was systematically searched to identify original peer-reviewed studies that explore the prevalence of permanent sensorineural or conductive HI in children. This review was carried out based on the guidelines of Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA P) 2015 statement. This includes the use of the PRISMA P checklist and following the methodological approaches recommended for systematic reviews based on the PRISMA statement (Moher et al. Citation2015; Shamseer et al. Citation2015).
The systematic literature search was carried out in November 2017, and updated in November 2018, within the following electronic databases: Embase, PubMed/Medline Ovid, Web of Science, Cochrane Central and Google Scholar. Search terms included: “prevalence”, “incidence”, “epidemiology”, “hearing disorder”, “hearing impairment”, “pediatric”, “preschool”, “newborn”, “infant”, “child”, which were used to develop a search string. These terms were combined in different ways using Boolean operators such as “AND” and “OR”. The complete search strings used have been attached in Appendix 1 (see Supplementary material). Although the search included infants up to 12 years of age, in this study we only consider articles that describe the prevalence of infants up to one year of age.
The articles obtained from the electronic databases were compiled using EndNote and duplicates were eliminated. All titles and abstracts were screened independently by three reviewers (A.B., H.H., A.M.). Studies were included in the literature review if they contained original data on prevalence rates of PHI among the general infant population or subgroups: infants admitted to the NICU and/or well babies (WB). Studies were excluded if they reported only on subgroups of infants (e.g. infants with syndromes), if the study population or threshold were poorly defined, if the diagnostic process was not described, if errors were made when calculating the prevalence rate, or if the full text was not available in English. Definitions of the terms used in this article are provided in .
Table 1. Definitions.
The remaining articles underwent a full-text review by two authors (A.B. and H.H.), and further selection was made based on a number of inclusion and exclusion criteria. Studies were included if they reported on sensorineural and permanent conductive or mixed HI, considered both bilateral and unilateral PHI or bilateral PHI only, and used hearing thresholds from 20 to 40 dB HL. PHI had to be diagnosed as a result of a screening programme or audiometric study and confirmed by means of ABR or ASSR. During the diagnostic process following a NHS programme, a number of tests may be needed to determine the definitive hearing thresholds. When studies reported on the variability of these results, the most definitive result obtained during the first year of life was used to calculate the prevalence rate. When more than one study reported on the same population, prevalence rates were used from the most recent study that adequately met all inclusion criteria.
Studies were excluded if they reported on transient HI only or when transient HI figures were not eliminated from the final reported prevalence rates. This selection also excluded all articles that reported prevalence on older children, the selection focussed only on the prevalence of PHI in infants. Disagreements about the interpretation of data were resolved by discussion, and when disagreements could not be resolved, A.G. acted as a referee.
Prevalence rates for a hearing threshold of 35 or 40 dB HL were used when this information was available. Some studies reported only on prevalence rates for thresholds of 20, 25 or 30 dB HL. All reported prevalence rates resulted from a study using OAE, aABR or ABR.
Selected studies
A data extraction sheet was developed to record specific information from the article (title, author, year), year(s) when the data were collected, information about the population (geographic, ethnic, socio-economic), general neonatal population or NICU/WB subgroups, population size, definition of PHI, screening and diagnostic tests, degree of PHI, unilateral or bilateral PHI, age at time of diagnosis, percentage lost to follow-up (LTFU), and prevalence rate of PHI.
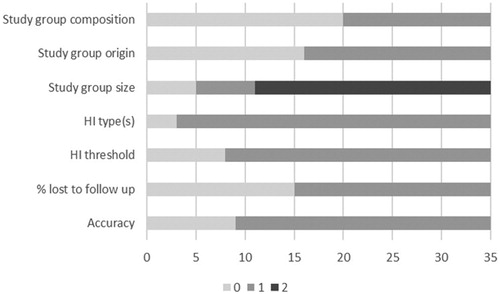
The Newcastle Ottawa Scale (Wells et al. Citation2018) is a quality assessment tool for cohort studies used to score the quality of studies included in reviews. Since not all subscales of the Newcastle Ottawa Scale were applicable to our set of data, we adapted the criteria to fit our study. This resulted in a list of seven items (). Criteria were scored on a two-point or three-point scale. For study group composition, a representative group was one in which no more than 10% of infants were admitted to the NICU (Harrison and Goodman Citation2015; Gijsen and Harbers Citation2015). The criterion “percentage of infants LTFU” was based on the recommendations of the JCIH to achieve a return-to-follow-up of 70% of infants or more (Joint Committee on Infant Hearing, American Academy of Pediatrics Citation2007; Joint Committee on Infant Hearing Citation2000; Prieve and Stevens Citation2000). Inadequate accuracy concerned ambiguity such as calculation uncertainties. The final quality score was calculated to a maximum of eight points and converted to a percentage. The cut-off value was set at 75% to distinguish between studies of low and high quality.
Table 2. List of criteria to indicate quality of the studies included for the meta-analysis.
The countries of the study populations were classified into five geographical areas: Europe, Asia, the Americas, Africa and Australia. Websites of The World Bank Group (Citation2018) and National Statistics Republic of China (Taiwan) for Taiwan (Citation2018) were consulted to classify each country as high, upper-middle, lower-middle or low-income country, and to record its Gross National Income (GNI) per capita in 2017.
Statistical analysis
A Bayesian random-effects meta-analysis was used to estimate the prevalence rate of both unilateral and bilateral HI in different populations. Prevalence rates were estimated for the general neonatal population and separately for WB and infants admitted to NICU for the studies in which these numbers were reported on.
The central idea of the Bayesian approach is to combine the likelihood (data) with our prior knowledge to result in a revised probability (posterior probability). Posterior distributions are typically summarised by the median and the 95% credible intervals, which are the counterparts to 95% confidence intervals used in classical statistics.
