Abstract
Objective
Previous research has linked recurrent otitis media (OM) during early childhood to reduced binaural masking level differences (BMLDs) in school-age children. How this finding relates to monaural processing abilities and the individual otologic history has not been investigated systematically. The current study, therefore, addressed these issues.
Design
Sensitivity to monaural and binaural phase information was assessed using a common test paradigm. To evaluate the influence of the otologic history, overall OM duration, OM onset age, and the time since the last OM episode were considered in the analyses.
Study sample
Children aged 6–13 years with a history of recurrent OM (N = 42) or without any previous ear diseases (N = 20).
Results
Compared to the controls, the OM children showed smaller BMLDs (p < 0.05) whereas their monaural and binaural detection thresholds were comparable (p > 0.05). After controlling for age, the otologic history factors failed to predict the BMLDs of the OM children. Their monaural detection thresholds were correlated with the binaural detection thresholds (r = ∼0.5, p < 0.05) but not the BMLDs.
Conclusions
The current study suggests that early-childhood OM can impair binaural processing abilities in school-age children.
1. Introduction
Otitis media (OM) is the most common cause of temporary hearing loss in early childhood (Boudewyns et al. Citation2011). Many children experience their first OM episode before 2 years of age and additional episodes afterwards (Atkinson, Wallis, and Coatesworth Citation2015). OM can lead to intermittent conductive hearing loss (CHL) of up to 40 decibel hearing level (dB HL), especially in the lower frequencies (Cai and McPherson Citation2017; Moore, Hartley, and Hogan Citation2003; Ungkanont, Charuluxananan, and Komoltri Citation2010). Asymmetric CHL associated with middle-ear disease may result in interaural asymmetries in middle-ear function, which may affect binaural processing abilities (Hall, Grose, and Pillsbury Citation1995). Auditory abilities are known to develop greatly during early childhood (Cameron et al. Citation2009; Hall et al. Citation2004). It is therefore possible that interruptions to auditory stimulation caused by CHL during this sensitive period result in longer-lasting auditory processing deficits, with potential consequences for language development and perception (Sininger, Doyle, and Moore Citation1999; Whitton and Polley Citation2011).
Binaural processing abilities are often studied using binaural masking level difference (BMLD) measurements. The BMLD is a measure of the ability to take advantage of interaural phase differences between the target and masker when detecting a tone in background noise (Moore Citation2012). Several studies have investigated the effects of CHL caused by OM during early childhood on binaural processing abilities (Gravel et al. Citation2006; Graydon et al. Citation2017; Hall, Grose, and Pillsbury Citation1995; Hogan and Moore Citation2003; Moore, Hutchings, and Meyer Citation1991; Pillsbury, Grose, and Hall Citation1991; Whitton and Polley Citation2011). Pillsbury, Grose, and Hall (Citation1991) found reduced BMLDs in children with OM (and associated CHL) during the disease as well as 1 and 3 months after ventilation tube (VT) surgery compared to normal-hearing (NH) controls. Furthermore, they reported a (non-significant) trend for the BMLDs of the OM children to increase over time. Moore, Hutchings, and Meyer (Citation1991) observed significantly smaller BMLDs in children with more than five OM episodes (as assessed using parental reports) compared to NH controls. Hall, Grose, and Pillsbury (Citation1995) measured BMLDs in OM children 3 months, 1, 2, 3, and 4 years after the CHL had disappeared. They observed reduced BMLDs that persisted for up to 2 years, leading them to propose that binaural function in OM children recovers slowly. Hogan and Moore (Citation2003) studied the effects of OM history on the BMLD in 6-year-old children. The middle-ear status and the degree of CHL associated with OM of the children were prospectively documented from birth to the time of testing. They found long-term negative effects of OM-related CHL on the BMLD, which was related to the number of experienced OM episodes. Using a prospective study design, Gravel et al. (Citation2006) investigated the long-term effects of CHL associated with OM in the first 3 years of life on the BMLD. In contrast to the aforementioned studies, these authors did not find a significant effect on the BMLD at school age. Graydon et al. (Citation2017) measured BMLDs in 6–13-year old children with a documented history of OM (and accompanying CHL) before age four. Consistent with Gravel et al. (Citation2006)’s study, these authors did not find any evidence for reduced BMLDs in OM children either. However, neither Gravel et al. (Citation2006) nor Graydon et al. (Citation2017) considered any details of the otologic records and thus the individual progression of the disease in their analyses.
Overall, the findings summarised above seem to imply that OM children are at risk of developing impaired binaural processing abilities and that these deficits will persist for several years after the middle-ear issues have disappeared. Nevertheless, because the binaural system receives its inputs from the two ascending monaural pathways (Gilkey and Anderson Citation2014) and because monaural processing abilities were not assessed in the aforementioned studies, it is unknown if the reduced BMLDs observed previously, in fact, had a monaural origin.
