Abstract
Objective
Baseline electroencephalography (EEG) alpha power, i.e. that measured prior to stimulus presentation, is a potential objective predictor of task performance. Here we assessed the predictive power of EEG alpha on performance accuracy in a digits-in-noise recognition task, factoring in hearing thresholds and age.
Design
EEG alpha power, recorded while participants listened to target digits presented in a noise background, was analysed during two different baseline periods: i) a pre-stimulus baseline (pre-STIM) free from any acoustic stimulus, and ii) a pre-target baseline (pre-TARG) recorded in background noise only.
Study sample
Eighty-five participants with either normal hearing or aided hearing impairment (age range: 55–85 years old, 42 male).
Results
Hierarchical multiple regression analyses indicated that i) lower hearing thresholds and, to a lesser extent, higher pre-STIM alpha power were associated with improved performance accuracy ii) alpha power in pre-STIM and pre-TARG were highly correlated across individuals but pre-TARG alpha power was not a significant predictor of performance accuracy.
Conclusion
Investigations of baseline EEG alpha power as a predictor of speech-in-noise performance accuracy should control for associations between hearing thresholds and measures of EEG baseline periods.
Introduction
Understanding speech presented against a background of noise can be challenging for both individuals with normal-hearing (NH) and individuals with hearing impairment (HI). However, individuals with HI report significantly more listening effort compared to age-matched NH controls (Alhanbali et al. Citation2017). Individuals with a similar degree of hearing impairment might also report different levels of listening effort (Pichora-Fuller et al. Citation2016). Even when performance on a speech recognition in noise task is equivalent, the amount of listening effort required by individuals to achieve a similar level of performance accuracy may differ (Gatehouse and Gordon Citation1990; Pichora-Fuller and Singh Citation2006; Ohlenforst et al. Citation2017).
Performance accuracy in a speech-in-noise task may depend not only on the sensory inputs during stimulus presentation but also on the brain state prior to stimulus presentation, i.e. during a baseline period. Pre-stimulus alpha activity has been hypothesised to influence task performance. For instance, it has been suggested that the association between increased pre-stimulus alpha and improved performance reflects the inhibition of task-irrelevant cortical regions, as suggested by the cortical idling hypothesis (Pfurtscheller and Lopes da Silva Citation1999). Alternatively, for internal information processing such as working memory tasks, increased pre-stimulus alpha has been hypothesised to reflect enhanced attentional control, particularly in parietal cortical regions (Palva and Palva Citation2007). Resting electroencephalography (EEG) alpha oscillations might reflect default network activity (Jann et al. Citation2009), or it may be an indicator of preparatory processing that facilitates improved performance (Doppelmayr et al. Citation2002). Furthermore, individuals with better cognitive abilities are expected to be more mentally aroused before stimulus presentation (i.e. have increased pre-stimulus alpha activity) and to be more prepared before the commencement of a new trial (Klimesch, Sauseng, and Hanslmayr Citation2007). Increased cortical arousal inferred from increased pre-stimulus alpha power has been found to predict better information processing (Klimesch, Vogt, and Doppelmayr Citation1999).
Baseline brain activity can be measured objectively using methods such as EEG or pupillometry. The baseline period could be defined during a resting period that is free from any sort of sensory stimulus presentation i.e. pre-stimulus activity (pre-STIM), for example, using pre-STIM EEG alpha (8–13 Hz) power (Steinmetzger and Rosen Citation2017). This approach aims to isolate preparatory processes from on-going auditory stimulus processing. Alternatively, a baseline measure of brain activity could be acquired during a period where a sensory stimulus other than the target stimulus is being presented, i.e. pre-target activity (pre-TARG).
Physiological measures of pre-STIM and pre-TARG EEG alpha oscillations (Henry and Obleser Citation2012; Ng, Schroeder, and Kayser Citation2012; Henry, Herrmann, and Obleser Citation2014; Kayser, McNair, and Kayser Citation2016; Steinmetzger and Rosen Citation2017; McNair, Kayser, and Kayser Citation2019; Wöstmann, Waschke, and Obleser Citation2019) have been linked with post-stimulus performance and metacognition in auditory tasks. Greater pre-STIM alpha power may indicate reduced attention as task difficulty increases (Steinmetzger and Rosen Citation2017; Wöstmann, Waschke, and Obleser Citation2019) or reduced confidence in ability to perform a “discrimination” task (Wöstmann, Waschke, and Obleser Citation2019). Increased pre-TARG EEG alpha power (∼8–13 Hz) may also index general processes related to the encoding of sensory information and decision-making processes, which can positively influence task performance (Kayser, McNair, and Kayser Citation2016; McNair, Kayser, and Kayser Citation2019).
Cognitive decline as a result of aging and/or hearing loss can influence cognitive processes needed for speech perception, including attention, memory, and speed of processing (Pichora-Fuller and Singh Citation2006; Pichora-Fuller et al. Citation2016; also see Wingfield and Stine-Morrow Citation2000; Wingfield and Tun Citation2001; for recent reviews see Lemke and Besser Citation2016; and Peelle Citation2018). Age-related differences in working memory tasks are more pronounced when the listening task involves the presentation of sentences rather than spoken digits (Pichora-Fuller Citation2003). However, digits-in-noise tasks tap into speech recognition and cognitive abilities, including verbal working memory (Moore et al. Citation2014). Previous work suggests that performance in a digits-in-noise task is sensitive to the effects of aging (Smits and Houtgast Citation2005; Moore et al. Citation2014) and hearing levels (Jansen et al. Citation2013; Smits, Theo Goverts, and Festen Citation2013; Heinrich, Henshaw, and Ferguson Citation2015). Performance on digits-in-noise tasks declines substantially during late middle age (50 years old and above) (Moore et al. Citation2014). Here we tested participants in the range of late middle age to old age (55–85 years).
