Abstract
Objectives
To inform and optimise a cochlear implant (CI) fitting software design through an analysis of big data to define array-specific comfort (C) level profiles, frequently-used MAP parameters, and the minimum number of Neural Response Telemetry thresholds (tNRT) needed to create an accurate profile. To evaluate the software’s ease of use and completion time for AutoNRT®s.
Design
MAPs analysis. Clinical study evaluating software use in creating MAPs, addressing sound-quality issues and setting patient goals.
Study sample
MAPs (N = 39,885); CI recipients (N = 47) and clinicians (N = 19).
Results
Distinct C-level profiles were observed for lateral-wall, contour, and slim-modiolar electrode arrays. Default settings were used for most MAP parameters (13/16) except for Pulse Width, Rate, and Maxima. Nine tNRT measurements were required for an accurate C-level profile. Measurement-time of nine tNRTs via the new algorithm was comparable to five tNRTs using the previous algorithm. Nearly all (99%) clinical tasks were completed by clinicians with the first use of the software. Most CI recipients (79.5%) rated goal-setting as valuable.
Conclusion
Custom Sound Pro fitting software developed based on big data analysis incorporates a guided fitting workflow and expected fitting ranges. It helps to improve clinical efficiency, is easy to use and supports patient-centred care.
Introduction
Cochlear Implants (CI) help improve speech perception and quality of life for adults and children who derive little or no benefit from hearing aids (WHO, Citation2021). The sound processor needs to be programmed to optimise sound quality for the individual to derive maximum benefit from the CI. In addition, the recipient needs to learn to use and manage the device effectively in daily life. The postoperative rehabilitative process involves ongoing appointments with the CI clinical team. The CI clinician plays a vital role in monitoring and improving the outcomes for CI recipients. Improvements in the primary tools used by the clinician can lead to better outcomes for patients. E.g. electronic health records systems (EHRs) are widely adopted in medicine due to their positive impact on workflow and patient outcomes (Evans Citation2016). The primary purpose of EHRs is to capture and distribute patient information. They also help in improving the outcomes by: 1) providing guidance where appropriate, 2) promoting best practice through standardised workflows, 3) being easy to learn and use (requiring less clinician focus on tool usage and more time directed towards patients), and 4) providing efficiencies and automation where possible. Although commercially available EHRs are used in many CI clinics, they are not the primary tools used by the CI clinician; rather, the fitting software is the primary tool used during postoperative CI appointments. Ideally, the CI fitting software should also incorporate the four areas described above to serve the clinician and help them improve patient outcomes.
Guidance
In the medical sector, checklists have been shown to improve the quality of medical care, adherence to evidence-based practice and to help reduce the frequency of errors or omission of key steps (Hales et al. Citation2008). Well-designed checklists in CI management are likely to include a standardised guided workflow that suggests the appropriate next steps while still allowing the flexibility for expert judgement. On this basis, Custom Sound Pro fitting software (CSPro) incorporates a guided workflow. Such a suggested workflow must be piloted and refined in a simulated clinical environment (Hales et al. Citation2008).
In addition to guided workflows, access to normative data during the clinical session is another way for clinicians to obtain helpful guidance. Normative data characterises what is usual for a population and is indispensable in almost all areas of medicine (O’Connor Citation1990). For CI clinicians, setting threshold (T) and comfort (C) levels are among the most frequent activities performed with the fitting software. Earlier studies have shown that incorrect C-levels have a relatively greater effect on performance and sound quality than T-levels (Busby and Arora Citation2015; Willeboer Citation2008). Normative data on where the C-levels would typically fall is not included in the current clinical fitting software. Incerti et al. Citation2018 had shown that the C-levels for young children (activated at or before 12 months of age) were conservatively set at significantly low levels at six months after initial activation compared to the levels set for the same children at three years of age. Wesarg et al. Citation2010 reported large differences in the C-level profiles across different CI centres and suggested a need for better methods to set C-levels consistently. Although normative data can seldom be used directly to provide individualised treatment, knowing the average C-levels of the population can provide clinicians with a valuable reference point for where the C-levels may need to be set for the individual and thus help reduce the risk of over- or under-stimulation. From this starting point, the clinician can do further personalisation as needed to optimise outcomes for the particular patient. Normative data for C-levels have been reported for Advanced Bionics CI by van der Beek, Briaire, and Frijns (Citation2015). However, they are not applicable for use with CochlearTM Nucleus® CI systems due to fundamental technical differences between these systems, such as strategy, the scale used for current units, and the interaction of levels with rate and pulse width (Vaerenberg, Govaerts, et al. Citation2014). Wesarg et al. Citation2010 reported average C-levels for Nucleus CI24RE implants; however, they were restricted to the Contour Advance® electrode array only. Given that the electrode array type, for example, lateral wall and perimodiolar, affects the T/C-levels and the shape of the profile, there is a need for normative data from and for each electrode array type, such as the lateral wall versus pre-curved electrode arrays. This will enable CSPro to provide electrode-specific guidance on the normative range of the C-levels in the form of different expected ranges.
Promote best practice
Best practice in almost all areas of medicine strives to provide patient-centred care (PCC), to improve outcomes, satisfaction for patients and clinicians and reduce healthcare costs (Constand et al. Citation2014). Optimisation of hearing through fitting is an essential part of providing PCC soon after initial activation of CI. However, as MAP levels are known to stabilise over the first year of CI use (Hughes et al. Citation2001, Henkin et al. Citation2003), fitting by itself may not be sufficient for PCC beyond the early period after CI. The challenges faced by CI recipients are not limited to those with listening and communication. The rehabilitation program needs to focus on the holistic needs of the individual and help them resolve their unique everyday life challenges related to their hearing. Surveys of CI recipients have revealed that they want a greater emphasis on PCC at their CI clinics (Athalye et al. Citation2015). For example, decisions about appointment schedules, provision of accessories and best treatment options are often driven by the implant team; however, recipients may prefer that such decisions are made jointly with them/their families (Athalye et al. Citation2015). The fact that most CI recipients return to their CI clinic for audiology appointments even after their MAPs have stabilised is a testament that some level of PCC occurs in their appointment; however, there is a need for structured activities that promote PCC.
The key elements involved in PCC are: 1) active listening; 2) open questions and reflective conversations; 3) involvement of family and friends; 4) understanding individual preferences; 5) empathy; and 6) shared goal setting and decision making (Ida Institute, n.d.). The Client Oriented Scale of Improvement (COSI), developed by Dillon, James, and Ginis (Citation1997), encompasses all the above-mentioned key elements of PCC. COSI has been incorporated within the CSPro software to promote the best practices for PCC.
