Abstract
Objective
We are currently undertaking a clinical investigation to evaluate the diagnostic capability of a system for detecting periods of pathological dizziness. This article presents an analysis of the data captured during an acute attack of Ménière’s disease.
Design
The Continuous Ambulatory Vestibular Assessment (CAVA) device is worn by patients in the community, and continuously records eye and head movement data (vestibular telemetry).
Study sample
A 53-year-old lady with a fifteen-year history of left-sided unilateral Ménière’s disease.
Results
The patient wore the device nearly continuously for thirty days. The data revealed a three-hour long attack of vertigo consisting of four separate phases of nystagmus. The duration, beat-direction and slow phase velocity of the nystagmus evolved through time. The first phase contained isolated nystagmus beats which preceded the patient’s record of the vertigo attack onset but coincided with anticipation of an impending vertigo attack.
Conclusions
CAVA provides a unique insight into the physiological parameters present during episodes of dizziness. Here, it has provided the first full example of an acute Ménière’s attack, including a period of prodrome. These findings have implications for the prediction of vertigo attack onset, for the diagnosis of Ménière’s disease and other diseases resulting in dizziness.
Introduction
Ménière’s disease is a chronic idiopathic condition affecting the inner ear that results in repeated episodes of vertigo (van et al. Citation2006). The lifetime prevalence of Ménière’s disease has been reported to be 190 per 100,000 people (Harris and Alexander Citation2010) with an annual incidence of 15.3 per 100,000 (Wladislavosky-Waserman et al. Citation1984). Characteristic episodes of vertigo last for between twenty minutes and twelve hours according to contemporary classification systems (Goebel Citation2016; Lopez-Escamez et al. Citation2015).
Little is known regarding the processes taking place within the vestibular system during a Ménière’s attack. This is because it is currently impossible to predict when an attack will occur and to put in place instrumentation to make appropriate measurements. However, there have been a handful of reports of patients becoming vertiginous whilst in a clinical environment, giving clinicians the opportunity to place Electronystagmography (ENG) or Videonystagmography (VNG) equipment onto the patient to make measurements during the attack (Watanabe Citation1996; Mcclure, Copp, and Lycett Citation1981; Nishikawa and Nishikawa Citation1986; Bance et al. Citation1991). There are also anecdotal reports of patients being asked to use a mobile phone to record their eye movements during an attack of dizziness. Such an approach is useful for confirming the presence of nystagmus but relies on patients to correctly identify attack onset and to operate their phone during an attack.
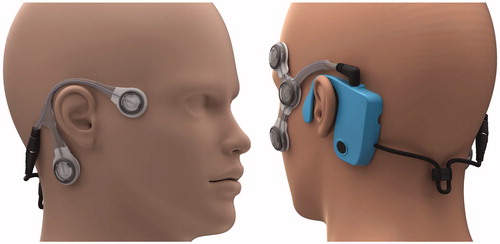
This report details the outcomes from preliminary trials using a novel diagnostic system and is part of a larger portfolio of work funded by the UK Medical Research Council. The system is composed of a piece of wearable technology (), plus the algorithms necessary to assess the data recorded by the device. Further detailed information regarding the system is available elsewhere (Phillips, Newman, and Cox Citation2019; Newman et al. Citation2019), but in essence, the device uses electrooculography for near-continuous monitoring of horizontal and vertical eye movements and also records three-dimensional head accelerations by individuals for thirty days at a time. As the device is worn near continuously, it does not rely on the patient to accurately detect attack onset, the end of the attack, or for the attack to coincide with a hospital visit. The extended monitoring duration also provides the opportunity to observe potentially informative pre- and post-attack data. Unlike patient-made video recordings which are corrupted by the patient closing their eyes, poor ambient lighting, and inconsistencies in head pose, this device is unaffected by these factors. A direct comparison may be drawn between this system and other ambulatory technologies employed in the fields of electrocardiology and electroencephalogy (Su, Borov, and Zrenner Citation2013; Seneviratne et al. Citation2013).
Figure 1. A picture showing how the CAVA® device looks when worn on the face. The device consists of a reusable logging module that sits behind the left ear, and two single-use electrode mounts that attach to the face by way of five ECG electrode pads. Two electrode pads are placed at the outer canthi of each eye to record horizontal eye movements, and two are placed above and below one eye to record vertical eye movements. A fifth electrode beneath the right ear provides a reference voltage.
During the development of our diagnostic system, we recorded an entire three-hour vertigo episode from prodrome to recovery. This is something that has not previously been possible and has therefore not been reported before. We describe the attack in detail, including a quantitative analysis of the nystagmus observed, and we discuss the findings and their implications for our understanding of acute attacks of vertigo in patients with Ménière’s disease.