We used an auditory version of the Sternberg paradigm (Sternberg Citation1966), which has been used to demonstrate the influence of hearing impairment on neural changes during the encoding and retention of spoken digits in working memory (Petersen et al. Citation2015). Petersen et al. (Citation2015) found that alpha power in the stimulus-free retention interval is dependent on the interplay between task demands and hearing loss: Alpha power increased with task difficulty for normal-hearing listeners and listeners with a mild hearing loss. However, lower alpha power was measured for listeners with a moderate hearing loss compared with listeners with a mild hearing loss. This pattern of results could be explained if listeners with a moderate hearing loss had reached the upper limit of mental resources they could deploy for the task, or lost motivation and gave up on the task because it was too difficult (Petersen et al. Citation2015). Alternatively, listeners with a moderate hearing loss may have used a different listening strategy to listeners with either normal hearing or a mild hearing loss (Dimitrijevic et al. Citation2019).
Based on Petersen et al. (Citation2015), we expected that hearing impairment would influence participants’ ability to perform a task that requires the encoding and retention of spoken digits presented under challenging listening conditions i.e. in background noise. Given the hypothesised association between pre-stimulus alpha activity and performance (Klimesch, Vogt, and Doppelmayr Citation1999), we predicted that variability in participants’ performance would be predicted by individual differences in alpha power during a pre-stimulus period. In our previous study (Alhanbali et al. Citation2019), incorporating multi-modal measures of listening effort, pre-STIM EEG alpha power was negatively correlated with hearing thresholds and positively correlated with the percentage of correctly recognised digits in a digits-in-noise task, i.e. “performance accuracy”. A positive correlation between pre-STIM EEG alpha power and cognition (including performance in memory, attention, and calculation tasks) has been previously reported (Klimesch, Vogt, and Doppelmayr Citation1999). However, including a measure of cognition was beyond the scope of Alhanbali et al. (Citation2019).
In our previous work (Alhanbali et al. Citation2019) we did not investigate whether measures of baseline alpha power (pre-STIM alpha and/or pre-TARG alpha) are unique predictors of listening effort or performance accuracy in the digits-in-noise listening task after controlling for potential confounding factors (age, hearing levels). Here, we performed secondary analyses of the data acquired in Alhanbali et al. (Citation2019) with the aim of investigating the potential of pre-STIM and pre-TARG EEG alpha power to predict self-reported listening effort and performance accuracy in a digits-in-noise task, after controlling for potential confounds of age and hearing level (Moore et al. Citation2014).
Materials and methods
Below we provide a summarised version of the methods section in Alhanbali et al. (Citation2019). Please refer to the original article for a detailed description of the methods.
Participants
Participants were native English speakers recruited from the databases of three UK National Health Service audiology departments, via flyers posted around the University of Manchester campus and through social groups such as the University of the Third Age and the Irish Social Group in Manchester. The participants’ age range was 55–85 years, (M: 70, SD: 7, 49% male). Older adults were recruited because they constitute the majority of the population of individuals with hearing impairment. The inclusion criteria was 55 years and above to ensure the presence of a wide range of age and hearing levels within recruited participants, as the severity of hearing impairment is known to increase with age (Schlauch and Nelson Citation2009). A total of 116 participants took part in the study. However, regression analyses aiming to identify significant predictors of performance accuracy were performed on a total of 85 participants. Thirty-one participants were excluded from the regression analyses: 12 participants had missing performance accuracy data due to technical errors, 8 participants had missed providing their age information on the information sheet that was completed before the testing session, while the rest had noisy EEG recordings.
Otoscopic examination was performed and both air and bone conduction hearing thresholds were measured at the start of the test session. No participants were found to have a conductive element to their hearing loss. Audiometry was conducted using a VIASYS SI Arrow audiometer coupled to TDH-39 supra-aural headphones.
Participants’ hearing levels ranged from “good/near normal” hearing (those who had hearing thresholds ≤30 dB HL at frequencies of 500, 1000, 2000, and 4000 Hz) through to severe hearing impairment, based on the criteria defined by the British Society of Audiology (Citation2018). Hearing thresholds were averaged across frequencies (500, 1000, 2000, and 4000 Hz) and ears to create a single hearing threshold for each participant. Participants who were hearing aid users (49 of 58 participants with hearing impairment) performed the listening task wearing their hearing aids. Details about hearing aid use and prescriptions can be found in Alhanbali et al. (Citation2019). The study was reviewed and approved by the National Research Ethics Services of South Central-Hampshire A, Research Ethics Committee reference: 15/SC/0113.
Listening task
The listening task was a modified version of the Sternberg paradigm (Sternberg Citation1966), in which participants had to listen to spoken digits and memorise the presented digits during a stimulus-free retention period, based on similar paradigms described by Obleser et al. (Citation2012) and Petersen et al. (Citation2015). The speech material used in the main listening task were sequences of 6 single digits (1–9 excluding 7) from the Whispered Voice Test (McShefferty et al. Citation2013) presented in unmodulated background noise. Digits were presented at a level of 65 dB(A) in the presence of stationary background noise that started 5 s before the first digit and ended 1 s after the end of the last digit. The digits were presented via two loudspeakers placed 1 m away from where the participant was sitting at ±45 degrees azimuth.
In order to minimise differences in performance across participants with different hearing levels, individualised SNRs were established using sequences of 3 digits (1–9, excluding 7) that participants had to memorise and recall in the correct order. A response was only considered correct if the participant correctly identified all three digits and in the correct order. In the first 10 trials, the level of the background noise was increased by 3 dB in the case of a correct response and decreased by 3 dB in the case of an incorrect response. In subsequent trials, the level of the noise varied in a 2-down, 1-up adaptive procedure to establish the 71% correct performance level. In the main listening task, 6 digits were presented in each trial. After listening to the 6 digits, there was a 3-s retention period during which participants had to mentally rehearse the digits they heard. Participants were then presented with a single digit (selected at random) that appeared on the computer screen in front of them. Using a button press, participants responded with “yes” if the number on the screen was one of the digits they heard and with “no” if it was not. The consequences of using 3 digits to calculate individualised SNRs and 6 digits in the main listening task has been addressed in Alhanbali et al. (Citation2019).
