Abstract
Objectives
Newborn hearing screening (NHS) varies regarding number and type of tests, location, age, professionals and funding. We compared the provision of existing screening programmes.
Design
A questionnaire containing nine domains: demography, administration, existing screening, coverage, tests, diagnosis, treatment, cost and adverse effects, was presented to hearing screening experts. Responses were verified. Clusters were identified based on number of screening steps and use of OAE or aABR, either for all infants or for well and high-risk infants (dual-protocol).
Study sample
Fifty-two experts completed the questionnaire sufficiently: 40 European countries, Russia, Malawi, Rwanda, India and China.
Results
It took considerable effort to find experts for all countries with sufficient time and knowledge. Data essential for evaluation are often not collected. Infants are first screened in maternity wards in most countries. Human development index and health expenditure were high among countries with dual protocols, three screening steps, including aABR, and low among countries without NHS and countries using OAE for all infants. Nationwide implementation of NHS took 6 years, on average.
Conclusion
The extent and complexity of NHS programmes are primarily related to health expenditure and HDI. Data collection should be improved to facilitate comparison of NHS programmes across borders.
Introduction
It has been 20 years since the first publications on the longstanding benefits of early detection of hearing impairment (HI) and intervention through newborn hearing screening (NHS) programmes (Yoshinaga-Itano and Apuzzo Citation1998a, Citation1998b; Yoshinaga-Itano et al. Citation1998). Prior to NHS, methods used to identify infants with HI included an inventory of risk factors or universal screening using the distraction test. The distraction test was not feasible until at least 7 months of age, and results were highly confounded by false positives and false negatives (Davis, Bamford, and Stevens Citation2001). In the 1990s, objective hearing tests such as otoacoustic emission (OAE) and automated auditory brain stem response (aABR) allowed screening to be performed at a much younger age. Using these tests reduced the cost per infant tested by half (Stevens et al. Citation1998; Uus, Bamford, and Taylor Citation2006). Reducing the age of hearing screening opened the possibility for earlier intervention, minimising the impact of HI on speech, language and general development and academic performance (Ching et al. Citation2018).
Many countries, regions and hospitals across the world have since implemented and sustained a universal NHS programme, in which infants eligible for screening are defined as all infants who are born in a certain country, region, or hospital. Variations exist across countries in how newborn hearing screening programs are implemented, which may make it difficult for policy makers and professionals in other locations to decide on best-practice protocols. International position statements provide some guidance for best practice (European Consensus Statement on Neonatal Hearing Screening Citation1999; Joint Committee on Infant Hearing Citation2007; World Health Organization Citation2009). Furthermore, differences exist in health care environments across countries. Hearing screening experts or policy makers may need to adapt guidelines and construct protocols for the successful implementation of NHS in their local health care environment (World Health Organization Citation2009). All NHS programmes aim to identify infants with HI early in life so that appropriate intervention may be provided, but different approaches are used to reach this shared goal (World Health Organization Citation2009; Sloot et al. Citation2015; Vos et al. Citation2016).
Existing NHS programmes have been compared in a number of studies. These studies differed in the methods used, the countries or regions included, and the scope of the study (World Health Organization Citation2009; White Citation2011; Sloot et al. Citation2015; Vos et al. Citation2016; Kanji, Khoza-Shangase, and Moroe Citation2018). These studies identified common screening sequences used across existing NHS programmes. An aABR protocol with multiple steps was most prominently used for infants with an increased risk for HI or retrocochlear dysfunction. An OAE protocol was most prominently used for infants without this risk. However, the central finding in these studies was that the choices made within NHS programme organisation and protocol design are diverse across countries. Within NHS, studies have not yet investigated the nature of the diversity of protocol design, the interaction of parameter choices, the country-specific factors behind the decisions that drive policy on NHS, and the possibility of harmonising best-practice guidelines within Europe.
A more detailed and essential overview of implemented NHS programmes is needed to inform policy makers on the considerations for planning and implementing NHS. When making the decision to implement an NHS programme, policy makers may consider the local (health care) circumstances, previous experiences, advice received from other programmes, or implementation research performed within the programme or elsewhere. The diversity between NHS programmes across regions and countries makes it difficult for policy makers to decide on how to optimise existing NHS programmes or how to implement a new NHS programme in their country.
The EUSCREEN study evaluates and compares the cost-effectiveness of vision and hearing screening programmes. A decision-analytic cost-effectiveness model is being developed to calculate the optimal, most cost-effective vision and hearing screening programme taking the local circumstances of a country into account. This model will be made available to health care policy makers to introduce, modify or disinvest screening programmes. For the development of this model, detailed information regarding hearing screening protocols, organisation of general preventive healthcare and local societal background was needed. We therefore carried out a comprehensive inventory of hearing screening in countries and regions, primarily in Europe.
This article and the accompanying article (Mackey et al. Citation2021) explore the results from this international inventory of hearing screening programmes. Specifically, these articles evaluate the existing approaches to NHS, its outcomes, and the possible features that influence NHS policy and performance. The current article inventories the provision, status and features of NHS in 45 countries to better understand the variability and the rationale for the diversity across programmes. The factors explored in this article include organisation, protocols, screening targets, referral pathway, infant age, location, screening professionals and funding. The accompanying article (Mackey et al. Citation2021) details the effectiveness of NHS programmes from screening quality measures. In these articles, NHS is defined as hearing screening performed from birth to age 6 months. A description of childhood hearing screening programmes after the newborn period will be reported on in a separate article.