The Bayesian approach is becoming more popular because it is intuitive and flexible due to recent advances in computational methods. In a meta-analysis framework, a Bayesian approach offers an advantage compared to the classical approach, especially when taking into account the limitations in misestimating the heterogeneity in a random-effects model (Sutton et al. Citation2000; Sutton and Abrams Citation2001). Statistical expertise has strongly recommended carrying out Bayesian meta-analyses (Higgins and Green Citation2011). In our model, random-effects were employed to take into account the heterogeneity between the studies. The SD of the random effects (tau) will be reported as the heterogeneity index.
In this study, we employed relatively non-informative priors for all parameters. We performed different relatively non-informative priors on random-effects’ variance (i.e. an inverted gamma-distribution with low values and a uniform prior to the random-effects SD) as a sensitivity analysis to check the robustness of our meta-analysis results.
We performed two other sensitivity analyses. These aimed to assess the impact of variations of information that were implemented into the analytical model. One sensitivity analysis was performed using only high-quality studies (defined as ≥75% on the quality score), and the other one was performed using only studies where NHS programmes targeted a strict threshold of 35 dB HL or worse.
A Bayesian random-effects meta-regression was performed to assess the relationship between the overall prevalence rate of HI and the GNI per capita, infants LTFU, and geographical area. The calculated exceedance probability describes the posterior probability that the estimated parameter is greater or smaller than zero. The exceedance probability is a counterpart to the p value that is used in classical statistics. Extreme values for the exceedance probability (i.e. <0.05) indicate a significant difference.
Computations and graphics were performed in R programme language (Dalgaard Citation2010). All Bayesian computations were performed using the Markov Chain Monte Carlo sampler through Jags (Plummer Citation2016) interface in R programme language. Markov Chain Monte Carlo sampling was run for each analysis for 50k iterations after discarding the first 50k iterations (burn-in) to reach the convergence. The 95% credible intervals use 95% of the highest posterior probability in the posterior distribution.
The Egger test was used to check the publication bias via the metafor package (Viechtbauer Citation2010) in R programme language.
Results
Our search strategy identified 7520 articles (). After duplicates were eliminated, 4497 articles remained. Titles and abstracts were screened, and a further 4117 were eliminated because the inclusion criteria were not met or full text was not available in English. Of the remaining 380 articles on childhood PHI, 243 focussed on infants in their first year of life. A total of 209 of these 243 articles were excluded because they did not meet the inclusion criteria. Although some of these excluded studies reported on the prevalence of neonatal PHI, they were not used in our review for a variety of reasons: the composition of the study group was not suitable, the screening procedure, tests, or number of ears tested were not described, the description of results of post-screen diagnostics were insufficient or absent, PHI was poorly or not defined, PHI thresholds were not mentioned, the study design was unclear, or obvious calculation errors were found. The update of this search in November 2018 identified 278 additional articles. One was included in the final analysis of 35 articles ().
Figure 1. Flowchart showing the process followed to identify, screen, determine eligibility and select articles for inclusion in this review. The number of articles for each step is displayed as (the original search + the search update).
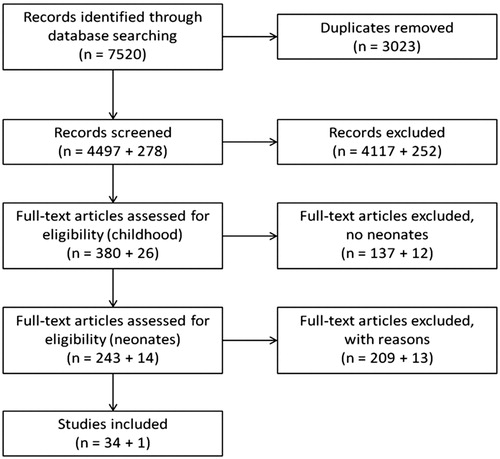
Table 3. Overview of the 35 studies in this review.
Most of the 35 studies included in the analysis were conducted in Europe, Asia, North America and Australia in the past 20 years. Only a few were conducted in Africa and South America. Twenty-five studies originate from high-income countries, eight from upper-middle-income countries and two from lower-middle-income countries. Thirteen studies had a quality score >75%. In particular, lower scores were achieved on the criteria composition of study group, local or national study group, and numbers LTFU ().
Prevalence rates were often readily presented in the article or were calculated from the supplied data. Only a few studies provided multiple thresholds obtained throughout the diagnostic process. Most studies reported prevalence rates with thresholds of 35 or 40 dB HL. However, for some studies only thresholds of 20, 25 or 30 dB HL were available. Notwithstanding this inconsistency, all studies screened for a target condition of 35 or 40 dB HL using OAE or aABR in a two-, three- or four-step screening programme. These techniques identify HI of 35–40 dB HL or worse, with a high specificity. How many cases of mild HI (20–40 dB HL) would actually be included in the prevalence rate estimations with a threshold of 20 dB HL depends on the specificity of the applied screening programme. Therefore, the impact of varying thresholds was evaluated in a sensitivity analysis.
displays the prevalence rates of PHI (bilateral and unilateral) and PHI bilateral only for the general population of infants, the WB subgroup and the NICU infant subgroup as they are reported in each article.
Table 4. Geographical areas from selected articles, reported prevalence rates of PHI (bi- and unilateral) and PHI bi (bilateral only), for general neonatal, WB and NICU populations, including HI threshold, quality score and LTFU.