The total number of trials was 50. Performance accuracy was calculated based on the percentage of the trials for which participants provided a correct answer. Mean performance accuracy in the main listening task ranged from 72% to 93% correct (M: 89%, SD: ± 6%). Only the trials for which correct responses were provided were included in the analyses. Physiological recordings were obtained while participants performed the listening task.
Electroencephalography
EEG was recorded with a sampling rate of 256 Hz using the Nexus-10 biofeedback physiological recording system using BioTrace+ (MindMedia Neuro and Biofeedback System). Three scalp electrodes were positioned at P3, P4, and Pz based on the international 10-20 system (Homan, Herman, and Purdy Citation1987). Two ground electrodes were placed at Cz and Fz. Detailed descriptions of the EEG data preprocessing including filtering, epoching, and rejection of trials containing artefacts are provided in Alhanbali et al. (Citation2019).
EEG analysis
The EEG data were filtered offline with a high-pass cut-off frequency of 5 Hz and a low-pass cut-off frequency of 45 Hz. For the trials where participants gave a correct response in the digits-in-noise task, alpha power was quantified from the EEG data collected at electrode Pz during 2 different baseline periods: i) a stimulus-free pre-STIM baseline period (−0.6 to −0.1 s prior to any auditory stimulus onset), ii) a pre-TARG baseline period (4.4–4.9 s after the start of a trial, see Alhanbali et al. (Citation2019)), where listeners were presented with an unmodulated noise only that preceded the presentation of the spoken digits.
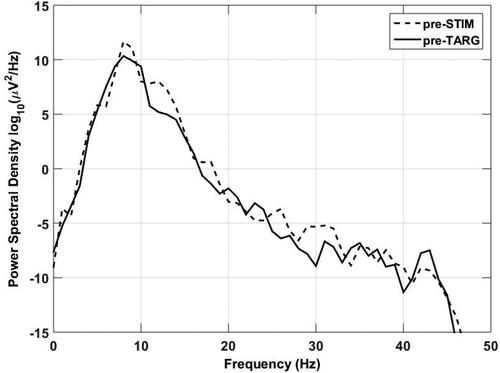
Alpha power was quantified for each individual participant in the centre frequencies ranging from 8 to 13 Hz using the power spectral density (PSD) calculation function (spectopo) in EEGlab tool box (Delorme and Makeig Citation2004). The EEGlab spectopo function, based on Welch’s method, used a 256-point Hamming window with no overlap, and 256-point FFT length, resulting in a frequency resolution of 1 Hz. Alpha power during the baseline periods was averaged across the centre frequencies for each participant. The logarithm of the mean power during the baseline periods was used for analysis ().
Self-reported listening effort
Self-reported listening effort was measured using the NASA Task Load Index (NASA-TLX; Hart and Staveland Citation1988). NASA-TLX has six items: mental demand, physical demand, temporal demand, perceived performance, effort, and frustration. After completion of the main listening task, participants provided responses on a 20-step scale, ranging from low demand to high demand for each dimension. The score of each item was converted to a percentage and the total score was calculated based on the mean score of the items used.
Statistical analysis
Individual results for performance accuracy were transformed into rationalised arcsine units (RAU) (Studebaker Citation1985) prior to statistical analyses.
Correlation analyses between the outcome and the predictor variables suggested that linear models were suitable for the regression analyses. This was confirmed with the curve estimation function on SPSS for both of the regression models (IBM statistics SPSS version 22).
Correlations between the outcome and the predictor variables were analysed using Pearson’s correlation coefficients. No corrections for multiple comparisons were applied because the correlation analyses were provided to support interpretation of the regression analyses.
Hierarchical linear regression models were used in which performance accuracy was the outcome variable. Since pre-STIM and pre-TARG alpha power were highly correlated (), separate regression models were used to investigate the predictive power of pre-STIM and pre-TARG alpha power on performance accuracy. In model 1, age, hearing thresholds and pre-STIM alpha power (mean across all trials) were entered as predictors. In model 2, age, hearing thresholds, and pre-TARG alpha power (mean across all trials) were entered as predictors.
Table 1. Correlation coefficients (Pearson’s r, 1-tailed significance) for the outcome (RAU performance accuracy) and predictor variables (PTA, Age, pre-STIM alpha, pre-TARG alpha, NASA-TLX, Hearing aid use, PTA x pre-STIM alpha, PTA x pre-TARG alpha) entered into the regression models.
Additional predictors were entered into regression models 1 and 2: i) the factor hearing aid use (entered as a categorical variable with two levels: Yes/No) was entered to investigate any effect that hearing aid use might have on performance accuracy; ii) the potential interaction between hearing thresholds and alpha power (Petersen et al. Citation2015) i.e. the interaction between hearing thresholds and pre-STIM alpha power (PTA x pre-STIM alpha power) and the interaction between hearing thresholds and pre-TARG alpha power (PTA × pre-TARG alpha power) were entered as predictors in model 1 and model 2, respectively.
A further two hierarchical linear regression models were used in which self-reported listening effort (NASA-TLX) was the outcome variable. In model 3, age, hearing thresholds and pre-STIM alpha power were entered as predictors. In model 4, age, hearing thresholds, and pre-TARG alpha power were entered as predictors.