Methods
Development of the questionnaire
A comprehensive questionnaire was developed to gather detailed information on general paediatric, vision and hearing screening programmes, following the success of a pilot study described in Sloot et al. (Citation2015). Within this pilot study, a short questionnaire containing 25 questions was sent out to screening experts in 38 countries to measure their response, and almost all participated. A focus group of hearing and screening experts formulated 191 questions on hearing screening. An additional 126 questions were formulated on vision screening and 82 questions on general screening. Questions were categorised into nine domains: demography and epidemiology, administration and general background, existing screening programmes, coverage and attendance, tests and devices, follow up and diagnostic assessment, treatment options, costs and benefits and adverse effects.
Three types of questions were used: open-ended, multiple-choice and yes-no questions. Most of the questions were followed by a sub-question about the source of the information provided. A respondent could choose between (a) Data unavailable, (b) I don’t know, (c) Rough estimate, (d) Real estimate from calculation, or (e) Actual data. The questionnaire then asked for the name and date of the data source if indicated.
The EUSCREEN questionnaire (Supplementary Appendix 1) was converted to a web-based questionnaire, accessible through the EUSCREEN website (www.euscreen.org). As a separate consortium partner within the EUSCREEN study, the EUS€REEN foundation served to collect the data. So called Country Representatives (CRs) were elected by a tender procedure as prescribed by EU regulations, one for vision, one for hearing and one for general screening for each country. They were awarded a remuneration up to €2000 after they had sufficiently completed their part of the questionnaire. CRs registered online and progressed through the tender procedure, in which their role in the local screening programme and rights to data were assessed. Once accepted, the CR could log in using a unique username and password.
Formation of the Country-Committees Joint-Partnership of EUS€REEN study consortium
The Country-Committees Joint-Partnership of EUS€REEN foundation had been formed as an international collaboration of CRs in hearing, vision and general screening. In each of these countries, CRs on hearing screening were actively searched through screening organisations, publications in peer reviewed journals on the subject, national audiology and ENT societies, existing professional contacts and other CRs who had already registered. CRs representing countries or regions outside of the original selection were not actively searched, though they were welcome to participate. The CRs were contacted through e-mail, telephone calls and in-person meetings during conferences. When CRs were unable to complete the questionnaire, measures were taken to find additional CRs.
Collection of data
Preliminary efforts were made to collect data representing hearing screening for an entire country, but in many instances hearing screening programmes were organised and managed regionally. In these circumstances, the questionnaire was answered by a regional CR and these data were then considered representative only for that regional screening programme.
The questionnaire was originally accessible on the EUSCREEN website from March 2017 until December 2018. Several CRs required significant time for receiving approval, calculating results, and sharing outcomes from internal registries so the deadline for data submission was extended to 30 June 2019 (Figure 1, Supplementary Appendix 2). CRs were encouraged to seek information and support from local contacts and resources in their home country or region to help fill in any unknown answers (e.g. costs of screening).
Multiple steps were taken to ensure the accuracy of the data. First, CRs were asked to agree to an audit to validate responses. Second, when multiple CRs agreed to answer the questionnaire for the same programme, the same questions were asked to all CRs. Third, the CRs were asked to cite the source of their answers in all relevant cases and provide a copy to the researchers if accessible, additional sources were searched for and accessed online. Source material was translated via Google Translate when necessary for verification purposes. In order to track any inconsistencies in the definitions of terms used in the questionnaire, a glossary was created and distributed (Supplementary Appendix 3).
Validation of answers and clarification
Each survey response went through a verification and validation process (Figure 2, Supplementary Appendix 2). First, each response was checked for completeness and was cross-checked internally. In cases where multiple CRs completed the questionnaire for one programme, the consistency of each answer was evaluated between respondents. In all cases, answers were evaluated to other similar answers provided by the same respondent. Second, answers were cross-checked externally with source material provided and acquired through a programme-specific literature search. Third, a list of clarification questions was prepared for each CR from any discrepancies noted in the verification process. This list contained any questions pertaining to: incomplete responses or inconsistencies noted during the verification stage, accessible source material that was not provided, responses based on a source that was not referenced, or responses of actual values or calculations where the details were not described or were inconsistent with the definition provided in the glossary. Finally, a report was drafted illustrating the current situation for childhood hearing screening in each participating country or region. Details of the definitions, source, and date of data collection were also described. The CRs were sent a copy of the report and provided their final comments and/or confirmed the final validity of the document.
Data management
Demographic data such as World Bank classification, gross-domestic product (GDP) per capita, health expenditure and human development index (HDI) were added. The HDI uses life expectancy, education, and per capita income indicators to rank countries (United Nations Development Programme Citation2019; The World Bank Citation2019; World Health Organization Citation2019).
Programmes that use one test protocol to screen all eligible infants, are referred to as single-protocol design. A dual-protocol design is where a different protocol, which includes direct referral without screening, may be used for infants with an increased risk of HI. This group mainly consists of infants admitted to the neonatal intensive care unit (NICU); the other risk factors used are described in detail in Table 1, Supplementary Appendix 2. Infants with an increased risk of HI are referred to as “high-risk” infants, and all other infants, infants born well, are referred to as “low-risk” infants. Programmes that only screen high-risk infants are defined as selective programmes.
