Abstract
Objective
To develop a method of visualising electrooculography data to improve the interpretability of nystagmus eye-movements captured using the Continuous Ambulatory Vestibular Assessment (CAVA®) device.
Design
We are currently undertaking a clinical investigation to evaluate the capabilities of the CAVA® device to detect periods of pathological nystagmus. The work presented here was undertaken using unblinded data obtained from the preliminary phase of this investigation.
Study sample
One patient with Ménière’s disease and one with Benign Paroxysmal Positional Vertigo.
Results
Using the electrooculography data captured by the CAVA® device, we reconstructed 2D animations of patients’ eye movements during attacks of vertigo. We were able to reanimate nystagmus produced as a consequence of two conditions. Concurrent video footage showed that the animations were visually very similar to the patient’s actual eye-movements, excepting torsional eye-movements.
Conclusions
The reconstructed animations provide an alternative presentation modality, enabling clinicians to largely interpret electrooculography data as if they were present during a vertigo attack. We were able to recreate nystagmus from attacks experienced in the community rather than a clinical setting. This information provides an objective record of a patient’s nystagmus and could be used to complement a full neurotologic history when considering diagnosis and treatment options.
Introduction
Dizziness is an extremely common symptom. One survey showed that over 20% of 18–64 year olds registered at four GP surgeries in London had experienced dizziness within the preceding month (Yardley et al. Citation1998). Patients with vertigo can experience violent spinning episodes for hours at a time (Bance et al. Citation1991). Vertigo can have many causes, and although a wide range of clinical tests are available, they are often undertaken in the absence of a dizzy attack (Molnar and McGee Citation2014). Furthermore, patient reporting of vertigo is subjective and imprecise (Newman-Toker et al. Citation2007). Therefore, dizziness can be challenging to diagnose and it would be beneficial to diagnose patients quickly and accurately, reducing the burden on health services.
Observation of eye movements is informative when assessing the dizzy patient. Vertigo is usually accompanied by nystagmus, and different patterns of nystagmus can help provide a diagnostic insight into the underlying cause of a patient’s symptoms (Roberts, Gans, and Kastner Citation2006; Jeffery et al. Citation2017). For example, nystagmus beating in the horizontal plane is often characteristic of problems in the inner ear, whereas nystagmus in the vertical plane can be suggestive of central causes (Büttner, Brandt, and Helmchen Citation1999). We have developed the Continuous Ambulatory Vestibular Assessment (CAVA®) device to provide valuable long-term eye movement data (), and computer algorithms for analysing the data collected by the device in the community. As with conventional electronystagmography, the CAVA® device records horizontal and vertical eye-movements but does not capture torsional eye-movement data. This system provides an objective record of the presence, duration and frequency of nystagmus, gathered over a period of thirty days.
Figure 1. The appearance of the CAVA® device when worn on the head. The device includes a reusable logging module and two, single-use electrode mounts. Five ECG electrodes are placed at specific sites on the face to record the corneo-retinal potential produced by the eyes. A button on the logging unit allows the patient to activate the device’s event marker, which causes the device to log the date and time of the button press. The button can also be used to initiate a status check of the device, the results of which are confirmed visually by the device’s status LED. The status checking feature provides feedback regarding battery level, the connection of the device’s electrodes, and confirmation of event marker activation.
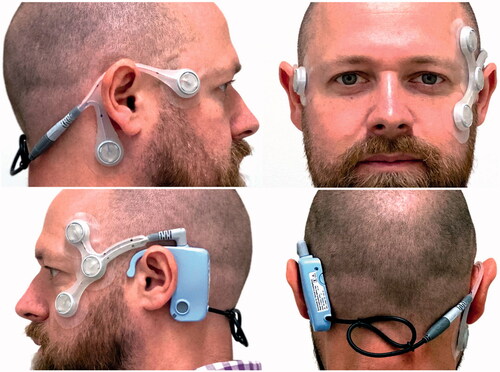
The CAVA® system has proven capable of detecting induced nystagmus and nystagmus in patients suffering from a variety of vestibular conditions (Phillips, Newman, and Cox Citation2019; Newman et al. Citation2019; Phillips et al. Citation2021; Phillips, Newman, and Cox Citationin press). The long-term data captured can provide an objective record of the presence of nystagmus, which patients are unlikely to report accurately by themselves. We have previously shown that nystagmus during a Ménière’s attack has unique temporal characteristics, which could be used to assist in diagnosing the condition (Phillips et al. Citation2021). Other conditions, such as Benign Paroxysmal Positional Vertigo (BPPV), are distinct in that they are motion-provoked, and nystagmus is short in duration. Nystagmus produced by BPPV typically beats torsionally and vertical upwards, although only the vertical component can be detected by the CAVA® system. The information provided by the CAVA® system is expected to supplement and complement a full neurotological history and examination, assisting the clinician to make or confirm a diagnosis (Phillips, Newman, and Cox Citationin press).
