Abstract
Objective
To date, no hearing-specific self-report tool is available in Dutch to give insight into how deficits in auditory skills are experienced by a child in daily life or to examine the impact of hearing loss on children’s quality of life. Therefore, we aimed to translate and validate the Speech, Spatial, and Qualities of Hearing Scale (SSQ) and the Hearing Environments and Reflection on Quality of Life (HEAR-QL) Questionnaire for children and adolescents into Dutch.
Design
Translation of the questionnaires into Dutch was conducted by means of the forward-backward procedure. Participants were invited to complete the questionnaires digitally. We examined discriminant validity, internal consistency, and test-retest reliability.
Study sample
A total of 121 subjects between 7 and 18 years old were included, of which 54 normal hearing and 67 bilaterally hearing-impaired subjects. Hearing-impaired subjects were fitted with hearing aids, bone conductive devices and/or cochlear implants.
Results
All questionnaires were shown to significantly discriminate between the normal hearing and the hearing-impaired group. Satisfying internal consistency and good test-retest reliability were found.
Conclusions
The Dutch SSQ and HEAR-QL questionnaires for children and adolescents appear to be valid and reliable self-report tools for management and follow-up of those with hearing loss.
1. Introduction
Hearing impairment in children is a challenging health problem, considering its wide prevalence, the broad diagnostic work-up and its major consequences (1). It can lead to delayed speech and language development, social isolation, emotional difficulties, psychopathology and impaired school performance, subsequently affecting relationships and professional opportunities later on (World Health Organization Citation2020; Tomblin et al. Citation2015; Elbeltagy Citation2020; Stevenson et al. Citation2015; Theunissen et al. Citation2014; Woodcock and Pole Citation2008). This emphasises the importance of early detection, adequate management and efficient follow-up of hearing loss in those affected.
Patient Reported Outcomes (PROs) are reports of the status of a patient’s health condition (like symptoms, functional status, quality of life, etc.), reported directly from the patient without the clinician’s interpretation (Weldring and Smith Citation2013). Patient Reported Outcome Measures (PROMs), thereupon, are the tools or instruments to measure these PROs, which are often self-completed questionnaires, either generic or condition-specific (Weldring and Smith Citation2013). They illuminate a patient’s own perspective, contributing to a patient-centred health care, and can potentially become an important part in the evaluation of hearing loss in children, exposing the auditory abilities in daily life circumstances and their impact on quality of life (QOL). They yield information which cannot be obtained by only taking into account a patient’s medical diagnosis and audiometric results. Furthermore, they can be used complementary to clinical performance tests in clinical decision making and in monitoring profits and losses of the rehabilitation of hearing loss.
Different self-report assessment tools have been developed for use specifically in hearing-impaired children questioning performance of specific auditory skills, either completed personally by the child or in the form of parent or caregiver reports (i.e., proxy questionnaires). For example, the Auditory Behaviour in Everyday Life (ABEL) questionnaire was developed by Purdy et al. in 2002 to assess parental perceptions of their children'sauditory behaviour (Purdy et al. Citation2002). Other parent or caregiver report tools, for example, are the LittlEARS Auditory Questionnaire (Coninx et al. Citation2009), the Parents’ Evaluation of Aural/Oral Performance of Children (PEACH) (Ching and Hill, Citation2005), and the Children’s Home Inventory for Listening Difficulties (CHILD) (Anderson and Smaldino Citation2000). These are, however, all parent or caregiver reports, of which only the LittlEARS Auditory Questionnaire has been translated and validated in Dutch (Berger, Schlüper, and Simon Citation2013). An important drawback of proxy questionnaires is the fact that a parent’s or caregiver’s perspective can be inaccurate and, furthermore, can be influenced by what is socially desirable. Report tools completed by children themselves can overcome this limitation, however, such paediatric self-report tools are scarce.
A valuable self-report tool questioning auditory abilities is the Speech, Spatial and Qualities of Hearing Scale (SSQ). It was initially developed for use in adults by Gatehouse and Noble (Gatehouse and Noble Citation2004) in 2004 and is a well-known and widely-used scale, revealing listening performance in everyday scenarios across the domains of speech perception, spatial hearing and other qualities of hearing. In 2013, it was adapted for use in the paediatric population by Galvin et al. (Galvin and Noble Citation2013), and besides the children’s report tool, separate versions directed to parents and teachers were provided as well. The parental version has been implemented in different studies investigating auditory performance after cochlear implantation (Ramos Macías et al. Citation2019; Hassepass et al. Citation2013; Cañete et al. Citation2017; Jang et al. Citation2019; Sparreboom, Snik, and Mylanus Citation2012; Beijen, Snik, and Mylanus Citation2007; Galvin, Hughes, and Mok Citation2010; Rauch et al. Citation2021). Studies implementing SSQ directed to the child, on the other hand, are less numerous. For instance, Sangen et al. (Sangen et al. Citation2017) and Rauch et al. (Rauch et al. Citation2021) implemented the children’s SSQ to investigate hearing performance in children with unilateral hearing loss. To date, the paediatric SSQ is the only hearing-related self-report tool assessing specific auditory skills available for children and adolescents, however, a translated and validated version in Dutch has not yet been provided.
