Abstract
Objective
To investigate listening skills in infant hearing aid users using the LittlEARS® Auditory Questionnaire (LEAQ).
Design
Caregivers completed the LEAQ, and hearing aid data logging was recorded, at infant age 3–7 months and 7–21 months.
Study sample
Seventy infant hearing aid users with permanent bilateral hearing loss, no developmental comorbidities, aged 3–7 months at first visit.
Results
Infants with mild and moderate losses tended to have scores within the normative range at the early time point (88%), but 29% were below the normative range when older. Thirty percent of infants with severe hearing loss were outside the normative range at the early time point and 60% outside the normative range when older. Infants with profound loss were almost always (95%) outside the normative range. At the later time point, and for infants with severe-to-profound loss, low LEAQ scores were associated with fewer daily hours hearing aid use. Scores were poorer than previous reports in the literature for infant hearing aid users.
Conclusions
This study provides further knowledge on infant listening performance and hearing aid use over time that can be used to guide management of individual cases and to develop and audit service quality improvements.
Introduction
The LittlEARS® Auditory Questionnaire (LEAQ; Coninx et al. Citation2009; Tsiakpini et al. Citation2004) is a validated questionnaire used to track infant listening skills over the first two years of life. It consists of 35 yes/no questions, answered by the parent/caregiver, designed to document a range of developmental auditory skills and language behaviour milestones, for example, “Does your child respond to a familiar voice?”. The result is a score out of 35, with one point for every question answered ‘yes’. Normative scores for LEAQ are consistent across a variety of cultures and languages (e.g. Bagatto et al. Citation2011; Coninx et al. Citation2009; Munro et al. Citation2019; Persson et al. Citation2019), making this a useful international tool for monitoring and comparing outcomes. Audiologists report that the LEAQ is a suitable tool for routine use in paediatric clinical audiology services (Moodie et al., Citation2011) and it has been widely recommended in paediatric audiology amplification protocols (American Academy of Audiology Citation2013; Bagatto et al. Citation2016, Citation2011).
Whilst the LEAQ is commonly used to assess outcomes in infants who wear hearing aids, there have been only a few reports of typical LEAQ scores in such cases (Bagatto et al., Citation2016, Citation2011; McCreery et al. Citation2015; Oropeza Citation2017). Oropeza (Citation2017) has published data on the Marion Downs Centre website of LEAQ scores measured in groups of US infant hearing aid users, with normal cognition scores, with differing severities of hearing loss and in different age groups (17–35 infants in each age/severity category). These data are from a well-respected source, but have yet to be published in the peer-reviewed literature. Infants with mean hearing thresholds no worse than 70 dB HL achieved age-appropriate scores in more than 90% of cases (). This finding of age-appropriate scores was based on comparison with LEAQ scores from well-established norms for infants with normal hearing (Bagatto et al. Citation2011). In contrast, 18% of infants with hearing thresholds >70 dB HL were in the age-appropriate range at age 8 and 15 months, improving to 33% at age 21 months.
Table 1. The percentage of infants with hearing loss with age-appropriate scores on LEAQ relative to infants with normal hearing (adapted from Oropeza Citation2017).
Bagatto et al. (Citation2011) reported that infants with hearing loss but no comorbidities (e.g. Down syndrome, cerebral palsy, genetic factors, prematurity) or complex factors related to hearing aid use (e.g. inconsistent hearing aid use or delayed hearing aid fitting) tended to perform within normative reference limits on LEAQ. Children with mild-to-moderate comorbidities tended to perform within normative reference limits up to age 12 months, with performance declining thereafter. Infants with severe comorbidities (indicated by the clinician to have a severe manifestation of a syndrome or disorder causing multiple issues and potentially affecting auditory performance) scored consistently low. However, the sample size was relatively small (e.g. 7 under 24 months without additional comorbidities or complex factors). The degree of loss in these cases was not reported in detail, but it appears cases were skewed towards mild to moderately-severe losses (20–70 dB HL average loss) and included unilateral losses. Bagatto et al. (Citation2016) reported updated outcome data form the Ontario programme, including LEAQ scores for 76 infant hearing aid users with varying degrees of unilateral/bilateral hearing loss. Of the 30 infants with no comorbidities or complex factors, 77% had LEAQ scores within the expected normative range.
McCreery et al. (Citation2015) reported longitudinal LEAQ scores from 96 infants with hearing loss, with mean pure tone thresholds (0.5, 1, and 2 kHz) in the better ear ranging from 20 to 92.5 dB HL (at approximately 12, 18 and 24 months). Most infants had better-ear mean thresholds of 75 dB HL or better, which was a criterion for entry to the study, though children with progressive losses were not excluded, hence the higher upper limit (Tomblin et al. Citation2015). Participants were mostly hearing aid users, though some with mild losses may not have worn hearing aids. Eighty percent of infants in this study had scores within the normative range at all time points, with 14% having low scores at each visit and 6% having scores that improved to within the normative range at subsequent visits. The validity of the LEAQ as a measure for monitoring auditory progress is supported by McCreery et al.’s data. They found that better LEAQ scores were associated with better hearing thresholds, higher aided audibility, more hours of daily hearing aid use (by parental report), higher word recognition and higher receptive language ability. LEAQ scores were not specifically broken down by degree of hearing loss, however. Participants were not representative of the broader population of children with hearing loss, as they were reported to have higher socioeconomic status on average than the general US population, were all from English-speaking homes, and had no additional developmental comorbidities. Data logging information reported in the paper showed that infants in this sample had a mean daily hearing aid use of 4.4 hours (Walker et al., Citation2015). Mehta et al. (Citation2017) reported similar values for infant daily hearing aid use (median 4.1–4.4 hours), as did Jones and Feilner (Citation2103) (mean 4.5 hours in a large sample of infants/toddlers, or 5.5 hours excluding very low/non-users).