The large quantity of data captured by the device makes manual inspection of the data impractical. To overcome this issue, we have previously developed and evaluated algorithms to automatically detect different patterns of nystagmus (Newman et al. Citation2019), for subsequent review and interpretation by a clinician. Eye movement data can be difficult to interpret from manual inspection of separate vertical and horizontal channels, especially if the eye movements are complex or rare (Califano et al. Citation2014). Additionally, some clinicians may have more experience examining the eyes than looking at signal traces. Patients themselves would better understand their own symptoms from a video representation of their eye-movements rather than complex signal traces. In this article, we describe an approach to reproduce animated eye movements from the long-term, horizontal and vertical electrooculography data provided by the CAVA® device. The recreated movements imitate the appearance of the eyes, excluding torsional eye-movements, and largely allow clinicians to review episodes of vertigo as if they were present with the patient during an attack. We demonstrate that the results obtained are visually comparable to actual video footage of the patient’s eyes. The data analysed exemplifies the challenges associated with interpreting eye-movement signals and highlights the benefits of reanimating them in 2D.
Materials and methods
We are currently undertaking a clinical investigation into the capabilities of our device to detect pathological nystagmus from patients with a range of vestibular conditions. This work is part of a larger portfolio of work funded by the UK Medical Research Council to develop a full medical system for diagnosing dizziness. During the “training” phase of this investigation, we have collected data from patients with vertigo which will be used to develop our computer algorithms for detecting nystagmus. The work presented here was undertaken using data from patients enrolled onto this training phase. This clinical investigation was reviewed and approved by the London-Dulwich Research Ethics Committee (IRAS Number: 261099).
Patients enrolled into our clinical trial wore the CAVA® device for thirty consecutive days. They attended three separate follow-up appointments during the trial. The first, on day five, was to check that the patient was getting on well with their device and to check for adverse skin reactions. The second, on day fifteen, was to change the device’s battery. After day thirty-one, patients returned their device and completed a questionnaire. In this article, we present data from two of the trial participants. Patient One was a 53-year-old female with a fifteen-year history of left-sided unilateral Ménière’s disease. Patient Two was a 56-year-old female with a three-year history of positional vertigo. For Patient One, we reanimated her nystagmus eye movements from a Ménière’s attack she experienced during her thirty-day trial, in her own home. For Patient Two, we reanimated her nystagmus during a Dix-Hallpike test, undertaken in a clinical setting at her day fourteen follow-up appointment. We also recorded concurrent video footage of Patient Two’s eyes during this test. The video was recorded using a Canon 200 D Digital SLR camera, at 1920 × 1080 pixels, at 60 frames per second.
The CAVA® device () records the corneo-retinal potential produced by the eyes, which is a proxy for eye movement. Horizontal eye movement is captured by way of two electrodes placed at the outer canthi of the eyes, and vertical eye movement by two electrodes placed above and below the left eye. A fifth electrode beneath the right ear provides a reference voltage. This technology is similar to electrooculography and electronystagmography, which have been used routinely for decades (Bhansali and Honrubia Citation1999), and as such the CAVA® device does not capture torsional eye-movements. Also, as with electronystagmography, the CAVA® device would require frequent recalibration in order to relate the signals recorded to precise eye-movements in degrees. As we are presently only interested in identifying nystagmus signals and comparing them qualitatively, here we present eye-movements using native device units. The device also contains an accelerometer which records three-axis head movements. Each channel of eye movement is sampled at 42.67 Hz and each acceleration channel is sampled at 20 Hz.