As age and hearing levels are known predictors of performance accuracy on a digits-in-noise task (Moore et al. Citation2014) and also known to influence the experience of listening effort (Ward et al. Citation2017; Alhanbali et al. Citation2019), they were entered in the first step of all the regression models (1–4). In Alhanbali et al. (Citation2019), PTA and SNR loaded equally to the same factor and were highly correlated. However, only PTA was included in the regression model, as previously done in Wang et al. (Citation2018). Pre-STIM/pre-TARG EEG alpha power, hearing aid use, and the mean-centered interactions between pre-STIM/pre-TARG EEG alpha power and hearing threshold were entered in the second step of the models in a stepwise manner, as the potentially unique contributions of these predictors to performance accuracy in a digits-in-noise task and/or self-report listening effort were unknown.
Before performing the regression analysis, case-wise diagnostics and collinearity diagnostics were carried out and the Durbin-Watson statistics were calculated (model 1 d = 1.76; model 2 d = 1.80; model 3 d = 1.80; model 4 d = 1.80). Case-wise diagnostics did not identify any outliers in models 1, 3, and 4 but did identify 1 participant outlier in model 2. Therefore, for model 2, regression analysis was performed both with and without this participant to rule out any effect they might have had on the overall results of the regression analysis. The results of the hierarchical regression analyses were not Bonferroni corrected.
Collinearity diagnostics did not suggest that the outcomes of the regression models were influenced by multicollinearity based on the variance inflation factor (VIF) (Bowerman and O’Connell Citation1990) (model 1 mean VIF = 1.08, maximum VIF = 1.14; model 2 mean VIF = 1.03, maximum VIF = 1.03; model 3 mean VIF = 1.04, maximum VIF = 1.04; model 4 mean VIF = 1.03, maximum VIF = 1.03).
Results
Correlation analyses
To aid interpretation of the regression analyses, provides Pearson’s correlation coefficients (not corrected for multiple comparisons) between the outcome and predictor variables entered into the regression models. Performance accuracy on the digits-in-noise task and PTA were significantly correlated (r = −0.44, p < 0.001); better hearing thresholds were associated with improved task performance. There were also significant correlations between pre-STIM alpha power and PTA (r = −0.29, p = 0.003), pre-STIM alpha power and performance accuracy in the listening task (r = 0.36, p < 0.001), pre-TARG alpha power and PTA (r = −0.29, p = 0.004), and pre-TARG alpha power and performance accuracy in the listening task (r = 0.26, p = 0.01). Participants with better hearing thresholds had higher pre-STIM/pre-TARG alpha power and higher pre-STIM/pre-TARG alpha power was associated with improved task performance. Pre-STIM and pre-TARG alpha power were highly correlated (r = 0.94, p < 0.001).
Predictors of performance accuracy on the digits-in-noise task
shows the results for regression model 1 used to assess predictors of performance accuracy in the digits-in-noise task. In model 1, performance accuracy was predicted by the first step of the model (R2 = 0.20, p < 0.001), which included PTA and age with PTA being the only significant predictor (β = −0.45, p < 0.001). illustrates that listeners with a higher PTA performed more poorly on the listening task. The second step of the model, which included pre-STIM alpha power, hearing aid use and the interaction between hearing thresholds and pre-STIM alpha power improved the model fit (R2 = 0.25, ΔR2 = 0.05, p = 0.02). However, the effects of hearing aid use (β = 0.06, p = 0.65) and the interaction term (β = 0.01, p = 0.92) were not significant. Pre-STIM alpha power (β = 0.25, p = 0.02) was the unique contributor to the improvement in model fit. shows that greater pre-STIM alpha power was associated with greater performance accuracy on the listening task. The difference in performance accuracy between participants in the top and bottom quartiles was about 13.5 RAU (87.4–99.9 RAU).
Figure 2. (a) Relation between partial regressions for RAU-transformed performance accuracy on the digits-in-noise recognition task and PTA in dB HL, when age is taken into account. Higher PTA is associated with poorer performance on the listening task. (b) Relation between partial regressions for RAU-transformed performance accuracy and pre-STIM alpha, when both age and PTA are controlled for. Greater pre-STIM alpha power is associated with better performance accuracy on the digits-in-noise task.
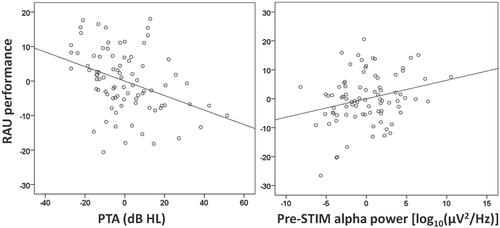
Table 2. Model 1: Linear model predictors (PTA, age, pre-STIM alpha) of performance accuracy on the digits-in-noise task.
In model 2, performance accuracy was predicted by the first step of the model (R2 = 0.20, p < 0.001), which included PTA and age with PTA being the only significant predictor (β = −0.45, p < 0.001). Pre-TARG alpha power, hearing aid use and the interaction between hearing thresholds and pre-TARG alpha power, added in the second step of the model, did not make a significant improvement to the model fit. There was no meaningful change in the results of regression model 2 when the participant outlier was removed from the analysis.
Note that the inclusion of incorrect trials in the regression analyses did not make any meaningful change to the results of model 1 and model 2 as the number of incorrect trials was relatively small (mean performance in the listening task was 89%, SD: ±6%).
Predictors of self-reported listening effort in a digits-in-noise task
Self-reported listening effort was not predicted by the first step of the model, which included age and PTA (model 3: R2 = 0.04, p = 0. 16; Model 4: R2 = 0.04, p = 0. 16). Neither pre-STIM alpha power nor pre-TARG alpha power, added in the second step of the models, made a significant improvement to the model fit.