To date, only a few studies have investigated the effects of early childhood OM on monaural processing abilities. Shetty and Koonoor (Citation2016) assessed amplitude modulation (AM) detection and frequency discrimination (FD) in OM children aged 7–10 years. They found that children with more than four OM episodes between 0.5 and 2 years of age had impaired AM detection and FD abilities. McKenna Benoit et al. (Citation2019) measured AM detection thresholds in 4–7-year old children with a history of CHL associated with OM before age three. They found poorer AM detection thresholds in OM children compared to NH controls. Moreover, Colella-Santos et al. (Citation2019) assessed pitch pattern (PP) and gap in noise (GIN) detection in two groups of 8–14-year old children with or without three documented episodes of OM and bilateral VT surgery at least once before age six. They also found a significant difference in the results of PP and GIN between their two groups of participants. Broadly speaking, these findings are consistent with sensory deprivation during early auditory development leading to impaired monaural sound encoding, which, in turn, could impair binaural processing and thus reduce BMLDs.
According to the research literature, the number of OM episodes experienced can play a role in the resultant auditory processing deficits, with more episodes seemingly leading to greater impairments (Moore, Hutchings, and Meyer Citation1991, Moore, Hartley, and Hogan Citation2003; Shetty and Koonoor Citation2016). From physiological studies, it is well known that there is a sensitive period for auditory development during which important neural changes take place that depends on sensory input (Caras and Sanes Citation2015; Insanally et al. Citation2009; Polley, Thompson, and Guo Citation2013). Therefore, the point in time when auditory deprivation occurs (rather than the number of occurrences per se) may be critical for auditory development. In other words, children with earlier versus later OM onset ages may be differentially affected by the disease. Furthermore, because the human brain remains malleable after the sensitive period, it can readjust itself to restored sensory input. In other words, auditory processing abilities may recover over time (Bose et al. Citation2010; Bures et al. Citation2018; Cai et al. Citation2010; Stephenson, Higson, and Haggard Citation1995). Given these principles of brain plasticity, it seems informative to consider the individual time course of the disease in the assessment of OM-related auditory processing deficits.
In general, a better understanding of the specific auditory deficits experienced by OM children could lead to improved diagnostics and treatment strategies for this population. To accomplish this, the current study investigated sensitivity to monaural and binaural phase information in school-age children with a history of OM. To that end, random frequency modulation detection (RFMD) and BMLD measurements were carried out in 6–13-year old children with or without a documented history of OM. To facilitate the comparison of the monaural and binaural measurements, the experimental task and stimuli used for them were very similar. Given that OM-induced CHL occurs mainly at lower frequencies (Moore, Hartley, and Hogan Citation2003; Ungkanont, Charuluxananan, and Komoltri Citation2010) and that low-frequency phase sensitivity is crucial for binaural processing abilities (Moore Citation2021; Neher Citation2017), all measurements were performed at 500 and 1000 Hz. Furthermore, based on the otologic records of the OM children, overall OM duration, age at first OM onset, and the time passed since the last OM episode was calculated and included in the analyses.
2. Materials and methods
Ethical approval for the current study was obtained from the Regional Committees on Health Research Ethics for Southern Denmark. For each participant, written informed consent was obtained from at least one parent. For the OM children, this included permission to obtain access to the medical records from the relevant otologist. At the end of the study, all participants received a gift card (corresponding to 120 Danish crowns per visit) for their participation.
2.1. Participants
Fifty-three children with a documented history of middle-ear infection or effusion (“OM group”) and 22 children without any previous ear diseases (“control group”) were recruited. Eleven children from the OM group and two children from the control group were excluded as they either did not pass all inclusion criteria (N = 9; see below) or because they withdrew from the study (N = 4). The remaining 42 OM children were aged 6–13 years (mean: 10.1 years; standard deviation, SD: 2.0 years; 21 male). The remaining 20 control children covered the same age range (mean: 10.1 years; SD: 1.9 years; 6 male). At the time of testing, all participants fulfilled the following inclusion criteria: (1) normal middle-ear function as indexed by type-A tympanograms, (2) pure-tone hearing thresholds ≤20 dB HL as averaged across 500, 1000, 2000, and 4000 Hz, or ≤25 dB HL at any one of these four frequencies, (3) word recognition scores in quiet >90%, and (4) normal language and cognitive development. Fulfillment of these criteria was assessed using tympanometry, standard pure-tone audiometry, monosyllabic word recognition measurements in quiet (Elberling, Ludvigsen, and Lyregaard Citation1989), and a parental questionnaire covering language and cognitive development. Moreover, a parental questionnaire that included questions related to the child’s mother tongue, if the child was monolingual, the level of education of the child’s parents, and their income was administered. This revealed that all participants were monolingual, native Danish speakers and came from families with middle to high incomes.
In addition to the inclusion criteria described above, all OM children were required to have had at least two episodes of middle-ear infection or effusion in at least one ear before age five. Children who had experienced middle-ear issues after age five but who otherwise fulfilled all inclusion criteria were also included in the current study (N = 21).
2.2. Otologic records
For each OM child, the history of the middle-ear diseases was extracted from the medical records from the relevant otologist. The otologic records contained information about the number of OM episodes, the age at which these episodes had occurred and how long they had lasted for, and the results of tympanometry and otoacoustic emission measurements (i.e. pass or refer) during and after the disease, but not the children’s hearing thresholds. All OM episodes were confirmed with type-B/C2 tympanograms with normal external ear canal volume (Jerger Citation1970). All 42 OM children had experienced recurrent acute OM or OM with effusion in both ears, starting with either the left ear, the right ear, or both ears at the same time. Furthermore, all of them had received VT treatment at least once.