The Bayesian random-effects meta-analysis of the prevalence rates of PHI in the general neonatal population resulted in an overall prevalence rate of 2.21 per 1000 with a credible interval of [1.71, 2.8] (Tau: 0.51 95% CI (0.34, 0.73)) (). The corresponding forest plot is depicted in , in which studies are ranked according to GNI per capita in the country of origin. The highest prevalence rates were found in Nigeria (Olusanya and Somefun Citation2009), India (Augustine et al. Citation2014), China (Hong-Kong) (Ng et al. Citation2004) and Iran (Farhat et al. Citation2015). The lowest prevalence rates were found in Ireland (Adelola et al. Citation2010), South Africa (Swanepoel et al. Citation2007), Brazil (Bevilacqua et al. Citation2010) and China (Shanghai) (Sun et al. Citation2009). The prevalence rate for bilateral PHI only (unilateral PHI excluded) was 1.33 per 1000 [1.01, 1.63], which is about 2/3 of the overall prevalence rate. The prevalence rate for unilateral PHI only (bilateral PHI excluded) was 0.78 per 1000 [0.51, 1.07] ().
Figure 3. Bayesian random-effects model, PHI prevalence rates (per 1000) of the general neonatal population sorted by GNI per capita. (Tau: 0.51 95% CI [0.34, 0.73]). (Event: number of infants with PHI; *Weighted prevalence for each study; **GNI: gross national income). Please note that the prevalence rates in this figure are based on the fitted random-effects model and thus differ from the prevalence rates in . This discrepancy is due to the so-called shrinkage estimates that occur in random-effects models.
![Figure 3. Bayesian random-effects model, PHI prevalence rates (per 1000) of the general neonatal population sorted by GNI per capita. (Tau: 0.51 95% CI [0.34, 0.73]). (Event: number of infants with PHI; *Weighted prevalence for each study; **GNI: gross national income). Please note that the prevalence rates in this figure are based on the fitted random-effects model and thus differ from the prevalence rates in Table 4. This discrepancy is due to the so-called shrinkage estimates that occur in random-effects models.](/cms/asset/45afccfc-9985-46dc-bc86-4437c2a9189d/iija_a_1716087_f0003_b.jpg)
Table 5. Overall prevalence rates in a Bayesian random-effects model.
Additional meta-analyses were performed for the studies reporting on NICU and WB populations. summarises the most relevant results. The prevalence rate in the WB population (1.93 [1.06, 2.98]) was lower than the prevalence rate for the general neonatal population (2.21 [1.71, 2.8]). As expected, a substantially higher prevalence rate was found for NICU infants (15.77 [4.65, 29.25]) when compared to both the general and WB population ().
The relationship between prevalence rates of unilateral PHI and all PHI (uni- and bilateral) was calculated for NICU infants and infants in the general neonatal population. Among the general infant population, 34% [25, 42%] of infants with PHI have a unilateral loss. Among NICU infants, 22% [12, 30%] of infants with PHI have a unilateral loss.
The first sensitivity analysis showed that, when only studies with a high-quality score (>75%) were included, the overall prevalence rate was 1.75 per 1000 [1.16, 2.37]. The second sensitivity analysis concerned the threshold of PHI. When a stricter criterion was applied for the threshold (35 dB HL), the overall prevalence rate was 2.14 per 1000 [1.47, 2.88]. Both sensitivity analyses found overall prevalence rates that did not differ significantly from the overall prevalence rate in the general population. We included all selected studies regardless of quality score and choice of threshold in the meta-analyses.
Analysis of variations in prevalence rates
For the analysis between geographical areas and prevalence rates of PHI, geographical areas were categorised into five continents. Despite the appearance of prevalence rates varying by geographical area, no statistically significant differences were found (Figure 4, See Supplementary Appendix 2 material) Prevalence rates seemed to be higher in Asia compared to Europe, but the difference was not statistically significant (p = 0.06). The relationship between PHI prevalence rate and GNI per capita was not statistically significant (p = 0.29) according to the Bayesian logistic regression analysis. No significant trend was observed between prevalence rate and the year in which the data had been collected (p = 0.12). Additionally, no significant association was observed between prevalence rate and the percentage of infants LTFU during the screening and diagnostic process (p = 0.16). Larger percentages LTFU were generally associated with lower GNI per capita, but this association did not reach statistical significance (p = 0.07).
Discussion
In this systematic review and meta-analysis, the prevalence of PHI in neonatal populations worldwide was investigated, as well as possible associations between prevalence and its determinants. The overall prevalence rate of PHI of 40 dB HL or worse in one or both ears, was 2.21 per 1000. As expected, the prevalence rate of PHI in NICU infants was much higher when compared to both WB and all infants, confirming earlier reports (Garinis et al. Citation2018; Wang et al. Citation2017).
Interestingly, a PHI is more likely to be bilateral in NICU infants compared to the general infant population. PHI in NICU infants is more likely to be acquired as the consequence of, among others, infections, hyperbilirubinemia, hypoxia or ototoxic medication. These risk factors for PHI would seem to have an effect on both ears, while other causes may affect one or both ears (Garinis et al. Citation2018; Wang et al. Citation2017; Hille et al. Citation2007).
The highest prevalence rates were reported by studies performed in Nigeria (Olusanya and Somefun Citation2009), India (Augustine et al. Citation2014), China (Hong-Kong) (Ng et al. Citation2004) and Iran (Farhat et al. Citation2015), but they concerned rather small populations. The lowest prevalence rates were found in Ireland (Adelola et al. Citation2010), South Africa (Swanepoel et al. Citation2007), Brazil (Bevilacqua et al. Citation2010) and China (Shanghai) (Sun et al. Citation2009).
Prevalence rates were higher in studies from Asia than Europe, however, this difference was not statistically significant. Countries with lower GNI per capita appeared to have higher but widely distributed, prevalence rates. A statistical association between prevalence and GNI was not found. Higher prevalence rates in developing countries and higher prevalence rates in Asia compared to Europe, were found by Stevens and Mathers in their WHO studies (Mathers, Smith, and Concha Citation2003; Stevens et al. Citation2013). These studies, however, concern older children, include conductive HI and apply various thresholds. The current study concerns neonates, PHI and strictly defined thresholds. The current study did not demonstrate a statistically significant association between the prevalence of PHI in the neonatal period and geographical region or low- and high-income countries, but such an association may still exist.