Discussion
The aim of this study was to investigate the potential of EEG alpha power, including pre-STIM EEG alpha power and/or pre-TARG EEG alpha power, to predict performance accuracy in a digits-in-noise task and task-related listening effort (NASA-TLX), after controlling for potential confounds of age and hearing level (Moore et al. Citation2014).
The results presented here suggest that performance accuracy on a digits-in-noise task is predicted mainly by PTA, and to a much lesser extent, by pre-STIM alpha power: lower hearing thresholds and higher pre-STIM alpha power were associated with greater performance accuracy. The effect size of the unique contribution of pre-STIM alpha power to performance accuracy was small. Alpha power in the pre-STIM baseline was highly correlated with that in the pre-TARG baseline () but pre-TARG alpha power was not a predictor of performance accuracy in the digits-in-noise task. As reported previously, self-reported listening effort was not associated with age (Alhanbali et al. Citation2017, Citation2019), hearing thresholds (Mackersie, MacPhee, and Heldt Citation2015; Alhanbali et al. Citation2017, Citation2019), or measures of baseline EEG alpha power (Alhanbali et al. Citation2019). The absence of effects of age and hearing loss on self-reported listening effort is also consistent with previous studies (Zekveld, Kramer, and Festen Citation2011).
The confounding effects of hearing thresholds on measures of EEG baselines
The present results also highlight the strong associations between PTA and baseline (pre-STIM and pre-TARG) measures of EEG alpha power. Direct correlations between i) performance accuracy and both pre-STIM and pre-TARG alpha power and also ii) PTA and both pre-STIM and pre-TARG alpha power were statistically significant (), even though participants with hearing impairment wore their hearing aids during the task. Changes in EEG alpha power may indicate deficits in supra-threshold processing that are associated with aging (Moore et al. Citation2012) and hearing loss (Alain, Roye, and Salloum Citation2014). Hearing aid amplification does not necessarily compensate for supra-threshold deficits in older adults with hearing impairment (Kortlang, Mauermann, and Ewert Citation2016).
Interestingly, PTA was correlated with both pre-STIM and pre-TARG (), suggesting that hearing levels are predictors of EEG alpha power even during silent periods where no acoustic stimulus was presented. Moreover, when PTA was controlled in the regression analyses, the relationship between pre-TARG alpha power and performance accuracy was no longer significant. Taken together, these results suggest that, although pre-STIM and pre-TARG are both considered measures of baseline alpha power, here pre-STIM and pre-TARG captured at least some differences in EEG alpha power during periods of no acoustic stimulation (pre-STIM) vs. presentation of noise only (pre-TARG).
Overall, these findings suggest that studies aiming to link pre-STIM, or pre-TARG, EEG alpha power with performance on an auditory task should consider the associations between measures of baseline EEG alpha power and PTA. This potentially confounding relationship may be more problematic when there is a wide range of hearing thresholds and/or ages across participants, for example, in studies that include a lifespan sample.
Associations between measures of baseline EEG alpha power and performance accuracy
The findings of the current study are consistent with previous research that has repeatedly reported associations between increased pre-STIM alpha and improved task performance (Vogt, Klimesch, and Doppelmayr Citation1998; Klimesch, Vogt, and Doppelmayr Citation1999; Doppelmayr et al. Citation2002). Consistent with the “cortical idling” hypothesis (Pfurtscheller and Lopes da Silva Citation1999), whereby increased alpha activity facilitates an individual’s ability to remain “on task” and avoid distractions when performing a task that involves processing sensory inputs, increased pre-STIM alpha power can be considered as a state of suppressing any irrelevant, non-task related brain activity that could later interfere with (visual) task performance (Dockree et al. Citation2007). Alternatively, increased pre-STIM might represent an alert state of effective anticipation, which facilitates improved task performance (Dockree et al. Citation2007). Given that pre-STIM alpha power was measured from occipito-parietal sensors in the present study, the positive relation between pre-STIM alpha power and performance accuracy is consistent with the idea that increased auditory attention may be associated with increased alpha power (Lim, Wöstmann, and Obleser Citation2015). Our results extend previous research findings by suggesting that the association between improved task performance and increased pre-STIM alpha power applies to a digits-in-noise listening task, and that this association may be moderated by hearing level.
Increased pre-STIM alpha power has been linked with cases of impaired performance on a speech recognition task (Steinmetzger and Rosen Citation2017) and may represent a state of reduced attention. In contrast with the present study, where the association between individual differences in mean pre-STIM alpha power across all trials and performance accuracy was determined, Steinmetzger and Rosen (Citation2017) measured pre-STIM alpha as a function of intelligibility where trials were sorted based on the number of correctly identified key words. Steinmetzger and Rosen (Citation2017) suggested that increased pre-STIM alpha power might be an indication of reduced attention as the difficulty of the task increases. However, increased pre-STIM alpha power corresponding to poor task performance might also indicate increased allocation of cognitive resources in an attempt to cope with task difficulty. Therefore, pre-STIM alpha may index increased allocation of cognitive resources that can potentially predict improved task performance. However, future research needs to consider establishing the extent to which performance accuracy can be predicted by pre-STIM alpha in tasks with varying levels of difficulty.
Alternatively, pre-TARG alpha activity may influence the sensory perception and coding of the presented stimuli (Kayser, McNair, and Kayser Citation2016). Kayser, McNair, and Kayser (Citation2016) asked participants to perform two auditory tasks, in which they had to discriminate between the intensity and the frequency of two brief tones presented in background noise. Increases in alpha/beta power, generated mainly from auditory cortex, reflected changes in the encoding of sensory gain. Later changes in the phase and power of alpha oscillations in fronto-parietal regions were found to influence general decision making. In the present work, EEG sensors were placed over the parietal lobe based on previous studies using tasks likely to induce listening effort (Obleser et al. Citation2012; Petersen et al. Citation2015). Given the clinical EEG setup used in the present study, together with the sensor-space analyses based on few EEG sensors, it is unclear whether the alpha activity recorded in the present study originated from the auditory or the parietal cortex.