The information from the otologic records was systematised by extracting the following variables: (1) the total duration for which a given child had experienced the middle-ear disease in years (“overall OM duration”), (2) the child’s age at the time of the first OM episode (“OM onset age”), and (3) the time interval between the last OM episode and the time of testing (“OM recovery period”). provides a summary of these three variables.
Table 1. Summary of the OM children’s otologic information.
2.3. Psychoacoustic measurements
The measurements of monaural and binaural phase sensitivity were based on those performed by Neher (Citation2017). To facilitate the comparison of the monaural and binaural measurements, the stimuli, tasks, and procedures used for them were very similar. In both cases, a 3-interval, 3-alternative, forced-choice (3-AFC) paradigm with a 1-up 2-down adaptive staircase procedure was applied. On each trial, all three intervals included a Gaussian noise with five equivalent rectangular bandwidths (Glasberg and Moore Citation1990). The noise was centred at the nominal test frequency fc (500 or 1000 Hz). The noise was presented at a fixed sound pressure level (SPL) of 65 dB. One randomly chosen interval contained the target stimulus. The duration of each interval was 500 ms, including 25-ms raised-cosine on- and offset ramps. Consecutive intervals were separated by 300 ms of silence. The participants were orally instructed to detect the interval which sounded different and to respond by pressing one of three buttons labelled “1”, “2”, or “3” on a touch screen. In case of doubts, they were encouraged to guess. Visual feedback was provided after each response. The measurements were controlled via customised MATLAB scripts that made use of the AFC toolbox (Ewert Citation2013). The stimuli were presented via an RME Fireface UC soundcard and free-field-equalized Sennheiser HDA200 headphones. All measurements were conducted in a large sound-attenuating booth. The participants were given a short break after finishing half of the measurements and whenever they felt tired.
2.3.1. Sensitivity to monaural phase information
To assess sensitivity to monaural phase information, RFMD threshold measurements similar to those described by Kortlang, Mauermann, and Ewert (Citation2016) were performed. For each participant, the left and right ears were tested separately and in random order. On any given trial, all three intervals contained a tone with random AM. In addition, the tone in the target interval contained random frequency modulation (FM). Random AM was applied to rule out the availability of AM cues, as caused by FM-to-AM conversion inside the cochlea (Zwicker Citation1962), for the detection of the FM-modulated target tones. The AM was carried out at a rate of 1–4 Hz with a root-mean-square (RMS) depth of 12 dB. The FM was also carried out at a rate of 1–4 Hz. The rate of the modulation changed randomly within this range. The FM depth was controlled by the RMS deviation of the instantaneous frequency finst from the nominal test frequency fc. The RMS FM depth was the ratio between the RMS frequency excursion and the nominal test frequency, ΔfRMS/fc. For example, for a tone with a nominal frequency of 500 Hz and an RMS frequency excursion of 50 Hz the RMS FM depth would correspond to 10%.
All tones were presented at an overall level of 68 dB SPL, corresponding to a fixed signal-to-noise ratio (SNR) of +3 dB. The starting FM depth was 20% relative to fc. Multiplicative step sizes of 2, 1.5, and 1.25 were used in the adaptive procedure. The step sizes changed from 2 to 1.5 at the third reversal, and they changed from 1.5 to 1.25 at the fifth reversal. The measurements were terminated after 10 reversals, and thresholds were calculated as the geometric mean of the adaptive variable at the last six reversals. Before the actual measurements, training runs starting with 30% FM depth were performed at both 500 and 1000 Hz for one randomly chosen ear to familiarise the participants with the procedure.
2.3.2. Sensitivity to binaural phase information
Sensitivity to binaural phase information was assessed using BMLD measurements. On any given trial, the three intervals contained a noise signal that was interaurally in-phase. One randomly chosen interval contained a 500- or 1000-Hz target tone that was either interaurally in-phase (“N0S0”) or π radians out of phase (“N0Sπ”). The order of the different test conditions was randomised across participants. The starting SNR was +1 dB with additive step sizes of 8, 4, and 2 dB. The step sizes changed from 8 to 4 dB at the third reversal, and they changed from 4 to 2 dB at the fifth reversal. After 10 reversals, the measurements were terminated, and the thresholds were calculated as the geometric mean of the adaptive variable at the last six reversals. The BMLD was calculated by taking the difference between the corresponding N0S0 and N0Sπ thresholds. Before the actual measurements, all participants completed a training run in the N0Sπ condition with a starting SNR of +7 dB at both 500 and 1000 Hz.
2.4. General procedure
The measurements were completed at two visits lasting for 45–60 min each in the audiological laboratory of the University of Southern Denmark. First, all children were screened to verify that they fulfilled the inclusion criteria. Next, they performed the psychoacoustic measurements. For each participant and type of measurement, a set of test and retest measurements was completed at the same visit. If a given retest measurement deviated by more than 3% (monaural measurements) or 3 dB (binaural measurements) from the corresponding test measurement, another (repeat) measurement was carried out. For the data analyses reported below, the median of each set of threshold measurements was used.
2.5. Statistical analyses
Initially, the raw data were inspected to identify unreliable measurements. First, the SD of the test, retest, and repeat measurements for a given measure (e.g. RFMD at 500 Hz, right ear) and the child were calculated. Next, boxplots of the SDs of all children for each measure were made to identify any outliers, that is, datapoints exceeding 1.5 × the interquartile range of the corresponding dataset. The data from children with SDs in excess of 1.5 × the interquartile range were excluded from the subsequent analyses. More specifically, out of more than 1000 measured thresholds 26 RFMD thresholds, three N0S0 thresholds, and seven N0Sπ thresholds were removed.