Unfortunately, both the current study and those by Stevens and Mathers contain a sparsity of data from low-income countries (Mathers, Smith, and Concha Citation2003; Stevens et al. Citation2013). Although this review includes studies from all over the world, the majority of the studies report on results of NHS programmes in high-income countries or large cities. Far less information is available from Africa and South America. The results of this review inevitably reflect a great extent the situation in high-income countries, which possibly causes an under- or overestimation of the overall prevalence rate. It is obvious that there is a strong need for more prevalence studies from low-income countries and regions.
The prevalence rate of PHI, as reported in a study, should be considered with caution. The reported prevalence rate in an approximation of the “real” prevalence rate and is not only determined by medical factors, but also by study factors. Medical factors determine the “real” prevalence, which is the factual (but unknown) number of infants affected in a population. A study uses methods (study factors) to reveal the “real” prevalence. Unfortunately, these methods may influence the outcome to an unknown extent.
Direct medical factors include genetic disorders, antenatal or postnatal infections, hyperbilirubinemia, hypoxia, medication (aminoglycosides, diuretics). Indirect medical factors include the availability and quality of health care provisions such as birth clinics, NICU and vaccination programmes, but also consanguinity and mortality rates. These factors are highly dependent on social, economic and cultural circumstances, and therefore, likely to vary considerably across countries. Congenital CMV is one of the most important non-genetic causes of PHI in neonates (Grosse, Ross, and Dollard Citation2008). It was found to be more prevalent in Nigeria, China, India and the Middle East (Zuhair et al. Citation2019). The incidence of infectious diseases and presence of vaccination programmes are likely to be associated with the economic situation of a country (Stevens et al. Citation2013), in contrast to global distribution of genes causing HI, and the prevalence of consanguineous marriages (Mathers, Smith, and Concha Citation2003; Stevens et al. Citation2013; Freeland, Jones, and Mohammed Citation2010). Consanguinity is especially common in North-Africa, the Middle East, South-West Asia and India (Black Citation2018). Neonatal PHI is assumed to be hereditary in more than 50% of cases, the majority being autosomal recessive (Grundfast Citation2002; Smith and Taggart Citation2004; Denoyelle et al. Citation1997; Zelante et al. Citation1997). As aetiological data on neonatal PHI mainly originate from developed countries, we cannot be certain whether this percentage also applies to the rest of the world. Study of the distribution of the numerous genes associated with HI across the world is complex, but more information is gradually becoming available (Chan and Chang Citation2014).
This systematic review targets “real” prevalence rates and how they depend on medical, economic and cultural circumstances. Nevertheless, the differences in study design and screening procedures between articles must be considered, as these factors influence the reported prevalence rates.
Study factors are the choice of the population studied, its size and composition, the definition of PHI, including the threshold, characteristics and quality of the audiometric testing and numbers of infants LTFU. The selected population determines to a large extent the reliability and relevance of a reported prevalence rate. The definition of PHI, the type and threshold used, has an effect on the reported prevalence rate. A poorly performed test will lead to more false “refers”, and thus more re-testing, which is likely to increase the number of infants LTFU. A high percentage LTFU will result in an underestimated prevalence rate because the denominator (the population) is usually defined as the number of neonates entering the screening programme or study group. The high percentage of infants LTFU may contribute to the reported low prevalence rate in the study by Sun in Shanghai (Sun et al. Citation2009). The number of infants LTFU may be especially high in developing countries, as the socio-economic circumstances too often impede the functioning and use of medical facilities. This implies that the “real” PHI prevalence rates in developing countries could be even higher than the reported ones. Although the assumption that LTFU may be associated with GNI, was not confirmed by the statistical analysis (p = 0.07), inclusion of more data from high-quality studies could possibly make a difference. The prevalence rate calculated for studies with a high-quality score was 1.75 per 1000, and the prevalence rate for all included studies was 2.21 per 1000. However, this result could have been biased by regional factors, as most high-quality studies were performed in Western Europe.
Proposal reporting prevalence
Several studies reported on prevalence rates but did not describe their results in a way that was useful for this study. There is clearly no uniform methodology for defining, calculating or reporting data on prevalence rates of HI. Therefore, it is proposed that future studies reporting on prevalence rates define the studied type(s) of HI in terms of sensorineural, permanent conductive, transient conductive and mixed HI, with a defined cut-off value (preferably 40 dB HL in neonates) for prevalence rate calculation; indicate if HI is bilateral, unilateral or both bi- and unilateral; use the most recent WHO (Citation2018) classification to report on the degree of HI; state the age at which the diagnosis is definitive, the audiometric tests used for screening and diagnostics, the study group size, composition, proportion NICU/WB, and number of infants LTFU.
Conclusion
Worldwide prevalence rates of HI in children of various age groups are becoming increasingly available, partially due to the ongoing implementation of universal NHS programmes. Much of the existing information concerns older children, and reported prevalence rates in neonates originate mainly from high-income countries. Our systematic review shows that approximately 2 per 1000 neonates worldwide are identified with neonatal PHI, of which approximately two-thirds are bilateral. Although no statistical significant correlations were found with demographic or study factors, prevalence rates in neonates tend to be higher in countries with lower incomes, and higher in Asia than in Europe. A marked lack of uniformity exists in studies, especially with regard to the definition of HI.