Whilst there are limited data about LEAQ scores in infant hearing aid users, there are considerable data on infant cochlear implant (CI) users. The data typically show very low (often at or near zero) scores before CI experience, with steadily increasing scores with CI experience, demonstrating the LEAQ is a good tool to monitor progress for infants with CIs (e.g. Liu et al., Citation2015; Long et al., Citation2018; Yang et al., Citation2020). Yang et al. (Citation2020) showed that early-implanted (<12 months) infant CIs users’ LEAQ scores had reached normative levels by at least 12 months post-operatively, in their sample of 89 children. Wie (Citation2010) showed that LEAQ scores from 21 infant CI users were equivalent to age-matched normal-hearing infants after 9 months CI experience (though some ceiling effects are likely to have come into play for the older children). LEAQ scores in infant CI users are associated with caregiver education level (Long et al. Citation2018; Liu et al. Citation2015) and age at implantation (Liu et al. Citation2015; May-Mederake et al. Citation2010). Some studies have shown better LEAQ scores for bilateral compared with unilateral infant CI users (Long et al., Citation2018), whilst others have not (Escorihuela García et al. Citation2016). Ganek et al. (Citation2020) compared LEAQ score trajectories in children with different devices and presentations. They found infants with CIs had lower initial scores than infants with hearing aids, but progressed more quickly, and infants with developmental delay progressed more slowly than those without delay. Hours of daily use were associated with better LEAQ scores.
The reports of LEAQ scores in infant hearing aid users described here are limited by: (i) lack of peer review (Oropeza Citation2017); (ii) small numbers in the relevant age range (Bagatto et al., Citation2011); (iii) limited breakdown of expected scores for differing degrees of loss (Bagatto et al. Citation2011, 2016; McCreery et al., Citation2015); and (iv) lack of reporting longitudinal outcomes (Bagatto et al. Citation2011, Citation2016; Oropeza Citation2017). The aim of the current study was to investigate longitudinal LEAQ scores for infants with a range of degrees of hearing loss recruited from a wide range of National Health Service (NHS) paediatric audiology services across the UK. Data were collected longitudinally in early infancy to investigate patterns of changing scores, primarily within the first year of life. The very young age of infants at the initial sample point (3–7 months) is also novel in terms of research literature reported for infant hearing aid users. A better understanding of LEAQ scores across the full age range for which the tool is used is important to help clinicians track progress across infancy and interpret scores appropriately. No limits were placed on the degree of permanent bilateral hearing loss for participants in the study, thus providing results for a wide range of losses, including pre-cochlear implantation. The findings will help clinicians understand the range of LEAQ scores typical in current UK practice, and establish a baseline for potential improvements.
Materials and methods
Participants
One hundred and three infants with permanent bilateral hearing loss (ranging from mild to profound) were recruited from 53 NHS paediatric audiology centres across the UK as part of a wider study investigating aided evoked potentials. All known NHS Trusts (i.e. geographic regions) providing paediatric audiology care in the UK (except Northern Ireland) were invited to join the study, with recruitment from around 50% of these. Caregivers completed the LEAQ, and data logging and hearing aid gain were recorded at two time points, which were opportunistic to coincide with participation in the wider study. Data from 70 of the 103 infants wearing 1 or 2 air conduction hearing aids at both time points were used in the final analysis (from 42 unique NHS centres). Some participants were candidates for future cochlear implantation, but were still hearing aid users for the duration of their study participation. Further participant details can be found in . Excluded cases were due to: likely/confirmed progressive/fluctuating loss (n = 19); missing questionnaire data (n = 5); lost-to-follow-up (n = 5); unaided at the initial time point (n = 1); bone conduction device (n = 1); hearing found to be within normal limits (n = 1); and developmental delay (n = 1). ‘Developmental delay that would likely significantly delay behavioural assessment (VRA)’ was an exclusion criterion for the wider study. Of those with missing questionnaire data, one was due to poor comprehension of the questionnaire, and the others were due to failure to return the questionnaire or complete all items. All questionnaires for included participants were completed in written English by participants with adequate written and spoken English comprehension (according to researcher’s report), with children being brought up in an oral language environment. Median infant age at the initial time point was 4.9 months (range 3.0–7.3) and median age at the later time point was 9.8 months (range 7.4–21.4). In cases of prematurity (affecting 5 of the 70 participants), ages were corrected at both sessions. The ages at which the infants were tested related to their participation in the wider study which involved aided EEG at 3–7 months old, and later aided behavioural testing when developmentally ready. Ethical approval was obtained from the North West National Research Ethics Service Ethics Committee (reference 15/NW/0736), and all caregivers gave written informed consent to participate. Most respondents (96%) were female caregivers. In all but 4 cases the same caregiver completed the questionnaire at both time points.
Table 2. Participant characteristics.
Procedures
Caregivers completed the LEAQ using pen and paper at the two time points. The research sessions took place outside of the families’ regular clinical care pathway, in a bespoke hearing research van that visited families close to their homes. Caregivers were instructed to answer the questions based on their child’s listening skills whilst wearing their hearing aids, and had the opportunity to ask the research audiologist for clarification if needed. At the same time, otoscopy, tympanometry and hearing aid checks were performed. Hearing aid checks included: hearing aid gain in a 2-cc coupler to 55, 65, 75 and 90 dB ISTS input signals (using the Interacoustics Callisto and TBS10 test box), visual inspection, listening check, reading the hearing aid settings into the manufacturer’s software, and noting data logging (daily hours hearing aid use) where possible.