Concerning quality of life questionnaires, several generic self-report tools exist for use in children, of which the Paediatric Quality of Life Inventory (PedsQL) (Varni, Seid, and Kurtin Citation2001) is one of the most-used scales applied in numerous studies. These generic tools, however, seem to be limited in their sensitivity to identify affected quality of life in children specifically due to hearing impairment (Ronner et al. Citation2020; Umansky, Jeffe, and Lieu Citation2011). Recently, Hoffman et al. designed the CI-specific Hearing Related Quality of Life (CI-HRQoL) instrument (Hoffman, Cejas, and Quittner Citation2019). As the title suggests, this questionnaire specifically applies to children with cochlear implants. The only other hearing-related quality of life questionnaire for use in children is the Hearing Environments and Reflection on Quality of Life (HEAR-QL) questionnaire (Umansky, Jeffe, and Lieu Citation2011). It was developed by Umansky et al. in 2011 and has shown to be a validated tool, accurate for assessing hearing-related quality of life in children with hearing-loss, regardless of hearing rehabilitation (Ronner et al. Citation2020; Umansky, Jeffe, and Lieu Citation2011; Griffin, Poissant, and Freyman Citation2019; Suneel, Davidson, and Lieu Citation2020). In 2014, Rachakonda et al. developed an adolescent version of the HEAR-QL (Rachakonda et al. Citation2014). As well as for the paediatric SSQ, no validated Dutch version of the paediatric HEAR-QL is available.
To date, no detailed hearing-specific self-report tool is available in Dutch to give insight into how deficits in specific auditory skills are experienced by a child in daily life or to examine the impact of hearing loss on children’s quality of life. Therefore, the primary purpose of this study was to translate and validate the SSQ and the HEAR-QL questionnaires for children and adolescents into Dutch, in order to introduce these valuable self-report tools in the management and follow-up of hearing-impaired children and adolescents in Dutch health care and audiological centres.
2. Methods
2.1. Original questionnaires
2.1.1. SSQ Children and Adolescents
The SSQ for children, developed by Galvin et al. (Galvin and Noble Citation2013) with the original adult version by Gatehouse and Noble (Gatehouse and Noble Citation2004) as reference, was used. It is a self-reporting questionnaire personally completed by children with a minimum age of 11 years old and it consists of 33 items, assessing hearing abilities across three subcategories. These comprise the domains of speech perception (i.e., perception in a variety of situations with various amounts of conversation companions), spatial hearing (i.e., identification of the location, direction and distance of different sounds), and quality of hearing (i.e., recognition and discrimination of sounds and listening efforts). A 10-point response scale, presented as a ruler, is used to rate their performance or experience in the described scenario. Alternative response options such as “would not hear it”, “do not know” and “not applicable” are appended, for the circumstance in which the responder feels unable to provide a rating. Means for the total scores and subscores are calculated, with higher scores indicating better performance. A minimum age of 11 years was stipulated by Galvin et al. (Galvin and Noble Citation2013), taking into account the length, the specificity of the items and the complexity of the response format.
2.1.2. HEAR-QL Children and Adolescents
Concerning the HEAR-QL questionnaire, the versions for children aged 7–12 years old by Umansky, Jeffe, and Lieu (Citation2011) as well as the one for adolescents aged 13–18 years old by Rachakonda et al. (Citation2014) were used. The paediatric version comprises 26 items, focussed on situations affecting interactions with family and friends, participation in social and school activities and impact of the hearing impairment on the child’s emotional well-being (Umansky, Jeffe, and Lieu Citation2011). The adolescent HEAR-QL version includes 28 items, addressing social, school and emotional issues specifically relevant to this age group (Rachakonda et al. Citation2014). In both versions responders are asked how frequently each described scenario has been a problem for them in the recent past, using a 5-point scale: “never” (1), “almost never” (2), “sometimes” (3), “often” (4), or “almost always” (5). Inquiry of the items are conducted by means of questions in the children’s form, whereas declarative statements are used in the adolescent form. For both surveys, scores are transformed to a 0 to 100 scale with 1 = 100, 2 = 75, 3 = 50, 4 = 25, and 5 = 0 points. Means for the total scores and subscores are calculated, with higher scores indicating better self-reported QOL.
2.2. Translation
To translate the SSQ and HEAR-QL questionnaires for children and adolescents into Dutch, the forward-backward procedure was applied. The forward translation was conducted by a bilingual investigator with Dutch as mother tongue, which was reviewed by two different Dutch-speaking clinicians of the department of Otorhinolaryngology, Head and Neck Surgery. An expert translator with Dutch as mother tongue independently performed the back translation to English. For the HEAR-QL Children and Adolescents, the back translated version was additionally sent to their developers for comparison with the original version (Umansky, Jeffe, and Lieu Citation2011; Rachakonda et al. Citation2014). Final modifications of the Dutch versions were executed by a cooperative panel of investigators. Concerning the HEAR-QL Children, the original 26-item questionnaire was reduced to a total of 25 items, for reason that the Dutch translation of item 21 and item 26 of the Feelings subscale were in such a way similar, that they were merged into one item (i.e., item 21) to prevent redundancy. Ultimately, the final versions were pretested in 4 subjects meeting the inclusion criteria. After completion, parents were asked to report (a) if parental support was needed, (b) if their child understood each questionnaire item, (c) if the response options were applicable, and (d) if they had other remarks. Since all questions were answered positively without any remarks, no further significant modifications were needed. A graphic representation of the translation process is represented in Supplementary Figure 4.