Author contributions
Andrea Bussé conceptualised and designed the study, was involved in the selection and analysis of the included articles, drafted the initial manuscript, reviewed and revised the manuscript. Hans Hoeve conceptualised and designed the study, was involved in the selection and analysis of the included articles, drafted the initial manuscript, reviewed and revised the manuscript. Kazem Nasserinejad performed the statistical analysis. Allison Mackey was involved in the selection and analysis of the included articles. André Goedegebure conceptualised and designed the study, was involved in the selection and analysis of the included articles, drafted the initial manuscript, reviewed and revised the manuscript. All authors critically revised the manuscript, approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
TIJA-2019-07-0273-File006.docx
Download MS Word (700.8 KB)TIJA-2019-07-0273-File005.docx
Download MS Word (13.5 KB)TIJA-2019-07-0273-File004.doc
Download MS Word (64 KB)Acknowledgments
We thank Wichor Bramer from the Medical Library of the Erasmus Medical Center for his help in designing and conducting the search strategy. This study sponsor had no role in the study design, or the collection, analysis and interpretation of the data or in the writing of the report or in the decision to submit the article for publication.
Disclosure statement
The authors have no conflicts of interest relevant to this article to disclose.
Additional information
Funding
References
- Adachi, N., K. Ito, H. Sakata, and T. Yamasoba. 2010. “Etiology and One-Year Follow-up Results of Hearing Loss Identified by Screening of Newborn Hearing in Japan.” Otolaryngology – Head and Neck Surgery 143 (1): 97–100. doi:10.1016/j.otohns.2010.02.008.
- Adelola, O. A., V. Papanikolaou, P. Gormley, J. Lang, and I. J. Keogh. 2010. “Newborn Hearing Screening: A Regional Example for National Care.” Irish Medical Journal 103 (5): 146–149.
- Antoni, M., I. Rouillon, F. Denoyelle, E. N. Garabédian, and N. Loundon. 2016. “Newborn Hearing Screening: Prevalence and Medical and Paramedical Treatment of Bilateral Hearing Loss in a Neonatal Series in the Île-de-France Region of France.” European Annals of Otorhinolaryngology, Head and Neck Diseases 133 (2): 95–99. doi:10.1016/j.anorl.2015.10.001.
- Augustine, A. M., A. K. Jana, K. A. Kuruvilla, S. Danda, A. Lepcha, J. Ebenezer, R. R. Paul, et al. 2014. “Neonatal Hearing Screening – Experience from a Tertiary Care Hospital in Southern India.” Indian Pediatrics 51 (3): 179–183. doi:10.1007/s13312-014-0380-5.
- Bailey, H. D., C. Bower, J. Krishnaswamy, and H. L. Coates. 2002. “Newborn Hearing Screening in Western Australia.” Medical Journal of Australia 177 (4): 180–185. doi:10.5694/j.1326-5377.2002.tb04728.x.
- Berninger, E., and B. Westling. 2011. “Outcome of a Universal Newborn Hearing-Screening Programme Based on Multiple Transient-Evoked Otoacoustic Emissions and Clinical Brainstem Response Audiometry.” Acta Oto-Laryngologica 131 (7): 728–739. doi:10.3109/00016489.2011.554440.
- Bevilacqua, M. C., K. D. F. Alvarenga, O. A. Costa, and A. L. M. Moret. 2010. “The Universal Newborn Hearing Screening in Brazil: From Identification to Intervention.” International Journal of Pediatric Otorhinolaryngology 74 (5): 510–515. doi:10.1016/j.ijporl.2010.02.009.
- Black, M. 2018. “Global prevalence of consanguinity.” Accessed 28 August 2017. http://consang.net/index.php/Global_prevalence.
- Calcutt, T. L., D. Dornan, R. Beswick, and D. I. Tudehope. 2016. “Newborn Hearing Screening in Queensland 2009–2011: Comparison of Hearing Screening and Diagnostic Audiological Assessment between Term and Preterm Infants.” Journal of Paediatrics and Child Health 52 (11): 995–1003. doi:10.1111/jpc.13281.
- Chan, D. K., and K. W. Chang. 2014. “GJB2-Associated Hearing Loss: Systematic Review of Worldwide Prevalence, Genotype, and Auditory Phenotype.” The Laryngoscope 124 (2): E34–E53. doi:10.1002/lary.24332.
- Dalgaard, P. 2010. R: A Language and Environment for Statistical Computing. Vienna: R Development Core Team, R Foundation for Statistical Computing.
- Dalzell, L., M. Orlando, M. MacDonald, A. Berg, M. Bradley, A. Cacace, D. Campbell, et al. 2000. “The New York State Universal Newborn Hearing Screening Demonstration Project: Ages of Hearing Loss Identification, Hearing Aid Fitting, and Enrollment in Early Intervention.” Ear and Hearing 21 (2): 118–130. doi:10.1097/00003446-200004000-00006.
- De Capua, B., D. Costantini, C. Martufi, G. Latini, M. Gentile, and C. De Felice. 2007. “Universal Neonatal Hearing Screening: The Siena (Italy) Experience on 19,700 Newborns.” Early Human Development 83 (9): 601–606. doi:10.1016/j.earlhumdev.2007.01.001.
- Denoyelle, F., D. Weil, M. A. Maw, S. A. Wilcox, N. J. Lench, D. R. Allen-Powell, A. H. Osborn, et al. 1997. “Prelingual Deafness: High Prevalence of a 30delG Mutation in the Connexin 26 Gene.” Human Molecular Genetics 6 (12): 2173–2177. doi:10.1093/hmg/6.12.2173.
- Downs, M. P., and C. Yoshinaga-Itano. 1999. “The Efficacy of Early Identification and Intervention for Children with Hearing Impairment.” Pediatric Clinics of North America 46 (1): 79–87. doi:10.1016/S0031-3955(05)70082-1.
- Farhat, A., M. M. Ghasemi, J. Akhondian, A. Mohammadzadeh, H. Esmaeili, R. Amiri, A. A. Raoof Saeb, et al. 2015. “Comparative Study of Hearing Impairment among Healthy and Intensive Care Unit Neonates in Mashhad.” Iranian Journal of Otorhinolaryngology 27 (81): 273–277. PMCID: PMC4710879.
