Abstract
Objective
To inventory provision and features of childhood hearing screening after the newborn period (CHS), primarily in Europe.
Design
From each participating country or region, experts provided information through an extensive questionnaire: implementation year, age at screening, test method, pass criteria, screening location, screener profession, and quality indicators: coverage, referral, follow-up and detection rates, supplemented by literature sources.
Study sample
Forty-two European countries or regions, plus Russia, Malawi, Rwanda, India, and China.
Results
CHS was performed universally with pure-tone audiometry screening (PTS) in 17 countries or regions, whereas non-universal CHS was performed in eight with PTS or whisper tests. All participating countries with universal PTS had newborn hearing screening. Coverage rate was provided from three countries, detection rate from one, and referral and follow-up rate from two. In four countries, universal PTS was performed at two ages. Earliest universal PTS was performed in a (pre)school setting by nurses (n = 9, median age: 5 years, range: 3–7), in a healthcare setting by doctors and nurses (n = 7, median age: 4.5 years, range: 4–7), or in both (n = 1).
Conclusions
Within universal CHS, PTS was mostly performed at 4–6 years by nurses. Insufficient collection of data and monitoring with quality indicators impedes evaluation of screening.
Introduction
Early detection of a hearing impairment (HI) and subsequent intervention have a positive effect on language, cognitive and social outcomes in children (Yoshinaga-Itano et al. Citation1998, Moeller Citation2000). To reach this goal, several guidelines and position statements recommend screening for HI among infants and children (Skarżyński and Piotrowska Citation2012; European Consensus Statement on Neonatal Hearing Screening Citation1999). Childhood hearing screening after the newborn period (CHS) uses a variety of methods. The distraction test (Ewing and Ewing Citation1944) was widely used in high-income countries to screen children for HI around the age of 7–9 months; however, it has since been replaced by universal newborn hearing screening (NHS). In a universal hearing screening programme, screening is provided to all children, regardless of their risk for HI. The whisper test can be administered to children from age 3 years and requires no equipment and little training, thereby making it feasible for lower-income settings (Pirozzo, Papinczak, and Glasziou Citation2003). Though, due to its poor reliability and a sensitivity of only 70–87% in children, its use is of limited value for CHS (Pirozzo, Papinczak, and Glasziou Citation2003). Pure-tone audiometry screening (PTS) can be performed with a frequency sweep or a limited series of frequencies and intensities which results in a pass or fail result. The earliest report of using PTS for children was published in 1955 (Ewing Citation1955). It has since become the standard practice for screening children aged 3 years and older (Yong et al. Citation2020b) because of its high sensitivity and reliability (Fortnum et al. Citation2016). PTS requires calibrated equipment, trained personnel, and a behavioural response from the child. Including tympanometry to evaluate middle ear function in the screening session can determine the appropriate referral pathway (i.e. medical or audiological) after a failed hearing test (American Academy of Audiology Citation2011). Automated methods are currently under development that will allow screening with, for example, speech stimuli to be performed via a smartphone or tablet (Denys et al. Citation2019; De Sousa et al. Citation2020).
The prevalence of permanent HI increases from 1–2 per 1000 at birth to 2–3 per 1000 by the age of 9 years (Le Clercq et al. Citation2017; Fortnum et al. Citation2001). Without screening, some children with a HI may remain undetected and lack appropriate intervention throughout important developmental and educational years (Watkin and Baldwin Citation2011). Children with a congenital HI may not be detected due to issues related to the NHS programme such as false negatives, loss to follow-up, parental refusal, or because they were not offered screening (Fortnum Citation2003; Prieve et al. Citation2015). Even a well-executed NHS programme may not be designed to target mild or unilateral HI, of which negative effects may become apparent particularly in early school years (Bess, Dodd-Murphy, and Parker Citation1998; Winiger, Alexander, and Diefendorf Citation2016). Many countries have targeted surveillance programmes after NHS, where children with risk factors are regularly scheduled for audiological assessment; however, there are limitations to this system, particularly the high loss to follow-up among children being monitored (Beswick et al. Citation2012).
Hearing impairment may be conductive or sensorineural, congenital or acquired, and permanent or transient. Most sensorineural HI detected after the newborn period is a delayed-onset HI. Delayed-onset HI has a prevalence of around 0.7 per 1000 children aged 3–7 (Lü et al. Citation2011) and may be caused by hereditary factors or pre- or perinatal infections, the most common being cytomegalovirus. Acquired sensorineural HI may be caused by ototoxic medications, trauma, or postnatal infection (Smith, Bale, and White Citation2005). The most common type of HI that is found among children is an acquired conductive HI caused by otitis media, a condition of the middle ear. Two types of otitis media are chronic suppurative otitis media (CSOM) and otitis media with effusion (OME).
CSOM is characterised by a discharge of fluid from the middle ear through a perforation in the tympanic membrane. The prevalence rate is approximately 2–2.5% in children aged 1–10 years, with no major differences across age (Monasta et al. Citation2012). CSOM is considered to be a particular burden for developing countries, and the most common cause of mild to moderate HI among children in these countries, with at least half the cases of CSOM resulting in associated HI (World Health Organization Citation2004). If left untreated, CSOM can cause permanent HI in over 90% of cases (Jensen, Koch, and Homøe Citation2013).