Clinical audiometric results
The clinics from which the infants were recruited provided hearing assessment details, that is, results from the infants’ Auditory Brainstem Response (ABR), Visual Reinforcement Audiometry (VRA) and tympanometry. From the infant audiograms, a best-estimate of the better-ear four-frequency average (4FA, averaged over 0.5, 1, 2 and 4 kHz) hearing threshold was made, using interpolated results where necessary. Where no response was seen at machine limits, the threshold was recorded as maximum level tested plus 15 dB. Where infants had flat tympanograms at the time of completing the second LEAQ, the best-estimate 4FA was made using audiograms obtained when the child also had flat tympanograms. Seventy percent of infants showed a bilateral pass on tympanograms at the early time point compared to 53% at the later time point.
Hours of hearing aid use and speech intelligibility index
Data logging was recorded as the daily hours use averaged between the two hearing aids, where infants wore bilateral aids, and from a single aid for those fit unilaterally. Data logging was available for n = 50 infants. The most common reason for missing data logging was technical error. Missing data logging could also be due to researcher failure to note the data logging, inconsistency with caregiver report suggesting aids being left switched on, or issues with the aids themselves, e.g. being unavailable or recently replaced or reviewed by a clinician. Data logging was compared for infants grouped by LEAQ scores falling within or outside the normative range. Estimated aided speech intelligibility index (SII) scores were additionally calculated, further details of which are provided in Supplementary Appendix 1. Hearing aid match-to-target was not calculated, as the researchers did not have access to the fitting parameters in use (e.g. exact audiogram used, measured RECD values, prescription) and hence any approximation would not have been a valid representation of what actually occurred in the fitting appointment with the clinician.
Statistical analysis
LEAQ scores were classified as ‘within’ or ‘outside’ the normative range (reference range for infants without hearing loss). A one-way ANOVA and post hoc t-tests were used to investigate differences in hearing threshold between groups categorised according to LEAQ trajectory (within/outside the normative range at early/late time points). Wilcoxon rank-sum tests were used to investigate differences in daily hearing aid use for infants falling within and outside the normative range. Finally, a linear mixed model was used to investigate factors impacting LEAQ score, including age, average hearing loss, hearing aid use, and age at hearing aid fitting.
Results
shows LEAQ scores, with degree of hearing loss in the better ear coded by colour (dark blue = mild; dark red = profound). Panel A shows results for all 70 participants, and subsequent panels show subsets of participants based on LEAQ trajectories. In 36 (51%) cases, scores were within the normative range at both time points, and these are shown in panel B. These infants typically had a mild-to-moderate hearing loss, as summarised in panel F (boxplot B). In 15 cases (21%), scores were within the normative range at the early time point but fell below the normative range at the later time point (panel C). These infants typically had moderate-severe losses (panel F, boxplot C), but covered a wide range from <40 dB HL to nearly 100 dB HL. Panel D shows 16 cases (23%) with scores outside the normative range at both time points. These infants typically had losses in the severe-to-profound range, though a small number of outliers were in the mild-to-moderate range (panel F). On review, the one infant with a mild underlying loss who performed outside the range at both points had persistent bilateral otitis media with conductive overlay throughout their clinical audiology appointments (but not on the day of the study). Hence our estimate of the hearing loss is likely to be better than the effective day-to-day loss. Of the three infants in the study with asymmetric losses (all with a profound loss in the poorer ear), two performed within the normative range at both time points (one mild–moderate and one moderate-severe loss in the better ear). The other had a mild-moderate loss in the better ear, and is one of the outlier cases in panel D, shown to be progressing in parallel with normative trajectories, but just below the normative range. The other case from panel D showing parallel but below normative progress did not have any notable circumstances in terms of hearing loss, age at fitting, daily usage, or complications. Only 3 infants (4%) had scores outside the normative range at the early time point and within the normative range at the later time point (Panel E). Due to the small number of cases in panel E, these are not included in the subsequent statistical analysis.
Figure 1. LEAQ scores as a function of age (panel A) and separated according to trajectory (panels B to E). The colour bar represents severity of hearing loss (better-ear 4FA in dB HL). The black lines show mean normative data and 95% normative range for infants with no hearing loss, as reported by Bagatto et al. (Citation2011). Panel A shows results for all participants (n = 70). Panel B shows those with scores within (or above) the normative range at both time points (n = 36). Panel C shows scores within the normative range at the initial time point, but outside of the normative range at the later time point (n = 15). Panel D shows those with scores outside the normative range at the both time points (n = 16). Panel E shows those outside the normative range at the initial time point only (n = 3). Panel F summarises the LEAQ trajectories from panels B, C and D as box plots.
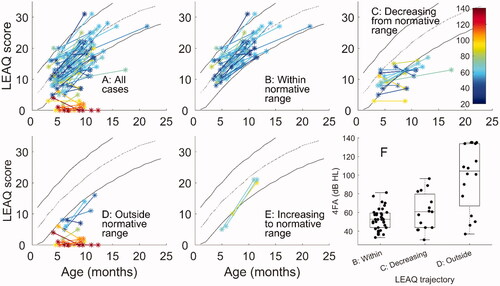
A one-way ANOVA, with LEAQ trajectory type as the independent variable and 4FA threshold as the dependent variable, revealed an overall significant difference in average hearing threshold for the three LEAQ trajectory patterns shown in panel F (F[2,64] = 23.92, p < 0.001). Post-hoc independent samples t-tests showed significant differences between trajectories ‘B: Within’ and ‘D: outside’ (t[50] = 6.78, p < 0.001) and trajectories ‘C: Decreasing’ and ‘D: outside’ (t[29] = 3.45, p = 0.002), with infants in the category ‘D: Outside’ having worse hearing thresholds. There was no statistically significant difference between trajectories ‘B: Within’ and ‘C: Decreasing’ (t[49] = 1.78, p = 0.081).