2.3. Subjects
Two groups of Dutch-speaking participants were included in the study, children with hearing loss and normal hearing children. Hearing impaired children were eligible for inclusion when (1): between 7 and 18 years of age (2); having hearing loss with a Pure Tone Average at the frequencies 500, 1000, 2000 and 4000 Hz of at least 35 dB HL in the best hearing ear (PTA not older than 1 year) (3); using a hearing aid (HA), bone conductive device (BCD) and/or cochlear implant (CI) since at least 6 months prior to inclusion. Bilaterally hearing-impaired children and adolescents were recruited from the Otorhinolaryngology outpatient clinic of the Erasmus MC Sophia Children’s Hospital and from the Auris audiological centre, both in Rotterdam (the Netherlands). Normal hearing children were eligible for inclusion when between 7 and 18 years of age and having no history of hearing problems or current complaints of such. The hearing needed to be regarded as normal by the child and parent(s). The normal hearing group consisted of normal hearing relatives and acquaintances of our ENT patients and of the researchers.
If comorbidity of sensorimotor or developmental disorders was present in any of both groups with a high probability of influencing the survey answers, independently of the auditory abilities, a subject was excluded. The same applies for circumstances in which a child had significant developmental or speech delay, compared to their peers. Dutch as mother tongue was not required, however, subjects needed to receive education in the Dutch language and should sufficiently master Dutch. This could be easily verified, since the investigators were acquainted with all subjects. Target age ranges for the SSQ, the HEAR-QL Children and the HEAR-QL Adolescents were 11–18, 7–12 and 13–18 years old, respectively, which were strictly respected. Full comprehension by the child of each item questioned needed to be obtained, regardless of the support given by a parent.
2.4. Questionnaire procedure
The questionnaires were completed by the child, whether or not with support of a parent, according to the child’s age. Parental support only concerned reading and understanding of the survey items. Concerning the hearing-impaired group, the described scenarios were interpreted using their hearing aids or cochlear implants. All questionnaires were designed and completed using an online open source survey tool LimeSurvey© (LimeSurvey GmbH, Hamburg, Germany). The software package GemsTracker© (GEneric Medical Survey Tracker, Erasmus MC and Equipe Zorgbedrijven, the Netherlands) was used for collecting the informed consent prior to participation and for survey distribution by means of automatically sent emails. The HEAR-QL surveys could not be submitted until all items were responded, so that no missing HEAR-QL data existed. For the SSQ, on the other hand, no required fields could be setup, since the SSQ provides the before mentioned alternative response options. In case an alternative response option was selected, the corresponding item was considered as missing data. Children’s full comprehension of the questionnaires was checked by sending a final brief survey.
2.5. Ethical considerations
The Medical Ethics Committee of the Erasmus MC has reviewed the research protocol and has judged that the rules laid down in the Medical Research Involving Human Subjects Act do not apply to this research proposal. The study was conducted according to the principles of the Declaration of Helsinki (64th WMA, 2013) and the general Data Protection Regulation.
2.6. Statistical analysis
All statistical analyses were completed using SPSS for Windows version 25 (Armonk, NY: IBM Corp.). Results were considered statistically significant at p < .05.
2.6.1. Discriminant validity
Computing mean values, missing data were deleted pairwise. Assumptions of normality were checked and verified using the Kolmogorov-Smirnov test, showing the majority of data being non-normally distributed. Therefore, non-parametric statistic tests were used. The ability of the questionnaires and their subscales to discriminate between normal hearing and hearing-impaired subjects was assessed by means of the Mann-Whitney U test. Likewise, we investigated if any significant difference could be found between children with conventional hearing aids and/or bone conductive devices (i.e., the hearing aid subgroup) and cochlear implants (i.e., the CI subgroup) within the hearing-impaired subject group. If a CI was present, regardless unilaterally of bilaterally, a subject was reckoned to the CI subgroup.
2.6.2. Internal consistency
Internal consistency was assessed for the hearing-impaired group as well as for the total subject group using item-subscale correlations, Cronbach’s alpha coefficients and Cronbach’s alpha if item deleted. Correlations ≥ 0.30 were found acceptable and a Cronbach’s alpha of at least 0.70 was considered sufficient (Nunnally and Berstein Citation1994; Streiner Citation2003). If the Cronbach’s alpha if item deleted value considerably exceeded the overall alpha value, one could suggest that deletion of the corresponding item would improve the overall internal consistency. Concerning the SSQ survey, missing data were deleted listwise, since a complete data set is required to compute internal consistency in SPSS.
2.6.3. Test-retest reliability
In order to estimate the test-retest reliability, questionnaires were completed twice with a time interval of minimum one week, which seems long enough to prevent recall, though short enough to ensure that clinical change has not occurred (Terwee et al. Citation2007). Test-retest reliability was quantified for both the total group and the hearing loss group, using the intraclass correlation coefficient (ICC) with a 95% confidence interval. An ICC value ≥ 0.70 was considered to indicate good test-retest reliability (Nunnally and Berstein Citation1994; Terwee et al. Citation2007).
3. Results
3.1. Subjects
In total, 121 subjects were included (64 females, 57 males), of which 54 normal hearing and 67 bilaterally hearing-impaired subjects. In Supplementary Figure 5, the completion process according to the different age ranges is graphically represented. In Supplementary Table 4, subject characteristics are presented for all subgroups (i.e., normal hearing and hearing-impaired subgroup of each questionnaire). In all subgroups a balanced gender distribution was present, except for the normal hearing responders of the HEAR-QL Adolescents, in which the female gender represented the majority (70.6% vs. 29.4%). The mean left and right PTA of the hearing loss subjects, CI users excluded, were consistent across all three scales with a range of 54.5–57.2 dB HL. Conventional hearing aid users dominated in number with a percentage around 55%. Subjects with cochlear implant varied from 36.8% to 44.8% and with bone conductive devices from 3.4% to 13.2%.