OME is characterised by the presence of fluid behind an intact tympanic membrane. OME is extremely common among young children. Prevalence rates range from 1% to 30% in children aged 1–8 years worldwide, with a peak at age 3–4 years (Casselbrant and Mandel Citation2003; Mandel et al. Citation2008). At least half of the children with OME will have an associated HI (Gravel Citation2003). Although most OME resolves within 3 months, up to 40% of affected children will have recurrent or longstanding OME requiring intervention (Rosenfeld et al. Citation2016). It is unclear whether targeting cases of OME in a CHS programme is cost-effective (Rosenfeld et al. Citation2016).
The EUSCREEN study compares the cost-effectiveness of vision and hearing screening programmes across participating countries, given the local circumstances in a country or region. In combination with our previous studies on NHS (Bussé et al. Citation2021; Mackey et al. Citation2021), results of this article will aid the development of a decision-analytic cost-effectiveness model produced within the EUSCREEN study. Unlike NHS, the importance of CHS has not been universally established. High quality information that is needed to make a conclusive judgement on the cost-effectiveness of CHS is currently lacking. This hampers the ability of policy makers and professionals to decide which programmes should be implemented and how. This study reports on the current provision, protocols, and outcomes of CHS for children after the newborn period via an international inventory of hearing screening programmes. It also compares its practice against local healthcare and school structure. This article evaluates the provision of CHS, funding source, age at which children are screened, test method, year of implementation, pass criteria, screening location, screener profession, quality measures (coverage, referral, follow-up and detection rate), and costs, supplemented by literature sources.
Methods
From each of the originally selected countries primarily in Europe, hearing screening experts, the so-called Country Representatives (CR), were recruited via professional networks, scientific journal articles, or existing professional connections. CRs from countries in Europe or with affiliated research programmes were actively sought. Participation in the study was open to any country, and experts from five non-European countries expressed interest in participation. Involvement of countries outside of Europe, such as from large, highly populated countries or low-income countries, could add information that would not be available from within a European-only context. CRs with the time available to complete the questionnaire were required to confirm their role and expertise in relation to their hearing screening programme. They formed the Country-Committee Joint-Partnership of EUS€REEN Foundation and were remunerated up to €2000 for sufficiently completing the questionnaire to formulate a country- or region-specific report detailing the strategies for screening.
Data collection
The EUSCREEN questionnaire contained 191 hearing questions, 51 of which related specifically to CHS. The remaining questions related to NHS or general early detection and intervention practice. Questions were a combination of multiple choice, yes-no, and open-ended questions and were subdivided into nine domains: demography and epidemiology, administration and general background, existing screening programmes, coverage and attendance, tests and devices, follow up and diagnostic assessment, treatment options, costs and benefits and adverse effects. A sub-question typically followed each question, asking the respondent to state the source of the information. Possible answers were (a) Data unavailable, (b) I don’t know, (c) Rough estimate, (d) Real estimate from calculation, or (e) Actual data. The name and date of the data source, in addition to source materials were requested if relevant. The questionnaire was made accessible to the CR through the EUSCREEN website (www.euscreen.org) via a unique username and password.
After submission, the questionnaire was checked for completeness and all answers went through a verification and validation process (Figure 1, Appendix). Answers were evaluated for completeness and cross-checked across similar questions. Any material provided by the CR or acquired via an online search were cross-checked to the provided answers. All discrepancies were sent to the CR for clarification. A country-specific report was written which was delivered back to the CR for final confirmation. Information on CHS after the newborn period was then extracted from the reports for evaluation.
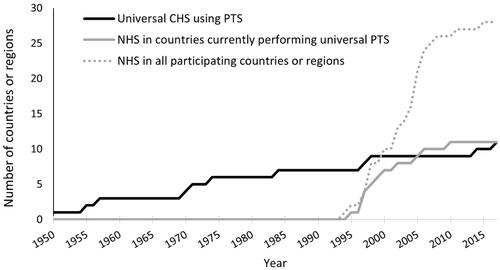
Figure 1. The cumulative number of participating countries or regions implementing newborn hearing screening (NHS) and universal childhood hearing screening (CHS) with pure-tone audiometry screening (PTS) according to the reported year of implementation. Information on implementation year was available for 28 NHS and 11 universal CHS programmes. All programmes with universal CHS used PTS. PTS started as early as 1950 with a slow spread across participating countries or regions. Only four implemented new programmes since 2000. Two programmes implemented PTS after NHS. No information was included about the use of other methods for CHS (e.g. the distraction test) in these countries. The first NHS programme was implemented in 1994 with a rapid increase from 1995 to 2010.
Quality indicators
Screening performance should be evaluated through predetermined quality measures. Data on four key quality measures were collected to assess CHS programmes (AMERICAN ACADEMY OF AUDIOLOGY Citation2011): coverage rate, referral rate, follow-up rate and detection rate. Coverage rate was the percentage of children screened out of all eligible children. Referral rate was the percentage of children referred from screening, either to a rescreening step or to diagnostic assessment, out of all children screened. Follow-up rate was the percentage of children who attend the diagnostic assessment out of all children referred. Detection rate was the percentage of children detected with a HI (targeted by the programme), out of all children screened. Additional measures may also be used to evaluate screening (e.g. sensitivity and specificity), but these are not reported in this article.