Materials and methods
The patient was a 53-year-old lady with a fifteen-year history of left-sided unilateral Ménière’s disease. Discrete episodes of rotatory vertigo lasting for hours associated with severe nausea and plugging in the affected ear had been recently reported. The patient wore the CAVA® device for thirty consecutive days. The patient experienced no technical issues with the device and reported no adverse events.
The patient was allocated one hour each day to remove the device, to wash themselves and to then reapply the device using a new pair of electrode mounts. The patient was instructed to activate the device’s event marker (a button on the device that records the time it was pressed) if she felt dizzy and to make an entry in her diary after the attack. No other patient intervention with the device was required. This clinical investigation was reviewed and approved by the London-Dulwich Research Ethics Committee (IRAS Number: 261099).
Results
Upon returning the device, the patient reported a Ménière’s attack lasting approximately three hours. The patient kept a record of this attack in her trial diary, and the onset of the attack was marked by the device’s event marker. The device data showed clear evidence of nystagmus during the period indicated by the patient—see . We performed an automated computer analysis of the data collected by the device, which was then validated and corrected by a Consultant Clinical Scientist with a special interest in balance disorders. We observed eight separate episodes of nystagmus occurring within a three-hour period ( and , and ). Nystagmus beats were considered to be part of the same episode if they occurred within five minutes of each other.
Figure 2. A thirty-second horizontal eye-movement trace from each of the eight separate episodes observed within the three-hour attack. Each trace is shown on the same x and y scales to aid comparison, although the y-scale is shifted appropriately to display the waveform in the centre of the panel. The nystagmus beats were automatically detected by a computer algorithm and then manually validated by an expert. Fast and slow phases are shown in red and green respectively.
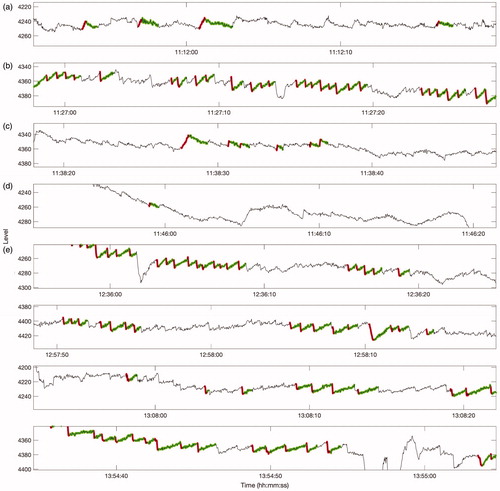
Figure 3. (a) Periods of right-beating and left-beating nystagmus are highlighted in red shaded and green shaded areas respectively. The duration of these periods was determined by manual inspection. Beats occuring within five minutes of each other were considered to be part of the same episode. Arrows mark the time at which the patient activated the device’s event maker, and the approximate start and end times of the attack as recorded in the patient’s trial diary. (b) Estimated mean and standard error of the slow phase velocities of the nystagmus in each episode. (c) Estimated mean and standard error of the fast phase amplitudes in each episode. (d) Estimated mean and standard error of the slow phase durations of each episode.
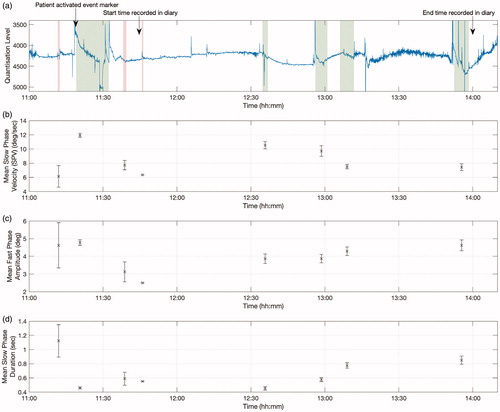
Table 1. Quantification of the nystagmus episodes within a three-hour, acute Ménière’s attack. The last three columns show the mean and standard error for their respective measures. This table relates to the episodes shown in and .
Approximately seven minutes prior to activation of the device’s event marker by the patient, we identified a thirty-six second period containing several isolated beats of nystagmus (a). This was suggestive of very minor, right-beating nystagmus. Following the patient’s activation of the event marker, left-beating nystagmus was evident, and persisted for over thirteen minutes (b). Approximately six minutes later, the direction reversed again, and continued for around one minute (c). Six minutes later, a further twenty second period of low-amplitude right-beating nystagmus was identified by manual inspection (d). A gap of around fifty minutes followed, during which no nystagmus was visible. The concurrent accelerometer data revealed that the patient was likely asleep during this period. Finally, the attack resumed and subsequently ended with left-beating nystagmus, occurring across four separate episodes (e).