Following the data cleaning, a test-retest analysis was performed. To that end, the within-subject SD (or measurement uncertainty) was calculated for the individual datasets from the different measures (e.g. RFMD at 500 Hz, right ear). The correlation between the test and retest results was also examined using Spearman’s correlation coefficient, rho. Children with more than two measurements (test, retest, repeat) were excluded from these analyses. More specifically, at 500 Hz 10 RFMD thresholds, 10 N0S0 thresholds, 15 N0Sπ thresholds, and 10 BMLD scores were excluded, while at 1000 Hz 10 RFMD thresholds, 8 N0S0 thresholds, 7 N0Sπ thresholds, and 11 BMLD scores were excluded. The measurement uncertainty was found to be <2% for all monaural measurements and <2 dB for all but one type of binaural measurement (see ). Moreover, rho ranged from 0.6 to 0.9 (all p < 0.001; ). Overall, the test-retest reliability of the collected data was therefore high.
Table 2. Within-subject standard deviations and Spearman’s correlation coefficients for the different monaural and binaural measurements.
Next, the RFMD thresholds were compared across right and left ears using a series of non-parametric Mann–Whitney U tests. No significant differences across ears were found (500 Hz: U = 1033.0, p > 0.3, effect size r = −0.13; 1000 Hz: U = 1321.5, p > 0.8, effect size r = −0.03). In addition, rho was calculated for the RFMD thresholds across left and right ears and found to be 0.68–0.69 (both p < 0.001). Thus, to simplify the subsequent analyses, the RFMD thresholds for each test frequency were averaged across the right and left ears of each child.
To examine the distributions of the resultant datasets, Shapiro-Wilk’s test, normal Q-Q plots, and boxplots were used. To verify the equality of variances, Levene’s test was used. Datasets that did not pass the assumption of normality were transformed (monaural measurements: reciprocal transform; binaural measurements: square-root transform), and the assumptions for parametric statistics were re-evaluated. Importantly, extreme values were not excluded at this stage because they were expected in the data of the OM children and thus considered genuine observations.
To compare the results of the OM and control group and to examine the effect of test frequency, repeated-measures multivariate analyses of variances (MANOVAs) with a significance level of 5% were applied. Because the two groups of children were closely matched in terms of age [independent t-test; t(52) = 0.21, p > 0.8, effect size r = 0.06], age was not included as a factor. In total, three models were built, that is, one per outcome type: (1) RFMD at 500 and 1000 Hz (NH controls, N = 13; OM children, N = 29; first model), (2) N0S0 and N0Sπ at 500 and 1000 Hz, and (3) BMLD at 500 and 1000 Hz (NH controls, N = 18; OM children, N = 36; second and third model).
To examine the influence of the otologic history variables (i.e. overall OM duration, OM onset age, and OM recovery period), significant MANOVAs were followed up with multiple linear regression analyses. In each case, the three otologic history variables were included, as was age to control for any changes in the participants’ auditory processing abilities that accompany higher age. The quality of the models was examined by checking the residuals. In that manner, the assumptions of homoscedasticity and normality were confirmed. Furthermore, a linear relationship between the outcome variables and the independent variables was verified. Finally, to assess the correlation between the different monaural and binaural measurements Spearman correlation coefficients were calculated based on the non-transformed data.
3. Results
3.1. Sensitivity to monaural phase information
and summarise the RFMD thresholds of the NH controls and OM children. At 500 Hz, the mean RFMD threshold was 3.2% for the NH controls and 2.4% for the OM children, while at 1000 Hz it was 2.6% and 1.9%, respectively. The repeated-measures MANOVA revealed a significant main effect of test frequency [F(1, 40) = 8.41, p = 0.006, effect size η = 0.174]. The effects of group (p = 0.07) and frequency × group were not significant (p = 0.71).
Figure 1. Boxplots showing the median, the 25th and 75th percentiles, the most extreme datapoints not considering outliers (whiskers), as well as any outliers (+) for the RFMD thresholds at 500 and 1000 Hz for the control and OM groups. RFMD stands for random frequency modulation detection.
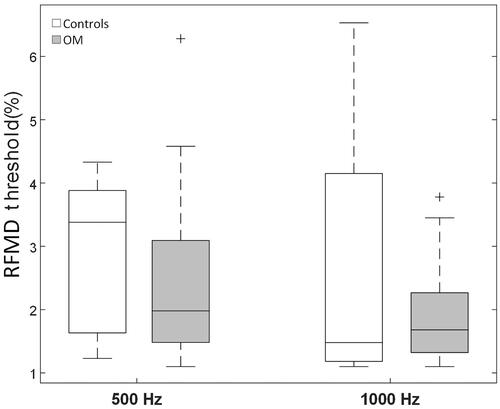
Table 3. Summary of the RFMD, N0S0, N0Sπ, and BMLD data of the NH controls and OM children for the two test frequencies.