Cluster analysis
A hierarchical agglomerative cluster analysis using a complete-linkage method was performed in SPSS (v.26.0, SPSS Inc, Chicago, IL), to identify groups of NHS programmes with similar protocol designs. Due to the relatively small sample, the number of variables that could be included in the cluster analysis were restricted. Factors such as screening location, professional, and infant age were not considered, as these decisions may be driven by the existing local structure of postnatal care. The diversity of decisions made on NHS protocol designs, however, was unexplained. Five variables were selected that comprehensively described the entire NHS protocol design: the programme type (no NHS programme, selective programme, single-protocol programme, or dual-protocol programme), the choice of using OAE only versus including aABR in the protocol for low-risk infants, the number of steps for low-risk infants, the choice of using only OAE in step 1 (i.e. without aABR/ABR) for high-risk infants, and the number of steps for high-risk infants. The use of aABR technology in any step (low-risk) or step 1 (high-risk) was based on strategies recommended by the Joint Committee on Infant Hearing (Citation2019). A category of “Not Applicable” was available for each protocol variable and was assigned to countries without an established NHS programme or with selective screening where appropriate. Note that the definition of a “step” is that screening is performed and a result (i.e. pass or refer) is obtained. When both OAE and aABR screening tests are performed to obtain one result (pass or refer), this is considered one step. Because insufficient information was provided regarding the different protocols used across India, assigning values for these variables was not possible and India was not included in the cluster analysis.
Clustering NHS programmes into groups, can reveal key factors that may be related to design choices. In an agglomerative method, clusters are built up into larger and larger groups until all cases are included. This is represented in a dendrogram (Table 2, Supplementary Appendix 2). The dendrogram displays how closely countries or regions are linked together. The complete-linkage method was used to create links between countries or regions, as this method can be applied to categorical (nominal) data. In the complete-linkage method, the maximum distance between two clusters is calculated to determine the dissimilarity between all cases, resulting in a dissimilarity matrix for all pairwise comparisons. A Chi-square measure was used to calculate this distance. The resulting distance between clusters is that between the furthest possible points (Defays Citation1977). The optimal number of clusters was validated using the stopping rule with the agglomeration coefficients (indicating the heterogeneity between clusters) and a comparison of silhouette coefficients. Silhouette coefficients measure the strength of cluster cohesion and separation. To apply the stopping rule, the agglomeration coefficient chart was visually inspected across an increasing number of clusters to identify the point before a large drop in value. Finally, the dendrogram was inspected to consider the utility of the clustering.
Kruskal–Wallis tests (a non-parametric test to compare independent samples) were performed to evaluate measures of health expenditure per capita and HDI across clusters. Health expenditure per capita was selected as it represents the potential financial resources available for NHS on a system level, and HDI was selected as it represents a broader measure of social and economic growth on an individual level (scale of 0–1). p-values were adjusted by Bonferroni correction for multiple pairwise comparisons where applicable.
Results
Recruitment of hearing screening experts
Recruiting a local CR with knowledge about the local screening programme was the preferred option for sharing and aggregating data. However, the level of access to information of local CRs needed to be established. It took considerable effort to identify a single representative across all countries with the breadth of knowledge and equally importantly the time to fill out the full extensive questionnaire. For many programmes, CRs consulted other professionals with knowledge of the requested information to help them complete unknown answers. If the questionnaire could not be completed fully, additional CRs were engaged to complete the remainder of the questionnaire. This enabled data supply from multiple sources covering elements such as costs, prevalence and intervention options.
Even for some highly developed screening programs, some information was not recorded within the programme or was not accessible by the CR. This information was regularly unavailable for questions on intervention, quality indicators, costs, prevalence, and sensitivity/specificity of screening. For questions on NHS organisation, screening professionals, and target age and conditions, over 90% of the countries or regions provided complete answers (Figure 3, Supplementary Appendix 2).
During the collection of information, regular contact was maintained with CRs to encourage the data completion. Furthermore, CRs were supported when technical difficulties occurred. When registered CRs encountered difficulties or did not have access to the requested information, new CRs had to be found. The first hearing CR registered on 12 April 2017, the last complete questionnaire was submitted on 30 June 2019. It took a considerable amount of time for CRs to collect all necessary information and fill out the questionnaire. It took a mean of 114 days between registration on the website and submission of the questionnaire (median of 44, range from 1 to 558 days) (Figure 1, Supplementary Appendix 2).
Participating countries, regions, and reporting CRs
The web-based questionnaire was completed sufficiently by a total of 52 CRs from 45 countries out of at least 85 CRs approached (). CRs from Norway and South Africa did not fill out the questionnaire sufficiently for inclusion in the analysis. The participation of countries that were geographically located within or affiliated with Europe was actively sought. Out of all countries fully or partially geographically located in Europe, 39 were represented in the analysis. Faroe Islands participated independently from Denmark. All member states of the European Union participated, in addition to Israel as an associated state.
Figure 1. Flow chart displaying the number of participating countries, the number of reports made and the number of countries with NHS implemented. In some cases, multiple CRs filled out the questionnaire for the same country, in other cases, more reports were made for regions within the same country.