Once the most important areas of difficulty are identified, the next step is to arrive at a differential diagnosis. History taking is of fundamental importance for differential diagnosis and clinical decision-making. The clinical history can provide sufficient information in the vast majority of cases to diagnose before performing any tests or examinations (Hampton et al. Citation1975). Easy access to this information, built over time, can support clinicians in not only providing individualised care but maintaining connection, trust, and engagement with their patients. Thus, having ready access to holistic information about the recipient is vital to the process. The fitting software is the repository of essential information collected during history taking over time. However, this information is typically presented in various locations. CSPro incorporates a dashboard that summarises all potentially relevant information in one place. It also promotes further capture of evolving hearing status and outcomes over time, like devices on each ear, audiogram, and historical session notes.
Ease of use
One of the nine design heuristics (principles) proposed by Nielsen (Citation1994) to improve the ease of use of any system is to use minimalistic features to reduce user error. As the number of features on a given screen increases, the user must also invest more time to figure out the distinction between features, diverting their attention away from the clinical task at hand.
In CI fitting software, the clinician has access to a range of software options and MAP parameters which are modified to address hearing-related issues. This flexibility is seen as important, especially for complex case management. Earlier studies (Vaerenberg, Govaerts, et al. Citation2014, Hemmingson and Messersmith Citation2018, Incerti et al. Citation2018, Browning et al. Citation2020 and Wathour, Govaerts, and Deggouj Citation2021) reported that for the majority of recipients, clinicians only use the default parameters provided. When clinicians use non-default settings, some parameters are changed more frequently than others, presumably based on the frequency of the issues that warrant the non-default settings. Therefore, software that makes the most commonly-used controls readily accessible while still maintaining access to the rarely-used controls if needed is desirable. This would require an accurate assessment of how frequently all MAP parameters are used.
The previous Custom Sound® fitting software allows the adjustment of 16 MAP parameters by the clinician. Earlier studies reported the frequency of use of 5 (Incerti et al. Citation2018) to 11 parameters (Vaerenberg, Smits, et al. Citation2014); however, no study yet provides the frequency of use of all 16 parameters.
Efficiencies
The software’s efficiency can be improved by automating or speeding up the processes that require minimal input from the recipient. The previous Custom Sound software already automated several tasks like compliance measurement, power management, and automatically saving MAPs to the database to improve efficiency. Among other clinician-initiated tasks requiring minimal recipient input, impedance measurement, followed by Neural Response Telemetry (NRT®) measurement, are the most frequently used (Vaerenberg, Smits, et al. Citation2014). NRT has already been automated with AutoNRT, and MAPs created using 5 NRT thresholds (tNRT) have been shown to provide equivalent performance to conventional fitting methods (Botros, Banna, and Maruthurkkara Citation2013, Müller-Deile et al. Citation2021). The measurement of 5 tNRTs takes several minutes, during which time the clinician cannot communicate verbally with the recipient. Müller-Deile et al. (Citation2021) reported that subjective sound quality ratings were better for MAPs created using 22 tNRTs than those using the default of 5 tNRTs. Measuring tNRT across all 22 electrodes requires a significantly longer measurement time, typically 18 minutes, reducing clinical efficiency. Therefore, an important design goal is to determine the minimum number of tNRT measurements that provide an acceptably accurate profile representation in the shortest testing time.
This paper describes the research conducted during the development and evaluation phases of CSPro fitting software. As part of the development phase, programming data from multiple clinical databases was analysed to address three critical design questions: 1) What is the average C-level range across different CI populations so that appropriate guidance can be provided? 2) Which software features are most frequently used and adjusted by clinicians so that the software is designed for easy access to these? 3) What is the minimum number of tNRTs required to obtain an accurate tNRT profile for the creation of MAPs?
CSPro software was developed based on the outputs of the development phase described below. In the evaluation phase, a clinical study was conducted to 1) Evaluate the software’s ease of use in a clinical scenario and 2) Compare the speed of making tNRT measurements using the optimised AutoNRT algorithm compared with the previous method.
Development phase: MAP analysis
Methods – development phase
According to HIPAA’s De-Identification Standard (45 CFR § 164.514), MAP data from 39885 MAP files from the USA were de-identified. HIPAA’s De-Identification Standard allows de-identified data to be used for secondary purposes, such as scientific analysis (45 CFR §164.502(d)). MAPs created for training purposes were excluded by removing MAPs with C < 50 CLs or T < 20 CLs. De-identified MAP data were analysed in the following three areas described below.
Population mean
This analysis was performed to obtain the average C-levels for the population to provide guidance within the software in an expected range for C-levels on the assumption that the incidence and presence of comorbidities unknown would have had a minimal effect statistically on the larger population data. Data from recipients younger than 12 years of age was removed so that data only represented responses from reliable respondents. The average C-levels from recipients younger than 12 years were compared with that of recipients 12 years and older for one implant (CI512) to evaluate the age effects on average C-levels. Data from CI22 series and CI24M series implants no longer implanted were excluded from the analysis. Data included represented the CI24RE family series and CI500 series. Only MAPs created with default programming parameters were included in the analysis, reflecting most MAPs created routinely (Vaerenberg, Smits, et al. Citation2014).
The population mean C-level profile was determined for each implant type by averaging the C-levels at each electrode. The default pulse width (PW) for all implants was 25 µs, except for Nucleus CI522 and CI422 devices, which use a default PW of 37 µs. While the PW used for the MAP can affect the overall stimulation levels, it usually has minimal impact on the shape of the profile due to the uniformity seen with monopolar stimulation modes (Skinner et al. Citation2000). Therefore, to aid in comparing the profile shapes, they were normalised by subtracting the average C-levels from each C-level profile.
Frequently used fitting parameters
MAPs from all available implants, including adults and children, were analysed to determine the frequency of use of various MAP parameters. The latest MAP in the database was used for the analysis for each implant. The proportion of MAPs that used the default setting, and the MAPs using the top three frequently used non-default settings, were analysed for each MAP parameter.
Minimum number of tNRT
Implant records from adults and children were identified, where tNRT was measured on all 22 electrodes. From this, a reduced set of the tNRT measurements was used to re-create a MAP profile. For example, 5 out of 22 tNRT values were sampled from evenly spaced electrodes across the electrode array, and spline interpolation was then applied to generate the tNRT values for the non-selected electrodes. The difference between the original and the interpolated tNRT was calculated for each implant and reported as the average absolute difference. For each implant, 18 interpolated tNRT profiles were generated, differing by the number of original tNRTs used for interpolation (which ranged from 3 to 21). The error introduced by interpolation was calculated for each of the 18 interpolated profiles. The derivative of the error was calculated as the number of originally measured electrodes increased to determine the rate of change in the error. An error of 4 CL was considered clinically insignificant based on the published test-retest error for AutoNRT measurements (Müller-Deile Citation2009). The proportion of implants where the error is less than 4CL was calculated. The above error calculations were performed with evenly spaced electrode distribution; however, the error may be further reduced if an optimised combination of measurement electrodes is used for interpolation. A brute-force search technique was used to generate 77520 possible combinations for selecting nine electrodes for tNRT measurements to determine the most optimal combination. The interpolation error for each nine-electrode combination was compared with the evenly spaced nine-electrode combination to identify the 9-electrode combination that produced the lowest error.