3.2. Sensitivity to binaural phase information
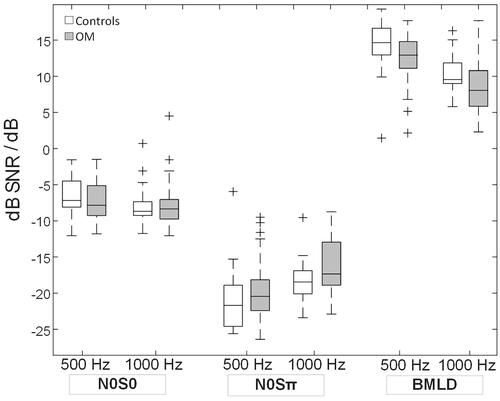
and summarise the binaural measurements of the NH controls and OM children. While the groups’ mean N0S0 detection thresholds were generally comparable, the mean N0Sπ detection thresholds were slightly higher and the BMLDs on average around 2 dB smaller in the OM children. The two repeated-measures MANOVAs showed a significant group difference in terms of mean BMLDs [F(1, 52) = 4.1, p = 0.047, effect size η = 0.074] but neither N0S0 nor N0Sπ detection thresholds (both p > 0.33). Furthermore, a significant effect of test frequency on the BMLDs [F(3, 52) = 55.2, p < 0.0001, effect size η = 0.515] but not the N0S0 or N0Sπ detection thresholds (both p > 0.20) was found. Neither were there any interactions between test frequency and group (p = 0.54).
Figure 2. Boxplots showing the median, the 25th and 75th percentiles, the most extreme datapoints not considering outliers (whiskers), as well as any outliers (+) for N0S0 and N0Sπ detection thresholds at 500 and 1000 Hz (in dB SNR) as well as BMLDs at 500 and 1000 Hz (in dB) for the control and OM groups. BMLD stands for binaural masking level difference.
The follow-up multiple regression analyses performed on the BMLD data of the OM group showed that neither age, overall OM duration, OM onset age, nor OM recovery period was a significant predictor of the BMLDs (all p > 0.05).
3.3. Relation between monaural and binaural measurements
shows the results of the correlation analysis performed on the monaural and binaural data of the OM children following Bonferroni (N = 6) correction. At 500 Hz, the RFMD thresholds were correlated with the N0Sπ thresholds, while at 1000 Hz they were correlated with the N0S0 thresholds. The correlations between the RFMD and N0S0 thresholds at 500 Hz and the RFMD and N0Sπ thresholds at 1000 Hz showed the same trend. No correlations were found between the RFMD thresholds and the BMLDs.
Table 4. Results of the correlation analysis performed on the monaural and binaural data after Bonferroni correction.
4. Discussion
The aim of the current study was to investigate the long-term effects of early-childhood OM on sensitivity to monaural and binaural phase information. Another aim was to investigate the effect of the individual OM history (i.e. overall OM duration, OM onset age, and OM recovery period). Children with a history of OM showed reduced BMLDs compared to children without any previous ear diseases. In contrast, the monaural and binaural detection thresholds of the OM children were comparable to those of the NH controls. Furthermore, significant correlations between the monaural and binaural detection thresholds of the OM group were found, whereas the RFMD detection thresholds and BMLD scores were not correlated with each other. In the following sections, these findings will be discussed.
4.1. Influence of OM on monaural and binaural processing abilities
The long-term effects of OM on binaural processing abilities have previously been studied. However, as pointed out in Section 1, only a few studies have dealt with the long-term effects of OM on monaural processing abilities so far. To recapitulate, Shetty and Koonoor (Citation2016) found impaired AM detection and FD abilities several years after OM correction in school-age children with a history of OM. McKenna Benoit et al. (Citation2019) also observed impaired AM detection in 4–5-year old OM children that recovered to normal performance levels at age 6–7. In addition, Colella-Santos et al. (Citation2019) found significant adverse effects of early-childhood OM on PP and GIN detection in 8–14-year old children with three documented episodes of OM and at least one bilateral VT surgery before age six. In the current study, the monaural FM detection abilities of the OM children were comparable to those of the NH controls. Possible explanations for this discrepancy will be discussed below.
Regarding binaural processing abilities, the literature contains reports of reduced BMLDs in OM children (Hall, Grose, and Pillsbury Citation1995; Moore, Hutchings, and Meyer Citation1991; Pillsbury, Grose, and Hall Citation1991). In line with these findings, the current study found BMLDs that were around 2 dB smaller compared to the NH controls. This result seems to provide further evidence for the adverse effects of OM on binaural processing abilities. In the study by Graydon et al. (Citation2017), however, where 6–13-year old children with or without a documented history of OM were compared using BMLD and “Listening in Spatialised Noise-Sentence” (LiSN-S) measurements, it was found that OM children obtained poorer LiSN-S results but not smaller BMLDs. The findings by Graydon et al. (Citation2017) support those by Stephenson, Higson, and Haggard (Citation1995) who did not find a significant difference in the BMLDs of young adults with a reported history of early-childhood OM and NH controls either.
Regarding monaural processing abilities, a possible explanation for the divergent findings across studies could be differences in terms of the study samples. The mean age of the participants tested here was higher compared to previous studies which found impaired monaural processing in OM children. The findings of the current study suggest that, through auditory recovery, monaural deficits disappear during the first years after the diseases while binaural deficits may normalise over a longer time period (Hogan, Meyer, and Moore Citation1996). While this would seem to imply that the auditory processing deficits of OM children eventually will disappear and thus do not require medical attention, early intervention (e.g. VT or hearing aid treatment) can be expected to minimise any adverse effects on, for example, academic achievement during the disease and should therefore be pursued.