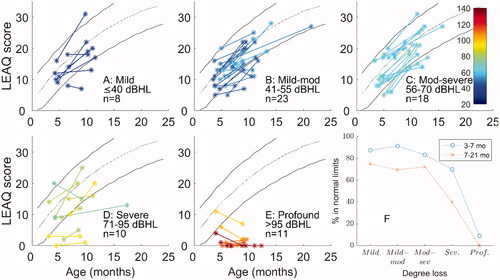
panels A to E show the same LEAQ score trajectories, but this time with severity of hearing loss in different panels. Panel F summarises these data in terms of percentage of scores within the normative range at the two test sessions. For mild-moderate losses, at the early time point, most infants (88%, panels A to C) performed within the 95% normative range. By the later time point, only 71% of infants with mild-moderate losses had scores within the normative range. For infants in the severe category (panel D) a wide range of performances were observed, with 70% within the normative range at the early time point and 40% within the normative range at the later time point. For those with profound losses, just 1 of 11 was within the normative range at the younger age, and all others were consistently outside normative reference limits.
Figure 2. LEAQ scores split by degree of hearing loss (panels A to E). Panel F shows percentage of cases within normative reference limits for different degrees of loss at both time points. Classification of loss was based on the better ear 4FA. Classifications are based on the British Society of Audiology definitions (BSA, Citation2018). Due to a large number of cases falling into the ‘moderate’ range (41–70 dB HL) this was split into two sub-categories of mild-moderate and moderate-severe. Colours in panels A-E represent degree of loss from mild (dark blue) to profound (dark red).
In total eight infants showed scores that decreased, in absolute terms, over time. Of these, four had profound losses, and could perhaps represent over-optimistic scoring at the initial time point. Two infants (one mild-moderate and one severe loss) were within/above the normative range at both time points. Of the remaining two cases, one had a mild underlying loss, and has been discussed above as a case significantly affected by otitis media with conductive overlay. The remaining case had a mild-moderate loss with their score decreasing by 3 points, to outside the normative range. This infant was diagnosed with enlarged vestibular aqueduct which is associated with progressive/variable loss. Clinical audiometric results did not indicate a progressive loss between the two test sessions, but it is possible that the infant was negatively impacted by variable loss.
summarises the hours of hearing aid use at 3–7 months (left panel) and 7–21 months (right panel). The blue bars show LEAQ scores falling inside, and the yellow outside, the normative range, for a total of 50 infants with complete data logging data. Median daily hearing aid use at the early time point was 6.1 hours for infants inside the normative range (lower and upper quartiles 4.4 and 9.8) and 6.0 hours for infants outside the normative range (lower and upper quartiles 3.7 and 8.6). At the later time point, median daily hearing aid use was 7.1 for infants inside the normative range (lower and upper quartiles 3.7 to 9.6) and 2.7 for infants outside (lower and upper quartiles 2.0 to 7.0). The Wilcoxon rank-sum test revealed a borderline significant difference in daily hearing aid use between infants falling within and outside the normative LEAQ range at the later time point (Z = 1.884, p = 0.060) and no significant difference at the early time point (Z = 0.250, p = 0.803). The data were then analysed separately for infants with mild-to-moderate losses (≤ 70 dB HL) (BSA, Citation2018) and infants with severe-to-profound losses (>70 dB HL). The results are summarised in . A significant difference was found for the severe to profound group only (again at the later time point), suggesting the effect is dominated by those with more significant degrees of hearing loss. Aided speech intelligibility index scores are provided and discussed in Supplementary Appendix 1. Most infants had aided SIIs falling within the expected 95% range for hearing aids meeting DSL V targets, and the few cases falling well below had demonstrable reasons.
Figure 3. Histograms showing daily hours’ hearing aid use at the two time points for infants with LEAQ scores inside the normative range (blue) and outside the normative range (yellow). Data shown from infants 3–7 months, within reference range, n = 34; 3–7 months outside reference range, n = 16; 7–21, months within reference range, n = 24; 7–21 months, outside reference range, n = 26.
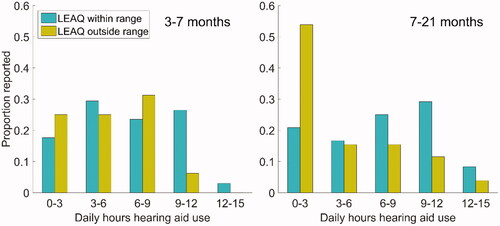
Table 3. Results from Wilcoxon rank-sum tests comparing daily hours hearing aid use in groups within and outside the LEAQ score reference range.
A linear mixed model was used to investigate factors impacting LEAQ score, with subject ID as the random factor. All variables were mean-centred. Fixed factors in the model included: age, average hearing loss, hearing aid use, and age at hearing aid fitting. The following interaction terms were also included as fixed factors: age*hearing aid use, age*average hearing loss, age*age at hearing aid fitting, hearing aid use*average hearing loss, hearing aid use*age at hearing aid fitting, and age at hearing aid fitting*average hearing loss. SII was not included in the model due to being highly correlated with degree of hearing loss.
Age (β = 0.909, p < 0.001) and average hearing loss (β = −0.114, p < 0.001) had a significant impact on LEAQ score, with higher LEAQ scores associated with older infants and those with lower hearing thresholds. Additionally, age*hearing aid use (β = 0.074, p = 0.022), age*average hearing loss (β = −0.013, p = 0.025) and age*age at hearing aid fitting (β = −0.040, p = 0.046) were significant. No other factors were significant (p > 0.05).