3.2. SSQ Children and Adolescents
For the total score and all subscales, significantly higher means were found for the normal hearing subjects compared to participants with hearing loss (p < .001), as presented in and . For the Spatial Hearing subscale, the hearing aid group scored significantly better that the CI group (p = .035) (). All subscales obtained an overall Cronbach’s alpha of at least 0.90 (). For item 10 of the Speech Comprehension subscale, the α if item deleted (i.e., 0.936) considerably exceeded the overall alpha value in the hearing loss group. For all items, the item-subscale correlations were found to be greater than 0.30. Regarding test-retest reliability, 46 subjects of the total group (75%) and 30 subjects of the hearing loss group (79%) completed the SSQ twice with a total mean time interval of 12 days, showing satisfactory test-retest reliability with all intraclass correlation coefficients being greater than 0.70 (Supplementary Table 5).
Figure 1. Boxplot of SSQ Children and Adolescents total scores and subscale scores for the NH group and the HL group.
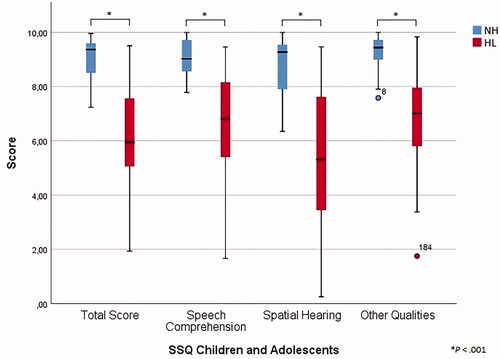
Table 1. Descriptive statistics (n, mean, SD and range) of the SSQ, the HEAR-QL Children and the HEAR-QL Adolescents with corresponding subscales: comparison between the normal hearing group and the hearing-impaired group.
Table 2. Descriptive statistics (n, mean, SD and range) of the SSQ, the HEAR-QL Children and the HEAR-QL Adolescents with corresponding subscales: comparison between hearing aid group and CI group.
Table 3. Internal consistency for all subscales of the SSQ, the HEAR-QL Children and the HEAR-QL Adolescents.
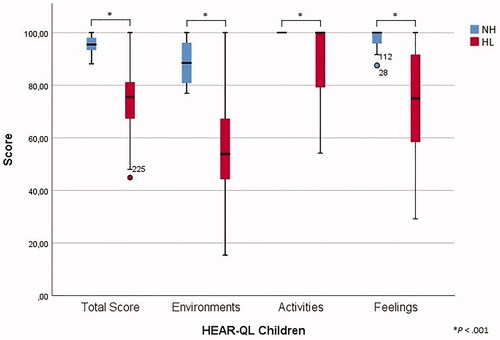
3.3. HEAR-QL Children
Descriptive statistics are presented in and . For the total score and all subscales, significantly higher means were found for the normal hearing group compared to the hearing-impaired group (p < .001). Furthermore, the hearing aid group showed significantly better perceived QOL than the CI group for the total score (p = .013) and the Activities subscale (p = .001) (). As presented in , the overall Cronbach’s α of each subscale was above 0.80, except for the subscale Feelings in the hearing loss group, for which an alpha value of 0.672 was found. For most items, the Cronbach’s α, if the corresponding item would be deleted, approached the overall value. However, item 19 of the Activities subscale exceeded the overall Cronbach’s alpha in both the total group and the hearing loss subgroup (0.900 and 0.886, respectively), which corresponded with lower item-subscale correlations (0.337 and 0.265, respectively). Furthermore, item 24 of the Feelings subscale acquired an α if item deleted of 0.841 and 0.727 in the total group and the hearing loss group with corresponding lower item-subscale correlations of 0.294 and 0.054, respectively. Finally, item 25 showed an α if item deleted of 0.699 in the hearing loss group with an item-subscale correlation of 0.185. Good test-retest reliability was concluded based on intraclass correlations coefficients all above 0.80. For this purpose, 59 children of the total group (79%) and 26 children of the hearing loss group (68%) completed the questionnaire a second time with a total mean time interval of 13 days (Supplementary Table 5).
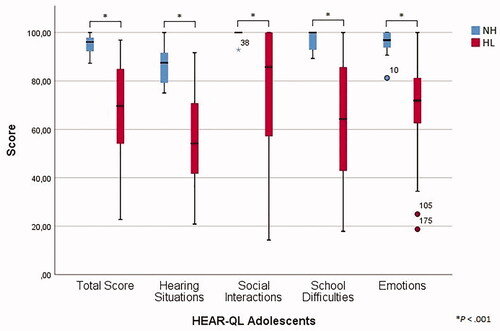
3.4. HEAR-QL Adolescents
In line with the other surveys, the normal hearing subjects scored significantly better than participants with hearing loss (p < .001), as presented in and . Significantly higher scores were found for the hearing aid group compared to the CI group for the Social Interactions subscale (p = .037) (). For all subscales, the overall Cronbach’s alpha was above 0.80 for both the total and the hearing-impaired group (). Other internal consistency measures were favourable as well, since no α if item deleted value considerably exceeded the overall alpha value and since all item-subscale correlations were above 0.30. The HEAR-QL Adolescents was completed twice by 38 participants of the total group (83%) and 23 of the hearing-impaired group (79%) with a total mean time interval of 11 days, showing good test-retest reliability with intraclass correlation coefficients ranging from 0.694 to 0.916 (Supplementary Table 5).