Results – development phase
Population mean
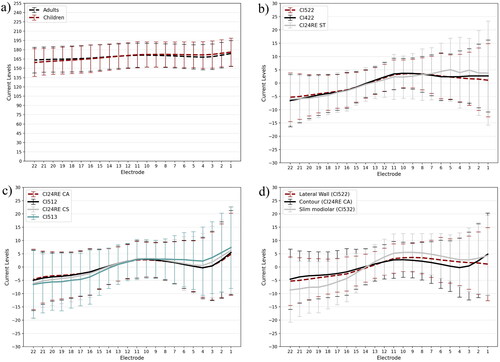
There was a negligible difference in the average C-levels and ± 1 SD from recipients younger than 12 years and that from recipients 12 years and older for the CI512 implant (). All implants with a lateral wall electrode array, CI522 (N = 4099), CI422 (N = 735), and CI24RE(ST) (N = 240), displayed a similar population mean C-level offset profile shape (). Similarly, implants with a Contour® electrode array, CI24RE(CA) (N = 9110), CI512 (N = 6783), CI24RE(CS) (N = 133), and CI513 (N = 157) displayed a similar population mean C-level offset profile shape (). The shape of the population mean offset profile for the slim modiolar electrode array, CI532 (N = 2814), was different to both the lateral wall and contour electrode arrays. Therefore, three distinct population mean C-level profile shapes were obtained for the recent Nucleus cochlear implants ().
Figure 1. (a) Mean C-levels for adults and children. (b,c) show the population mean C-level offset profiles for lateral wall and contour/contour advance electrode arrays, respectively. (d) compares the population mean C-level offset profiles between lateral wall, contour, and slim modiolar electrode arrays. Error bars indicate standard deviation.
Frequently used fitting parameters
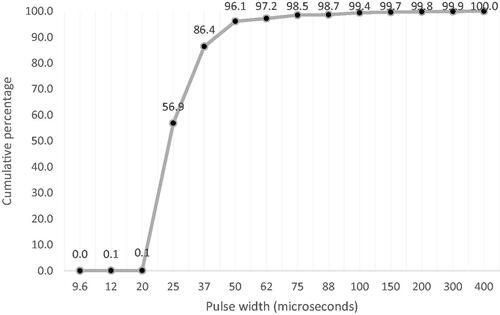
Analysis was completed on the latest MAPs from all available implants (N = 39885), including adults/teenagers (12 and above, 71%) and children (under 12, 29%). For most of the MAP parameters included (13/16), the default setting was used for greater than 94% of recipients. shows the prevalence of the parameters used in the MAPs. Hybrid MAPs (2.2%) were removed while analysing the frequency allocation tables as they always use custom values. Unlike Rate and Maxima, higher PW is suitable for recipients using lower PWs; thus, a cumulative percentage graph () can be shown for PW.
Table 1. Frequently used parameters (N = 39885).
The minimum number of tNRT measurements
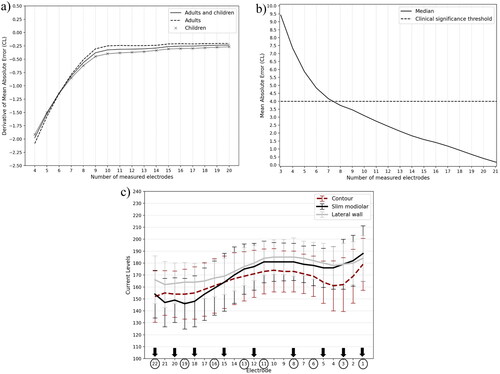
Data were analysed from 4718 implants where tNRT was available on all 22 electrodes. The dataset included CI532, CI512, CI24RE (CA), CI422, and CI522 implants from adults/teenagers (12 and above, 41.75%) and children (under 12, 58.25%). shows the mean and standard deviation of tNRTs across electrodes for these implants. The variability in tNRT was highest for apical and basal electrodes. The mean absolute error (MAE) between the complete and interpolated profiles was greatest when only three tNRT generated the interpolated profile (median error = 9.42 CL). The median error reduced to 5.84 CL as the number of tNRTs used for interpolation increased to five electrodes and reduced again to 3.46 CL when nine tNRTs were used (). A subgroup analysis based on electrode array type showed that the median error with nine measurements was below 4CL for implants with lateral wall/contour (3.45 CL) and slim modiolar (3.50 CL) electrode arrays. With more than nine tNRT measurements available for interpolation, the rate of change in the error between the complete and interpolated tNRT profiles reached an asymptote for adults, children, or the whole population, as shown in . With the nine tNRT interpolated profile, the absolute error was less than the 4 CL clinically significant cut-off for 59.31% of implants compared to 25.56% when five electrodes were used. The brute-force analysis (to determine the ideal combination of electrodes) showed that when tNRT is measured on nine electrodes, the lowest error can be obtained if more measurements are made in apical (18, 20 & 22) and basal electrodes (1, 3, 5 & 8) and fewer measurements are made at middle electrodes (12 & 15) as shown in .
Figure 3. (a) Rate of Change of Mean absolute error between interpolated and complete profile, (b) Mean Absolute error for interpolated electrodes, (c) Mean and standard deviation of tNRT from contour, slim modiolar and lateral wall electrode arrays. Circles and arrows show the evenly spaced and optimum electrodes for measuring nine tNRTs, respectively.
Clinical evaluation phase: Clinical study
Material and methods – clinical evaluation phase
Study design
This prospective, single-centre study used repeated-measures and usability study designs. The study assessed the usability of CSPro CI fitting software for clinicians in terms of learnability, memorability, and efficiency. The time taken for AutoNRT measurements with CSPro was compared with previous Custom Sound software. The study received ethics approval at a single site in Sydney (Ref: 5201800322).
Research participants
Study participants included both CI recipients and CI clinicians. Nucleus CI recipients who lived in Sydney and had provided prior consent to be contacted by Cochlear for research were invited to participate in the study via email. Inclusion criteria for these participants were: Adults (≥18 years), implanted with the CI500 series, Freedom series, Nucleus 24 Series, or Nucleus 22 series cochlear implants in one or both ears. Inclusion criteria for clinicians were tertiary education in audiology and at least two months of experience working with people with hearing impairment. A total of 47 recipients (using 64 implants) and 19 clinicians were enrolled in the study. provides the demographic details of the participants.