Assessment of CHS
The questions on the status of CHS included whether it was provided, the year it was first implemented, and the funding source (e.g. parents, health insurance, state). Questions on the protocol covered the age at screening (open-ended), the test method (e.g. pure-tone screening, whisper test), the pass criteria and the number of rescreening tests performed prior to referral for audiological or ENT assessment if pass criteria were not met. Pass criteria for PTS were defined as a valid response at a minimum intensity (dB HL) across specified frequencies, in either one ear or both ears.
An open-ended question on screener profession (e.g. nurse, audiologist) was evaluated, in addition to a multiple-choice question on the screening location (e.g. child health clinic, school, kindergarten), where several options may be selected including an open choice, under the heading of ‘other’. The term for early education prior to primary school varied across countries and for children of different ages. For this article, pre-school was defined as all pre-primary school systems up to primary-school start. The questions on costs included both the total cost of the screening programme and the cost per child screened.
Supplemental literature data on coverage for CHS
High coverage of CHS can be ensured by combining screening with school attendance, healthcare appointments or with vaccination. Data from supplemental literature were aggregated to investigate the context for ensuring high coverage rates for CHS for all countries and regions that participated in data collection, including those without CHS. Indicators of school and routine child healthcare during childhood were compiled from the following databases: European Commission/Eacea/Eurydice (Citation2015), UNESCO (Citation2020), UNICEF (Citation2012), and World Health Organisation (Citation2020, 2019a, 2019b). These included the age, location, and coverage rate of the second dose of measles-containing vaccine; the out-of-school rate for primary school-age children; and the participation rate in pre-school.
Results
Out of 49 countries or regions, the questionnaire was sufficiently completed in 47, which were included in the overall study. Two countries were excluded because the information supplied was not sufficient to complete a descriptive report (South Africa and Norway). Among the included countries were 30 high income (HIC), 10 upper-middle income, 3 lower-middle income and 2 low income, according to the World Bank classification system The World Bank (Citation2019) (Table 1, Appendix).
Table 1. Age at screening, test methods, pass criteria, screening profession and location for the 17 countries or regions performing universal childhood hearing screening with pure-tone audiometry screening (PTS).
Provision of CHS
and provide an overview of the provision of CHS in 17 participating countries or regions with universal CHS and 8 countries or regions that offer non-universal or non-routine CHS. In all 25 countries or regions where CHS is offered, NHS is also performed. CRs from the remaining 22 countries or regions indicated that their country did not offer CHS. In 20 programmes, the type of funding for CHS was reported: 14 were funded by either the regional or national government and six by health insurance.
Table 2. Description of childhood hearing screening after the newborn period among 8 countries or regions that offered non-routine or non-universal screening.
The distraction test at 7–9 months of age was not reported to be used by any programme. The whisper test was used by three countries or regions for children aged 5–8 years of which one also used PTS. PTS was used for screening children aged 3–8 years in 20 countries or regions, alone or in combination with tympanometry, speech test or a tuning fork test (). The test method was unknown for three countries.
In eight countries or regions, limited or variable screening was reported, which took place during short periods (i.e. project-based), during paediatric doctors’ appointments (i.e. non-systematic), or intermittently during screening camps (). In 17 countries or regions, there was universal screening, all using PTS (). With the exception of Bulgaria and Serbia, all were high-income countries. The following sections provide findings from these 17 programmes with universal CHS, all performing PTS.
Implementation of PTS
Universal PTS started in 1950, well before NHS (). In 2016, the Flanders region of Belgium stopped screening universally at age 3, and instead, screening was only performed on children with risk factors for delayed-onset HI. Universal screening is still performed at age 5–6. In 2015 in England, expansion of screening in other health care districts was not recommended while existing CHS could continue to operate. In 2012, Iceland terminated their CHS programme.
Quality indicators of PTS
Coverage rates were only provided by CRs in three out of the 17 countries or regions with universal screening. Referral rates from screening and follow-up rates to the diagnostic assessment were only provided by CRs from two countries. Data on detection rate after PTS were reported from one country.
Coverage rates were provided from Israel, Luxembourg and Serbia. A 97% coverage rate was reported from Israel, based on a 2015 audit, and 99% from Luxembourg based on data from 2018. In Serbia, the coverage rate in 2017 was 45% for the entire country and 92% for the Belgrade Region. CRs from four additional programmes reported rough estimations of their coverage rates, ranging from 90 to 100%.
Referral rates from screening and follow-up rate to the diagnostic assessment were reported from Luxembourg and Israel. In Luxembourg, 10% did not pass the first screening and were invited to rescreening, after which 7.6% of all infants first screened were referred to diagnostic assessment in 2018. Out of these children, 58% followed up to diagnostic assessment. In Israel, 7.9% were referred from screening to diagnostic assessment in 2015. A follow-up rate of 77% was found by a survey among parents.
The detection rate after PTS was only reported from Israel; 0.012% of infants screened were diagnosed with a previously undetected permanent HI ≥25 dB HL. Little information was provided on OME or CSOM. Five CRs provided estimated prevalence rates; however, the estimated rates varied dramatically (3.5% of all children to 35% of pre-school-age children).