4. Discussion
Hearing-specific Patient Reported Outcome Measures (PROMs) offer valuable information about the auditory performance and well-being of a child with hearing loss and can contribute to the management and follow-up of hearing loss and its daily-life impact. The SSQ gives insight into how deficits in specific auditory skills are experienced by a child in daily life, and the HEAR-QL examines the impact of hearing loss on children’s quality of life. Therefore, these questionnaires enable us to obtain a more holistic view of the child and are a valuable addition to clinical performance tests. Difficulties, unnoticed by parents or too complex to be brought up by a child him- or herself, could nevertheless be detected. Furthermore, these PROMs can be used as a guide to conduct an intake or follow-up consultation. To use these PROMs as clinical outcome measures, they must be validated. Therefore, the aim of this study was to translate and validate the SSQ scale and HEAR-QL questionnaires for children and adolescents into Dutch.
4.1. SSQ
The Dutch SSQ for children and adolescents showed significantly better auditory performance for the normal hearing participants compared to the hearing-impaired subject group, as expected. Furthermore, the hearing aid group scored significantly better than the CI group for the Spatial Hearing subscale. This could be ascribed to the fact that all participants of the hearing aid group used bilateral hearing devices, in contrast to the CI group. Approximately half of CI users were only unilaterally implanted without hearing aids on the contralateral side, missing the benefits of binaural hearing in spatial hearing abilities (Avan, Giraudet, and Büki Citation2015). However, investigating the Spatial Hearing subscores, no clear contrast could be found between those unilaterally and those bilaterally implanted. Another possible explanation is the fact that subjects implanted with CI in general suffer greater hearing losses than those using an hearing aid or bone conductive device. Other studies publishing data of the self-completed paediatric SSQ are scarce. Sangen et al. (Citation2017) found that 22 children with unaided congenital sensorineural unilateral hearing loss scored significantly worse than their normal hearing peers. Although the inclusion of unaided subjects impedes comparison with our study, results for both subject groups correspond considerably with our findings, except for the Spatial Hearing subscale. For this subscale, the hearing-impaired subjects of Sangen et al. showed an evidently lower absolute average score (i.e., slightly above 3, by means of visual inspection of the presented boxplot) compared to our hearing loss participants (i.e., 5.4). Compared to our CI subgroup, however, these spatial hearing results correspond better. Rauch et al. (Citation2021) investigated the subjective benefit of cochlear implantation in 11 children with congenital single sided deafness and especially in the Spatial Hearing section, median scores increased after implantation. Regarding internal consistency, the Dutch SSQ showed favourable outcomes. Only item 10 of the Speech subscale (“Is it easy for you to talk on the telephone with a friend or your Mum or Dad?”) yielded an α if item deleted exceeding the overall alpha value. A possible explanation is the different quotation of this item, compared to the other nine items of the Speech subscale, in which a situation is described followed by the question how well one can follow a conversation or what has been said by someone.
4.2. HEAR-QL Children
The Dutch HEAR-QL Children showed to be able to adequately differentiate the normal hearing children from the children with hearing loss, consistent with the original study by Umansky, Jeffe, and Lieu (Citation2011) Furthermore, the hearing aid group showed significantly better perceived QOL than the CI group for the total score and the Activities subscale. Again, this can be attributed to CI subjects suffering greater hearing loss than hearing aid subjects. Investigating subject characteristics, only few CI subjects were unilaterally implanted, nevertheless, these subjects scored evidently worse than those bilaterally implanted. Furthermore, nearly all subjects in the hearing aid group were bilaterally fitted. This could contribute to the significant differences between the CI and hearing aid subgroup as well.
Besides Umansky, Jeffe, and Lieu (Citation2011), several other studies consistently found significantly lower perceived QOL for hearing-impaired children compared to their normal hearing peers using the paediatric HEAR-QL (Ronner et al. Citation2020; Griffin, Poissant, and Freyman Citation2019; Suneel, Davidson, and Lieu Citation2020). Despite of various inclusion criteria used for the hearing loss group, nearly all previously referred studies found absolute mean values for the total HEAR-QL score and subscales very well corresponding with our mean values, for both the normal hearing and hearing-impaired subject group. In our study, internal consistency was excellent based on Cronbach’s alpha values according to Umansky, Jeffe, and Lieu (Citation2011). Item 19 of the Activities subscale (“Do your parents not let you do certain things because of your hearing?”) showed, however, only little contribution to internal consistency. The mean score for this item did not drop out compared to the other items of the Activities subscale. A possible explanation is the fact that, according to the child’s age, a parent is present to give support reading and understanding the survey and, therefore, this item could be answered differently. Internal consistency results were likewise less favourable for item 25 and 26 of the Feelings subscale (“Do you worry about your hearing loss getting worse?” and “If you can’t hear someone, do you have a hard time asking them to speak louder or repeat what they said?”, respectively). The mean value of item 25 in the hearing-impaired group was quite high compared to the other items of the Feelings subscale, which could suggest that children with hearing loss possibly worry less about their hearing loss getting worse. The mean value of item 26 was, on the other hand, quite low, which could suggest that hearing-impaired children worry more about asking others to speak louder or repeat something they did not hear. Possibly, these emotionally loaded items are experienced more differently between subjects in comparison with the other items of the Feelings subscale.