Discussion
Overview
The LEAQ is widely used across the world to assess infant outcomes in paediatric audiology (e.g. Bagatto et al. Citation2011, Citation2016). This study presents patterns of results from 70 infants with a range of hearing losses and no significant developmental comorbidities, to build on the knowledge base of expected LEAQ scores for infants with hearing loss. An advantage of the present study over earlier studies is the trajectory of functional performance over time. This revealed that many infants performed well with reference to the normative values at the early time point but fell outside the normative range at the later time point. An important aspect of the study compared to previous published data is that data from a large number of infants, including from very young age of 3–7 months, are broken down by degree of hearing loss. Whilst the overall impact of degree of loss is clear, there is considerable variability within each category as well as significant overlap across categories. Therefore, clinicians should be mindful not to see average performance for any degree of hearing loss as a limitation on what can be achieved in any individual case. An important limitation of the study is that detailed information on hearing aid fittings and adherence to best practice were not available, since the research did not take place within the clinical setting. This limits the degree to which results can be generalised outside of the population from which they were drawn.
Comparison with previous data
The results show positive outcomes for infants with mild-to-moderate losses, with performance close to that of infants with normal hearing at the early time point. However, at the later time point, the functional performance was below the normative range for 29% of these cases. This is in contrast to the results from Oropeza (Citation2017) who reported functional performance within the normative range at 8, 15 and 21 months of age (the percentage scoring within normal limits varied from 85 to 100% in these groups). Oropeza retrospectively analysed LEAQ data for children with hearing loss enrolled in the Colorado Home Intervention Program. McCreery et al. (Citation2015) also reported better overall LEAQ outcome scores than seen in the present data, with 80% of their infants with mild-to-severe losses scoring within the normative range at all time points. Excluding 11 cases from our data with profound losses (>90 dB HL), leaves 36 of 59 (61%) scoring within normal limits at both time points. If we exclude infants with hearing losses of >75 dB HL (as per the original inclusion criterion in McCreery et al. Citation2015), we find 34 of 51 (67%) scored within the normative range at both time points. In the McCreery data, six children who scored outside the normative range at early visits scored within the normative range at later visits, and none showed decreasing scores with reference to normative data over time. This is in contrast to the current study in which 21% showed scores decreasing with respect to normative values over time. This difference is likely due to the early initial time point at which infants were sampled: 3–7 months in the present data compared to 12 months in McCreery et al. Bagatto et al. (Citation2016) reported 77% of 30 typically developing infants without complex factors were scoring within the LEAQ normative range. They also reported that excluding the few cases with severe-profound loss did not have a significant impact on the overall pattern observed. Combining data from the current sample across time points and for all degrees of loss, 64% of scores were within normative limits. Excluding cases with losses >70 dB HL in the current data, 80% of points were within the normative range: a value close to that reported by Bagatto et al. (Citation2016). It may therefore be that the discrepancy between our full dataset and that of Bagatto et al. is due to relatively few severe-profound cases in the Bagatto data that also included cases of unilateral loss, which were excluded in the present data set.
Further possible reasons for differences compared to previous data
It is unclear why performance appears to be worse overall in the present group compared to some previous data. Caregivers taking part in the present study were volunteers taking part in a wider study, so likely to be amongst the most motivated caregivers, which, if anything, may lead us to expect better functional performance than in the population as a whole. We would not expect cultural differences to affect the scores, as the LEAQ has been shown to give consistent results in a range of international settings, at least for infants with normal hearing (Bagatto et al. Citation2011; Coninx et al. Citation2009; Munro et al. Citation2019; Persson et al. Citation2019). The results from Oropeza (Citation2017), which contrast most starkly to the present data, were collected from a unified program (the Colorado Home Intervention Program) which has closely followed best-practice guidance, including early intervention with 95% successful match-to-target, early behavioural assessment and re-fitting, use of LENA (Language Environment Analysis) technology (wearable devices giving feedback on the child’s language environment), using this data to counsel parents (Aragon and Yoshinaga-Itano, Citation2012), and weekly family sessions with a language specialist (Yoshinaga-Itano, personal communication). Participants in the current study were pooled from 42 centres spread across the UK, so it is likely there will be more variation in audiological practice and wider early intervention support (e.g., teacher of the deaf practices). McCreery, Bentler, and Roush (Citation2013) showed an effect of clinic location with poorer match-to-target for infants recruited from a wide range of sites than those recruited from a single large centre. This highlights the potential for improving outcomes with a more unified approach, though there are resource implications in providing the level of care associated with the Colorado program. Another factor could be the LEAQ being delivered outside of the clinical protocol in the present study, by researchers, not local clinicians. The consistent timing and delivery of the questionnaire in the Colorado data could have had a positive influence on outcomes, particularly in terms of fitting in with other clinical procedures and caregiver consultations. This could also be viewed as a bias towards more positive scores than would be seen on a typical day.
Details of match-to-target and other best-practice information are not available in the current data due to limitations in what information could be gathered and accurately synthesised from the many individual clinics with separate local protocols. It is therefore unknown to what extent adherence, or lack thereof, to best practice may have influenced outcomes. This is a limitation of the study and somewhat limits the extent to which the results are generalisable to a wider context. Future research will be of benefit that reports on large numbers of LEAQ scores for hearing and users, breaks them down by degree of hearing loss, and additionally reports on adherence to best practice including match-to-target, use of real-ear measurements, and early behavioural assessment with appropriate fitting adjustments. Updated UK guidance on infant hearing aid fitting best practice is in development by the British Society of Audiology and should serve as a benchmark against which to record best practice for such future research in the NHS. The timing of the study visits meant that some infants had their hearing aid settings updated between study visits. Twenty one percent of infants had the same hearing aid settings at both time points. Early behavioural testing and appropriate hearing aid adjustment ensures infants have the most suitable settings to achieve optimum outcomes. Without access to further information on the timing and purpose of hearing aid adjustments we are unable to comment further on the effect this may have had on the present data, but this could be an area for future research to address.