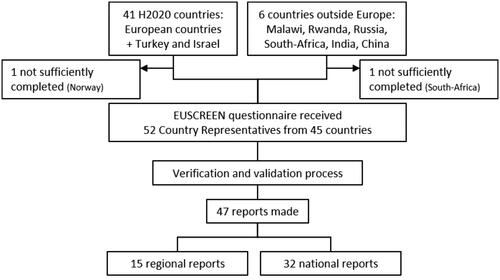
Despite the predominance of participating countries in or affiliated with Europe, participation in this study was open to any country. The additional countries supplied information that could not be collected from within Europe, i.e. from very large or highly populated countries and from low-income countries.
The participating countries included two low-income, three lower-middle income, 10 upper-middle income, and 30 high-income countries, according to the World Bank classification system (The World Bank Citation2019). The GDP per capita ranged from €195 to €92,600 (median: €16,560), and annual health expenditure per capita ranged from €30 to €8575 (World Health Organization Citation2019). HDI ranged from 0.477 to 0.944 (median: 0.870) (United Nations Development Programme Citation2019) (Table 2, Supplementary Appendix 2).
CRs were predominantly medical professionals, of which the majority were otorhinolaryngologists. Of all CRs, 30 were involved in the organisation of the screening programme, two supervised screening, 18 were involved in diagnostic assessment and intervention of infants failing the screening and two were involved in other health care provision.
Current status and presence of NHS programmes
A total of 42 established NHS programmes were included in the analysis. A complete list of participating countries and regions with their corresponding NHS programme (if applicable) can be found in , including the variety of protocols, organisational authority and reach. Because of the multi-step verification and validation process performed, there is a high level of confidence in the information reported in this article.
Table 1. Programme reach and protocols described for low and high-risk infants in each country or region.
Nationwide universal NHS is lacking in more low- and middle-income countries than high-income countries. For the 30 high-income countries surveyed, four do not have nationwide universal NHS, while for the 15 low- and middle-income countries surveyed, 12 lack nationwide universal NHS. These 12 countries are among the low- and middle-income countries with the lowest health care expenditure per capita and GDP per capita. Out of these 12 countries, five do not have an established NHS programme, however, most perform some sort of screening. NHS may be project-based, only performed in private hospitals or only some hospitals provide selective screening.
NHS in the EU was first implemented in the mid-1990s, and a sharp incline in NHS implementation can be observed in the mid-2000s. It took an average of 6 years (median of three years, range of 0–18 years) for countries to achieve nationwide reach after implementation (Figure 4, Supplementary Appendix 2).
Programme features
A dual protocol is reported to be followed in 29 programmes, which means that high-risk infants are screened using a different protocol as compared to low-risk infants. For nine NHS programmes, the same protocol is used for all infants, i.e. a single-protocol design. In Finland, a single or dual protocol may be used depending on the hospital. Malta and North Macedonia have selective programmes. All protocols are described in for individual programmes and summarised in .
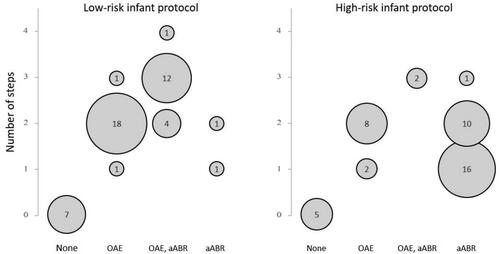
Figure 2. Type of protocol against number of steps. Protocols for low- and high-risk infants were combined with single-protocol design, in which the same protocol is used for both low- and high-risk infants. High-risk programmes that directly refer to diagnostic assessment without screening and programmes with varying protocols across a country or region were omitted. OAE, aABR: only the final step includes aABR, aABR for high-risk infants: aABR may be performed alone or together with OAE (OAE + aABR). A step is defined as that screening is performed and a result of pass or refer is obtained.
Variability was found across the indicators used to classify risk among participating NHS programmes with dual-protocol designs (Table 1, Supplementary Appendix 2). Admittance to the NICU is commonly used as an indicator for high risk, although, the minimum length of stay in the NICU varies. For many NHS programmes additional risk indicators were reported (range of 0–12, average of 6), which may cover the infants admitted to the NICU (e.g. assisted ventilation, infections) or not (e.g. family history, caregiver concern).
The type of test and the number of steps for both low and high-risk infants are displayed in . Some protocols were grouped together for the purpose of this comparison. Among protocols used for low-risk infants, a two-step protocol or three-step protocol is most frequent ( and ). In Hungary and Russia, a one-step protocol is used; however, repeat screening is allowed prior to discharge (i.e. counted in the same step). Among protocols used for high-risk infants, a one-step protocol or two-step protocol is most common ( and ). In the Wallonia-Brussels Federation (Belgium), high-risk infants are referred to diagnostics without screening. In Poland and Russia, high-risk infants are referred to diagnostics regardless of screening outcome. Fewer steps tend to be used when screening for HI among high-risk infants than for low-risk infants. The screening protocol for high-risk infants more often includes aABR to screen for retrocochlear disorders.