4.3. HEAR-QL Adolescents
Concerning the Dutch HEAR-QL Adolescents, normal hearing subjects scored significantly better than the hearing-impaired responders, showing mean values consistent with the original study of Rachakonda et al. (Citation2014). For the Social subscale, our results showed significantly higher HEAR-QL values for the hearing aid group compared to the CI group. Similarly as for the SSQ and the HEAR-QL Children questionnaire, this could be attributed to the more severe hearing loss of CI-implanted subjects. All hearing aid subjects were bilaterally rehabilitated, whereas about half of CI-subjects were only unilaterally implanted. Investigating the Social subscale scores, however, the unilaterally implanted CI-subjects did not evidently score worse than those bilaterally implanted. Ronner et al. (Citation2020) and Leonard et al. (Citation2020) also reported significantly worse perceived QOL for their hearing-impaired subject group compared to normal hearing peers. All subjects of Leonard et al. (Citation2020), normal hearing and hearing-impaired, were postoperative cholesteatoma patients. Interestingly, the HEAR-QL scale was sensitive enough to detect significant differences between the normal hearing cholesteatoma patients of Leonard et al. (Citation2020) (PTA < 30 dB HL) and the normal hearing group of Rachakonda et al. (Citation2014) without history of cholesteatoma (PTA < 20 dB HL at 500, 1000 and 2000 Hz, PTA < 30 dB HL at 4000 Hz). Concerning internal consistency, our Dutch HEAR-QL Adolescents showed favourable outcomes for all subscales, for which all Cronbach’s alpha values were above 0.80, consistent with Rachakonda et al. (Citation2014).
4.4. Strengths and limitations
Our study provides three valuable paediatric hearing-specific self-report tools in Dutch at once, showing data consistent with the available literature. Furthermore, our findings are probably well generalisable to the target population (i.e., hearing-impaired children and adolescents in Dutch health care and audiological centres), showing balanced patient characteristics (Supplementary Table 4).
Several limitations of our study should be mentioned as well. The main drawback is the relatively small number of participants in all subgroups, especially the normal hearing subjects. For the normal hearing subgroups, the relevance of this drawback is possibly limited, since normal hearing subjects show less variation in their responses and since hearing-impaired patients represent the clinical target group for these surveys. Second, the risk of selection bias exists. For example, low-income families without electronic equipment could be missed, since completion of the surveys were conducted digitally. Inevitably, self-selection bias can be present, since subjects and/or their parents decide themselves whether or not they participate. One could suppose that parents of children with less successful hearing rehabilitation are more motivated to participate with the hope for an opportunity to further improve their rehabilitation. On the other hand, children with poor quality of life due to their hearing-impairment possibly could avoid participation, because it concerns a sensitive subject.
Future research with the Dutch SSQ and HEAR-QL for children and adolescents should comprise larger sample sizes to confirm the findings of the present study and to further investigate the sensitivity of these valuable questionnaires.
5. Conclusions
In conclusion, the present study provides validated SSQ and HEAR-QL questionnaires for hearing-impaired children and adolescents in the Dutch language for use in clinical management and follow-up of these children in health clinic and audiological centres. Overall, these translated questionnaires correlated well with previously published data and showed good discriminant validity, adequate internal consistency and proper test-retest reliability.
| Abbreviations | ||
| ABEL | = | auditory behaviour in everyday life questionnaire |
| BCD | = | bone conductive device |
| CHILD | = | children's home inventory for listening difficulties |
| CI | = | cochlear implant |
| dB HL | = | decibels hearing level |
| ENT | = | ear, nose and throat |
| Erasmus MC | = | Erasmus University Medical Centre Rotterdam |
| HEAR-QL | = | hearing environments and reflection on quality of life questionnaire |
| HL | = | hearing loss group |
| Hz | = | hertz |
| ICC | = | intraclass correlation coefficient |
| NH | = | normal hearing group |
| PEACH | = | parents' evaluation of aural/oral performance of children |
| PedsQL | = | paediatric quality of life inventory |
| PTA | = | pure tone average (500, 1000, 2000 and 4000 Hz) |
| QOL | = | quality of life |
| SD | = | standard deviation |
| SEM | = | standard error of measurement |
| SSQ | = | speech, spatial, and qualities of hearing scale |
| T | = | total subject group |
| WHO | = | World Health Organisation |
TIJA-2021-08-0355-File008.docx
Download MS Word (488.5 KB)TIJA-2021-08-0355-File007.jpg
Download JPEG Image (1.2 MB)TIJA-2021-08-0355-File006.jpg
Download JPEG Image (823.9 KB)TIJA-2021-08-0355-File005.jpg
Download JPEG Image (491.6 KB)TIJA-2021-08-0355-File004.jpg
Download JPEG Image (426.2 KB)TIJA-2021-08-0355-File003.jpg
Download JPEG Image (477.7 KB)Acknowledgements
The authors thank the children and adolescents, and their parents, for participation in the study. The authors also express their thanks to Marian Rodenburg of Auris audiological centre Rotterdam, for her assistance in recruiting hearing-impaired subjects, and to Anouk Heine, for her assistance in the development and setup of the questionnaires using LimeSurvey© and GemsTracker©.
Disclosure statement
There were no conflicts of interest.