Demographic differences in the study samples could potentially contribute to differences in LEAQ outcomes across studies (Vandam et al. Citation2012), though there is no evidence to suggest systematic differences between our sample and those in previous studies. McCreery et al. (Citation2015) reported their participants had high socio-economic status, were users of spoken English, and did not have additional disabilities, which may have elevated the scores compared to expected averages. In our sample, all parents were also proficient in spoken and written English, and infants did not have significant developmental delay. Fifty of the 103 participants recruited to the current study did complete an ethnographic survey, though the results were anonymised and not matched to individual participants. Of these, 66% were university-educated, suggesting they were similarly advantaged compared to the McCreery participants (51% college graduates and 32% having post-secondary education) (Tomblin et al., Citation2015), though categories used and education systems differ.
Change in outcomes over time
One possible explanation for several infants performing within the normative range at the early but not the later time points is that the LEAQ is likely to become more sensitive to inter-subject differences as infants get older and are expected to display a wider range of listening behaviours. Yoshinaga-Itano et al. (Citation2010) described this phenomenon as “gap opening”, whereby the gap between typical performance and the performance of children with hearing loss becomes more evident as children get older. Oropeza (Citation2017) outlined which skills on the LEAQ had been acquired by 80% of infants in their sample of infants with hearing loss. For example, infants with mild loss achieved 13 of the 35 items (items 1–8 and 10–14) at 8 months; 24 of the 35 items by 15 months (items 1–23 and 33); and 33 of the items by 21 months (items 1–33). Similar analysis could not be done on the present dataset due to the varying ages at which the infants were seen. With a wider range of behaviours typically demonstrated in older groups, a wider range of questions become pertinent for highlighting cases that fall outside the normative range.
At the later time point, the data showed higher daily hours data logging for infants falling within, compared to outside of, the LEAQ normative range for infants with severe-to-profound degree of loss but not for those with mild-to-moderate loss. It is important to note that this effect does not imply causality. It is not known to what degree parents used the hearing aids less because they were not observing benefit, or lack of use had an impact on LEAQ outcome. Furthermore, hours of hearing aid use in young children are known to be confounded with other factors that affect outcomes, such as maternal education and socioeconomic status (Marnane and Ching Citation2015). McCreery et al. (Citation2015) did show a positive effect of increased hours of hearing aid use on LEAQ scores in their children, scored at 18 or 24 months, so older than those in the present study. Results of the mixed-model in the current dataset did not show an effect of hearing aid use on LEAQ score in isolation, but did show an interaction between age and hearing aid use on LEAQ score.
Factors impacting LEAQ score
The linear mixed model showed, as expected, that age and degree of loss both have a significant impact on LEAQ score, with older children and those with less severe hearing losses performing better. The significant interaction terms paint a complex picture of how age interacts with many other factors to impact LEAQ score. Further investigation revealed hearing aid use more positively impacts LEAQ score for older children. This ties in with the data shown in , showing increased variability in hearing aid use for older children. The interactions between age*degree of hearing loss and age*age at hearing aid fitting do not lend themselves to simple explanations, and may themselves be impacted by many multiple factors.
Limitations
One limitation of the present study is that the clinical audiogram data were not collected by the researchers themselves, but were passed to the research team from the NHS audiology centres across the UK who were responsible for the routine care of the infant. The researchers made their best estimations of the hearing thresholds from the data received, but it was not always known how reliable the clinicians estimated the ABR and VRA threshold data to be, and the clinical threshold data were not collected at the same time as the study data. In some cases, the effect of middle ear effusion on hearing thresholds may not have accurately captured the effect at the time of the LEAQ, and the effect may have varied across the infant’s lifetime. Seventy percent of infants showed a bilateral pass on tympanograms at the early time point compared to 53% at the later time point. The hearing levels which the LEAQ scores were compared to were the best estimates of hearing at the second time point, including any conductive overlay. This may mean that hearing loss was slightly overestimated at the initial time point. Future studies could look directly at whether the onset of middle ear dysfunction affects LEAQ score trajectories in infants. As discussed above, basing data collection in a clinical environment in the future would also enable data collection on adherence to best-practice protocols which will further help to address possible reasons for low LEAQ scores and to monitor improvements.
A further potential limitation of the present dataset is that infants were not all seen at the same age, but rather over a range of ages with a relatively small sample of infants tested at each age point and for each degree of hearing loss. Further research would be of great value to establish a robust dataset of LEAQ scores for infants with a range of hearing losses, over several time points, aiming to gather data at similar times for all infants. This would allow normative data to be gathered which may help audiologists in interpreting scores and counselling families, and to determine how scores change over time and how they are affected by family factors such as socioeconomic status and hearing aid factors such as SII. Whilst data were collected from a range of centres to give good representation at a population level, families may still have been somewhat advantaged compared to the population as a whole, with data suggesting relatively high maternal education level and English language ability within the sample, as well as sampling infants without developmental delay. It should also be noted that while the LEAQ is a well-validated tool, it is still a subjective assessment and susceptible to caregiver bias.
Many caregivers struggle to achieve consistent hearing aid use during all waking hours for their infant children (Moeller et al. Citation2009). It is still unknown to what degree either LEAQ scores in infancy, or daily hours hearing aid use in infancy, are predictive of later speech and language development. Whilst there are data to show that consistent hearing aid use in slightly older children positively impacts language outcomes (Tomblin et al. Citation2015), and that early amplification has positive language impacts (Yoshinaga-Itano et al. Citation1998), there are minimal data regarding the impact of consistent hearing aid use in infancy on later outcomes. This would be valuable data to gather in future research that could significantly impact how parents are counselled regarding hearing aid use and expectations for their children. Such a project is likely to require a multicentre study to gather sufficient data to answer these complex questions.