As noted in Wöstmann, Waschke, and Obleser (Citation2019), interpretations of effects related to baseline alpha power should take into account both the topographic distribution and the timing of changes in alpha power relative to the stimulus. The results presented here suggest that, in addition to topography and timing of alpha power modulation, the choice of the baseline period (pre-STIM or pre-TARG), and the hearing thresholds of the participants, may affect relations between baseline alpha power and performance accuracy.
A limitation of the present study was our inability to assess whether pre-stimulus alpha power is related to attention/sensitivity (Steinmetzger and Rosen Citation2017) or confidence (Wöstmann, Waschke, and Obleser Citation2019) because of the limited number of trials (50 trials) used and the performance level (72–93% correct) achieved by participants. Furthermore, given that a data analysis in terms of Signal Detection Theory (Green and Swets Citation1966) was not an aim in Alhanbali et al. (Citation2019), we did not record the digits series that were presented to the participants in each trial and only recorded whether participants gave a correct or incorrect response.
Implications for future work
Our results suggest a weak association between pre-STIM EEG alpha power and performance accuracy in a digits-in-noise task. This result has implications for future work considering individual differences in pre-STIM/pre-TARG activity when using some physiological measures of listening effort. Listening effort studies using EEG often report results within a pre-defined period of interest, e.g. a retention period (Petersen et al. Citation2015), relative to a baseline period. Here, pre-STIM and pre-TARG alpha power were also found to correlate with hearing thresholds, even though participants with hearing impairment wore their hearings aids during testing. If studies run comparisons between groups of listeners with “normal hearing” and listeners with a known hearing impairment, differences in measures of baseline alpha power may obscure potential differences in post-stimulus periods of interest.
Conclusions
Pre-STIM EEG alpha power, i.e. prior to stimulus presentation, was found to be weakly associated with performance accuracy in a digits-in-noise recognition task. For a digits-in-noise recognition task, results suggested that increased pre-STIM alpha power might represent increased attention and/or sensory gain that facilitates improved performance. However, analyses of associations between baseline alpha power and performance on a listening task should factor in hearing levels.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Alain, C., A. Roye, and C. Salloum. 2014. “Effects of Age-Related Hearing Loss and Background Noise on Neuromagnetic Activity from Auditory Cortex.” Frontiers in Systems Neuroscience 8: 8.
- Alhanbali, S., P. Dawes, S. Lloyd, and K. J. Munro. 2017. “Self-Reported Listening-Related Effort and Fatigue in Hearing-Impaired Adults.” Ear and Hearing 38 (1): e39–e48. doi:https://doi.org/10.1097/AUD.0000000000000361.
- Alhanbali, S., P. Dawes, R. E. Millman, and K. J. Munro. 2019. “Measures of Listening Effort Are Multidimensional.” Ear and Hearing 40 (5): 1084–1097. doi:https://doi.org/10.1097/AUD.0000000000000697
- Bowerman, B. L., & R. T. O'Connell. 1990. Linear Statistical Models: An Applied Approach (2nd ed.). Belmont, CA: Duxbury.
- British Society of Audiology. 2018. Pure Tone Air and Bone Conduction Threshold Audiometry with and Without Masking [Online]. https://www.thebsa.org.uk/wp-content/uploads/2018/11/Recommended-Procedure-Pure-Tone-Audiometry-August-2018-FINAL.pdf
- Delorme, A., and S. Makeig. 2004. “EEGLAB: An Open Source Toolbox for Analysis of Single-Trial EEG Dynamics Including Independent Component Analysis.” Journal of Neuroscience Methods 134 (1): 9–21. doi:https://doi.org/10.1016/j.jneumeth.2003.10.009.
- Dimitrijevic, A., M. Smith, D. Kadis, and D. Moore. 2019. “Neural Indices of Listening Effort in Noisy Environments.” Scientific Reports 9 (1): 1–10. doi:https://doi.org/10.1038/s41598-019-47643-1.
- Dockree, P. M., S. P. Kelly, J. J. Foxe, R. B. Reilly, and I. H. Robertson. 2007. “Optimal Sustained Attention Is Linked to the Spectral Content of Background EEG Activity: Greater Ongoing Tonic Alpha (approximately 10 Hz) Power Supports Successful Phasic Goal Activation.” The European Journal of Neuroscience 25 (3): 900–907. doi:https://doi.org/10.1111/j.1460-9568.2007.05324.x.
- Doppelmayr, M., W. Klimesch, W. Stadler, D. Pöllhuber, and C. Heine. 2002. “EEG Alpha Power and Intelligence.” Intelligence 30 (3): 289–302. doi:https://doi.org/10.1016/S0160-2896(01)00101-5.
- Gatehouse, S., and J. Gordon. 1990. “Response Times to Speech Stimuli as Measures of Benefit from Amplification.” British Journal of Audiology 24 (1): 63–68. doi:https://doi.org/10.3109/03005369009077843
- Green, D. M., and J. A. Swets. 1966. Signal Detection Theory and Psychophysics. New York, NY: John Wiley and Sons.
- Hart, S. G., and L. E. Staveland. 1988. “Development of NASA-TLX (Task Load Index): Results of Empirical and Theoretical Research.” Advances in Psychology 52: 139–183. doi:https://doi.org/10.1016/S0166-4115(08)62386-9
- Heinrich, A., H. Henshaw, and M. Ferguson. 2015. “The Relationship of Speech Intelligibility with Hearing Sensitivity, Cognition, and Perceived Hearing Difficulties Varies for Different Speech Perception Tests.” Frontiers in Psychology 6: 782.