The episodes of right-beating nystagmus, which were beating away from the affected ear, were noticeably different to the episodes containing left-beating nystagmus. Specifically, the right-beating nystagmus was shorter in duration and its slow phase velocities were consistently low. The characteristics of the nystagmus also varied temporally. For the left-beating nystagmus, the slow phase duration increased with time, while the slow phase velocity decreased through time.
The patient provided the following account of their attack:
Before the attack I was walking around the garden. I experienced an aura and felt very hot, indicating that an attack was about to start. I had minor, sporadic dizziness for about half an hour but thought it might go away and so didn’t press the event marker. The dizziness then became too much, it took on a spinning quality, I pressed the event marker and I had to lie down. From memory, I was lying down with my eyes shut during the entire attack, and I fell asleep. I would occasionally open my eyes to see if the spinning had stopped. I tried to keep still and to not stand up, as moving feels like it makes the dizziness worse. After the attack, I completed the diary and then went for a walk.
Discussion
The data captured by our device has given us a unique insight into the mechanisms at play within the vestibular system during an acute Ménière’s attack. The device captured the data from a full attack that took place in the patient’s home: a clinician was not present during the attack.
Until now, it has commonly been reported that the acute episode of Ménière’s disease is associated with three phases: an “irritative phase”, a “paralytic phase” and then a “recovery” phase (Watanabe Citation1996; Nishikawa and Nishikawa Citation1986; Luryi, Morse, and Michaelides Citation2019). Previous studies of acute Ménière’s attacks have resulted from occurrences of attacks that, fortuitously, took place within a clinical environment, enabling a clinician to make measurements on the resulting nystagmus. However, such circumstances are very rare and require the patient and clinician to correctly identify the onset of the attack. These problems have led some studies to observe only two phases of nystagmus during a Ménière’s attack (Mcclure, Copp, and Lycett Citation1981; Bance et al. Citation1991). Our device overcomes this problem by recording data continuously; before, during and after an attack. It is for this reason that we have been able to capture the first full example of an acute attack, including a period of prodrome. During this prodromal phase of the patient’s attack, which preceded their activation of the device’s event marker, the patient reported feeling slight dizziness and an expectation that a full vertigo attack was imminent. This observation is consistent with the timeline of events typically reported by patients with Ménière’s disease.
The exact pathophysiology of Ménière’s disease remains unknown despite many historical (Schuknecht Citation1968) and contemporary theories (Gibson Citation2019; Phillips and Prinsley Citation2009). Having authentic objective physiological data from a patient experiencing an entire vertigo attack provides a unique opportunity to revisit these theories.
In the 1960s, Shucknecht proposed that blockage of the endolymphatic duct was responsible for the rupture of a distended Reissner’s membrane, which leads to subsequent leakage of potassium into the perilymph (Schuknecht Citation1968). This in turn amplifies afferent nerve firing and leads to episodes of vertigo until the relative ionic concentrations can be restored. Whilst this theory has been recently challenged, there had been a great deal of historical support for this theory, as well as for performing surgical procedures on the endolymphatic sac. More recently, focus has moved to mechanisms whereby there is a sudden release of endolymph from the pars inferior (cochlea) into the pars superior (utricle and semicircular canals). It is considered that it is this sudden shift of fluid that is responsible for increased output of afferent vestibular nerve activity during a Ménière’s attack (Gibson Citation2019). This may be due to a malfunction of the valve of Bast that resides between the pars inferior and pars superior (Gibson Citation2019) or due to an obstruction in this area due to the presence of otoconial debris (Phillips and Prinsley Citation2009).
Hypothetically, one could propose that whilst pressure builds and before it is released from the pars inferior to the pars superior (prodromal phase), patients will experience a paralytic form of nystagmus (contralateral nystagmus). Once the valve of Bast opens, or any obstructive debris is cleared, the flow of endolymph into the pars superior will be associated with increased vestibular afferent activity and the start of the patient's vertigo attack (irritative phase with ipsilateral nystagmus).
This leaves two further phases, phase three (contralateral nystagmus) and phase four (ipsilateral nystagmus). These two phases could be explained by the process of redressing equilibrium between the pars inferior and pars superior. This process could be caused by further fluid shift, electrophysiological rectification, and/or by central compensation via processes such a cerebellar clamping. This would take place via a process of under and overcorrection before homeostasis is achieved.
In conclusion, the results provided by our patient present the first ever complete report of what occurs during an entire Ménière’s attack. In contrast to previous studies reporting three phases of nystagmus, the attack described here contained four phases. The first phase preceded the patient’s vertigo symptoms, demonstrating the potential to predict and subsequently warn a patient of impending vertigo. In our ongoing work, we intend to determine if the four phases identified here are a consistent finding among other patients suffering from Ménière’s disease. We will also continue to explore the correlation between eye movements and vertiginous events that might uncover the true underlying pathophysiology of Ménière’s disease.