Table 2. Demographic details of participants.
Custom sound pro description
CSPro software has been designed considering the usability heuristics (principles) published by Nielsen (Citation1994), namely; visibility of system status, a match between the system and the real world, user control and freedom, consistency and standards, error prevention, recognition rather than recall, flexibility and efficiency of use, aesthetic and minimalist design, and helping users recognise, diagnose, and recover from errors. Below are the key features of the software.
User interface
The software provides a guided workflow of routine programming tasks highlighted visually at the bottom right of all screens as a sequential checklist suggesting the next logical step a clinician might want to take. The guided workflow is optional; the clinician can choose to follow their preferred workflow or change it based on the recipient’s needs. All settings and controls available in previous software versions remain available in CSPro. The clinician can navigate to any screen using the top navigation panel. The software attempts to stay on the air as much as possible throughout the fitting session so the clinician can interact seamlessly with the patient. For example, on the dashboard, the software goes on air with the active MAP in the patient’s processor; it goes off the air during impedance measurement, sweeps, and writing MAPs but comes back on air after the task. The software goes off-air on both ears during threshold measures to aid the threshold task. The clinician can swap between the open MAPs using a drop-down menu and test them on air. The ease of access of different functions on each screen is designed based on the frequency of their use. The functions that are used in every session are available on every screen. Less frequently used functions that may be needed for some, but not all recipients, are accessed via the cog icon on each screen. The cog icon also provides access to the MAP parameters that might need to be changed for a minority of patients. The functions that are rarely used are placed under manufacturer settings. The clinicians can also set their preferred settings under preferences. The software is touch-friendly for tablet use with almost all screens. In the adjust screens, swipe controls can be used in place of buttons so the clinician can change the levels without looking at the screen.
The software alerts when changes are applied to a MAP parameter, which impacts another parameter. For example, when increasing PW results in a decrease of maxima number. It also shows messages when it prevents the clinician from accessing a function and suggests what steps to take to access that control. The clinician can capture notes throughout the application to remind them what activities were completed and any planned follow-ups. The notes from the previous session are displayed on the dashboard.
The Fitting Assistant feature provides rank-ordered recommendations to resolve various issues reported by recipients. The guided workflow enables easy access to the appropriate screen to perform the step. The software is designed to better support bilateral fitting with the ability to go on-air bilaterally on every screen. On certain screens like the global adjustments and finalise screens, the MAP levels or settings can be viewed for both ears, and changes can be applied together. When certain tasks are performed on one ear alone, for example, sweep on comfort, NRT, or threshold measurement, the software automatically goes off-air on the contralateral ear to streamline the process.
Dashboard
The dashboard is designed to provide the clinician with a view of important information to support patient management. The interactive dashboard includes patient and device information such as age, sex, implant and sound processor devices, history, and notes captured in the previous appointment. In addition, the dashboard also provides access to clinically useful information such as audiograms and impedance results, usage data logs, and patient hearing goals. The user can retrieve further details by clicking through from the dashboard.
An onscreen alert to indicate sound processor usage provides a visual reminder for counselling about sound processor upgrades when appropriate. When the sound processor is connected to the software, it automatically opens the dashboard screen for that patient and all MAPs saved to the patient’s sound processor will be uploaded. The processor will go immediately ‘on air’ with the last used MAP to facilitate discussions at the start of the session. The patient goals feature is based on the Client-Oriented Scale of Improvement (COSI™) clinician questionnaire (Dillon, James, and Ginis Citation1997). It allows the clinician to create individualised patient hearing goals and monitor progress between appointments. The goals feature is intended to be utilised on an ongoing basis, rather than just administered pre and post device fitting. Clinicians can document the agreed action plan with the patient to achieve their individual goals.
Impedance screen
Since clinicians make impedance measurements in every session, the software automatically runs impedance on connection to the implant. The impedance screen shows the results of the latest impedance measurement and the status of electrodes if they are enabled or turned off, including why they are turned off. The clinician can also turn off electrodes in this screen, for instance, when the surgical report states a partial electrode array insertion to prevent future impedance measurements and stimulation on those electrodes. A more detailed impedance view is accessible to review historical impedance measurements.
AutoNRT screen
A clinician can measure AutoNRT in Custom Sound Pro software using a new algorithm optimised to improve measurement speed. As with NRT algorithms used in previous software, stimulation progressively increases until an NRT response is obtained at two consecutive levels. Then a threshold is identified by using a descending test run. A key difference to previous NRT algorithms is that unnecessary measurements at sub-threshold levels are minimised by calculating the minimum tNRT level obtained after the first three measurements and then using that as the starting level for the remaining measures.
Select MAP screen
Allows opening existing MAPs or creating new MAPs using multiple methods. The starting profile of the MAP can be created using Thresholds, Population mean, latest AutoNRT, or other measures like electrical stapedius reflex thresholds or intraoperative AutoNRT. Once the starting profile is created using these methods, the guided workflow suggests that C-levels are adjusted globally in live speech until loud and comfortable sound.
Global adjustment screen
This allows the clinician to make global adjustments to C-levels or C & T levels using Master Volume (MV), Bass, and Treble controls in live speech. The screen also allows adjustment of global T-levels using MV. As levels increase, profile scaling, an algorithm that progressively flattens the profile, is applied to both T and C-levels. Due to the broader spread of excitation with increasing stimulation, psychophysically measured C-level profiles are flatter than T-level profiles. Profile scaling takes into account this progressive flattening of the profile at higher levels (Botros and Psarros Citation2010). When a profile is created using few measurements, the levels at unmeasured electrodes are set using spline interpolation to obtain a closer approximation to measured levels (Müller-Deile et al. Citation2021). In global, comfort, and threshold screens, the previous levels are displayed using faded lines so that the clinician can quickly see how much change has been made in the session. On these screens, the audiometric frequencies the electrodes relate to are displayed. This enables the clinician to easily identify the relevant electrodes to work on, especially in response to outcomes from audiometric tests like aided audiograms. Optionally the NRT markers, electrode numbers, and compliance limits can be displayed on the screen. In the global and comfort screen, the expected range for the C-level is displayed so the clinician can use it as a guide to setting the C-levels. The expected range is based on tNRT for NRT-based MAPs and the average C-levels of the population ± 1 standard deviation for other MAPs (). The software displays an onscreen explanation of the expected range: “This guide is based on Cochlear’s population mean data and can assist you in setting levels. Approximately, 68% of patients have their C level set within this range. Some patients have their C-level above or below this range”. The expected range displayed depends on the electrode array type for that implant, rate of stimulation and PW used in the MAP. If the MAP levels at one or more channels are above the compliance limits, the software prompts to increase the PW on all channels for compatible implants. The MAP T- and C-levels are reduced to a softer level while maintaining the shape of the profile. The acoustic screen allows the clinician to program acoustic component for Hybrid MAPs.