Protocol for PTS
Across the 17 universal PTS programmes, universal screening was performed at two different ages in four programmes: at 3–5 years and again at 6–7 years. The age of children at the earliest screen was 3–4 years (4 programmes), 4–5 years (5 programmes), 5–6 years (4 programmes), and 6–7 years (4 programmes).
In all 17 programmes with universal PTS, both ears were screened. In all programmes except for that in Latvia, children were referred for diagnostic assessment if suspected with a unilateral or bilateral HI. In 12 programmes, children who did not pass initial PTS were referred to an audiology or ENT clinic for diagnostic testing. In the other five programmes, one or two rescreening tests were offered before referral to diagnostic testing. The intensity (in dB) used as a pass criteria for PTS was available for all 17 programmes and ranged from 20 to 40 dB. In most programmes, the frequencies 500–4000 Hz were used. In two programmes 500 Hz was excluded, and in two others there is higher pass intensity at 500 Hz. The median pass intensity was 30 dB for PTS performed on children aged 3–4 years, and 25 dB for PTS on children over age 4.
Professionals and locations for PTS
CRs from all 17 universal PTS programmes provided information on location and professionals (). For nine programmes, nurses screened children aged 3–7 years (median: 5) in a (pre)school setting. For seven programmes, doctors and nurses screened children aged 4–7 years (median: 4.5) in a healthcare setting. In the Netherlands, nurses performed screening in either a healthcare or school setting.
Costs of PTS
Most CRs could not report on costs of screening. The reported cost per child screened ranged from 5 to 45 euros; the specific costs included in each estimation were not specified (e.g. equipment, disposables, salary). Therefore, these values may have included varying aspects of the screening programme.
Supplemental literature data on coverage for CHS
Table 1 in the Appendix lists the enrolment in pre-school and primary school education together with economic status, age and coverage of measles-containing vaccine. The median participation rate in the final year of pre-school education for 10 low- and middle income countries (LMIC) was 83% compared to 98% for 31 HICs (UNESCO. Citation2020). In contrast, the median participation rate for primary school differed only slightly between participating LMICs (95%) and HICs (98%). Twenty-five participating countries (16 HICs and nine LMICs) offered the second dose of the measles-containing vaccine during the pre-school or school-entry years (3–7 years) (World Health Organization Citation2020). According to the World Health Organisation, out of these nine LMICs, five offered the vaccine in schools (The European Observatory on Health Systems and Policies Citation2018; World Health Organization Citation2019b).
Discussion
This study revealed that PTS is performed only in some countries that also have NHS, and most are high-income. The distraction test at 9 months was not reported to be used in any participating country. The whisper test, easy to perform but with low sensitivity, is only performed by three countries or regions. In 20 countries or regions, PTS is used alone or in combination with tympanometry, speech testing, or tuning fork tests, 17 of which are universal programmes. Universal PTS is performed by nurses in a (pre)school setting in nine programmes for children at a median age of 5 years (range 3–7 years), in a healthcare setting by nurses and doctors in seven programmes for children at a median age of 4.5 years (range: 4–7 years), and in both for one programme.
Although we originally aimed to compare the effectiveness of CHS programmes in this study, coverage rates could only be provided by three programmes, referral and follow-up rates by two and detection rates by one. It is apparent that data are not routinely collected and that monitoring and quality control are not performed in the majority of screening programmes. This makes it impossible to compare the effectiveness of hearing screening programmes and perpetuates the variety in screening programmes.
When implementing a CHS programme, healthcare policy makers have to make some key decisions within the context in their country, including the screening location, the professional performing the screening, the test method, and the age at which a child is eligible for screening. The location of screening may be a healthcare centre or school. A (pre)school setting is a viable place for routine health services to ensure high coverage if enrolment rates are favourable and lack disparity, which may not be the case in some countries in Europe (UNICEF. Citation2012). Integrating screening with other healthcare services, such as immunisation, also leads to high coverage rates and equitable care (Okwo-Bele Citation2012). The screening professionals could typically be nurses, nurse assistants, doctors, audiologists or speech-language therapists. When deciding on a screening professional, costs of experienced professionals and extensive training may be justified if it ensures high specificity of the screening.
When deciding on the test method, policy makers must consider that the feasibility of various hearing screening tests for CHS depends on the age of the child and the costs. Before 3 years, PTS is typically not possible, as many children this age are not mature enough to learn the task in a short time or maintain attention throughout the screening. From 3 years onward, PTS is the most commonly used method for CHS, because it has high sensitivity and specificity under ideal conditions (Fortnum et al. Citation2016). PTS will detect both sensorineural and conductive HI, including conductive HI caused by OME or CSOM. Tympanometry supplements PTS in two programmes and provides an indication of the presence or absence of fluid in the middle ear. PTS requires a quiet location, best realised through a sound-proof room. PTS also requires well-trained screeners and a screening audiometer, which needs regular calibration. All of these prerequisites may be especially scarce in lower income or more remote areas (Yong et al. Citation2020b). The whisper test is still performed in some countries in this study, which requires no equipment and little training; however, reliability and sensitivity are poor (Pirozzo, Papinczak, and Glasziou Citation2003). None of the participating countries reported use of new automated technologies for performing CHS with speech stimuli via a smartphone or tablet, that are based on hearing screening tests for adults using speech perception in noise (Denys et al. Citation2019; De Sousa et al. Citation2020).