In addition, Whitton and Polley (Citation2011) reviewed the literature on both animal and human studies of early auditory deprivation. They provided a meta-analysis and concluded that when looking at long-term effects of OM without considering the degree of hearing loss, the effect on auditory processing was unclear, with nine studies finding an effect and eight other studies finding no effect. However, when looking at these studies with the degree of hearing loss taken into consideration, an effect was found in most studies. In the current study, the otologic records did not contain information that would have allowed quantifying the degree of CHL during the middle-ear disease. This could be why the current study failed to demonstrate the long-term adverse effects of OM on monaural processing.
Another possible explanation for the lack of a group difference in the monaural measurements performed in the current study could be that the auditory impairments of the OM children tested here occurred at a higher level of auditory processing. In that case, they would not be detectable using a measure of monaural phase sensitivity, suggesting that a measure of binaural phase sensitivity would be more suited for assessing the long-term effects of OM.
4.2. Relation between monaural and binaural measurements
The current study found correlations of moderate magnitude between the RFMD thresholds and the N0S0 and N0Sπ thresholds (). In contrast, the RFMD thresholds were not correlated with the BMLDs. Broadly speaking, these findings are consistent with the results of Neher (Citation2017) who tested older hearing-impaired listeners and who also found RFMD thresholds to be correlated with binaural detection thresholds but not with BMLDs. Previous studies of BMLDs in OM children did not assess monaural processing abilities, which is why they were unable to test for monaural and binaural contributions to tone-in-noise performance. In the current study, the correlations observed between RFMD and binaural tone in noise detection suggest an involvement of the monaural ascending pathways in the N0S0 and N0Sπ detection thresholds of the OM children. The lack of a correlation between RFMD and BMLD, on the other hand, suggests that monaural phase sensitivity does not necessarily predict binaural unmasking abilities.
4.3. Influence of the individual otologic history
The current study did not find overall OM duration, OM onset age, or OM recovery period to be significant predictors of the BMLD in OM children. A link between OM onset age and binaural processing abilities was found in the study by Tomlin and Rance (Citation2014), lending support to the idea of a sensitive period for auditory development (Caras and Sanes Citation2015; Insanally et al. Citation2009; Polley, Thompson, and Guo Citation2013). Tomlin and Rance (Citation2014) assessed binaural processing in children with a reported history of OM using the LiSN-S test, which involves higher-level (i.e. cortical) auditory processing and streaming (Cameron and Dillon Citation2008). In comparison, the BMLD is assumed to reflect lower-level (i.e. brainstem) processing (Jerger and Musiek Citation2000). It could therefore be that the sensitive period for auditory development plays a greater role for cortical compared to brainstem processing.
The lack of an effect of overall OM duration is in agreement with Tomlin and Rance (Citation2014) who did not observe a relationship with long-term binaural processing abilities in OM children either. In the study by Zumach et al. (Citation2009), however, the total number of OM episodes between 0 and 24 months of age was associated with speech perception abilities in noise at age seven. In their study, the OM episodes were monitored prospectively while the OM episodes in the current study and the study by Tomlin and Rance (Citation2014) were monitored retrospectively. The possibility of missing information regarding OM episodes in retrospective studies cannot be completely ruled out. It is therefore possible that the total number of OM episodes in Zumach et al. (Citation2009)’s study was more reliably quantified than the overall OM duration in the aforementioned retrospective studies. Prospective study designs are thus recommended for similar future research.
4.4. Limitations and future directions
As mentioned in Section 4.1, in their review of perceptual and physiological consequences of auditory deprivation during critical developmental periods, Whitton and Polley (Citation2011) suggested that the degree of CHL (rather than the presence of OM per se) is decisive for the occurrence of central auditory deficits. As is a standard clinical practice in Denmark, the OM children tested here had received VT treatment at least once. VT treatment can be expected to alleviate the effects of auditory deprivation due to CHL. As such, it is possible that the effects of early-childhood OM observed here would be more pronounced in other countries where VT treatment is less common. Furthermore, the current study focused on specific psychoacoustic abilities but did not consider speech recognition in noise or language development, which are important factors for academic achievement. Ideally, these issues should be considered in future research to provide optimal guidance for the hearing rehabilitation of OM children.
Author contributions
S.K.: investigation, data curation, formal analysis, and writing—original draft. J.H.S.: project administration. T.N.: conceptualisation, formal analysis, project administration, funding acquisition, and supervision. All authors: methodology, writing—review, and editing.
Acknowledgements
The authors wish to thank Signe Hjorth Fogh for help with the data collection and all the participants and parents for their efforts.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Atkinson, H., S. Wallis, and A. P. Coatesworth. 2015. “Otitis Media with Effusion.” Postgraduate Medicine 127 (4): 381–385. doi:10.1080/00325481.2015.1028317.
- Bose, M., P. Munoz-Llancao, S. Roychowdhury, J. A. Nichols, V. Jakkamsetti, B. Porter, R. Byrapureddy, et al. 2010. “Effect of the Environment on the Dendritic Morphology of the Rat Auditory Cortex.” Synapse 64 (2): 97–110. doi:10.1002/syn.20710.