Within all 29 dual-protocol design programmes participating, OAE is used in step 1 for all low-risk protocols, and aABR is used in step 1 for 24 high-risk protocols. An aABR screen may be performed alone or together with an OAE screen in the same step (i.e. OAE + aABR). There are only two participating single-protocol programmes in which all infants are screened with aABR using either one or two steps. For the remaining seven single-protocol programmes, all infants are screened using OAE for steps 1 and 2. Two of these also have a third step using aABR.
Other NHS programme features such as screening professionals, screening location and the age of the infant at each step of screening varied across participating programmes (Table 3, Supplementary Appendix 2). A large array of professionals perform screening, including nurses, audiologists, midwives, otorhinolaryngologists, paediatricians, dedicated screeners, and health care workers or technicians. Infants in most NHS programmes typically complete step 1 in the hospital (maternity ward or NICU) before discharge. Low-risk infants typically leave the maternity hospital between 24 h and 72 h after birth, steps 2 and 3 are usually performed after discharge. High-risk infants stay in the NICU longer and steps 2 and 3 can take place before discharge if needed.
All 42 programmes are funded by the government or health insurance except for Cyprus and Poland, where the national programme is funded predominantly by charity. All participating NHS programmes are free for parents except in the Wallonia-Brussels Federation in Belgium where a fee is billed to parents upon discharge from the maternity hospital to supplement the costs. When infants are delivered in private maternity hospitals, parents would be charged for NHS by the hospital.
Aim of screening programme and criteria for referral
The Joint Committee on Infant Hearing (Citation2007) makes recommendations on target age: completion of screening by 1 month of age, completion of diagnostics by 3 months of age and initiation of intervention by 6 months of age. CRs from 36 programmes indicated that they follow an existing local guideline (Table 4, Supplementary Appendix 2). The typical age for hearing aid fitting was reported to be 0–6 months of age for 35 out of 42 NHS programmes. However, typical age of hearing aid fitting was reported to be above 6 months for four programmes without universal NHS and not specified for three programmes.
In addition to age, target conditions are also reported for the severity of HI for both screening and intervention. However, it is important to realise that target conditions are specified as a hearing threshold (dB HL) while OAE and aABR are screening technologies that estimate the integrity of auditory function. For screening, over half of the reporting programmes described a target condition of 20 dB HL or greater. Bilateral and unilateral HI are the reported target conditions for 22 low-risk infant and 17 high-risk infant programmes. Bilateral HI only is reported as the target condition for nine low-risk infant programmes and four high-risk programmes (Table 4, Supplementary Appendix 2).
In most cases, devices from one manufacturer are often selected for an entire NHS programme. A total of 13 different models across seven manufacturers were reported by 25 NHS programmes.
For most participating programmes, hearing aids are fit when a HI exceeds 30 to 40 dB HL (25 out of 41), though the range varied across programmes from ≥21 to ≥60 dB HL. For about half of the participating programmes, unilateral HI is fitted, even if on a case-by-case basis; for the other half, only bilateral HI is fitted. Despite this fact, most still refer infants that fail screening in one ear only.
Cluster analysis: screening protocols
Five clusters were optimally generated for 45 protocols of included countries or regions, except India. Silhouette values for four, five and six clusters were calculated to be 0.37, 0.58 and 0.62. It was not clear from the silhouette values alone whether to select a five- or six-cluster solution. The stopping rule was applied, which signified a stopping point at five clusters. Further inspection of the dendrogram (Table 2, Supplementary Appendix 2) confirmed this approach. The dendrogram revealed that, for a six-cluster solution, only two countries (Estonia and Faroe Islands) would form the sixth cluster. Because these two countries would ultimately be excluded from further analyses of health expenditure per capita and HDI in a six-cluster solution, the five-cluster solution was optimal for evaluation.
describes each of the five clusters. All countries without an established NHS programme were grouped into the first cluster: No NHS. Countries with selective screening (Malta and North Macedonia) were grouped into the second cluster: Selective NHS. The cluster analysis grouped countries and regions with existing universal NHS programmes into the remaining three clusters. In the Single OAE-only cluster, all infants (including high-risk) are screened using only OAE in step 1, and a single protocol design for both high- and low-risk infants is used in seven out of the nine programmes. In the Dual OAE-only cluster, an OAE-only protocol is used for low-risk infants in all 14 programmes, eleven of which have two steps. An aABR is used in step 1 for high-risk infants. Similarly, in the Dual including aABR cluster, aABR is used for high-risk infants; however, aABR is also used for low-risk infants either for initial screening or for rescreening. Ten programmes have three steps. In general, the number of steps were greater for low-risk protocols than for high-risk protocols. This is also revealed in , which displays the protocols for low- and high-risk infants across both single- and dual-protocol designs. It displays a preference for two-step OAE and three-step OAE, aABR protocols for low-risk protocols, while no obvious preference is revealed for high-risk protocols. All protocols are described in for individual programmes.
Table 2. Results of the cluster analysis.