After choosing the test method, the age at which screening is provided and the pass criteria must be decided upon. The advantage of screening from age 3 to 4 is so that intervention can be supplied as early as possible. However, sensitivity and specificity may be lower when screening is performed at 3–4 years because of difficulty with sustained attention and less reliable responses to a low intensity tone in the presence of background noise (Browning Citation2000). This may also explain the 5 dB difference in the reported pass criterion between younger and older ages. Additionally, OME is highly prevalent at this age (Browning Citation2000) which will lead to higher referral rates of children with a conductive HI. Although not consistent across all studies (e.g. Fitzpatrick, Whittingham, and Durieux-Smith Citation2014), permanent mild HI may have a negative effect on speech and language development and school performance (Bess, Dodd-Murphy, and Parker Citation1998; Winiger, Alexander, and Diefendorf Citation2016). It may be crucial to identify children with mild permanent HI and persistent OME before or soon after they start school, so that audiological intervention and accommodation in the classroom can be provided (McKay Gravel and Tharpe. Citation2008).
With PTS, a lower pass intensity will increase the number of cases detected with mild HI, while a higher pass intensity may more efficiently identify the more severe cases, which could be particularly resourceful in countries with limited resources (Mahomed-Asmail, Swanepoel, and Eikelboom Citation2016). The use of a low-frequency tone (250 and 500 Hz) was not consistent practice across participating programmes. Lower pass intensity and inclusion of low frequencies will also capture many children with CSOM or OME, which often presents as a mild HI in the lower frequencies (Silman, Silverman, and Arick Citation1994). Environmental noise may interfere with a low-frequency tone, which can result in a higher number of false positives (McPherson et al. Citation2010). The decision to include low frequencies may depend on local circumstances, including the likelihood of environmental noise interference in the chosen screening locations, the existing intervention pathways, and the local prevalence rates of OME and CSOM at the age when screening is performed. Including low frequencies in the test protocol improves the sensitivity of the screen, capturing children with a low-frequency HI (McPherson et al. Citation2010).
In contrast to universal NHS for which cost-effectiveness has largely been established (Sharma et al. Citation2019), that of CHS is not clear (Fortnum et al. Citation2016). NHS has seen rapid widespread implementation since 1995. However, children with a HI that develops after the neonatal period or a mild HI that was not detected by NHS, may subsequently remain undetected if no additional screening or risk factor surveillance is done (Lü et al. Citation2011). Whereas universal CHS has been implemented in 17 countries since 1950, the CHS programme in Flanders (Belgium) was scaled down in 2016 and terminated in Iceland in 2012. Similarly, in 2015, national expansion of CHS was no longer recommended in England until more information would become available on its cost-effectiveness.
For parts of the world where NHS is not available, CHS could be cost-effective when it is the first screening or the only screening available. CHS may also be cost-effective when local circumstances are taken into account. In countries where CSOM is prevalent and not detected or treated at a young age, CHS may contribute to its detection (Yong et al. Citation2020a). Therefore, the World Health Organisation (2004) recommends performing CHS in developing countries to reduce the burden of CSOM. Surgical and medical treatment can be provided to mitigate hearing problems as well as prevent permanent HI and its associated costs (Nguyen et al. Citation2015). In sub-Saharan Africa and South East Asia, a modelling study showed that PTS in combination with the provision of hearing aids among school-aged children would be cost effective. NHS was not included in that model, however (Baltussen and Smith Citation2012).
This study set out to compare the cost-effectiveness of CHS by evaluating screening quality indicators: coverage, referral rate, follow-up rate and detection rate across programmes. Unfortunately, most Country Representatives could not report these data. One may argue that the lack of available data may have been due to limited access of the participating Country Representatives who were predominantly involved in NHS. However, in several countries, data were not sufficiently collected or shared across district or regional programmes.
Consequently, when basic determinants like coverage, referral rate, follow-up rate and detection rate are not collected, cost-effectiveness cannot be calculated or compared with that of other countries and, hence, the large diversity between screening programmes remains. It would be advantageous if all systems for data collection, monitoring and evaluation of screening were uniform across regions of a nation and across countries. When the data collected and reported from CHS programmes can be compared across borders, it will be possible to calculate whether CHS would be cost-effective (alone or in combination with NHS), and optimal conditions like choices on location, personnel, test method and age of testing can be formulated.
TIJA-2020-11-0573-File004.docx
Download MS Word (61 KB)Acknowledgements
Members of the EUS€REEN Foundation contributed information from their local screening programmes. The following are members of the EUS€REEN Foundation contributing to this work: B. Qirjazi; D. Holzinger; L. Stappaert; B. Vos; F. Brkić; P. Rouev; X. Peng; M. Velepic; C. Thodi; J. Drsata; T. Ovesen; M. Bambus; M. Lepplaan; B. Ellefsen; R. Niemensivu; T. Willberg; F. Denoyelle; P. Matulat; T. Nikolopoulos; A. Gáborján; I. Hinriksdóttir; Z. Chaudhurri; G. Norman; L. Rubin; A. Martini; D. Spanca; M. Audere; S. Kušķe; N. Drazdiene, E. Lesinskas; J.M. Hild; M. Cakar; T. Fenech; W. Mulwafu; D. Chiaburu; T. Kujundžić; E. Zvrko; A. Meuwese; A. Goedegebure; H. Hoeve; V. Nagaraj; G. Greczka; L. Monteiro; M. Georgescu; G. Tavartkiladze; L. Gouma; S. Filipovic; G. Jokovic; L. Langova; I. Sebova; S. Battelino; D.W. Swanepoel; F. Núñez-Batalla, J.M. Sequi-Canet; I. Uhlén; B. Nora; M. Baydan; J. McCall.