- Henry, M., and J. Obleser. 2012. “Frequency Modulation Entrains Slow Neural Oscillations and Optimizes Human Listening Behavior.” Proceedings of the National Academy of Sciences of the United States of America 109 (49): 20095–20100. doi:https://doi.org/10.1073/pnas.1213390109.
- Henry, M. J., B. Herrmann, and J. Obleser. 2014. “Entrained Neural Oscillations in Multiple Frequency Bands Comodulate Behavior.” Proceedings of the National Academy of Sciences of the United States of America 111 (41): 14935–14940. doi:https://doi.org/10.1073/pnas.1408741111.
- Homan, R. W., J. Herman, and P. Purdy. 1987. “Cerebral Location of International 10–20 System Electrode Placement.” Electroencephalography and Clinical Neurophysiology 66 (4): 376–382. doi:https://doi.org/10.1016/0013-4694(87)90206-9
- Jann, K., T. Dierks, C. Boesch, M. Kottlow, W. Strik, and T. Koenig. 2009. “BOLD Correlates of EEG Alpha Phase-Locking and the fMRI Default Mode Network.” NeuroImage 45 (3): 903–916. doi:https://doi.org/10.1016/j.neuroimage.2009.01.001
- Jansen, S., H. Luts, P. Dejonckere, A. van Wieringen, and J. Wouters. 2013. “Efficient Hearing Screening in Noise-Exposed Listeners Using the Digit Triplet Test.” Ear and Hearing 34 (6): 773–778. doi:https://doi.org/10.1097/AUD.0b013e318297920b.
- Kayser, S. J., S. W. McNair, and C. Kayser. 2016. “Prestimulus Influences on Auditory Perception from Sensory Representations and Decision Processes.” Proceedings of the National Academy of Sciences of the United States of America 113 (17): 4842–4847. doi:https://doi.org/10.1073/pnas.1524087113.
- Klimesch, W., P. Sauseng, and S. Hanslmayr. 2007. “EEG Alpha Oscillations: The Inhibition–Timing Hypothesis.” Brain Research Reviews 53 (1): 63–88. doi:https://doi.org/10.1016/j.brainresrev.2006.06.003.
- Klimesch, W., F. Vogt, and M. Doppelmayr. 1999. “Interindividual Differences in Alpha and Theta Power Reflect Memory Performance.” Intelligence 27 (4): 347–362. doi:https://doi.org/10.1016/S0160-2896(99)00027-6.
- Kortlang, S., M. Mauermann, and S. D. Ewert. 2016. “Suprathreshold Auditory Processing Deficits in Noise: Effects of Hearing Loss and Age.” Hearing Research 331: 27–40. doi:https://doi.org/10.1016/j.heares.2015.10.004
- Lemke, U., and J. Besser. 2016. “Cognitive Load and Listening Effort: Concepts and Age-Related Considerations.” Ear and Hearing 37 (1): 77S–84S. doi:https://doi.org/10.1097/AUD.0000000000000304.
- Lim, S. J., M. Wöstmann, and J. Obleser. 2015. “Selective Attention to Auditory Memory Neurally Enhances Perceptual Precision.” The Journal of Neuroscience : The Official Journal of the Society for Neuroscience 35 (49): 16094–16104. doi:https://doi.org/10.1523/JNEUROSCI.2674-15.2015.
- Mackersie, C. L., I. X. MacPhee, and E. W. Heldt. 2015. “Effects of Hearing Loss on Heart Rate Variability and Skin Conductance Measured During Sentence Recognition in Noise.” Ear and Hearing 36 (1): 145–154. doi:https://doi.org/10.1097/AUD.0000000000000091.
- McNair, S. W., S. J. Kayser, and C. Kayser. 2019. “Consistent Pre-Stimulus Influences on Auditory Perception Across the Lifespan.” NeuroImage 186: 22–32. doi:https://doi.org/10.1016/j.neuroimage.2018.10.085.
- McShefferty, D., W. M. Whitmer, I. R. C. Swan, and M. A. Akeroyd. 2013. “The Effect of Experience on the Sensitivity and Specificity of the Whispered Voice Test: A Diagnostic Accuracy Study.” BMJ Open 3 (4): e002394. doi:https://doi.org/10.1136/bmjopen-2012-002394.
- Moore, B. C. J., B. R. Glasberg, M. Stoev, C. Füllgrabe, and K. Hopkins. 2012. “The Influence of Age and High-Frequency Hearing Loss on Sensitivity to Temporal Fine Structure at Low Frequencies (L).” The Journal of the Acoustical Society of America 131 (2): 1003–1006. doi:https://doi.org/10.1121/1.3672808.
- Moore, D. R., M. Edmondson-Jones, P. Dawes, H. Fortnum, A. McCormack, R. H. Pierzycki, and K. J. Munro. 2014. “Relation Between Speech-in-Noise Threshold, Hearing Loss and Cognition from 40–69 Years of Age.” PLoS One. 9 (9): e107720. doi:https://doi.org/10.1371/journal.pone.0107720.
- Ng, B. S., T. Schroeder, and C. Kayser. 2012. “A Precluding but Not Ensuring Role of Entrained Low-Frequency Oscillations for Auditory Perception.” Journal of Neuroscience 32 (35): 12268–12276. [Database] doi:https://doi.org/10.1523/JNEUROSCI.1877-12.2012.
- Obleser, J., M. Wöstmann, N. Hellbernd, A. Wilsch, and B. Maess. 2012. “Adverse Listening Conditions and Memory Load Drive a Common α Oscillatory Network.” The Journal of Neuroscience : The Official Journal of the Society for Neuroscience 32 (36): 12376–12383. doi:https://doi.org/10.1523/JNEUROSCI.4908-11.2012.