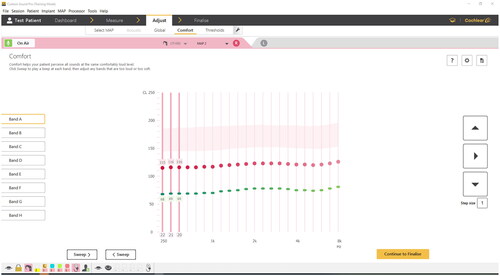
Comfort screen
The comfort screen allows stimulation at C-levels at individual electrodes or in three-channel bands to check the comfort and loudness balance of a MAP (). The three-channel beep presents stimulation at three adjacent channels in quick succession. The three-channel beep is designed to provide increased loudness summation compared to a single-channel beep. The clinician can test individual electrodes and turn them off to resolve issues like facial nerve stimulation.
Thresholds screen
This allows the measurement of thresholds on individual channels. After the first measurement, a global shift is applied to the T-levels profile. After subsequent measurements, interpolation is automatically applied to set the thresholds on unmeasured electrodes based on the measured electrodes. The electrodes where thresholds were measured in the previous session can be highlighted for easy reference.
Set levels screen
is included for working with MAPs with <10 electrodes enabled or where interpolation is not possible due to mixed PW or modes, non-monopolar modes (i.e. common ground, bipolar, pseudomonopolar typically used with N22 implants or double arrays). The other adjustment screens impose a minimum dynamic range (DR) of 10 CL; if a recipient requires less than 10CL DR, the set levels screen will need to be used. This screen also allows programming non-default channel to electrode MAPs typically used only with auditory brainstem implants. Ninety percent of the functions available in the set levels screen are accessible in the alternate programming screens in CSPro. This screen provides access to the 10% of functions like adjusting gains, adjusting T/C-levels using non-default sensitivity, viewing dynamic range, measuring C-levels using psychophysical beeps, predicting levels when the rate is changed, hug C profile with T profile and sweeping at different % DR other than 50% and 100% C-levels.
Finalise screen
enables the clinician to modify program and processor settings before writing them to the sound processor. The screen also provides battery life estimates and important notifications that the clinician might want to counsel the recipient about at the end of the session. The clinician can set progressively louder MAPs, without reaching compliance limits, for recipients who would benefit from trying louder MAPs over time, especially in the early part of their CI journey.
Study procedures
Formal informed consent was obtained for all CI recipients and clinicians before study enrolment and participation. Once written informed consent was obtained from both the recipient and clinician-participants, they were enrolled in the study. Recipients attended only a single session in the study, and clinicians attended up to four sessions where they worked with different recipients. Clinician-participants had no prior training or exposure to CSPro before the session. An opportunity to preview the software was provided to all clinician-participants. The investigator prepared test MAPs that would have sound quality issues perceptible by the recipient-participant so that the clinician-participant could address them via the software. The investigator created test MAPs by applying random modifications to the recipient-participant’s everyday MAP by lowering Master Volume, Bass, Treble, or the last Comfort band. Both the recipient and clinician-participants were blinded to the MAP modifications made. The clinician-participant was asked to complete three tasks:
To gather feedback from the recipient-participants and adjust the MAPs to resolve the perceived sound quality issues. This task was to emulate a typical follow-up fitting session.
To create a new patient record and a new AutoNRT based MAP for the recipient-participant using the CSPro software to emulate an initial activation session. This included measuring AutoNRT at nine electrodes.
To use the patient goals feature to set goals for the recipient-participants’ current situation in life.
An observer/investigator rated the ease with which they could use each screen to complete the primary tasks using a five-point rating scale (1-Unable to complete, 2-Completed after using help, 3-Completed after trying an incorrect option, 4-Completed after some exploration and 5-Completed in the first attempt). AutoNRT was measured at nine electrodes using the comparator Custom Sound 5.1 software (CS) by the investigator. Screen recording of the software was performed to calculate the time taken to complete tasks and the time to measure AutoNRT. Recipient-participants were asked to provide feedback on their experience of being fitted with CSPro in a customised questionnaire. Likewise, clinician-participants were asked to give feedback on their experience during fitting and their confidence in using the new software without any prior training.
Results - Clinical evaluation phase
Ease of use
Learnability
was defined as the ease with which clinician-participants accomplished tasks the first time they encountered the design. The usability ratings showed that the 19 clinicians could complete 99% of the tasks during the first use of the software. In this session, 64% of tasks were completed at the first attempt, with 33% completed following some exploration or after initially trying an incorrect option (24% and 9%, respectively), while 2% of tasks were completed after using help. Only 1% of the tasks (comfort check on individual channels and thresholds) could not be completed by some clinician-participants in their first session.
Memorability
was assessed by comparing usability ratings during the first and second sessions using CSPro. Four clinicians did not conduct a second session, and the remaining 15 clinicians completed 110 tasks. Overall, the ratings for the second session were significantly higher (p = 0.005) than for the first session. Almost half the tasks (49% or 54/110) were scored at the maximum of 5 in both sessions. For another 5% of tasks (6/110), no change in the rating of 4 was seen across sessions. For 33% of tasks (36/110), the usability ratings for the second session were better than the first session. For 13% of tasks (14/110), the rating during the second session was lower than for the first session. At the end of each session, the clinicians were asked, “How confident are you with using the software?” on a five-point rating scale. The median rating provided by clinicians overall and after the first, second, third, and fourth session was “4-somewhat confident” (range 2-Little confidence to 5-very confident). Questionnaire responses were available from 13 of 15 clinicians who had conducted multiple sessions and provided feedback on this question. For six clinicians, their confidence rating remained the same across multiple sessions. For four clinicians, the confidence rating improved, and for three, it decreased over multiple sessions.
Efficiency
was evaluated by the time taken to complete the tasks. The median duration to create a new MAP after measurement of tNRT was 10 min 42 secs (range 2:46 to 27:52 min:sec). The median value for the total time taken to create a complete MAP from scratch, including measurement of 5 tNRT with CSPro followed by adjustment using MVBT, was 13 min 19 sec (range 6:17 to 29:58 min:sec).