Disclosure statement
The authors have no conflicts of interest relevant to this article to disclose.
Additional information
Funding
References
- American Academy of Audiology. 2011. Childhood Hearing Screening, Clinical Practice Guidelines [Online]. American Academy of Audiology. Available: https://audiology-web.s3.amazonaws.com/migrated/ChildhoodScreeningGuidelines.pdf_5399751c9ec216.42663963.pdf [Accessed 16 November 2020].
- Baltussen, R., and A. Smith. 2012. “Cost Effectiveness of Strategies to Combat Vision and Hearing Loss in Sub-Saharan Africa and South East Asia: Mathematical Modelling Study.” Bmj 344 (mar02 1): e615–e615. doi:https://doi.org/10.1136/bmj.e615.
- Bess, F. H., J. Dodd-Murphy, and R. A. Parker. 1998. “Children with Minimal Sensorineural Hearing Loss: Prevalence, Educational Performance, and Functional Status.” Ear and Hearing 19: 339–354. doi:https://doi.org/10.1097/00003446-199810000-00001
- Beswick, R., C. Driscoll, J. Kei, and S. Glennon. 2012. “Targeted Surveillance for Postnatal Hearing Loss: A Program Evaluation.” International Journal of Pediatric Otorhinolaryngology 76 (7): 1046–1056. doi:https://doi.org/10.1016/j.ijporl.2012.04.004.
- Browning, G. G. 2000. “Influence of Age, Type of Audiometry and Child's Concentration on Hearing Thresholds.” British Journal of Audiology 34 (4): 231–240. doi:https://doi.org/10.3109/03005364000000133.
- Bussé, A. M. L., A. R. Mackey, H. L. Hoeve, A. Goedegebure, G. Carr, I. M. Uhlèn, and H. J. Simonsz. 2021. Assessment of hearing screening programmes across 47 countries or regions I: Provision of newborn hearing screening. International Journal of Audiology, in press.
- Casselbrant, M. L., and E. M. Mandel. 2003. “Chapter 10 Epidemiology.” In Evidence-Based Otitis Media, edited by Rosenfeld, R. M. & Bluestone, C. D. 2nd ed. London: BC Decker Inc Hamilton.
- De Sousa, K. C., D. W. Swanepoel, D. R. Moore, H. C. Myburgh, and C. Smits. 2020. “Improving Sensitivity of the Digits-In-Noise Test Using Antiphasic Stimuli.” Ear and Hearing 41 (2): 442–450. doi:https://doi.org/10.1097/AUD.0000000000000775.
- Denys, S., J. DE Laat, W. Dreschler, M. Hofmann, A. VAN Wieringen, and J. Wouters. 2019. “Language-Independent Hearing Screening Based on Masked Recognition of Ecological Sounds.” Trends in Hearing 23: 2331216519866566. doi:https://doi.org/10.1177/2331216519866566.
- European Commission/Eacea/Eurydice. 2015. “Early Childhood Education and Care Systems in Europe.” National Information Sheets – 2014/15. In: EURYDICE FACTS AND FIGURES (ed.). Luxembourg: Publications Office of the European Union.
- European Consensus Statement on Neonatal Hearing Screening. 1999. “European Consensus Statement on Neonatal Hearing Screening. Finalized at the European Consensus Development Conference on Neonatal Hearing Screening.” Acta Paediatrica 88: 107–108. doi:https://doi.org/10.1080/08035259950170745
- Ewing, A. W. 1955. “The Sweep-Frequency Method of Making Screening Tests of the Hearing of Schoolchildren .” British Medical Journal 1 (4904): 41–42. doi:https://doi.org/10.1136/bmj.1.4904.41.
- Ewing, I. R., and A. W. G. Ewing. 1944. “The Ascertainment of Deafness in Infancy and Early Childhood.” The Journal of Laryngology & Otology 59 (9): 309–333. doi:https://doi.org/10.1017/S0022215100007465.
- Fitzpatrick, E. M., J. Whittingham, and A. Durieux-Smith. 2014. “Mild Bilateral and Unilateral Hearing Loss in Childhood: A 20-Year View of Hearing Characteristics, and Audiologic Practices before and after Newborn Hearing Screening.” Ear and Hearing 35 (1): 10–18. doi:https://doi.org/10.1097/AUD.0b013e31829e1ed9.
- Fortnum, H. 2003. “Epidemiology of Permanent Childhood Hearing Impairment: Implications for Neonatal Hearing Screening.” Audiological Medicine 1 (3): 155–164. doi:https://doi.org/10.1080/16513860310001997.
- Fortnum, H. M., A. Q. Summerfield, D. H. Marshall, A. C. Davis, and J. M. Bamford. 2001. “Prevalence of Permanent Childhood Hearing Impairment in the United Kingdom and Implications for Universal Neonatal Hearing Screening: Questionnaire Based Ascertainment Study.” BMJ (Clinical Research ed.) 323 (7312): 536–540. doi:https://doi.org/10.1136/bmj.323.7312.536.