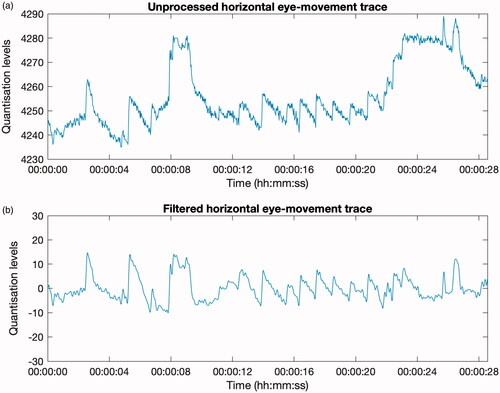
We developed software to simultaneously visualise the two-channel eye-movement data provided by the CAVA® device, the video data (for Patient Two), and a 2D reconstructed animation of the patients’ eye movements. This software, which was created using Mathwork’s MATLAB, generates a video file showing the three modalities playing in real-time: vertical and horizontal eye-movement traces, an animated reconstruction of the eyes and concurrent video footage of the patient’s eyes. Prior to visualisation and reconstruction, each eye movement channel was pre-processed to remove signal drift and high frequency noise, such as interference from mains electricity, both common issues when working with electrooculography data (Bulling et al. Citation2007). To achieve this, a bandpass filter was applied to the data (). The filter used a high-pass threshold of 0.20 Hz and a low-pass threshold of 6 Hz.
Figure 2. Panels (a) and (b) display a 28-second, horizontal eye-movement trace captured by the CAVA® device. The waveforms show an extract of nystagmus produced by a patient with Ménière’s disease. In panel (a), the signal has not been filtered, and shows evidence of signal drift and high frequency noise. In (b), the signal has been filtered using a bandpass filter, with a passband of 0.20 Hz to 6 Hz. The filtered signal retains the characteristic sawtooth nystagmus waveform shape whilst discarding the signal drift and the higher frequency noise.
Animated eye-movements were reconstructed by plotting the filtered horizontal and vertical eye-movement channels onto two separate 2D plots, designed to imitate the appearance of the pupils of the eyes. Although the CAVA® device does not record left and right eye movements independently, both eyes are shown in order to generate a more realistic and interpretable animation. The axes limits of the reconstructed plots were selected such that all of the patient’s eye-movement data would be visible within the plots. The video data was temporally aligned with the eye-movement data using activation of the CAVA® device’s event marker as a reference point. As the device and video data were captured at different sample rates, care was taken to maintain alignment of these data channels during playback.
Results
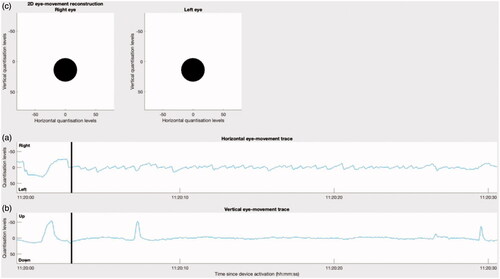
Patient One returned their device after thirty days and reported an acute Ménière’s attack consisting of an episode of rotary vertigo lasting approximately three hours. Analysis of the eye-movement traces from the CAVA® device showed clear evidence of sporadic, left- and right-beating jerk nystagmus throughout the period indicated by the patient. The full details of this attack have been reported previously (Phillips et al. Citation2021). A thirty-second extract of the device data was processed by our software to generate a new video showing the horizontal and vertical eye-movement traces alongside a 2D-reconstruction of the patient’s eye movements ( and Supplemental Movie 1). The reconstruction confirmed the presence of left-beating, jerk nystagmus during the reported vertigo attack.
Figure 3. The initial frame of a video showing the eye-movements of a patient experiencing an acute attack of Ménière’s disease. The signal shown is an extract from an attack which lasted for approximately three hours, in total. (a) and (b) show the horizontal and vertical eye movement traces as captured by the CAVA® device. The black, vertical line marks the current timestamp. (c) Shows the animated reconstruction of the eye movements in 2D.
Patient Two returned after thirty days, reporting several short episodes of motion-provoked vertigo during the trial. At Patient Two’s second follow-up visit, three right-sided Dix-Hallpike manoeuvres were performed. Visual observation of the patient’s eyes during these tests showed nystagmus beating torsionally with the upper pole of the eye to the right-hand side, and vertically upward. The nystagmus lasted for less than thirty seconds. These observations confirmed right posterior canal, BPPV canalithiasis. Consistent with the response “fatigue” that has been reported previously, the first manoeuvre yielded the strongest nystagmus response (Haynes et al. Citation2002). To compare the patient’s actual eye-movements with those recreated from the device data, we recorded video of the patient’s eyes whilst they underwent the manoeuvre. The video and device data were processed by our software to generate a new video showing these modalities alongside a 2D-reconstruction of the patient’s eye movements ( and Supplemental Movie 2). Observation of the CAVA® device’s horizontal and vertical eye movement data showed evidence of up-beating nystagmus in the vertical channel ().