- Freeland, A., J. Jones, and N. K. Mohammed. 2010. “Sensorineural Deafness in Tanzanian Children – Is Ototoxicity a Significant Cause? A Pilot Study.” International Journal of Pediatric Otorhinolaryngology 74 (5): 516–519. doi:10.1016/j.ijporl.2010.02.010.
- Galambos, R., M. J. Wilson, and P. D. Silva. 1994. “Identifying Hearing Loss in the Intensive Care Nursery: A 20-Year Summary.” Journal of the American Academy of Audiology 5 (3): 151–162. PMID: 8075411.
- Garinis, A. C., A. Kemph, A. M. Tharpe, J. H. Weitkamp, C. McEvoy, and P. S. Steyger. 2018. “Monitoring Neonates for Ototoxicity.” International Journal of Audiology 57 (sup4): S41–S48. doi:10.1080/14992027.2017.1339130.
- Gijsen, R., and M. Harbers. 2015. “Opnamen neonatale intensive care unit.” Accessed 20 November 2018. https://www.volksgezondheidenzorg.info/onderwerp/zorg-rond-de-geboorte/cijfers-context/gebruik#!node-opnamen-neonatale-intensive-care-unit.
- Global Research on Developmental Disabilities Collaborators. 2018. “Developmental Disabilities among Children Younger than 5 Years in 195 Countries and Territories, 1990–2016: A Systematic Analysis for the Global Burden of Disease Study 2016.” The Lancet. Global Health 6 (10): e1100–e1121. doi:10.1016/S2214-109X(18)30309-7.
- Grosse, S. D., D. S. Ross, and S. C. Dollard. 2008. “Congenital Cytomegalovirus (CMV) Infection as a Cause of Permanent Bilateral Hearing Loss: A Quantitative Assessment.” Journal of Clinical Virology 41 (2): 57–62. doi:10.1016/j.jcv.2007.09.004.
- Grundfast, K. M. 2002. “Chapter 16. Hearing Loss.” In Pediatric Otolaryngology, edited by C. D. Bluestone and S. E. Stool. Philadelphia, PA: W.B. Saunders.
- Harrison, W., and D. Goodman. 2015. “Epidemiologic Trends in Neonatal Intensive Care, 2007–2012.” JAMA Pediatrics 169 (9): 855–862. doi:10.1001/jamapediatrics.2015.1305.
- Higgins, J. P. T., and S. Green, eds. 2011. “Cochrane handbook for systematic reviews of interventions.” Accessed 14 November 2011. www.cochrane-handbook.org.
- Hille, E. T., H. I. van Straaten, P. H. Verkerk, and Dutch NICU Neonatal Hearing Screening Working Group. 2007. “Prevalence and Independent Risk Factors for Hearing Loss in NICU Infants.” Acta Paediatrica 96 (8): 1155–1158. doi:10.1111/j.1651-2227.2007.00398.x.
- Hsu, H. C., F. P. Lee, and H. M. Huang. 2013. “Results of a 1-Year Government-Funded Newborn Hearing Screening Program in Taiwan.” Laryngoscope 123 (5): 1275–1278. doi:10.1002/lary.23713.
- Iwasaki, S., Y. Hayashi, A. Seki, M. Nagura, Y. Hashimoto, G. Oshima, T. Hoshino, et al. 2003. “A Model of Two-Stage Newborn Hearing Screening with Automated Auditory Brainstem Response.” International Journal of Pediatric Otorhinolaryngology 67 (10): 1099–1104. doi:10.1016/S0165-5876(03)00199-X.
- Joint Committee on Infant Hearing. 2000. “Year 2000 Position Statement: Principles and Guidelines for Early Hearing Detection and Intervention Programs.” American Journal of Audiology 9 (1): 9–29. doi:10.1044/1059-0889(2000/005).
- Joint Committee on Infant Hearing, American Academy of Pediatrics. 2007. “Year 2007 Position Statement: Principles and Guidelines for Early Hearing Detection and Intervention Programs.” Pediatrics 120 (4): 898–921. doi:10.1542/peds.2007-2333.
- Kanji, A., K. Khoza-Shangase, and N. Moroe. 2018. “Newborn Hearing Screening Protocols and Their Outcomes: A Systematic Review.” International Journal of Pediatric Otorhinolaryngology 115: 104–109. doi:10.1016/j.ijporl.2018.09.026.
- Kennedy, C. R., L. Kimm, D. Cafarelli Dees, M. J. Campbell, A. R. D. Thornton, J. Bamber, V. Innes, et al. 1998. “Controlled Trial of Universal Neonatal Screening for Early Identification of Permanent Childhood Hearing Impairment.” The Lancet 352 (9145): 1957–1964. doi:10.1016/S0140-6736(98)06359-4.
- Magnani, C., G. Bacchi, A. M. Borghini, D. Delmonte, G. Fava, A. M. Occasio, A. Sarti, et al. 2015. “Universal Newborn Hearing Screening: The Experience of the University Hospital of Parma.” Acta BioMedica 86 (3): 273–277. PMID: 26694155.
- Mason, J. A., and K. R. Herrmann. 1998. “Universal Infant Hearing Screening by Automated Auditory Brainstem Response Measurement.” Pediatrics 101 (2): 221–228. doi:10.1542/peds.101.2.221.
- Mathers, C. D., A. Smith, and M. Concha. 2003. Global Burden of Hearing Loss in the Year 2000. Geneva: World Health Organization.
- Mehl, A. L., and V. Thomson. 2002. “The Colorado Newborn Hearing Screening Project, 1992–1999: On the Threshold of Effective Population-Based Universal Newborn Hearing Screening.” Pediatrics 109 (1): E7. doi:10.1542/peds.109.1.e7.
- Moher, D., L. Shamseer, M. Clarke, D. Ghersi, A. Liberati, M. Petticrew, P. Shekelle, et al. 2015. “Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015 Statement.” Systematic Reviews 4 (1): 1–9. doi:10.1186/2046-4053-4-1.