Additional information
Funding
References
- Anderson, K. L., and J. J. Smaldino. 2000. Children's home inventory of listening difficulties. http://www.kandersonaudconsulting.com/uploads/child_questionnaire.pdf
- Avan, P., F. Giraudet, and B. Büki. 2015. “Importance of Binaural Hearing.” Audiology & Neuro-Otology 20(Suppl 1):3–6. doi:10.1159/000380741.
- Beijen, J. W., A. F. Snik, and E. A. Mylanus. 2007. “Sound Localization Ability of Young Children with Bilateral Cochlear Implants.” Otology & Neurotology 28 (4): 479–485. doi:10.1097/MAO.0b013e3180430179.
- Berger, L., K. Schlüper, and S. Simon. The LittlEARS Early Speech Production Questionnaire: vertaling, validatie en normering 2013. https://hbo-kennisbank.nl/details/sharekit_zuyd:oai:surfsharekit.nl:7abe6999-f23a-4f56-a225-4cd9cf598a99.
- Cañete, O. M., S. C. Purdy, M. Neeff, C. R. S. Brown, and P. R. Thorne. 2017. “Cortical Auditory Evoked Potential (CAEP) and Behavioural Measures of Auditory Function in a Child with a Single-Sided Deafness.” Cochlear Implants International 18 (6): 335–346. doi:10.1080/14670100.2017.1373499.
- Ching, T. Y., and M. Hill. 2005. The Parents' Evaluation of Aural/Oral Performance of Children (PEACH) Rating Scale. Chatswood, NSW: Australian Hearing. http://www.ouctomes.nal.gov.au/LOCHI%20assessments.html
- Coninx, F., V. Weichbold, L. Tsiakpini, E. Autrique, G. Bescond, L. Tamas, A. Compernol, et al. 2009. “Validation of the LittlEARS((R)) Auditory Questionnaire in Children with Normal Hearing.” International Journal of Pediatric Otorhinolaryngology 73 (12): 1761–1768. doi:10.1016/j.ijporl.2009.09.036.
- Elbeltagy, R. 2020. “Prevalence of Mild Hearing Loss in Schoolchildren and Its Association with Their School Performance.” International Archives of Otorhinolaryngology 24 (1): e93–e8. doi:10.1055/s-0039-1695024.
- Galvin, K. L., and W. Noble. 2013. “Adaptation of the Speech, Spatial, and Qualities of Hearing Scale for Use with Children, Parents, and Teachers.” Cochlear Implants International 14 (3): 135–141. doi:10.1179/1754762812Y.0000000014.
- Galvin, K. L., K. C. Hughes, and M. Mok. 2010. “Can Adolescents and Young Adults with Prelingual Hearing Loss Benefit from a Second, Sequential Cochlear Implant?” International Journal of Audiology 49 (5): 368–377. doi:10.3109/14992020903470767.
- Gatehouse, S., and W. Noble. 2004. “The Speech, Spatial and Qualities of Hearing Scale (SSQ).” International Journal of Audiology 43 (2): 85–99. doi:10.1080/14992020400050014.
- Griffin, A. M., S. F. Poissant, and R. L. Freyman. 2019. “Speech-in-Noise and Quality-of-Life Measures in School-Aged Children with Normal Hearing and with Unilateral Hearing Loss.” Ear and Hearing 40 (4): 887–904. doi:10.1097/AUD.0000000000000667.
- Hassepass, Frederike, Antje Aschendorff, Thomas Wesarg, Stefanie Kröger, Roland Laszig, Rainer L. Beck, Christian Schild, et al. 2013. “Unilateral Deafness in Children: audiologic and Subjective Assessment of Hearing Ability after Cochlear implantation.” Otology & Neurotology 34 (1): 53–60. doi:10.1097/MAO.0b013e31827850f0.
- Hoffman, M. F., I. Cejas, and A. L. Quittner. 2019. “Health-Related Quality of Life Instruments for Children with Cochlear Implants: Development of Child and Parent-Proxy Measures.” Ear and Hearing 40 (3): 592–604. doi:10.1097/AUD.0000000000000631.
- Jang, Jeong Hun., Ji-Min Roh, Oak Sung Choo, You-Jeong Kim, Hantai Kim, Hun Yi Park, and Yun-Hoon Choung. 2019. “Critical Factors for Binaural Hearing in Children with Bilateral Sequential Cochlear Implantation: First Implant Performance and Inter-Implant Interval.” Audiology and Neurotology 24 (4): 174–182. doi:10.1159/000500700.
- Leonard, C. G., P. R. Dixon, S. Cushing, B. C. Papsin, K. A. Gordon, and A. L. James. 2020. “Measurement Properties of the Hearing Environments and Reflection of Quality of Life (HEAR-QL) 28-Item Questionnaire in Cholesteatoma.” Otology & Neurotology. 42(3):e304–e310.
- Nunnally, J. C., and I. H. Berstein. 1994. Psychometric Theory. 3rd ed. New York: McGraw-Hill;
- Purdy, S. C., D. R. Farrington, C. A. Moran, L. L. Chard, and S. A. Hodgson. 2002. “A Parental Questionnaire to Evaluate Children's Auditory Behavior in Everyday Life (ABEL).” American Journal of Audiology 11 (2): 72–82. doi:10.1044/1059-0889(2002/010).