- Fortnum, Heather, Obioha C. Ukoumunne, Chris Hyde, Rod S. Taylor, Mara Ozolins, Sam Errington, Zhivko Zhelev, et al. 2016. “A Programme of Studies Including Assessment of Diagnostic Accuracy of School Hearing Screening Tests and a Cost-Effectiveness Model of School Entry Hearing Screening Programmes.” Health Technology Assessment 20 (36): 1–178. doi:https://doi.org/10.3310/hta20360.
- Gravel, J. S. 2003. “Hearing and Auditory Function.” In Evidence-Based Otitis Media, edited by Rosenfeld, R. M. & Bluestone, C. D. 2nd ed. London: BC Decker Inc Hamilton.
- Jensen, R. G., A. Koch, and P. Homøe. 2013. “The Risk of Hearing Loss in a Population with a High Prevalence of Chronic Suppurative Otitis Media.” International Journal of Pediatric Otorhinolaryngology 77 (9): 1530–1535. doi:https://doi.org/10.1016/j.ijporl.2013.06.025.
- Le Clercq, C. M. P., G. VAN Ingen, L. Ruytjens, A. Goedegebure, H. A. Moll, H. Raat, V. W. V. Jaddoe, R. J. Baatenburg DE Jong, and M. P. VAN DER Schroeff. 2017. “Prevalence of Hearing Loss among Children 9 to 11 Years Old: The Generation R Study.” JAMA Otolaryngology- Head & Neck Surgery 143 (9): 928–934. doi:https://doi.org/10.1001/jamaoto.2017.1068.
- Lü, J., Z. Huang, T. Yang, Y. Li, L. Mei, M. Xiang, Y. Chai, et al. 2011. “Screening for Delayed-Onset Hearing Loss in Preschool Children Who Previously Passed the Newborn Hearing Screening.” International Journal of Pediatric Otorhinolaryngology 75 (8): 1045–1049. doi:https://doi.org/10.1016/j.ijporl.2011.05.022.
- Mackey, A. R., A. M. L. Bussé, H. L. Hoeve, A. Goedegebure, G. Carr, H. J. Simonsz, and I. M. Uhlèn. 2021. Assessment of hearing screening programmes across 47 countries or regions II: Coverage, referral, follow-up and detection rates from newborn hearing screening. International Journal of Audiology, in press.
- Mahomed-Asmail, F., D. W. Swanepoel, and R. H. Eikelboom. 2016. “Referral Criteria for School-Based Hearing Screening in South Africa: Considerations for Resource-Limited Contexts.” Health SA Gesondheid 21: 96–102. doi:https://doi.org/10.1016/j.hsag.2015.11.003.
- Mandel, E. M., W. J. Doyle, B. Winther, and C. M. Alper. 2008. “The Incidence, Prevalence and Burden of OM in Unselected Children Aged 1-8 Years Followed by Weekly Otoscopy through the "Common Cold" Season.” International Journal of Pediatric Otorhinolaryngology 72 (4): 491–499. doi:https://doi.org/10.1016/j.ijporl.2007.12.008.
- Mckay, S., J. S. Gravel, and A. M. Tharpe. 2008. “Amplification Considerations for Children with Minimal or Mild Bilateral Hearing Loss and Unilateral Hearing Loss.” Trends in Amplification 12 (1): 43–54. doi:https://doi.org/10.1177/1084713807313570.
- Mcpherson, B., M. Law, and M. Wong. 2010. “Hearing Screening for School Children: Comparison of Low‐Cost, Computer‐Based and Conventional Audiometry.” Child: Care, Health and Development 36: 323–331. doi:https://doi.org/10.1111/j.1365-2214.2010.01079.x
- Moeller, M. P. 2000. “Early Intervention and Language Development in Children Who Are Deaf and Hard of Hearing.” Pediatrics 106 (3): E43. doi:https://doi.org/10.1542/peds.106.3.e43.
- Monasta, L., L. Ronfani, F. Marchetti, M. Montico, L. Vecchi Brumatti, A. Bavcar, D. Grasso, C. Barbiero, and G. Tamburlini. 2012. “Burden of Disease Caused by Otitis Media: Systematic Review and Global Estimates.” PLoS One 7 (4): e36226. doi:https://doi.org/10.1371/journal.pone.0036226.
- Nguyen, K.-H., A. C. Smith, N. R. Armfield, M. Bensink, and P. A. J. P. O. Scuffham. 2015. “Cost-Effectiveness Analysis of a Mobile Ear Screening and Surveillance Service versus an Outreach Screening, Surveillance and Surgical Service for Indigenous Children in Australia.” PLoS One 10 (9): e0138369. doi:https://doi.org/10.1371/journal.pone.0138369.
- Okwo-Bele, J. M. 2012. “Integrating Immunization with Other Health Interventions for Greater Impact: The Right Strategic Choice.” Journal of Infectious Diseases 205 (Suppl 1): S4–S5. doi:https://doi.org/10.1093/infdis/jir800.