Conclusion
LEAQ scores from infant hearing aid users recruited from multiple centres around the UK were poorer than scores reported in published data from centres in the US and Canada (Bagatto et al. Citation2016, Citation2011; McCreery et al. Citation2015; Oropeza Citation2017). There may therefore be potential to improve infant functional outcomes with more unified best-practice protocols, though results may also be affected by more complex factors including availability of resources to deliver successful early intervention, and the mode of delivery of LEAQ, which in this study was outside of clinical protocols. There was a trend for poorer scores in later infancy to be associated with fewer hours of hearing aid use, but there may be confounding factors which were not fully measured in this study, such as socio-economic status or maternal education. Infants with greater degrees of hearing loss were significantly more likely to show consistently low LEAQ scores across both time points. The results provide a baseline for current normative LEAQ scores for infants with a range of hearing losses in the UK, from which service improvements can be monitored. Further work would be of benefit to better define performance for children with different degrees of hearing loss across different age categories whilst monitoring adherence to best-practice, and to investigate differences in LEAQ scores in different centres, such as those reported in the literature, and any reasons for these differences.
TIJA-2021-10-0444-File007.docx
Download MS Word (49.6 KB)Acknowledgements
Thanks to Jo Brooks, Caroline Hudson, Amber Roughley and Rhiannon Morgan for help collecting study data. Thanks to Michael Stone for advice on the SII calculations. We are grateful to all the families who took part and all the audiologists and teachers of the deaf around the UK who helped to recruit families to the study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- American Academy of Audiology. 2013. “American Academy of Audiology Clinical Practice Guidelines: Pediatric Amplification.” American Academy of Audiology. https://audiology-web.s3.amazonaws.com/migrated/PediatricAmplificationGuidelines.pdf_539975b3e7e9f1.74471798.pdf
- Aragon, M., and C. Yoshinaga-Itano. 2012. “Using Language ENvironment Analysis to Improve Outcomes for Children Who Are Deaf or Hard of Hearing.” Seminars in Speech and Language 33 (04): 340–353. doi:10.1055/s-0032-1326918.
- Bagatto, M., S. Moodie, C. Brown, A. Malandrino, F. Richert, D. Clench, and S. Scollie. 2016. “Prescribing and Verifying Hearing AIDS Applying the American Academy of Audiology Pediatric Amplification Guideline: Protocols and Outcomes from the Ontario Infant Hearing Program.” Journal of the American Academy of Audiology 27 (03): 188–203. doi:10.3766/jaaa.15051.
- Bagatto, M. P., C. L. Brown, S. T. Moodie, and S. D. Scollie. 2011. “External Validation of the LittlEARS® Auditory Questionnaire with English-Speaking Families of Canadian Children with Normal Hearing.” International Journal of Pediatric Otorhinolaryngology 75 (6): 815–817. doi:10.1016/j.ijporl.2011.03.014.
- Bagatto, M. P., S. T. Moodie, A. C. Malandrino, F. M. Richert, D. A. Clench, and S. D. Scollie. 2011. “The University of Western Ontario Pediatric Audiological Monitoring Protocol (UWO PedAMP).” Trends in Amplification 15 (1): 57–76. doi:10.1177/1084713811420304.
- BSA. 2018. “Recommended Procedure. Pure-Tone Air-Conduction and Bone-Conduction Threshold Audiometry with and without Masking.” British Society of Audiology. https://www.thebsa.org.uk/wp-content/uploads/2018/11/OD104-32-Recommended-Procedure-Pure-Tone-Audiometry-August-2018-FINAL.pdf
- Coninx, F., V. Weichbold, L. Tsiakpini, E. Autrique, G. Bescond, L. Tamas, A. Compernol, et al. 2009. “Validation of the LittlEARS((R)) Auditory Questionnaire in Children with Normal Hearing.” International Journal of Pediatric Otorhinolaryngology 73 (12): 1761–1768. doi:10.1016/j.ijporl.2009.09.036.
- Escorihuela García, V., M. I. Pitarch Ribas, I. Llópez Carratalá, E. Latorre Monteagudo, A. Morant Ventura, and J. Marco Algarra. 2016. “Comparative Study between Unilateral and Bilateral Cochlear Implantation in Children of 1 and 2 Years of Age.” Acta Otorrinolaringologica (English Edition) 67 (3): 148–155. doi:10.1016/j.otoeng.2016.04.011.
- Ganek, H., A. James, V. Papaioannou, and K. Gordon. 2020. “Can Differences in Early Hearing Development Be Distinguished by the LittlEARs Auditory Questionnaire?” Ear and Hearing 41(4):998–1008. doi:10.1097/AUD.0000000000000821.
- Jones, C., and M. Feilner. 2103. “What Do We Know about the Fitting and Daily Life Usage of Hearing Instruments in Pediatrics?.” A Sound Foundation through Early Amplification: Proceedings of the 2013 International Conference. Chicago, IL: Phonak AG, 97–103.
- Liu, H., X. Jin, J. Li, L. Liu, Y. Zhou, J. Zhang, W. Ge, and X. Ni. 2015. “Early Auditory Preverbal Skills Development in Mandarin Speaking Children with Cochlear Implants.” International Journal of Pediatric Otorhinolaryngology 79 (1): 71–75. doi:10.1016/j.ijporl.2014.11.010.
- Long, Y., H. Liu, Y. Li, X. Jin, Y. Zhou, J. Li, Z. Zheng, et al. 2018. “Early Auditory Skills Development in Mandarin Speaking Children after Bilateral Cochlear Implantation.” International Journal of Pediatric Otorhinolaryngology 114: 153–158. doi:10.1016/j.ijporl.2018.08.039.
- Marnane, V., and T. Y. C. Ching. 2015. “Hearing Aid and Cochlear Implant Use in Children with Hearing Loss at Three Years of Age: Predictors of Use and Predictors of Changes in Use.” International Journal of Audiology 54 (8): 544–551. doi:10.3109/14992027.2015.1017660.