Figure 4. The initial frame of a video showing the eye-movements of a patient undergoing a Dix-Hallpike manourvre. (a) and (b) show the horizontal and vertical eye movement traces as captured by the CAVA® device. The black line marks the current timestamp, and event marker activations are shown as orange lines. (c) Shows an animated reconstruction of the patient’s eye movements in 2D. The video in (d) shows the clinician activating the device’s event marker, performing the Dix-Hallpike manourvre on the patient, followed by a closeup of the patient’s eyes. The first event marker activation allowed the eye-movement channels to be aligned temporally with the video of the patient undergoing to the procedure. The second activation was at the first sign of nystagmus, which coincided with the patient reporting the onset of vertigo, and the final event mark was deployed after the nystagmus had ceased. The green line in (b) is the point at which the video in (d) is rotated by 180 degrees, so that they are presented in the same orientation.
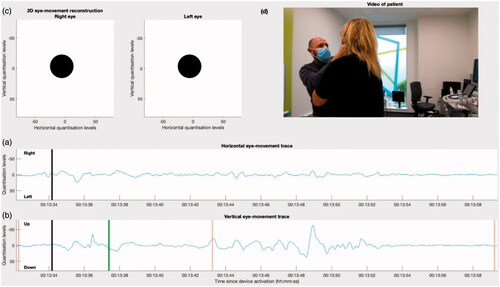
The 2D animated reconstruction of Patient Two’s eye-movements revealed an obvious visual correlation between the reconstruction and the video footage (). At about 00:13:36, the patient can be seen to blink (), and this appears as a fast, upward eye excursion in both the eye movement traces () and the reconstruction (). From the reconstruction, Patient Two’s eyes can also be seen to move in an arching motion towards the right-hand side of her face. This motion could not easily be identified from the eye-movement traces alone, but is clearly visible both in the reconstruction and in the video footage. As purely torsional eye-movements cannot be captured using electrode-based electronystagmography, no torsional movements are visible in the reconstruction, despite being visible in the video footage. The device’s accelerometer data confirmed that the patient underwent a right-sided Dix-Hallpike test. Both the reconstruction and the video showed clear evidence of nystagmus starting approximately seven seconds after the patient was placed into a supine position, which lasted for approximately nine seconds. Up-beating nystagmus is visible in the vertical channel and the trace has an oscillatory appearance. After the nystagmus had subsided, the patients’ eyes were visibly stationary.
Discussion
The results presented here have provided proof-of-concept that signals captured by the CAVA® device largely reflect the actual eye-movements displayed by patients. We were able to use reconstructed animated eye movements to observe the up-beating component of nystagmus present in a patient undergoing a Dix-Hallpike manoeuvre and also the jerk nystagmus experienced by a patient experiencing an attack of Ménière’s disease, in her own home. In the absence of a video recording of a patient’s eyes or without being physically present with a patient during a vertigo attack, an animated 2D reconstruction would enable clinicians to retrospectively evaluate the presence and characteristics of nystagmus, and would aid discussions with the patient regarding their vertigo. Clinicians may favour the animated 2D reconstruction because they are familiar with observing physical eye movements, or as highlighted here, because of the complexity of the signal under interrogation.
This study is the first to recreate eye-movements from the long-term data provided by the CAVA® device for the purpose of assessing dizziness. Due to the emergence of videonystagmography technology, electrooculography has decreased in popularity for recording nystagmus in clinical settings (Ascherfeld Citation2020). Interpreting eye movements from electrooculography (EOG) data remains an active area of research in the field of Human-Computer-Interaction (HCI). Applications of EOG in HCI tend to focus on the automatic detection of specific eye-movement gestures to facilitate some kind of computer-based activity, often to assist people with quadriplegia to interact with a computer (Chang Citation2019).
There is a clear similarity between the data captured by the CAVA® device and the actual eye-movements experienced by patients. This relationship is sufficient to allow a qualitative comparison between nystagmus from different patients and between nystagmus resulting from different conditions. There are several issues which make discriminating the characteristics of nystagmus more challenging. As with ENG, the relationship between eye-movements and native device units is only linear up to ± 30 degrees (Diez Citation2018). This is an unavoidable limitation of electrode-based systems, so care should be taken when visualising large excursions of the eye. It is also not possible to record pure torsional eye-movements using electrode-based systems. Such eye-movements are common with BPPV, although they are usually accompanied by a vertical component.