AutoNRT comparisons
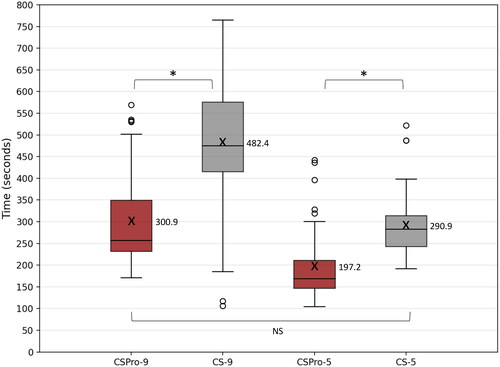
AutoNRT measurement was available from 50 implanted ears in 47 recipients. AutoNRT could not be completed for three recipients as no responses were found. Paired t-test showed that AutoNRT measurements on 9 electrodes was completed in significantly less time (p < 0.0001) with CSPro (median = 257 secs, range = 171 to 569) compared to CS (median = 475 secs, range = 106 to 765). Similarly, AutoNRT measurements on 5 electrodes was completed in significantly less (p < 0.0001) time with CSPro (median = 169 secs, range = 105 to 442) compared to CS (median = 283 secs, range = 192 to 522). There was no significant difference (p = 0.558) between the time taken to measure tNRT on nine electrodes with CSPro and five with CS. A paired t-test showed no significant difference between tNRT thresholds obtained with CSPro and CS (p = 0.085). shows the box plots for the time taken with CSPro and CS.
Figure 5. Box plots of time taken for AutoNRT with CSPro at 9 electrodes (CSPro-9) and 5 electrodes (CSPro-5) compared to the time taken for AutoNRT with CS at 9 electrodes (CS-9) and 5 electrodes (CS-5). X and adjacent values indicate the average time taken. NS: No significant difference; *: significant difference.
Patient goals
The median number of goals set by clinicians in consultation with the patient was two (range 0 to 3 goals). The median time to discuss and set patient goals was 15 min 38 secs (range 6:13 to 27:51 min:sec). The median time to set a single goal was 8 min 10 secs. When recipients were asked, “To what extent was the goal-setting activity valuable to you?” The majority, 79.5% (35/44) of recipient-participants who responded, rated goal setting as valuable or extremely valuable. Six recipients rated it as neither valuable nor not valuable. When recipient-participants were asked, “Thinking about a few months after your initial switch-on, how valuable do you think the goal-setting activity would have been at that point?” almost all, 88.6% (39/44) recipient-participants rated goal setting as valuable or extremely valuable. Four recipients rated it as neither valuable nor not valuable.
Discussion
CSPro was designed to: 1) provide guidance, 2) help promote PCC, 3) be easy to use, and, 4) be efficient for the clinician managing their recipients.
Guidance
CSPro was designed to provide guidance in the form of a normative population mean data to set C-levels and a guided path for initial activation and a follow-up fitting session. This paper describes the normative data for C-levels based on a large retrospective clinical dataset for three different electrode types: lateral wall, contour, and slim modiolar electrode arrays. This normative data matches published analysis requirements (O’Connor Citation1990) which specify the data be extracted from 1) a large population, 2) of broadly defined patients, 3) in several care/service settings, 4) accounting for age effects, and 5) for epoch effects. van der Beek, Briaire, and Frijns (Citation2015) have reported normative data for Advanced Bionics (California, USA) cochlear implants. However, because of the differences in stimulation and fitting paradigms, these data are also not transferable to guide fitting with devices from other CI manufacturers. Additionally, a limitation of that study was using data from a single site, which could be confounded by the programming practices used within that clinic. In the current study, data were acquired from multiple clinics, thus less likely to be affected by clinic-specific programming practices. Wesarg et al. Citation2010 reported the average C-level profiles from Nucleus CI recipients across 10 centres; however, the C-levels from MAPs using different rates were combined. The same study reports that the C-levels change based on the stimulation rate. Furthermore, the study also restricted the analysis to contour electrode arrays only, as it is well known that the electrode array affects the shape of the C-level profile (Saunders et al. Citation2002, Garaycochea et al., Citation2020); the data are not applicable to users with alternative electrode types. Hence in our study, data from the different electrode types were separately analysed to provide normative data for each electrode type. For obtaining the population mean C-levels, we have used only data from CI recipients 12 years of age or older who are likely to respond reliably, given that earlier studies (Incerti et al. Citation2018) have shown that clinicians tend to use conservative programming practices for young children. Additionally, about 12% of the paediatric population may have neural and structural cochlear lesions (Liddle et al. Citation2022), which might affect the C-levels (Incerti et al. Citation2018). In our analysis for one implant type (CI512), there was a negligible difference between the average C-levels from adult and paediatric CI recipients. Thus, as per the FDA guidance (FDA, 2016), normative data obtained from adults is still applicable to children as the effects of the device, and the course of the disease is similar for all age groups. As with normative data in any field of medicine, the clinician will need to use it as a guide and consider the aetiology, history and other clinical outcome measures holistically in providing individual treatment for their patients. As reported in earlier reports (Saunders et al. Citation2002, van der Beek, Briaire, and Frijns Citation2015), our data show an increase in the C-levels at the basal end of the electrode array; further, we have demonstrated variations in the C-level profile rise that is dependent on the electrode array type. CSPro displays the average C-levels ± 1SD of the population, based on the electrode array type used as a shaded area called the expected range to guide where C-levels are likely to range for 68% of the population. The shape of the MAP profile derived from population statistics has been used to produce a starting profile for the creation of new MAPs in both the Nucleus Fitting Software (NFS) (Botros, Banna, and Maruthurkkara Citation2013) and the Fitting to Outcomes Expert (FOX) system (Govaerts et al., Citation2010). In both NFS and FOX, the same profile is used irrespective of the electrode array type. Our study results show that the C-level profile shape depends on the electrode array type in use. CSPro improves upon previous work and guidance by providing electrode array-specific starting profiles for initial activation. The availability of guidance in the form of an expected range for C-levels can help clinicians prevent underfitting, especially in young children, as reported earlier by Incerti et al. (Citation2018). Nevertheless, clinicians would need to consider the onscreen caution that “Some patients have their C-level above or below this range” and be driven ultimately “by perception rather than by prescription"(Schweitzer, Mortz, and Vaughan Citation1999).
Promoting patient-centred care
CSPro aims to encourage best clinical practice in supporting PCC by including client goals based on the COSI framework. Although all study participants had at least one year of experience with their CI, most participants (79.5%) reported goal setting as a valuable activity reflected with at least one goal being set for almost all (91%) participants. An even greater proportion of recipients (88.6%) felt that goal setting would have been a valuable activity in the first few months after initial activation. Although the participating clinicians were using the integrated goals feature for the first time, they could successfully complete it at first use, demonstrating its ease of use. The median time taken to set goals was 8 min 10 secs, and the median number of goals set for recipients was 2. Thus, goals were set in under 16 minutes in 50% of the sessions, suggesting that this valuable activity can be completed without significantly increasing the session duration.