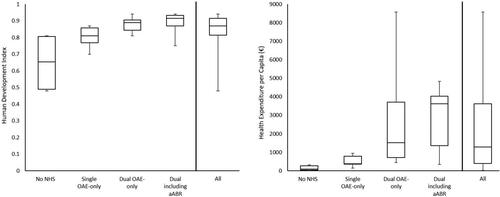
Kruskal–Wallis tests revealed significant differences in health expenditure per capita (p < 0.001) and HDI (p = 0.001) across all clusters except the Selective NHS cluster. These differences, including the percentiles for each metric across clusters are illustrated in . Because the Selective NHS cluster contained only two countries (Malta and North Macedonia), it was excluded from the analysis. Post hoc pairwise comparisons for health expenditure per capita revealed significant differences between the Single OAE-only and the Dual including aABR cluster (p = 0.016), between the No NHS and Dual including aABR cluster (p < 0.001), and between the No NHS and Dual OAE-only cluster (p = 0.006). Significant and near significant differences were also found for HDI between the Single OAE-only and Dual including aABR cluster (p = 0.013), between the No NHS and Dual including aABR cluster (p = 0.003) and between the No NHS and Dual OAE-only cluster (p = 0.055). Although some of the reported protocols were region-specific, health expenditure per capita and HDI were only available for the whole country. Values were not available for Faroe Islands, so this country was excluded from the Kruskal-Wallis analyses.
Figure 3. Box plots displaying the Human Development index and health expenditure per capita separately for each cluster expect the Selective NHS cluster and for all five clusters combined. The selective NHS cluster is not displayed because only two countries are included, Malta and North Macedonia. Health expenditure per capita and human development index for each country are available in , Supplementary Appendix 2. The No NHS cluster contains countries without established NHS. Single OAE only contains mostly protocols that screen infants with low and high risk for HI with the same protocol (OAE only). In Dual OAE-only and Dual including aABR different protocols are used to screen infants with low and high risk for HI. For protocols in Dual OAE-only, only OAE is used to screen low-risk infants; protocols in Dual including aABR include aABR. The box plots represent the median (centre line), the 25th and 75th percentile (length of the box) with whiskers indicating the minimum and maximum values.
Discussion
Results of this study show that, in most participating countries, the first screening step takes place 24–72 h after birth, before discharge from the maternity hospital. Countries with the lowest health expenditure and HDI do not have NHS yet. Among countries with NHS programmes, those with the lowest health expenditure and HDI use OAE only to screen all infants and use fewer screening steps. Screening is performed by a diversity of screening professionals. Implementation of NHS takes 6 years on average to scale up to nationwide screening.
In this study, a comprehensive survey was undertaken investigating all aspects of childhood hearing screening, from its organisation, protocol, intervention, outcomes and costs. The extensive international network of screening experts covered almost all countries in Europe. The information received was verified and validated ensuring high accuracy of the data reported. The original purpose of this study was to gather data about hearing screening programmes to populate a cost-effectiveness model, which is the main product of the EUSCREEN project. This model compares the cost-effectiveness of hearing screening programmes across countries, taking local circumstances into account.
However, much of the data essential for evaluation of cost-effectiveness, particularly the prevalence of HI, hearing screening outcomes, and costs, were not provided even by some countries with highly developed hearing screening programmes (Mackey et al. Citation2021). Data were sometimes not compiled in a central database, or data governance regulations prevented CRs from accessing or sharing the required information. Moreover, in some cases, CRs were well informed about one part of the hearing screening programme (e.g. screening low-risk infants) but were less familiar with other parts. It was a considerable time commitment to gather the requested information, both for the CRs and the researchers. Regular phone and email reminders, engagement with additional experts, and full-time technical support were needed to complete a study of this scale across countries where sustainable methods for quality control were often lacking. The lack of data that can be compared across countries may perpetuate the lack of uniformity of hearing screening programmes in Europe. In the future, a cross-border exchange of data can only be feasible if data collection, monitoring and quality control exist across all programmes.
From our data, we can derive at least five essential questions with which policy makers are faced. First, they must decide on the location of screening, which may be connected to the age at which an infant is screened. Screening can take place before discharge from the maternity hospital within the first days after birth or later, e.g. in the hospital, healthcare centre or at home. Next, the professional performing the screening should be decided on; this could be someone already employed at the screening location (e.g. nurse, midwife or physician) or someone specially hired and trained to only perform screening. Third, the type of screening device(s), either OAE, aABR or a combination of both, and fourth, the number of steps, make up the screening protocol. Finally, policy makers must decide if a separate protocol will be used for high-risk infants. It is unclear and debateable if one set of guidelines for NHS implementation would be ideal or even beneficial among countries with high variability across postnatal care, resources available and health care organisation, as certain decisions on NHS policy are made based on local circumstances.
In countries where the majority of infants are born in a maternity hospital, performing the first step of screening before discharge ensures high coverage. Completing all screening steps in the maternity hospital can avoid loss to follow-up that could otherwise occur between screening steps. However, residual amniotic fluid in the middle ear reduces the number of infants that pass screening when screening is performed within the first days after birth (Berninger and Westling Citation2011). In some countries, screening professionals are already involved in postnatal care, ranging from nurses to physicians, whereas other countries use dedicated screeners for NHS. Although professionals with a medical background may require less initial training, which lowers initial costs, combining screening with other work can result in less practice and higher overall referral rates, compared to dedicated screeners (Vohr et al. Citation2001).