A further challenge shared with conventional electrode-based electronystagmography is that frequent device calibration is usually required to determine precise eye-movements in degrees. This is due to the variability of the corneo-retinal potential, which is the bioelectrical signal used by both systems as a proxy for eye-movement. Calibration can be performed by the subject performing a calibration task, such as predefined eye movements (e.g. moving the eyes by ± 30 degrees), in order to calculate the number of native device units per degree of eye movement. Alternatively, an average calibration value could be used, with a known margin of error. We did not perform these steps here as it is possible to determine the presence of nystagmus, its duration and beat-direction without prior calibration.
BPPV is the most common cause of vertigo, and by far the most common cause of motion-provoked vertigo. Thus, nystagmus correlated with head-movements, as confirmed by the CAVA® device’s accelerometer data, would provide a likely first indication of BPPV. Further supporting data is provided by the fact that the onset of nystagmus is delayed for BPPV but not for vertigo with central causes, and the nystagmus produced as a consequence of BPPV is fatigable whereas for central causes it is not. Posterior canalithiasis is the most common form of BPPV, accounting for around 90% of cases. Therefore, in the majority of cases, the side the person lies on would indicate the affected side and the affected canal. Confirmation of motion-provoked vertigo fulfilling these criteria could be used in conjunction with a full neurotological history and examination to supplement a clinician’s diagnosis.
We have shown here that eye blinks are reconstructed as short duration, vertical eye movements, which could be misinterpreted as genuine, vertical eye-movements. Some vertical movement can occur during blinking (Bell’s phenomenon), but this is unlikely to fully account for the signals captured (Fraco and Doba Citation1984). In light of this, it could be useful to develop ways to automatically detect blinks in the data, to alert the clinician of their presence.
The method of reconstructing eye movements presented here provides an alternative representation of the data captured by the CAVA® device, allowing easier interpretation of the patient’s eye movements and confirming the presence of nystagmus to a clinician. Our ultimate goal is to further develop this system from one that detects nystagmus to one that can provide useful insights regarding the nature of the nystagmus detected. If deployed into routine medical care, we expect that the CAVA® system would be provided to patients in secondary care settings, most likely to provide an objective confirmation of vertigo and to aid the discrimination of possible vestibular causes. The system could provide insight into conditions with a degree of overlap, such as Ménière’s disease and vestibular migraine, or for patients with coexistent conditions, such a dual diagnosis of BPPV and Ménière’s disease. A combination of a full neurotologic history, examination and the nystagmus recorded over thirty-days would then guide a clinician towards the most likely diagnosis and treatment options. For example, hearing loss combined with prolonged episodes of direction-changing vertigo might suggest Ménière’s disease. Prolonged nystagmus in the vertical plane might suggest a central cause. BPPV might be suggested by short durations of up-beating nystagmus, with a latency following a provocative head-movement, and this would be confirmed by a Dix-Hallpike test. As we work towards this goal, we next intend to undertake a clinical trial to determine whether the nystagmus signals captured by the CAVA® device are sufficient by themselves to differentiate some of the most common inner-ear causes of vertigo.
TIJA-2020-10-0497-File005.mp4
Download MP4 Video (8.5 MB)TIJA-2020-10-0497-File004.mp4
Download MP4 Video (2.1 MB)Disclosure statement
All three authors are listed as inventors on a patent application for the CAVA® device.
Data availability statement
The data presented here is available upon reasonable request.
Additional information
Funding
References
- Ascherfeld, K. R. Revisiting Simultaneous Irrigation for Videonystagmography. The University of Arizona.; 2020. http://hdl.handle.net/10150/641739
- Bance, M., M. Mai, D. Tomlinson, and J. Rutka. 1991. “The Changing Direction of Nystagmus in Acute Menière’s Disease: Pathophysiological Implications.” The Laryngoscope 101 (2): 197–201. doi:https://doi.org/10.1288/00005537-199102000-00017.
- Bhansali, S. A., and V. Honrubia. 1999. “Current Status of Electronystagmography Testing.” Otolaryngology–Head and Neck Surgery 120 (3): 419–426. doi:https://doi.org/10.1016/S0194-5998(99)70286-X.
- Bulling, A., P. Herter, M. Wirz, and G. Tröster. Automatic Artefact Compensation in EOG Signals. In: In Adjacent Proceeding of the 2nd European Conference on Smart Sensing and Context (EuroSSC 2007. 12–13.
- Büttner, U., Th Brandt, and Ch Helmchen. 1999. “The Direction of Nystagmus is Important for the Diagnosis of Central Paroxysmal Positioning Nystagmus (cPPV).” Neuro-Ophthalmology 21 (2): 97–104. doi:https://doi.org/10.1076/noph.21.2.97.3919.