- Ohlenforst, B., A. A. Zekveld, T. Lunner, et al. 2017. “Impact of Stimulus-Related Factors and Hearing Impairment on Listening Effort as Indicated by Pupil Dilation.” Hearing Research 351: 68–79. doi:https://doi.org/10.1016/j.heares.2017.05.012
- Palva, S., and J. Palva. 2007. “New Vistas for α-Frequency Band Oscillations.” Trends in Neurosciences 30 (4): 150–158. doi:https://doi.org/10.1016/j.tins.2007.02.001
- Peelle, J. 2018. “Listening Effort: How the Cognitive Consequences of Acoustic Challenge Are Reflected in Brain and Behavior.” Ear and Hearing 39 (2): 204–214. doi:https://doi.org/10.1097/AUD.0000000000000494.
- Petersen, E. B., M. Wöstmann, J. Obleser, et al. 2015. “Hearing Loss Impacts Neural Alpha Oscillations under Adverse Listening Conditions.” Frontiers in Psychology 6: 177.
- Pfurtscheller, G., and F. H. Lopes da Silva. 1999. “Event-Related EEG-MEG Synchronization and Desynchronization: Basic Principles.” Clinical Neurophysiology : Official Journal of the International Federation of Clinical Neurophysiology 110 (11): 1842–1857. doi:https://doi.org/10.1016/S1388-2457(99)00141-8.
- Pichora-Fuller, M. K., S. E. Kramer, M. A. Eckert, B. Edwards, B. W. Y. Hornsby, L. E. Humes, U. Lemke, et al. 2016. “Hearing Impairment and Cognitive Energy: The Framework for Understanding Effortful Listening (FUEL).” Ear and Hearing 37 (1): 5S–27S. doi:https://doi.org/10.1097/AUD.0000000000000312.
- Pichora-Fuller, M., and G. Singh. 2006. “Effects of Age on Auditory and Cognitive Processing: Implications for Hearing Aid Fitting and Audiologic Rehabilitation.” Trends in Amplification 10 (1): 29–59. doi:https://doi.org/10.1177/108471380601000103.
- Pichora-Fuller, M. 2003. “Cognitive Aging and Auditory Information Processing.” International Journal of Audiology 42 (Suppl 2): 26–32. doi:https://doi.org/10.3109/14992020309074641.
- Schlauch, R. S., and P. Nelson. 2009. “Puretone Evaluation.” In Handbook of Clinical Audiology, edited by J. Katz. Philadelphia, PA: Lippincott Williams & Wilkins.
- Smits, C., and T. Houtgast. 2005. “Results from the Dutch Speech-in-Noise Screening Test by Telephone.” Ear and Hearing 26 (1): 89–95. doi:https://doi.org/10.1097/00003446-200502000-00008
- Smits, C., S. Theo Goverts, and J. Festen. 2013. “The Digits-in-Noise Test: Assessing Auditory Speech Recognition Abilities in Noise.” The Journal of the Acoustical Society of America 133 (3): 1693–1706. doi:https://doi.org/10.1121/1.4789933.
- Steinmetzger, K., and S. Rosen. 2017. “Effects of Acoustic Periodicity, Intelligibility, and Pre-Stimulus Alpha Power on the Event-Related Potentials in Response to Speech.” Brain and Language 164: 1–8. doi:https://doi.org/10.1016/j.bandl.2016.09.008.
- Sternberg, S. 1966. “High-Speed Scanning in Human Memory.” Science (New York, N.Y.) 153 (3736): 652–654. doi:https://doi.org/10.1126/science.153.3736.652.
- Studebaker, G. A. 1985. “A ‘Rationalized’ Arcsine Transform.” Journal of Speech and Hearing Research 28 (3): 455–462. doi:https://doi.org/10.1044/jshr.2803.455.
- Vogt, F., W. Klimesch, and M. Doppelmayr. 1998. “High-Frequency Components in the Alpha Band and Memory Performance.” Journal of Clinical Neurophysiology : Official Publication of the American Electroencephalographic Society 15 (2): 167–172. doi:https://doi.org/10.1097/00004691-199803000-00011.
- Wang, Y., G. Naylor, S. E. Kramer, A. A. Zekveld, D. Wendt, B. Ohlenforst, and T. Lunner. 2018. “Relations Between Self-Reported Daily-Life Fatigue, Hearing Status, and Pupil Dilation During a Speech Perception in Noise Task.” Ear and Hearing 39 (3): 573–582. doi:https://doi.org/10.1097/AUD.0000000000000512.
- Ward, K., J. Shen, P. Souza, and T. Grieco-Calub. 2017. “Age-Related Differences in Listening Effort during Degraded Speech Recognition.” Ear & Hearing 38 (1): 74–84. doi:https://doi.org/10.1097/AUD.0000000000000355.
- Wingfield, A., and P. Tun. 2001. “Spoken Language Comprehension in Older Adults: Interactions Between Sensory and Cognitive Change in Normal Aging.” Seminars in Hearing 22 (03): 287–302. doi:https://doi.org/10.1055/s-2001-15632.
- Wingfield, A., and E. A. L. Stine-Morrow. 2000. “Language and Speech.” In The Handbook of Aging and Cognition, edited by F. I. M. Craik and T. A. Salthouse, 359–416. 2nd ed. Mahwah, NJ: Erlbaum.
- Wöstmann, M., L. Waschke, and J. Obleser. 2019. “Prestimulus Neural Alpha Power Predicts Confidence in Discriminating Identical Auditory Stimuli.” The European Journal of Neuroscience 49 (1): 94–105. doi:https://doi.org/10.1111/ejn.14226.
- Zekveld, A., S. Kramer, and J. Festen. 2011. “Cognitive Load During Speech Perception in Noise: The Influence of Age, Hearing Loss, and Cognition on the Pupil Response. Ear and Hearing, 32, 498–510.