This study found that the provision of NHS and the screening protocol – the use of both OAE and aABR, the number of screening steps, and screening high-risk infants with a separate protocol – are related to health expenditure and HDI. In middle- or low-income countries with fewer resources available for preventive healthcare, a single screening protocol using only OAE may be chosen because of initial cost savings. The cost for aABR screening, including the device, consumables, screening time and training, is up to two times higher than the cost for OAE screening (Boshuizen et al. Citation2001; Vohr et al. Citation2001). Policy makers should be aware that excluding aABR from the protocol for both low- and high-risk infants, may put these countries at a long-term disadvantage. Screening with aABR is less influenced by middle ear fluid, and including aABR in the protocol reduces the total number of false positives (Caluraud et al. Citation2015). Reducing the number of referrals eases the burden on diagnostic centres and can reduce the number of infants lost to follow-up (Mackey et al. Citation2021). Thereby, the overall effectiveness of the NHS programme may be higher when including aABR, particularly in countries where loss to follow-up is a concern. Furthermore, because of the higher prevalence of retrocochlear disorders among high-risk infants, aABR is the recommended technology for screening among the high-risk population (EFCNI (European Standards of Care for Newborn Health project report) Citation2018; Joint Committee on Infant Hearing Citation2019). Despite these points, an NHS programme with OAE screening may be the preferred choice for low- and middle-income countries with limited resources particularly during early stages of implementation.
It proved to be difficult to gather detailed data, and in many countries essential data on NHS needed for evaluation could not be reported on a regional or national scale. When screening outcomes are not collected on a programme-wide level, a screening programme cannot be evaluated, nor can it be compared to screening programmes of other countries. This lack of monitoring and evaluation likely perpetuates the diversity in screening protocols across countries and regions. To be able to exchange information routinely and reliably, NHS programmes need good, sustainable monitoring systems.
Supplemental Material
Download MS Word (21 KB)Supplemental Material
Download MS Word (269.7 KB)Supplemental Material
Download MS Word (32 KB)Acknowledgements
Members of the EUS€REEN foundation contributed information from their local screening programmes. The following are members of the EUS€REEN Foundation contributing to this work: B. Qirjazi, D. Holzinger, L. Stappaert, B. Vos, F. Brkić, P. Rouev, X. Peng, M. Velepic, C. Thodi, J. Drsata, T. Ovesen, M. Bambus, M. Lepplaan, B. Ellefsen, R. Niemensivu, T. Willberg, F. Denoyelle, P. Matulat, T. Nikolopoulos, A. Gáborján, I. Hinriksdóttir, Z. Chaudhurri, G. Norman, L. Rubin, A. Martini, D. Spanca, M. Audere, S. Kušķe, N. Drazdiene, E. Lesinskas, J. M. Hild, M. Cakar, T. Fenech, W. Mulwafu, D. Chiaburu, T. Kujundžić, E. Zvrko, A. Meuwese, A. Goedegebure, H. Hoeve, V. Nagaraj, G. Greczka, L. Monteiro, M. Georgescu, G. Tavartkiladze, L. Gouma, S. Filipovic, G. Jokovic, L. Langova, I. Sebova, S. Battelino, D. W. Swanepoel, F. Núñez-Batalla, J. M. Sequi-Canet, I. Uhlén, B. Nora, M. Baydan and J. McCall.
Disclosure statement
The authors have no conflicts of interest relevant to this article to disclose.
Additional information
Funding
References
- Berninger, E., and B. Westling. 2011. “Outcome of a Universal Newborn Hearing-Screening Programme Based on Multiple Transient-Evoked Otoacoustic Emissions and Clinical Brainstem Response Audiometry.” Acta Oto-Laryngologica 131 (7): 728–739. doi:https://doi.org/10.3109/00016489.2011.554440.
- Boshuizen, H. C., G. J. Van Der Lem, D. E. Kauffman, M. A. Boer, G. A. VAN Zanten, A. M. Oudesluys-Murphy, and P. H. Verkerk. 2001. “Costs of Different Strategies for Neonatal Hearing Screening: A Modelling Approach.” Archives of Disease in Childhood. Fetal and Neonatal Edition 85 (3): F177–F181. doi:https://doi.org/10.1136/fn.85.3.f177.
- Caluraud, S., A. Marcolla-Bouchetemble, A. DE Barros, F. Moreau-Lenoir, E. DE Sevin, S. Rerolle, E. Charriere, et al. 2015. “Newborn Hearing Screening: Analysis and Outcomes after 100,000 Births in Upper-Normandy French Region.” International Journal of Pediatric Otorhinolaryngology 79 (6): 829–833. doi:https://doi.org/10.1016/j.ijporl.2015.03.012.
- Ching, T. Y. C., H. Dillon, G. Leigh, and L. Cupples. 2018. “Learning from the Longitudinal Outcomes of Children with Hearing Impairment (LOCHI) Study: Summary of 5-Year Findings and Implications.” International Journal of Audiology 57 (Suppl 2): S105–S111. doi:https://doi.org/10.1080/14992027.2017.1385865.
- Davis, A., J. Bamford, and J. Stevens. 2001. “Performance of Neonatal and Infant Hearing Screens: Sensitivity and Specificity.” British Journal of Audiology 35 (1): 3–15. doi:https://doi.org/10.1080/03005364.2001.11742727.
- Defays, D. 1977. “An Efficient Algorithm for a Complete Link Method.” The Computer Journal 20 (4): 364–366. doi:https://doi.org/10.1093/comjnl/20.4.364.