- Califano, L., F. Salafia, S. Mazzone, M. G. Melillo, and M. Califano. 2014. “Anterior Canal BPPV and Apogeotropic Posterior Canal BPPV: Two Rare Forms of Vertical Canalolithiasis.” Acta Otorhinolaryngologica Italica: Organo Ufficiale Della Societa Italiana di Otorinolaringologia e Chirurgia Cervico-Facciale 34 (3): 189–197.
- Chang, W.-D. 2019. “Electrooculograms for Human–Computer Interaction: A Review.” Sensors 19 (12): 2690. doi:https://doi.org/10.3390/s19122690.
- Diez, P. 2018. “Smart Wheelchairs and Brain-Computer Interfaces.” In: Mobile Assistive Technologies, 382. Cambridge, Massachusetts: Academic Press.
- Fraco, I. C. F., and J. A. L. Doba. 1984. “Bell’s Phenomenon: A Study of 508 Patients.” Australian and New Zealand Journal of Ophthalmology 12 (1): 15–21. doi:https://doi.org/10.1111/j.1442-9071.1984.tb01119.x.
- Haynes, David S., John R. Resser, Robert F. Labadie, Christopher R. Girasole, Bradley T. Kovach, Luis E. Scheker, Donald C. Walker, et al. 2002. “Treatment of Benign Positional Vertigo Using the Semont Maneuver: Efficacy in Patients Presenting without Nystagmus.” The Laryngoscope 112 (5): 796–801. doi:https://doi.org/10.1097/00005537-200205000-00006.
- Jeffery, H., M. Hopkins, R. Anderson, V. Patel, and J. Rogers. 2017. “The Interpretation of Static Positional Nystagmus in a Balance Clinic.” International Journal of Audiology 56 (12): 958–966. doi:https://doi.org/10.1080/14992027.2017.1357841.
- Molnar, A., and S. McGee. 2014. “Diagnosing and Treating Dizziness.” The Medical Clinics of North America 98 (3): 583–596. doi:https://doi.org/10.1016/j.mcna.2014.01.014.
- Newman, J. L., J. S. Phillips, S. J. Cox, J. FitzGerald, and A. Bath. 2019. “Automatic Nystagmus Detection and Quantification in Long-Term Continuous Eye-Movement Data.” Computers in Biology and Medicine 114: 103448. doi:https://doi.org/10.1016/j.compbiomed.2019.103448.
- Newman-Toker, D. E., L. M. Cannon, M. E. Stofferahn, R. E. Rothman, Y.-H. Hsieh, and D. S. Zee. 2007. “Imprecision in Patient Reports of Dizziness Symptom Quality: A Cross-Sectional Study Conducted in an Acute Care Setting.” Mayo Clinic Proceedings 82 (11): 1329–1340. doi:https://doi.org/10.4065/82.11.1329.
- Phillips, J. S., J. L. Newman, and S. J. Cox. 2019. “An Investigation into the Diagnostic Accuracy, Reliability, Acceptability and Safety of a Novel Device for Continuous Ambulatory Vestibular Assessment (CAVA).” Scientific Reports 9 (1): 10452. doi:https://doi.org/10.1038/s41598-019-46970-7.
- Phillips, J. S., J. L. Newman, and S. J. Cox. in press. “Towards Providing an Automated Approach to Differentiating the Nystagmus of Ménière’s Disease, Vestibular Migraine and Benign Paroxysmal Positional Vertigo.” Otology & Neurotology.
- Phillips, J. S., J. L. Newman, S. J. Cox, and J. FitzGerald. 2021. “Nystagmus during an Acute Ménière’s Attack: From Prodrome to Recovery.” International Journal of Audiology 60 (1): 70–75. doi:https://doi.org/10.1080/14992027.2020.1799252.
- Roberts, R. A., R. E. Gans, and A. H. Kastner. 2006. “Differentiation of Migrainous Positional Vertigo (MPV) from Horizontal Canal Benign Paroxysmal Positional Vertigo (HC-BPPV).” International Journal of Audiology 45 (4): 224–226. doi:https://doi.org/10.1080/14992020500429658.
- Yardley, L., N. Owen, I. Nazareth, and L. Luxon. 1998. “Prevalence and Presentation of Dizziness in a General Practice Community Sample of Working Age People.” British Journal of General Practice 48 (429): 1131.