- Rachakonda, Tara, Donna B. Jeffe, Jennifer J. Shin, Leila Mankarious, Robert J. Fanning, Marci M. Lesperance, Judith E. C. Lieu, et al. 2014. “Validity, Discriminative Ability, and Reliability of the Hearing-Related Quality of Life Questionnaire for Adolescents.” The Laryngoscope 124 (2): 570–578. doi:10.1002/lary.24336.
- Ramos Macías, Á., S. A. Borkoski-Barreiro, J. C. Falcón González, I. de Miguel Martínez, and Á. Ramos de Miguel. 2019. “Single-Sided Deafness and Cochlear Implantation in Congenital and Acquired Hearing Loss in Children.” Clinical Otolaryngology 44 (2): 138–143. doi:10.1111/coa.13245.
- Rauch, Ann-Kathrin, Susan Arndt, Antje Aschendorff, Rainer Beck, Iva Speck, Manuel Christoph Ketterer, Till Fabian Jakob, et al. 2021. “Long-Term Results of Cochlear Implantation in Children with Congenital Single-Sided Deafness.” European Archives of Oto-Rhino-Laryngology 278 (9): 3245–3255. doi:10.1007/s00405-020-06409-6.
- Ronner, E. A., L. Benchetrit, P. Levesque, R. A. Basonbul, and M. S. Cohen. 2020. “Quality of Life in Children with Sensorineural Hearing Loss.” Otolaryngology–Head and Neck Surgery 162 (1): 129–136. doi:10.1177/0194599819886122.
- Sangen, A., L. Royackers, C. Desloovere, J. Wouters, and A. van Wieringen. 2017. “Single-Sided Deafness Affects Language and Auditory Development - a Case-Control Study.” Clinical Otolaryngology 42 (5): 979–987. doi:10.1111/coa.12826.
- Sparreboom, M., A. F. Snik, and E. A. Mylanus. 2012. “Sequential Bilateral Cochlear Implantation in Children: quality of Life.” Archives of Otolaryngology–Head & Neck Surgery 138 (2): 134–141. doi:10.1001/archoto.2011.229.
- Stevenson, J., J. Kreppner, H. Pimperton, S. Worsfold, and C. Kennedy. 2015. “Emotional and Behavioural Difficulties in Children and Adolescents with Hearing Impairment: A Systematic Review and Meta-Analysis.” European Child & Adolescent Psychiatry 24 (5): 477–496. doi:10.1007/s00787-015-0697-1.
- Streiner, D. L. 2003. “Starting at the Beginning: An Introduction to Coefficient Alpha and Internal Consistency.” Journal of Personality Assessment 80 (1): 99–103. doi:10.1207/S15327752JPA8001_18.
- Suneel, D., L. S. Davidson, and J. Lieu. 2020. “Self-Reported Hearing Quality of Life Measures in Pediatric Cochlear Implant Recipients with Bilateral Input.” Cochlear Implants International 21 (2): 83–91. doi:10.1080/14670100.2019.1670486.
- Terwee, Caroline B., Sandra D. M. Bot, Michael R. de Boer, Daniëlle A. W. M. van der Windt, Dirk L. Knol, Joost Dekker, Lex M. Bouter, et al. 2007. “Quality Criteria Were Proposed for Measurement Properties of Health Status Questionnaires.” Journal of Clinical Epidemiology 60 (1): 34–42. doi:10.1016/j.jclinepi.2006.03.012.
- Theunissen, Stephanie C. P. M., Carolien Rieffe, Anouk P. Netten, Jeroen J. Briaire, Wim Soede, Jan W. Schoones, and Johan H. M. Frijns. 2014. “Psychopathology and Its Risk and Protective Factors in Hearing-Impaired Children and Adolescents: A Systematic Review.” JAMA Pediatrics 168 (2): 170–177. doi:10.1001/jamapediatrics.2013.3974.
- Tomblin, J. B., M. Harrison, S. E. Ambrose, E. A. Walker, J. J. Oleson, and M. P. Moeller. 2015. “Language Outcomes in Young Children with Mild to Severe Hearing Loss.” Ear and Hearing 36(Suppl 1): 76S–91S. doi:10.1097/AUD.0000000000000219.
- Umansky, A. M., D. B. Jeffe, and J. E. Lieu. 2011. “The HEAR-QL: quality of Life Questionnaire for Children with Hearing Loss.” Journal of the American Academy of Audiology 22 (10): 644–653. doi:10.3766/jaaa.22.10.3.
- Varni, J. W., M. Seid, and P. S. Kurtin. 2001. “PedsQL 4.0: reliability and Validity of the Pediatric Quality of Life Inventory Version 4.0 Generic Core Scales in Healthy and Patient Populations.” Medical Care 39 (8): 800–812. doi:10.1097/00005650-200108000-00006
- Weldring, T., and S. M. Smith. 2013. “Patient-Reported Outcomes (PROs) and Patient-Reported Outcome Measures (PROMs).” Health Services Insights 6: 61–68.
- Woodcock, K., and J. D. Pole. 2008. “Educational Attainment, Labour Force Status and Injury: A Comparison of Canadians with and without Deafness and Hearing Loss.” International Journal of Rehabilitation Research. Internationale Zeitschrift Fur Rehabilitationsforschung. Revue Internationale de Recherches de Readaptation 31 (4): 297–304. doi:10.1097/MRR.0b013e3282fb7d4d.
- World Health Organization 2020. Deafness and hearing loss: Key facts. Available from: https://www.who.int/news-room/fact-sheets/detail/deafness-and-hearing-loss