Ease of use
CSPro was designed to incorporate human factors engineering principles and usability heuristics to improve the software’s ease of use for clinicians. To achieve a minimalistic design that is also flexible and efficient, it was necessary to determine the frequency with which clinicians typically use the MAP parameters. The MAP parameters analysis showed that the default was the most used setting for each parameter. In our analysis of MAPs from adults and children combined, changes to default settings occurred most frequently with PW, maxima and rate. Our findings are similar to an earlier paediatric-only study (Incerti et al. Citation2018), which reports that these parameters are changed based on aetiology and age at implantation. This suggests that these options should be more readily accessible to clinicians in the software. Less frequently adjusted parameters were confirmed as: Frequency table allocation, TSPL, CSPL, and loudness growth settings, and they are also the settings that, when incorrectly set, can cause harm. These settings have been relocated to “manufacture’s settings” in the software menu. The software has been designed based on the assumption that clinicians use their best judgement to select the optimal parameters for recipients. In the future, the software and the fitting process could be further improved if the fitting and selection of parameters are made based on objective outcome measures rather than clinical judgement.
In addition, CSPro introduces new screens to improve the ease of use of some routine fitting tasks. In the clinical evaluation, the clinician-participants, who included clinicians with prior CI fitting experience and new-to-CI clinicians, completed 99% of the tasks in the first session. Most (88%) of the tasks were completed at the first attempt or after some exploration, demonstrating the software’s learnability. In the second session, the clinicians completed the tasks at the first attempt or with greater ease in most tasks (81%). Following a second session using the software, the overall ratings were significantly higher than for the first session across most tasks, demonstrating the software’s memorability.
Efficiency
CSPro introduces several features to improve the efficiency of fitting tasks, for example, automatically measuring impedances, opening the last active MAP, and going on air. CSPro implemented an optimised AutoNRT algorithm that measures tNRTs in significantly less time than previous CS software. New MAPs created with five tNRTs have been shown to provide satisfactory results (Botros, Banna, and Maruthurkkara Citation2013); a recent study (Müller-Deile et al. Citation2021) confirmed this finding and has suggested that the subjective feedback may be improved by using 22 tNRTs. The analysis of tNRTs in the development phase showed that as more tNRTs are measured, the interpolation error, compared to the full 22 tNRT-based profile, reduces. The rate of change of the error reaches a plateau after nine tNRTs. Measuring more than nine tNRTs leads to diminishing returns in error improvement. In 59.31% of cases, the error with nine tNRTs is less than the absolute test-retest error for AutoNRT (4 CL). The AutoNRT algorithm in CSPro now enables the measurement of 9 tNRTs within the same time it took to measure five tNRTs with CS. CSPro can improve the accuracy of the tNRT profile without the loss of efficiency. Combining AutoNRT measurements and global MAP adjustments using CSPro allows a new MAP to be completed for 50% of the recipients in under 15 minutes.
Conclusion
Custom Sound Pro software is designed to provide guidance to clinicians when fitting MAPs for CI recipients through a guided workflow and normative data derived from big data analysis. CSPro supports clinicians in delivering patient-centred care by embedding COSI-based patient goals within the software that is easy to use and has proven to be highly valuable for most recipients. Optimisation of the CSPro user interface, software automation and enhanced AutoNRT, further improve clinical efficiency and patient management capability.
Author contributors
SM was involved in the design of CSPro, study design, ethics submission, data collection, data analysis, and write-up of the draft manuscript. CB was involved in data analysis for the development phase. Both authors reviewed the draft manuscript and provided their approval for submission for publication.
Acknowledgements
The authors thank the CI recipients and clinicians who participated in the study. The authors also thank Bernadette Pickering, Esti Nel, Suzanne Hayley and Toby Cumming for their involvement in the conduct of the study. Thanks to Beejal Vyas-Price, Ben Fernee, Janine Del Dot and Josie Wyss for review of the manuscript. Cochlear Limited sponsored this study.
Disclosure statement
SM and CB are employees of Cochlear Ltd.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
References
- Athalye, S., S. Archbold, I. Mulla, M. Lutman, and T. Nokolopoulous. 2015. “Exploring views on current and future cochlear implant service delivery: the perspectives of users, parents and professionals at cochlear implant centres and in the community.” Cochlear Implants International 16 (5):241–253. doi:10.1179/1754762815Y.0000000003
- Botros, A., R. Banna, and S. Maruthurkkara. 2013. “The next generation of Nucleus(®) fitting: A multiplatform approach towards universal cochlear implant management.” International Journal of Audiology 52 (7):485–494. doi:10.3109/14992027.2013.781277.
- Botros, A., and C. Psarros. 2010. “Neural response telemetry reconsidered: I. The relevance of ECAP threshold profiles and scaled profiles to cochlear implant fitting.” Ear and Hearing 31 (3):367–379. doi:10.1097/AUD.0b013e3181c9fd86.
- Browning, L. M., Y. Nie, A. Rout, and M. Heiner. 2020. “Audiologists’ preferences in programming cochlear implants: A preliminary report.” Cochlear Implants International 21 (4):179–191. doi:10.1080/14670100.2019.1708553.
- Busby, P. A., and K. Arora. 2015. “Effects of Threshold Adjustment on Speech Perception in Nucleus Cochlear Implant Recipients.” Ear and Hearing; 37 (3):303–311. doi:10.1097/AUD.0000000000000248.
- Constand, M. K., J. C. MacDermid, V. Dal Bello-Haas, and M. Law. 2014. “Scoping review of patient-centered care approaches in healthcare.” BMC Health Services Research 14 (1):271–279. doi:10.1186/1472-6963-14-271.
- Dillon, H., A. James, and J. Ginis. 1997. “Client Oriented Scale of Improvement (COSI) and Its Relationship to Several Other Measures of Benefit and Satisfaction Provided by Hearing Aids.” Journal of the American Academy of Audiology 8 (1):27–43. http://www.audiology.org/sites/default/files/journal/JAAA_08_01_04.pdf.
- Evans, R. S. 2016. “Electronic Health Records: Then, Now, and in the Future.” Yearbook of Medical Informatics, 25 (S 01): S48–S61. doi:10.15265/IYS-2016-s006.
- Garaycochea, O., R. Manrique-Huarte, C. Lazaro, A. Huarte, C. Prieto, M. Alvarez de Linera Alperi, and M. Manrique. 2020. “Comparative study of two different perimodiolar and a straight cochlear implant electrode array: surgical and audiological outcomes.” European Archives of Oto-Rhino-Laryngology 277 (1):69–76. doi:10.1007/s00405-019-05680-6.