- Pirozzo, S., T. Papinczak, and P. Glasziou. 2003. “Whispered Voice Test for Screening for Hearing Impairment in Adults and Children: Systematic Review.” BMJ (Clinical Research ed.) 327 (7421): 967. doi:https://doi.org/10.1136/bmj.327.7421.967.
- Prieve, B. A., T. Schooling, R. Venediktov, and N. Franceschini. 2015. “An Evidence-Based Systematic Review on the Diagnostic Accuracy of Hearing Screening Instruments for Preschool- and School-Age Children.” American Journal of Audiology 24 (2): 250–267. doi:https://doi.org/10.1044/2015_AJA-14-0065.
- Rosenfeld, R. M., J. J. Shin, S. R. Schwartz, R. Coggins, L. Gagnon, J. M. Hackell, D. Hoelting, et al. 2016. “Clinical Practice Guideline: Otitis Media with Effusion (Update).” Otolaryngology–Head and Neck Surgery 154 (1_suppl): S1–S41. doi:https://doi.org/10.1177/0194599815623467.
- Sharma, R., Y. Gu, T. Y. C. Ching, V. Marnane, and B. Parkinson. 2019. “Economic Evaluations of Childhood Hearing Loss Screening Programmes: A Systematic Review and Critique.” Applied Health Economics and Health Policy 17 (3): 331–357. doi:https://doi.org/10.1007/s40258-018-00456-1.
- Silman, S., C. Silverman, and D. S. Arick. 1994. “Pure-Tone Assessment and Screening of Children with Middle-Ear Effusion.” Journal of the American Academy of Audiology 5 (3): 173.
- Skarżyński, Henryk, and Anna Piotrowska. 2012. “Screening for Pre-School and School-Age Hearing Problems: European Consensus Statement.” International Journal of Pediatric Otorhinolaryngology 76 (1): 120–121. doi:https://doi.org/10.1016/j.ijporl.2011.10.016.
- Smith, R. J., J. F. Bale, JR., and K. R. White. 2005. “Sensorineural Hearing Loss in Children.” Lancet (London, England) 365 (9462): 879–890. doi:https://doi.org/10.1016/S0140-6736(05)71047-3.
- The European Observatory on Health Systems and Policies. 2018. The Organization and Delivery of Vaccination Services in the European Union. United Kingdom: World Health Organisation.
- The World Bank. 2019. World Bank Country and Lending Groups [Online]. The World Bank. Available: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups [Accessed 03-06-2019].
- UNESCO. 2020. UNESCO Institute for Statistics [Online]. UNESCO. Available: http://data.uis.unesco.org/ [Accessed 05-06-2020].
- UNICEF. 2012. The Right of Roma Children to education, Position Paper [Online]. UNICEF. Available: https://www.unicef.org/eca/reports/right-roma-children-education [Accessed 05-06-2020].
- Watkin, P. M., and M. Baldwin. 2011. “Identifying Deafness in Early Childhood: Requirements after the Newborn Hearing Screen.” Archives of Disease in Childhood 96 (1): 62–66. doi:https://doi.org/10.1136/adc.2010.185819.
- Winiger, A. M., J. M. Alexander, and A. O. Diefendorf. 2016. “Minimal Hearing Loss: From a Failure-Based Approach to Evidence-Based Practice.” American Journal of Audiology 25 (3): 232–245. doi:https://doi.org/10.1044/2016_AJA-15-0060.
- World Health Organization. 2004. Chronic Suppurative Otitis Media: Burden of Illness and Management Options. Geneva, Switzerland: World Health Organization.
- World Health Organization. 2019a. Measles, 2nd dose (MCV2), Immunization coverage estimates by country [Online]. World Health Organization. Available: https://apps.who.int/gho/data/node.main.MCV2n [Accessed 05-06-2020].
- World Health Organization. 2019b. Vaccines and immunization, Measles and rubella country profile [Online]. World Health Organization. Available: http://www.euro.who.int/en/health-topics/disease-prevention/vaccines-and-immunization/publications/surveillance-and-data/measles-and-rubella-elimination-country-profiles/2019 [Accessed 05-06-2020].
- World Health Organization. 2020. WHO vaccine-preventable diseases: monitoring system. 2019 global summary [Online]. World Health Organization,. Available: https://apps.who.int/immunization_monitoring/globalsummary/schedules [Accessed 05-06-2020].
- Yong, M., J. Liang, J. Ballreich, J. Lea, B. D. Westerberg, and S. D. Emmett. 2020a. “Cost-Effectiveness of School Hearing Screening Programs: A Scoping Review.” Otolaryngology-Head and Neck Surgery : Official Journal of American Academy of Otolaryngology-Head and Neck Surgery 162 (6): 826–838. 194599820913507. doi:https://doi.org/10.1177/0194599820913507.
- Yong, M., N. Panth, C. M. Mcmahon, P. R. Thorne, and S. D. Emmett. 2020b. “How the World’s Children Hear: A Narrative Review of School Hearing Screening Programs Globally.” OTO Open 4 (2): 2473974X2092358. doi:https://doi.org/10.1177/2473974X20923580.
- Yoshinaga-Itano, C., A. L. Sedey, D. K. Coulter, and A. L. Mehl. 1998. “Language of Early- and Later-Identified Children with Hearing Loss.” Pediatrics 102 (5): 1161–1171. doi:https://doi.org/10.1542/peds.102.5.1161