- May-Mederake, B., H. Kuehn, A. Vogel, A. Keilmann, A. Bohnert, S. Mueller, G. Witt, et al. 2010. “Evaluation of Auditory Development in Infants and Toddlers Who Received Cochlear Implants under the Age of 24 Months with the LittlEARS® Auditory Questionnaire.” International Journal of Pediatric Otorhinolaryngology 74 (10): 1149–1155. doi:10.1016/j.ijporl.2010.07.003.
- McCreery, R. W., R. A. Bentler, and P. A. Roush. 2013. “Characteristics of Hearing Aid Fittings in Infants and Young Children.” Ear and Hearing 34 (6): 701–710. doi:10.1097/AUD.0b013e31828f1033.
- McCreery, R. W., E. A. Walker, M. Spratford, J. Oleson, R. Bentler, L. Holte, and P. Roush. 2015. “Speech Recognition and Parent Ratings from Auditory Development Questionnaires in Children Who Are Hard of Hearing.” Ear and Hearing 36 Suppl 1(01):60S–75S. doi:10.1097/AUD.0000000000000213.
- Mehta, K., P. Watkin, M. Baldwin, J. Marriage, M. Mahon, and D. Vickers. 2017. “Role of Cortical Auditory Evoked Potentials in Reducing the Age at Hearing Aid Fitting in Children with Hearing Loss Identified by Newborn Hearing Screening.” Trends in Hearing 21: 2331216517744094. doi:10.1177/2331216517744094.
- Moeller, M. P., B. Hoover, B. Peterson, and P. Stelmachowicz. 2009. “Consistency of Hearing Aid Use in Infants with Early-Identified Hearing Loss.” American Journal of Audiology 18 (1): 14–23. doi:10.1044/1059-0889(2008/08-0010).
- Moodie, Sheila T., Marlene P. Bagatto, Linda T. Miller, Anita Kothari, Richard Seewald, and Susan D. Scollie. 2011. “An Integrated Knowledge Translation Experience: Use of the Network of Pediatric Audiologists of Canada to Facilitate the Development of the University of Western Ontario Pediatric Audiological Monitoring Protocol (UWO PedAMP v1.0).” Trends in Amplification 15 (1): 34–56. doi:10.1177/1084713811417634.
- Munro, K. J., S. C. Purdy, K. Uus, A. Visram, R. Ward, I. A. Bruce, A. Marsden, M. A. Stone, and B. Van Dun. 2019. “Recording Obligatory Cortical Auditory Evoked Potentials in Infants.” Ear and Hearing 41 (3): 630–639. doi:10.1097/aud.0000000000000789.
- Oropeza, J. 2017. LittlEars Auditory Questionnaire Project. http://mdcresearch.net/index.php/rerc/parent-questionnaires-of-auditory-skill-development/little-ears/
- Persson, A., C. Miniscalco, A. Lohmander, and T. Flynn. 2019. “Validation of the Swedish Version of the LittlEARS® Auditory Questionnaire in Children with Normal Hearing–a Longitudinal Study.” International Journal of Audiology 58 (10): 635–642. doi:10.1080/14992027.2019.1621397.
- Tomblin, J. B., M. Harrison, S. E. Ambrose, E. A. Walker, J. J. Oleson, and M. P. Moeller. 2015. “Language Outcomes in Young Children with Mild to Severe Hearing Loss.” Ear and Hearing doi:10.1097/AUD.0000000000000219.
- Tomblin, J. B., E. A. Walker, R. W. McCreery, R. M. Arenas, M. Harrison, and M. P. Moeller. 2015. “Outcomes of Children with Hearing Loss: Data Collection and Methods.” Ear and Hearing 36 Suppl 1(0 1):14S-23S. doi:10.1097/AUD.0000000000000212.
- Tsiakpini, L., V. Weichbold, H. Kuehn-Inacker, F. Coninx, P. D’Haese, and S. Almadin. 2004. LittlEARS Auditory Questionnaire. Innsbruck, Austria: MED-EL.
- Vandam, M., S. E. Ambrose, and M. P. Moeller. 2012. “Quantity of Parental Language in the Home Environments of Hard-of-Hearing 2-Year-Olds.” Journal of Deaf Studies and Deaf Education 17 (4): 402–420. doi:10.1093/deafed/ens025.
- Walker, E. A., R. W. McCreery, M. Spratford, J. J. Oleson, J. Van Buren, R. Bentler, P. Roush, and M. P. Moeller. 2015. “Trends and Predictors of Longitudinal Hearing Aid Use for Children Who Are Hard of Hearing.” Ear and Hearing 36 Suppl 1(0 1):38S-47S. doi:10.1097/AUD.0000000000000208.
- Wie, O. B. 2010. “Language Development in Children after Receiving Bilateral Cochlear Implants between 5 and 18 Months.” International Journal of Pediatric Otorhinolaryngology 74 (11): 1258–1266. doi:10.1016/j.ijporl.2010.07.026.
- Yang, Y.,. M. Chen, J. Zheng, J. Hao, B. Liu, W. Liu, B. Li, et al. 2020. “Clinical Evaluation of Cochlear Implantation in Children Younger than 12 Months of Age.” Pediatric Investigation 4 (2): 99–103. doi:10.1002/ped4.12202.
- Yoshinaga-Itano, C., A. L. Sedey, D. K. Coulter, and A. L. Mehl. 1998. “Language of Early- and Later-identified Children with Hearing Loss.” Pediatrics 102 (5): 1161–1171. doi:10.1542/peds.102.5.1161.
- Yoshinaga-Itano, Christine, R. L. Baca, and A. L. Sedey. 2010. “Describing the Trajectory of Language Development in the Presence of Severe-to-Profound Hearing Loss: A Closer Look at Children with Cochlear Implants versus Hearing Aids.” Otology and Neurotology 31(8):1268–74. doi:10.1097/MAO.0b013e3181f1ce07.