- National Statistics Republic of China (Taiwan). 2018. “Latest Indicators, per Capita GNI (US Dollars).” https://eng.stat.gov.tw/point.asp?index=1.
- Neumann, K., M. Gross, P. Böttcher, H. A. Euler, M. Spormann-Lagodzinski, and M. Polzer. 2006. “Effectiveness and Efficiency of a Universal Newborn Hearing Screening in Germany.” Folia Phoniatrica et Logopaedica 58 (6): 440–455. doi:10.1159/000095004.
- Ng, P. K., Y. Hui, B. C. C. Lam, W. H. S. Goh, and C. Y. Yeung. 2004. “Feasibility of Implementing a Universal Neonatal Hearing Screening Programme Using Distortion Product Otoacoustic Emission Detection at a University Hospital in Hong Kong.” Hong Kong Medical Journal = Xianggang yi Xue za Zhi 10 (1): 6–13. PMID: 14967849.
- Olusanya, B. O., and A. O. Somefun. 2009. “Place of Birth and Characteristics of Infants with Congenital and Early-Onset Hearing Loss in a Developing Country.” International Journal of Pediatric Otorhinolaryngology 73 (9): 1263–1269. doi:10.1016/j.ijporl.2009.05.018.
- Pastorino, G., P. Sergi, M. Mastrangelo, P. Ravazzani, G. Tognola, M. Parazzini, F. Mosca, et al. 2007. “The Milan Project: A Newborn Hearing Screening Programme.” Acta Paediatrica 94 (4): 458–463. doi:10.1111/j.1651-2227.2005.tb01918.x.
- Plummer, M. 2016. “rjags: Bayesian Graphical Models Using MCMC. R package version 4-6.” https://CRAN.R-project.org/package=rjags.
- Prieve, B. A., and F. Stevens. 2000. “The New York State Universal Newborn Hearing Screening Demonstration Project: Introduction and Overview.” Ear and Hearing 21 (2): 85–91. doi:10.1097/00003446-200004000-00003.
- Qi, B., X. Cheng, H. En, B. Liu, S. Peng, Y. Zhen, Z. Cai, et al. 2013. “Assessment of the Feasibility and Coverage of a Modified Universal Hearing Screening Protocol for Use with Newborn Babies of Migrant Workers in Beijing.” BMC Pediatrics 13 (1): 116. doi:10.1186/1471-2431-13-116.
- Shamseer, L., D. Moher, M. Clarke, D. Ghersi, A. Liberati, M. Petticrew, P. Shekelle, et al. 2015. “Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) Elaboration and Explanation.” BMJ 350: g7647. doi:10.1136/bmj.g7647.
- Sloot, F., H. L. Hoeve, M. L. de Kroon, A. Goedegebure, J. Carlton, H. J. Griffiths, H. J. Simonsz, et al. 2015. “Inventory of Current EU Paediatric Vision and Hearing Screening Programmes.” Journal of Medical Screening 22 (2): 55–64. doi:10.1177/0969141315572403.
- Smith, A. W. 2008. “Demographics of Hearing Loss in Developing Countries.” In Audiology in Developing Countries, edited by B. McPherson and R. Brouillette, 21–50. New York: Nova Science Publishers, Inc.
- Smith, S. D., and R. T. Taggart. 2004. “Chapter 5. Genetic Hearing Loss with No Associated Abnormalities.” In Hereditary Hearing Loss and Its Syndromes, edited by H. V. Toriello, W. Reardon, and R. J. Gorlin. New York: Oxford University Press.
- Stevens, G., S. Flaxman, E. Brunskill, M. Mascarenhas, C. D. Mathers, and M. Finucane. 2013. “Global and Regional Hearing Impairment Prevalence: An Analysis of 42 Studies in 29 Countries.” European Journal of Public Health 23 (1): 146–152. doi:10.1093/eurpub/ckr176.
- Sun, X., X. Shen, D. Zakus, J. Lv, Z. Xu, H. Wu, W. Hsiao, et al. 2009. “Development of an Effective Public Health Screening Program to Assess Hearing Disabilities among Newborns in Shanghai: A Prospective Cohort Study.” World Health and Population 11 (1): 14–23. PMID: 20039591.
- Sutton, A. J., and K. R. Abrams. 2001. “Bayesian Methods in Meta-Analysis and Evidence Synthesis.” Statistical Methods in Medical Research 10 (4): 277–303. doi:10.1177/096228020101000404.
- Sutton, A. J., S. J. Duval, R. L. Tweedie, K. R. Abrams, and D. R. Jones. 2000. “Empirical Assessment of Effect of Publication Bias on Meta-Analyses.” BMJ 320 (7249): 1574–1577. doi:10.1136/bmj.320.7249.1574.
- Swanepoel, D., S. Ebrahim, A. Joseph, and P. L. Friedland. 2007. “Newborn Hearing Screening in a South African Private Health Care Hospital.” International Journal of Pediatric Otorhinolaryngology 71 (6): 881–887. doi:10.1016/j.ijporl.2007.02.009.
- Tajik, S., and M. Ahmadpour-Kacho. 2016. “Early Diagnosis and Intervention for Hearing Loss in Newborns Discharged from Intensive Care Units: A Four-Year Follow-up Study in North of Iran.” International Journal of Pediatrics-Mashhad 4 (8): 3283–3291. doi:10.22038/IJP.2016.7154.
- Uus, K., and J. Bamford. 2006. “Effectiveness of Population-Based Newborn Hearing Screening in England: Ages of Interventions and Profile of Cases.” Pediatrics 117 (5): e887–e893. doi:10.1542/peds.2005-1064.