- European Consensus Statement on Neonatal Hearing Screening. 1999. “European Consensus Statement on Neonatal Hearing Screening. Finalized at the European Consensus Development Conference on Neonatal Hearing Screening.” Acta Paediatrica 88: 107–108. doi:https://doi.org/10.1080/08035259950170745
- Joint Committee on Infant Hearing. 2007. “Year 2007 Position Statement: Principles and Guidelines for Early Hearing Detection and Intervention Programs.” Pediatrics 120: 898–921. doi:https://doi.org/10.1542/peds.2007-2333
- Joint Committee on Infant Hearing. 2019. “Year 2019 Position Statement: Principles and Guidelines for Early Hearing Detection and Intervention Programs.” The Journal of Early Hearing Detection and Intervention 4: 1–44.
- Kanji, A., K. Khoza-Shangase, and N. Moroe. 2018. “Newborn Hearing Screening Protocols and Their Outcomes: A Systematic Review.” International Journal of Pediatric Otorhinolaryngology 115: 104–109. doi:https://doi.org/10.1016/j.ijporl.2018.09.026.
- Mackey, A. R., A. M. L. Bussé, H. L. Hoeve, A. Goedegebure, G. Carr, H. J. Simonsz, and I. M. Uhlèn. 2021. Assessment of hearing screening programmes across 47 countries or regions II: Coverage, referral, follow-up and detection rates from newborn hearing screening Submitted for publication.
- EFCNI. 2018. European Standards of Care for Newborn Health: Hearing screening [Online]. Mader, S., Thiele, N., Walz, J. M., eds. Available: https://newborn-health-standards.org/hearing-screening/ [Accessed December 5 2019].
- Sloot, F., H. L. Hoeve, M. L. DE Kroon, A. Goedegebure, J. Carlton, H. J. Griffiths, and H. J. Simonsz, EUS€REEN Study Group. 2015. “Inventory of Current EU Paediatric Vision and Hearing Screening Programmes.” Journal of Medical Screening 22 (2): 55–64. doi:https://doi.org/10.1177/0969141315572403.
- Stevens, J., D. M. B. Hall, A. Davis, C. M. Davies, and S. Dixon. 1998. “The Costs of Early Hearing Screening in England and Wales.” Archives of Disease in Childhood 78 (1): 14–19. doi:https://doi.org/10.1136/adc.78.1.14.
- The World Bank. 2019. World Bank Country and Lending Groups [Online]. The World Bank. Available: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups [Accessed 03-06-2019 2019].
- United Nations Development Programme. 2019. Global Human Development Indicators [Online]. United Nations Development Programme. Available: http://hdr.undp.org/en/countries [Accessed 12-09-2019 2019].
- Uus, K., J. Bamford, and R. Taylor. 2006. “An Analysis of the Costs of Implementing the National Newborn Hearing Screening Programme in England.” Journal of Medical Screening 13 (1): 14–19. doi:https://doi.org/10.1258/096914106776179764.
- Vohr, B. R., W. Oh, E. J. Stewart, J. D. Bentkover, S. Gabbard, J. Lemons, L. A. Papile, and R. Pye. 2001. “Comparison of Costs and Referral Rates of 3 Universal Newborn Hearing Screening Protocols.” Journal of Pediatrics 139 (2): 238–244. doi:https://doi.org/10.1067/mpd.2001.115971.
- Vos, B., C. Senterre, R. Lagasse, G. Tognola, and A. Leveque. 2016. “Organisation of Newborn Hearing Screening Programmes in the European Union: Widely Implemented, Differently Performed.” European Journal of Public Health 26 (3): 505–510. doi:https://doi.org/10.1093/eurpub/ckw020.
- White, K. R. (2011). “Universal Infant Hearing Screening: Successes and Continuing Challenges. Keynote Address.” Proceedings of Phonak's 5th International Pediatric Conference: A Sound Foundation through Early Amplification. Chicago, IL: November 8–10, 2010
- World Health Organization. 2009. Newborn and infant hearing screening [Online]. WHO. Available: https://www.who.int/blindness/publications/Newborn_and_Infant_Hearing_Screening_Report.pdf?ua=1 [Accessed 13-03-2019 2019].
- World Health Organization. 2019. Global Health Expenditure Database [Online]. World Health Organisation. Available: http://apps.who.int/nha/database/Select/Indicators/en [Accessed 03-06-2019 2019].
- Yoshinaga-Itano, C., and M. L. Apuzzo. 1998a. “The Development of Deaf and Hard of Hearing Children Identified Early through the High-Risk Registry.” American Annals of the Deaf 143 (5): 416–424. doi:https://doi.org/10.1353/aad.2012.0118.
- Yoshinaga-Itano, C., and M. L. Apuzzo. 1998b. “Identification of Hearing Loss after Age 18 Months is Not Early Enough.” American Annals of the Deaf 143 (5): 380–387. doi:https://doi.org/10.1353/aad.2012.0151.
- Yoshinaga-Itano, C., A. L. Sedey, D. K. Coulter, and A. L. Mehl. 1998. “Language of Early- and Later-Identified Children with Hearing Loss.” Pediatrics 102 (5): 1161–1171. doi:https://doi.org/10.1542/peds.102.5.1161