- Govaerts, P. J., B. Vaerenberg, G. De Ceulaer, K. Daemers, C. De Beukelaer, and K. Schauwers. 2010. “Development of a software tool using deterministic logic for the optimization of cochlear implant processor programming.” Otology & Neurotology 31 (6):908–918. doi:10.1097/MAO.0b013e3181dd160b.
- Hales, B., M. Terblanche, R. Fowler, and W. Sibbald. 2008. “Development of medical checklists for improved quality of patient care.” International Journal for Quality in Health Care 20 (1):22–30. doi:10.1093/intqhc/mzm062.
- Hampton, J. R., M. J. G. Harrison, J. R. A. Mitchell, J. S. Prichard, and C. Seymour. 1975. “Medical Education Relative Contributions of History-taking, Physical Examination, and Laboratory Investigation to Diagnosis and Management of Medical Outpatients,” British Medical Journal. 2 (5969):486–489. doi:10.1136/bmj.2.5969.486
- Hemmingson, C., and J. J. Messersmith. 2018. “Cochlear Implant Practice Patterns: The U.S. Trends with Pediatric Patients.” Journal of the American Academy of Audiology 29 (8):722–733. doi:10.3766/jaaa.17011.
- Henkin, Y., R. Kaplan-Neeman, C. Muchnik, J. Kronenberg, and M. Hildesheimer. 2003. “Changes over time in the psycho-electric parameters in children with cochlear implants.” International Journal of Audiology 42 (5):274–278. doi:10.3109/14992020309078346.
- Hughes, M. L., K. R. Vander Werff, C. J. Brown, P. J. Abbas, D. M. Kelsay, H. F. Teagle, and M. W. Lowder. 2001. “A longitudinal study of electrode impedance, the electrically evoked compound action potential, and behavioral measures in nucleus 24 cochlear implant users.” Ear and Hearing 22 (6):471–486. doi:10.1097/00003446-200112000-00004.
- IDA Institute. n.d. Understanding the Elements of person-centered care. https://learninghall.idainstitute.com/content
- Incerti, P. V., T. Y. C. Ching, S. Hou, P. Van Buynder, C. Flynn, and R. Cowan. 2018. “Programming characteristics of cochlear implants in children: effects of aetiology and age at implantation.” International Journal of Audiology 57 (Sup2):S27–S40. doi:10.1080/14992027.2017.1370139.
- Liddle, K., R. Beswick, E. J. Fitzgibbons, and C. Driscoll. 2022. “Aetiology of permanent childhood hearing loss at a population level.” Journal of Paediatrics and Child Health 58 (3):440–447. doi:10.1111/jpc.15738.
- Müller-Deile, J. 2009. Verfahren zur Anpassung und Evaluation von Cochlear Implant Sprachprozessoren (1st ed.). Heidelberg, Germany: Median Verlag von Killisch-Horn GmbH.
- Müller-Deile, J., N. Neben, N. Dillier, A. Büchner, A. Mewes, F. Junge, W. Lai, M. Schuessler, and M. Hey. 2021. “Comparisons of electrophysiological and psychophysical fitting methods for cochlear implants.” International Journal of Audiology 1–11. doi:10.1080/14992027.2021.2015543.
- Nielsen, J. 1994. “Enhancing the Explanatory Power of Usability Heuristics.” In Proceedings of the SIGCHI conference on Human factors in computing systems, 152–158. Boston, MA: ACM Press. doi:10.1145/191666.191729.
- O’Connor, P. J. 1990. “Normative data: their definition, interpretation, and importance for primary care physicians.” Family Medicine 22 (4):307–311. http://www.ncbi.nlm.nih.gov/pubmed/2200734.
- Saunders, E., L. Cohen, A. Aschendorff, W. Shapiro, M. Knight, M. Stecker, B. Richter, S. Waltzman, M. Tykocinski, T. Roland, et al. 2002. “Threshold, comfortable level and impedance changes as a function of electrode-modiolar distance.” Ear and Hearing 23 (1 Suppl):28–40. doi:10.1097/00003446-200202001-00004.
- Schweitzer, C., M. Mortz, and N. Vaughan. 1999. “Perhaps not by prescription, but by perception.” Hearing Journal 3:58–62.
- Skinner, M. W., L. K. Holden, T. A. Holden, and M. E. Demorest. 2000. “Effect of stimulation rate on cochlear implant recipients’ thresholds and maximum acceptable loudness levels.” Journal of the American Academy of Audiology 11 (4):203–213. http://www.ncbi.nlm.nih.gov/pubmed/10783923. doi:10.1055/s-0042-1748046.
- Vaerenberg, B., P. J. Govaerts, T. Stainsby, P. Nopp, A. Gault, and D. Gnansia. 2014. “A Uniform Graphical Representation of Intensity Coding in Current-Generation Cochlear Implant Systems.” Ear and Hearing 3:1. doi:10.1097/AUD.0000000000000039.
- Vaerenberg, B., C. Smits, G. De Ceulaer, E. Zir, S. Harman, N. Jaspers, Y. Tam, M. Dillon, T. Wesarg, D. Martin-Bonniot, et al. 2014. “Cochlear implant programming: a global survey on the state of the art.” TheScientificWorldJournal 2014:501738. 2014(August 2013), doi:10.1155/2014/501738.
- van der Beek, F. B., J. J. Briaire, and J. H. M. Frijns. 2015. “Population-Based Prediction of Fitting Levels for Individual Cochlear Implant Recipients.” Audiology & Neuro-Otology 20 (1):1–16. doi:10.1159/000362779.
- Wathour, J., P. J. Govaerts, and N. Deggouj. 2021. “Variability of fitting parameters across cochlear implant centres.” European Archives of Oto-Rhino-Laryngology 278 (12):4671–4679. doi:10.1007/s00405-020-06572-w.
- Wesarg, T., R. Battmer, L. Cavall, N. Dillier, L. Garcia-Ib, M. Hey, A. H. Irujo, A. Morsnowski, F. Erwin, and F. Guido. 2010. Effect of changing pulse rate on profile parameters of perceptual thresholds and loudness comfort levels and relation to ECAP thresholds in recipients of the Nucleus CI24RE device. 775–787. doi:10.3109/14992027.2010.4924.
- Willeboer, C. 2008. Simplifying cochlear implant speech processor fitting. Veenendaal, Netherlands: Universal Press.
- United States Department of Health and Human Services (US HHS). Guidance regarding methods for de-identification of protected health information in accordance with the Health Insurance Portability and Accountability Act (HIPAA) Privacy Rule. HHS.gov. https://www.hhs.gov/hipaa/for-professionals/privacy/special-topics/de-identification/index.html
- WHO 2021. World report on hearing. Geneva: World Health Organization. https://www.who.int/publications/i/item/world-report-on-hearing