- Van Der Ploeg, C. P. B., N. N. Uilenburg, M. A. Kauffman-De Boer, A. M. Oudesluys-Murphy, and P. H. Verkerk. 2012. “Newborn Hearing Screening in Youth Health Care in The Netherlands: National Results of Implementation and Follow-up.” International Journal of Audiology 51 (8): 584–590. doi:10.3109/14992027.2012.684402.
- Van Dommelen, P., A. D. Mohangoo, P. H. Verkerk, C. P. Van Der Ploeg, H. L. Van Straaten, and The Dutch NICU Neonatal Hearing Screening Working Group. 2010. “Risk Indicators for Hearing Loss in Infants Treated in Different Neonatal Intensive Care Units.” Acta Paediatrica 99 (3): 344–349. doi:10.1111/j.1651-2227.2009.01614.x.
- Van Kerschaver, E., A. N. Boudewyns, F. Declau, P. H. Van de Heyning, and F. L. Wuyts. 2013. “Socio-Demographic Determinants of Hearing Impairment Studied in 103,835 Term Babies.” The European Journal of Public Health 23 (1): 55–60. doi:10.1093/eurpub/cks010.
- Van Straaten, H. L., E. T. Hille, J. H. Kok, P. H. Verkerk, and Dutch NICU Neonatal Hearing Screening Working Group. 2007. “Implementation of a Nation-Wide Automated Auditory Brainstem Response Hearing Screening Programme in Neonatal Intensive Care Units.” Acta Paediatrica 92 (3): 332–338. doi:10.1111/j.1651-2227.2003.tb00555.x.
- Viechtbauer, W. 2010. “Conducting Meta-Analyses in R with the metafor Package.” Journal of Statistical Software 36 (3): 1–48. doi:10.18637/jss.v036.i03.
- Vos, B., C. Senterre, R. Lagasse, G. Tognola, and A. Leveque. 2016. “Organisation of Newborn Hearing Screening Programmes in the European Union: Widely Implemented, Differently Performed.” The European Journal of Public Health 26 (3): 505–510. doi:10.1093/eurpub/ckw020.
- Wang, C.-H., C.-Y. Yang, R. Lien, S.-M. Chu, J.-F. Hsu, R.-H. Fu, M.-C. Chiang, et al. 2017. “Prevalence and Independent Risk Factors for Hearing Impairment among Very Low Birth Weight Infants.” International Journal of Pediatric Otorhinolaryngology 93: 123–127. doi:10.1016/j.ijporl.2016.12.029.
- Watkin, P. M., and M. Baldwin. 1999. “Confirmation of Deafness in Infancy.” Archives of Disease in Childhood 81 (5): 380–389. doi:10.1136/adc.81.5.380.
- Watson, D. R., R. J. McClelland, and D. A. Adams. 1996. “Auditory Brainstem Response Screening for Hearing Loss in High Risk Neonates.” International Journal of Pediatric Otorhinolaryngology 36 (2): 147–183. doi:10.1016/0165-5876(96)01352-3.
- Wells, G., B. Shea, D. O’Connell, J. Peterson, V. Welch, M. Losos, and P. Tugwell. 2018. “The Newcastle-Ottawa Scale (NOS) for assessing the quality of non-randomised studies in meta-analysis.” Accessed 23 July 2019. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- Wenjin, W., T. Xiangrong, L. Yun, L. Jingrong, C. Jianyong, W. Xueling, H. Zhiwu, et al. 2018. “Neonatal Hearing Screening in Remote Areas of China: A Comparison between Rural and Urban Populations.” Journal of International Medical Research 46 (2): 637–651. doi:10.1177/0300060517706643.
- Wolff, R., J. Hommerich, R. Riemsma, G. Antes, S. Lange, and J. Kleijnen. 2010. “Hearing Screening in Newborns: Systematic Review of Accuracy, Effectiveness, and Effects of Interventions after Screening.” Archives of Disease in Childhood 95 (2): 130–135. doi:10.1136/adc.2008.151092.
- World Bank Group. 2018. “GNI per Capita, PPP (Current International $).” Accessed 22 November 2018. https://data.worldbank.org/indicator/NY.GNP.PCAP.PP.CD.
- World Health Organization. 2018. “Prevention of blindness and deafness – grades of hearing impairment.” https://www.who.int/pbd/deafness/hearing_impairment_grades/en/.
- Wroblewska-Seniuk, K., G. Greczka, P. Dabrowski, W. Szyfter, and J. Mazela. 2017. “The Results of newborn hearing Screening by Means of Transient Otoacoustic Emissions – Has Anything Changed over 10 Years?” International Journal of Pediatric Otorhinolaryngology 96: 4–10. doi:10.1016/j.ijporl.2017.02.021.
- Yoshinaga-Itano, C., and M. L. Apuzzo. 1998a. “The Development of Deaf and Hard of Hearing Children Identified Early through the High-Risk Registry.” American Annals of the Deaf 143 (5): 416–424. doi:10.1353/aad.2012.0118.
- Yoshinaga-Itano, C., and M. L. Apuzzo. 1998b. “Identification of Hearing Loss after Age 18 Months Is Not Early Enough.” American Annals of the Deaf 143 (5): 380–387. doi:10.1353/aad.2012.0151.
- Zelante, L., P. Gasparini, X. Estivill, S. Melchionda, L. D’Agruma, N. Govea, M. Milá, et al. 1997. “Connexin26 Mutations Associated with the Most Common Form of Non-Syndromic Neurosensory Autosomal Recessive Deafness (DFNB1) in Mediterranean.” Human Molecular Genetics 6 (9): 1605–1609. doi:10.1093/hmg/6.9.1605.
- Zuhair, M., G. S. A. Smit, G. Wallis, F. Jabbar, C. Smith, B. Devleesschauwer, and P. Griffiths. 2019. “Estimation of the Worldwide Seroprevalence of Cytomegalovirus: A Systematic Review and Meta-Analysis.” Reviews in Medical Virology 29 (3): e2034. doi:10.1002/rmv.2034.