Acknowledgements
We would like to thank Dr Art Mallinson and Dr Neil Longridge for their constructive input on these findings.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data presented here is available upon reasonable request.
Additional information
Funding
References
- Bance, M., M. Mai, D. Tomlinson, and J. Rutka. 1991. “The Changing Direction of Nystagmus in Acute Menière’s Disease: Pathophysiological Implications.” The Laryngoscope 101 (2): 197–201. doi:10.1288/00005537-199102000-00017.
- Gibson, W. P. R. 2019. “Meniere’s Disease.” Adv Otorhinolaryngol 82: 77–86. doi:10.1159/000490274.
- Goebel, J. A. 2016. “2015 Equilibrium Committee Amendment to the 1995 AAO-HNS Guidelines for the Definition of Ménière's Disease.” Otolaryngology-Head and Neck Surgery: Official Journal of American Academy of Otolaryngology-Head and Neck Surgery 154 (3): 403–404. doi:10.1177/0194599816628524.
- Harris, J. P., and T. H. Alexander. 2010. “Current-day Prevalence of Ménière's Syndrome.” Audiology & Neuro-Otology 15 (5): 318–322. doi:10.1159/000286213.
- Lopez-Escamez, Jose A., John Carey, Won-Ho Chung, Joel A. Goebel, Måns Magnusson, Marco Mandalà, David E. Newman-Toker, et al. 2015. “Diagnostic Criteria for Menière’s disease.” Journal of Vestibular Research: Equilibrium & Orientation 25 (1): 1–7. doi:10.3233/VES-150549.
- Luryi, A., E. Morse, and E. Michaelides. 2019. “Pathophysiology and Diagnosis of Meniere’s Disease.” In Diagnosis and Treatment of Vestibular Disorders, 165–188. Springer. doi:10.1007/978-3-319-97858-1_13.
- Mcclure, J. A., J. C. Copp, and P. Lycett. 1981. “Recovery Nystagmus in Ménière's disease.” The Laryngoscope 91 (10): 1727–1737. doi:10.1288/00005537-198110000-00019.
- Newman, J. L., J. S. Phillips, S. J. Cox, J. FitzGerald, and A. Bath. 2019. “Automatic Nystagmus Detection and Quantification in Long-Term Continuous Eye-Movement Data.” Computers in Biology and Medicine 114: 103448. Published online . doi:10.1016/j.compbiomed.2019.103448.
- Nishikawa, K., and M. Nishikawa. 1986. “Nystagmus during Attack in Meniere’s Disease.” Auris, Nasus, Larynx 13 (Suppl 2): S147–S151. doi:10.1016/S0385-8146(86)80068-2.
- Phillips, J. S., and P. R. Prinsley. 2009. “A Unified Hypothesis for Vestibular Dysfunction?” Otolaryngology-Head and Neck Surgery: Official Journal of American Academy of Otolaryngology-Head and Neck Surgery 140 (4): 477–479. doi:10.1016/j.otohns.2008.12.028.
- Phillips, J. S., J. L. Newman, and S. J. Cox. 2019. “An Investigation into the Diagnostic Accuracy, Reliability, Acceptability and Safety of a Novel Device for Continuous Ambulatory Vestibular Assessment (CAVA).” Scientific Reports 9 (1): 10452. doi:10.1038/s41598-019-46970-7.
- Schuknecht, H. 1968. “Correlation of Pathology with Symptoms of Meniere’s Disease.” Otolaryngol Clin North Am 1: 433–440.
- Seneviratne, Udaya, Armin Mohamed, Mark Cook, and Wendyl D'Souza. 2013. “The Utility of Ambulatory Electroencephalography in Routine Clinical Practice: A Critical Review.” Epilepsy Research 105 (1–2): 1–12. doi:10.1016/j.eplepsyres.2013.02.004.
- Su, L., S. Borov, and B. Zrenner. 2013. “12-lead Holter Electrocardiography. Review of the Literature and Clinical Application update.” Herzschrittmachertherapie & Elektrophysiologie 24 (2): 92–96. doi:10.1007/s00399-013-0268-4.
- van, Cruijsen N., J. P. C. Jaspers, Wiel H. B. M. van de, H. P. Wit, and F. W. J. Albers. 2006. “Psychological Assessment of Patients with Menière’s Disease.” International Journal of Audiology 45 (9): 496–502. doi:10.1080/14992020600753239.
- Watanabe, T. Nystagmus during an acute attack of Meniere’s disease. ENG Report. Published online 1996. 1–3.
- Wladislavosky-Waserman, P., G. W. Facer, B. Mokri, and L. T. Kurland. 1984. “ Meniere’s Disease: A 30-Year Epidemiologic and Clinical study in Rochester, Mn, 1951–1980 .” The Laryngoscope 94 (8): 1098–1102. doi:10.1288/00005537-198408000-00020.