- Boudewyns, A., F. Declau, J. Van den Ende, E. Van Kerschaver, S. Dirckx, A. Hofkens-Van den Brandt, and P. Van de Heyning. 2011. “Otitis Media with Effusion: An Underestimated Cause of Hearing Loss in Infants.” Otology & Neurotology 32 (5): 799–804. doi:10.1097/MAO.0b013e31821b0d07.
- Bures, Z., K. Pysanenko, J. Lindovsky, and J. Syka. 2018. “Acoustical Enrichment during Early Development Improves Response Reliability in the Adult Auditory Cortex of the Rat.” Neural Plasticity 2018: 5903720. doi:10.1155/2018/5903720.
- Cai, R., X. Zhou, F. Guo, J. Xu, J. Zhang, and X. Sun. 2010. “Maintenance of Enriched Environment-Induced Changes of Auditory Spatial Sensitivity and Expression of GABAA, NMDA, and AMPA Receptor Subunits in Rat Auditory Cortex.” Neurobiology of Learning and Memory 94 (4): 452–460. doi:10.1016/j.nlm.2010.08.008.
- Cai, T., and B. McPherson. 2017. “Hearing Loss in Children with Otitis Media with Effusion: A Systematic Review.” International Journal of Audiology 56 (2): 65–76. doi:10.1080/14992027.2016.1250960.
- Cameron, S., D. Brown, R. Keith, J. Martin, C. Watson, and H. Dillon. 2009. “Development of the North American Listening in Spatialized Noise-Sentences test (NA LiSN-S): Sentence Equivalence, Normative Data, and Test-Retest Reliability Studies.” Journal of the American Academy of Audiology 20 (2): 128–146. doi:10.3766/jaaa.20.2.6.
- Cameron, S., and H. Dillon. 2008. “The Listening in Spatialized Noise-Sentences Test (LISN-S): Comparison to the Prototype LISN and Results from Children with Either a Suspected (Central) Auditory Processing Disorder or a Confirmed Language Disorder.” Journal of the American Academy of Audiology 19 (5): 377–391. doi:10.3766/jaaa.19.5.2.
- Caras, M. L., and D. H. Sanes. 2015. “Sustained Perceptual Deficits from Transient Sensory Deprivation.” The Journal of Neuroscience 35 (30): 10831–10842. doi:10.1523/JNEUROSCI.0837-15.2015.
- Colella-Santos, M. F., C. Donadon, M. D. Sanfins, and L. R. Borges. 2019. “Otitis Media: Long-Term Effect on Central Auditory Nervous System.” Biomed Research International 2019: 1–10. doi:10.1155/2019/8930904.
- Elberling, C., C. Ludvigsen, and P. E. Lyregaard. 1989. “Dantale: A New Danish Speech Material.” Scandinavian Audiology 18 (3): 169–175. doi:10.3109/01050398909070742.
- Ewert, S. D. 2013. “AFC—A Modular Framework for Running Psychoacoustic Experiments and Computational Perception Models.” Proceedings of the international conference on acoustics AIA-DAGA, Merano, Italy, 18–21 March 2013, 1326–1329.
- Gilkey, R., and T. R. Anderson. 2014. Binaural and Spatial Hearing in Real and Virtual Environments. Psychology Press.
- Glasberg, B. R., and B. C. Moore. 1990. “Derivation of Auditory Filter Shapes from Notched-Noise Data.” Hearing Research 47 (1–2): 103–138. doi:10.1016/0378-5955(90)90170-T.
- Gravel, J. S., J. E. Roberts, J. Roush, J. Grose, J. Besing, M. Burchinal, E. Neebe, I. F. Wallace, and S. Zeisel. 2006. “Early Otitis Media with Effusion, Hearing Loss, and Auditory Processes at School Age.” Ear and Hearing 27 (4): 353–368. doi:10.1097/01.aud.0000224727.45342.e9.
- Graydon, K., G. Rance, R. Dowell, and B. Van Dun. 2017. “Consequences of Early Conductive Hearing Loss on Long-Term Binaural Processing.” Ear & Hearing 38 (5): 621–627. doi:10.1097/AUD.0000000000000431.
- Hall, J. W., E. Buss, J. H. Grose, and M. B. Dev. 2004. “Developmental Effects in the Masking-Level Difference.” Journal of Speech, Language, and Hearing Research 47 (1): 13–20. doi:10.1044/1092-4388(2004/002).
- Hall, J. W., J. H. Grose, and H. C. Pillsbury. 1995. “Long-Term Effects of Chronic Otitis Media on Binaural Hearing in Children.” Archives of Otolaryngology-Head & Neck Surgery 121 (8): 847–852. doi:10.1001/archotol.1995.01890080017003.
- Hogan, S. C., S. E. Meyer, and D. R. Moore. 1996. “Binaural Unmasking Returns to Normal in Teenagers Who Had Otitis Media in Infancy.” Audiology & Neuro-Otology 1 (2): 104–111. doi:10.1159/000259189.
- Hogan, S. M., and D. R. Moore. 2003. “Impaired Binaural Hearing in Children Produced by a Threshold Level of Middle Ear Disease.” Journal of the Association for Research in Otolaryngology 4 (2): 123–129. 45342.e9 doi:10.1097/01.aud.0000224727..
- Insanally, M. N., H. Kover, H. Kim, and S. Bao. 2009. “Feature-Dependent Sensitive Periods in the Development of Complex Sound Representation.” The Journal of Neuroscience 29 (17): 5456–5462. doi:10.1523/JNEUROSCI.5311-08.2009.
- Jerger, J. 1970. “Clinical Experience with Impedance Audiometry.” Archives of Otolaryngology 92 (4): 311–324. doi:10.1001/archotol.1970.04310040005002.
- Jerger, J., and F. Musiek. 2000. “Report of the Consensus Conference on the Diagnosis of Auditory Processing Disorders in School-Aged Children.” The Journal of the American Academy of Audiology 11 (9): 467–474.
- Kortlang, S., M. Mauermann, and S. D. Ewert. 2016. “Suprathreshold Auditory Processing Deficits in Noise: Effects of Hearing Loss and Age.” Hearing Research 331: 27–40. doi:10.1016/j.heares.2015.10.004.
- McKenna Benoit, M.,. M. Orlando, K. Henry, and P. Allen. 2019. “Amplitude Modulation Detection in Children with a History of Temporary Conductive Hearing Loss Remains Impaired for Years after Restoration of Normal Hearing.” Journal of the Association for Research in Otolaryngology 20 (1): 89–98. doi:10.1007/s10162-018-00699-8.
- Moore, B. C. 2012. An Introduction to the Psychology of Hearing. Brill.
- Moore, B. C. J. 2021. “Effects of Hearing Loss and Age on the Binaural Processing of Temporal Envelope and Temporal Fine Structure Information.” Hearing Research 402: 107991. doi:10.1016/j.heares.2020.107991.
- Moore, D. R., D. E. Hartley, and S. C. Hogan. 2003. “Effects of Otitis Media with Effusion (OME) on Central Auditory Function.” International Journal of Pediatric Otorhinolaryngology 67 (Suppl 1): S63–S67. doi:10.1016/j.ijporl.2003.08.015.
- Moore, D. R., M. E. Hutchings, and S. E. Meyer. 1991. “Binaural Masking Level Differences in Children with a History of Otitis Media.” Audiology 30 (2): 91–101. doi:10.3109/00206099109072874.
- Neher, T. 2017. “Characterizing the Binaural Contribution to Speech-in-Noise Reception in Elderly Hearing-Impaired Listeners.” The Journal of the Acoustical Society of America 141 (2): EL159–EL163. doi:10.1121/1.4976327.
- Pillsbury, H. C., J. H. Grose, and J. W. Hall. 1991. “Otitis Media with Effusion in Children. Binaural Hearing Before and After Corrective Surgery.” Archives of Otolaryngology-Head & Neck Surgery 117 (7): 718–723. doi:10.1001/archotol.1991.01870190030008.
- Polley, D. B., J. H. Thompson, and W. Guo. 2013. “Brief Hearing Loss Disrupts Binaural Integration During Two Early Critical Periods of Auditory Cortex Development.” Nature Communications 4 (1): 2547. doi:10.1038/ncomms3547.
- Shetty, H. N., and V. Koonoor. 2016. “Sensory Deprivation Due to Otitis Media Episodes in Early Childhood and Its Effect at Later Age: A Psychoacoustic and Speech Perception measure.” International Journal of Pediatric Otorhinolaryngology 90: 181–187. doi:10.1016/j.ijporl.2016.09.022.
- Sininger, Y. S., K. J. Doyle, and J. K. Moore. 1999. “The Case for Early Identification of Hearing Loss in Children. Auditory System Development, Experimental Auditory Deprivation, and Development of Speech Perception and Hearing.” Pediatric Clinics of North America 46 (1): 1–14. doi:10.1016/S0031-3955(05)70077-8.
- Stephenson, H., J. Higson, and M. Haggard. 1995. “Binaural Hearing in Adults with Histories of Otitis Media in Childhood.” Audiology 34 (3): 113–123. doi:10.3109/00206099509071905.
- Tomlin, D., and G. Rance. 2014. “Long-Term Hearing Deficits after Childhood Middle Ear Disease.” Ear & Hearing 35 (6): e233–e242. doi:10.1097/AUD.0000000000000065.
- Ungkanont, K., S. Charuluxananan, and C. Komoltri. 2010. “Association of Otoscopic Findings and Hearing Level in Pediatric Patients with Otitis Media with Effusion.” International Journal of Pediatric Otorhinolaryngology 74 (9): 1063–1066. doi:10.1016/j.ijporl.2010.06.006.
- Whitton, J. P., and D. B. Polley. 2011. “Evaluating the Perceptual and Pathophysiological Consequences of Auditory Deprivation in Early Postnatal Life: A Comparison of Basic and Clinical Studies.” Journal of the Association for Research in Otolaryngology 12 (5): 535–547. doi:10.1007/s10162-011-0271-6.
- Zumach, A., E. Gerrits, M. N. Chenault, and L. J. C. Anteunis. 2009. “Otitis Media and Speech-in-Noise Recognition in School-Aged Children.” Audiology & Neuro-Otology 14 (2): 121–129. doi:10.1159/000162664.
- Zwicker, E. 1962. “Direct Comparisons Between the Sensations Produced by Frequency Modulation and Amplitude Modulation.” The Journal of the Acoustical Society of America 34 (9B): 1425–1430. doi:10.1121/1.1918362.
